Abstract
Intense activation of skeletal muscle results in fatigue development, which involves impaired function of the muscle cells resulting in weaker and slower contractions. Intense muscle activity also results in increased heat production and muscle temperature may rise by up to ∼6°C. Hyperthermia is associated with impaired exercise performance in vivo and recent studies have shown contractile dysfunction and premature fatigue development in easily fatigued muscle fibres stimulated at high temperatures and these defects were attributed to oxidative stress. Here we studied whether fatigue-resistant soleus fibres stimulated at increased temperature show premature fatigue development and whether increasing the level of oxidative stress accelerates fatigue development. Intact single fibres or small bundles of soleus fibres were fatigued by 600 ms tetani given at 2 s intervals at 37°C and 43°C, which is the highest temperature the muscle would experience in vivo. Tetanic force in the unfatigued state was not significantly different at the two temperatures. With 100 fatiguing tetani, force decreased by ∼15% at both temperatures; the free cytosolic [Ca2+] (assessed with indo-1) showed a similar ∼10% decrease at both temperatures. The oxidative stress during fatigue at 43°C was increased by application of 10 μm hydrogen peroxide or tert-butyl hydroperoxide and this did not cause premature fatigue development. In summary, fatigue-resistant muscle fibres do not display impaired contractility and fatigue resistance at the highest temperature that mammals, including humans, would experience in vivo. Thus, intrinsic defects in fatigue-resistant muscle fibres cannot explain the decreased physical performance at high temperatures.
Muscle contractions result in an increased muscle metabolism and endogenous heat production. Indeed, exercise has been shown to induce muscle temperature to rise by 3–5°C to attain values as high as 41°C in human vastus lateralis muscle (Saltin et al. 1972; Gonzalez-Alonso et al. 1999; Nybo & Nielsen, 2001) and up to 44°C in rat thigh muscle after exhaustive exercise (Brooks et al. 1971). During repeated or prolonged muscle contractions, there is a reduction in force production, i.e. fatigue develops (for a review see Allen et al. 2008b). In vitro studies of intracellular fatigue mechanisms have often been performed at room temperatures and fatigue properties may be different at higher physiological temperatures of mammalian muscle. For example, it has been shown that while acidosis has large negative effects on contractile function at low temperatures, the effects are limited at higher, more physiological temperatures (Ranatunga, 1987; Pate et al. 1995; Westerblad et al. 1997). Similarly, the depressant effects of inorganic phosphate on force production are considerably less at near-physiological temperatures than at lower temperatures (Coupland et al. 2001).
Numerous studies have shown oxidative stress and an increased production of reactive oxygen and nitrogen species (ROS) during physical exercise both in humans and animal models and this has been associated with impaired contractile function (for recent review see Powers & Jackson, 2008). An important functional role of ROS during exercise is supported by recent studies showing that endurance training results in an improved endogenous ROS defence in muscle (Brooks et al. 2008; Ristow et al. 2009). Recently, premature fatigue development was observed when intact single fibres of mouse flexor digitorum brevis (FDB) muscles were fatigued at 37°C (Moopanar & Allen, 2005, 2006), which is about 6°C above the in situ temperature of this muscle (Bruton et al. 1998). Moreover, a marked force depression has been shown in skinned extensor digitorum longus fibres at a temperature about 6°C above the normal in situ temperature (van der Poel & Stephenson, 2002). These deleterious effects at high temperature were prevented by treatment with antioxidant and therefore they were attributed to an increased ROS production (van der Poel & Stephenson, 2002; Moopanar & Allen, 2005). However, it should be noted that not all in vitro studies show premature fatigue development at higher temperatures (Cifelli et al. 2007; Roots et al. 2009).
We have now studied contractile consequences of increased temperature in isolated fibres of mouse soleus muscle, which is a deeply located and tonically active calf muscle composed of fatigue resistant type I and type IIa fibres (Hennig & Lømo, 1985; Marechal & Beckers-Bleukx, 1993). Contracting muscles generate heat and the soleus muscle is intensely activated during leg exercise (e.g. evidenced by impaired excitability after prolonged running exercise; Racinais et al. 2007) and hence it can experience markedly increased temperatures in vivo. We hypothesized (1) that fatigue would occur more rapidly at increased (43°C) than at normal (37°C) temperature and (2) that the early fatigue development is due to oxidative stress resulting in decreased myofibrillar Ca2+ sensitivity, which is a process highly sensitive to ROS in mouse muscle fibres (Andrade et al. 2001; Moopanar & Allen, 2005). To test these hypotheses, we simultaneously measured force and the free cytosolic [Ca2+] ([Ca2+]i) during fatigue induced by repeated tetanic stimulation. Unexpectedly, the results show no premature fatigue in soleus fibres at increased temperature. We then performed additional experiments at increased temperature where the oxidative stress was increased by application of peroxides, but the force decrease during fatiguing stimulation was still not accelerated.
Methods
Ethical approval
The studies were approved by the Stockholm North local ethical committee. Adult male mice (NMRI; weight 30–38 g) were housed at room temperature and fed ad libitum. Animals were killed by rapid neck disarticulation and muscles were thereafter removed.
Isolation of muscle preparations
Intact single fibres or bundles consisting of up to four muscle fibres were dissected from isolated soleus muscles as previously described (Bruton et al. 2003). Fibres were mounted horizontally between an adjustable hook and an Akers AE801 force transducer (resonance frequency 3.5 kHz) as close as possible to the thin glass coverslip bottom of the perfusion channel of a muscle bath. Fibres were flanked by platinum plate electrodes, which were used to stimulate the fibres with supramaximal current pulses (duration 0.5 ms). The preparations were stretched to the length at which maximum tetanic force was obtained. Contractions were evoked using stimulus trains with a duration of 600 ms. A control force–frequency (10–120 Hz) curve was obtained by stimulating the fibres at 1 min intervals. Fibres were then stimulated every 2 s with 100 tetani at 100 Hz. In a few experiments, fibres were subjected to two fatigue runs separated by 60 min. Additional experiments were performed on easily fatigued fibres from FDB muscles, which are mainly composed of IIa and IIx fibres (Allen et al. 1993; Gonzalez et al. 2003). FDB fibres were studied using the same protocol as for soleus fibres with the exception that tetanic duration was 350 ms and fatiguing stimulation continued until force was decreased to 40% of the original. Biochemical fibre typing of the cells used in the present study was not performed.
Solutions
Muscle fibres were superfused with a Tyrode solution containing (mm): NaCl 121, KCl 5, MgCl2 0.5, Na2HPO4 0.4, CaCl2 1.8, EDTA 0.1 (to chelate trace amounts of metal ions), NaHCO3 24 and glucose 5.5; and also fetal calf serum (0.2%, Gibco). This solution was bubbled with 95% O2–5% CO2 (pH 7.4). The temperature of the solution flowing through the muscle bath was kept constant by passing it through the inner glass coil of a heated Graham condenser. The temperature of the bath solution was routinely measured in front of the hook furthest from the solution inflow. The temperatures used were 37°C and 43°C for soleus fibres and 31°C and 37°C for FDB fibres, which reflects the in vivo temperatures at rest and the temperatures that could be reached during intense exercise in these muscles (Brooks et al. 1971; Saltin et al. 1972; Bruton et al. 1998; Gonzalez-Alonso et al. 1999; Nybo & Nielsen, 2001; Zhang et al. 2006a). Fibres were equilibrated at each temperature for up to 30 min before the start of force measurement. In some experiments at 43°C, soleus fibres were exposed to 10 μm hydrogen peroxide (H2O2) or 10 μm tert-butyl hydroperoxide (t-BOOH) for 5 min and then fatigued.
Measurement of myoplasmic [Ca2+]i
Single dissected soleus fibres were pressure-injected with the fluorescent indicator indo-1 in order to measure [Ca2+]i. Following injection of the dye, fibres were left for a further 45 min before any measurements were made. The dye was excited with light at 360 ± 5 nm, and the light emitted at 405 ± 5 and 495 ± 5 nm was measured with two photomultiplier tubes. The fluorescence ratio of the light emitted at 405 nm to that emitted at 495 nm (R) is monotonically related to [Ca2+]i according the following equation (Grynkiewicz et al. 1985):
where KD is the apparent dissociation constant of indo-1, β is the ratio of the 495 nm signals at very low and saturating [Ca2+]i, Rmin and Rmax are the ratios at very low and saturating [Ca2+]i, respectively. β, Rmin and Rmax vary between experimental set-ups (e.g. due to light source, filters and detection devices) and they are not the same in simple salt solutions and in the intracellular environment. KD should not depend on the experimental set-up but is affected by the intracellular environment. We have not performed intracellular calibration experiments at the different temperatures used in the present study and therefore data are presented as changes in R. One potential problem with using indo-1 to measure tetanic [Ca2+]i is that it is a high Ca2+ affinity indicator with a KD of ∼300 nm in skeletal muscle cells (Andrade et al. 1998) and the tetanic R might then be close to Rmax. If this were the case, minor changes in R would reflect large changes in [Ca2+]i and hence changes in [Ca2+]i during fatigue would be underestimated. However, increasing the stimulation frequency in unfatigued fibres from 100 to 120 Hz resulted in a significant increase in the tetanic R by 7 ± 2% at 37°C and 10 ± 2% at 43°C (n= 5, P < 0.05). Thus, indo-1 was not close to Ca2+ saturation during the 100 Hz tetani used to induce fatigue and relative changes in tetanic R would then translate to similar relative changes in [Ca2+]i.
Statistics
Values are expressed as means ±s.e.m. Two-way repeated measures ANOVA (SigmaStat 3.11, Systat Software) was used when comparing repeated measurements (time or frequency and temperature) and when this showed a significant difference, the Holm–Sidak post hoc test was performed. Student's paired or unpaired t tests were also used when appropriate. P < 0.05 was considered to indicate statistical significance.
Results
Force and [Ca2+]i in unfatigued muscle fibres
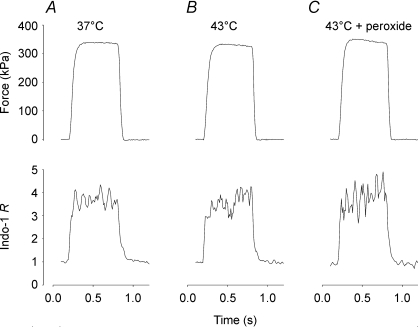
Figure 1 shows that increasing the temperature from 37°C (A) to 43°C (B) had little effect on the amplitude of force and indo-1 R (reflecting [Ca2+]i) during 100 Hz tetani produced in an unfatigued soleus fibres. Mean data show no significant difference between force in 100 Hz tetani at 37°C (323 ± 32 kPa, n= 4) and 43°C (373 ± 26 kPa, n= 5) (P > 0.05). Increasing the temperature from 37 to 43°C did not increase resting force in any of the fibres tested. The indo-1 R during 100 Hz tetani was similar at 37°C (3.14 ± 0.32, n= 7) and 43°C (2.60 ± 0.27, n= 7) (P > 0.05).
Figure 1. Increasing the temperature from 37 to 43°C had little effect on tetanic force and [Ca2+]i.
Original force (upper part) and indo-1 R (reflecting [Ca2+]i; lower part) records from 100 Hz tetani produced in a soleus fibre at 37°C (A) and subsequently at 43°C (B). The fibre was finally exposed to H2O2 (10 μm) for 5 min (C).
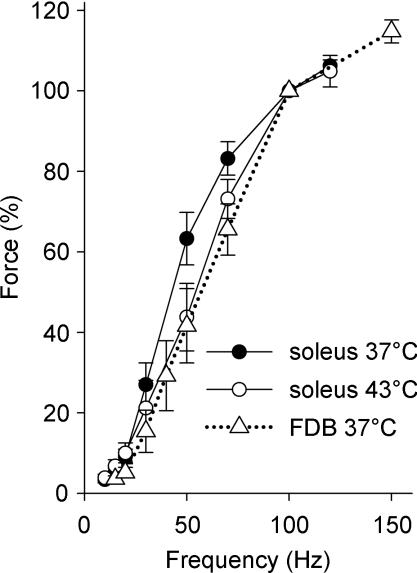
The force–frequency relationship of soleus fibres was shifted to higher frequencies at 43°C as compared to 37°C (Fig. 2) and the mean force at 50 Hz stimulation was 30% lower at 43°C than at 37°C (P < 0.05). For comparison, Fig. 2 also shows force–frequency data of FDB fibres at 37°C (15–150 Hz) and these are similar to those for soleus at 43°C. Mean values of the half-contraction time (51 ± 6 vs. 46 ± 5 ms, n= 8–9) and half-relaxation time (40 ± 6 vs. 27 ± 2 ms) were longer at 37°C than at 43°C, although the differences were not statistically significant (P > 0.05). Taken together, the contractile speed of soleus fibres increased slightly with increasing temperature.
Figure 2. The force–frequency relationship was shifted to higher frequencies in soleus fibres at 43°C as compared to 37°C.
Mean (±s.e.m.) data from soleus fibres stimulated at 37°C (•, n= 8) and 43°C (○, n= 7). For comparison, data from FDB fibres at 37°C are also included (▵, n= 11, dotted line). The force at 100 Hz was set to 100% in each fibre.
Figure 1C shows representative tetanic force and indo-1 R records obtained in a soleus fibre exposed to 10 μm H2O2 for 5 min at 43°C. Mean data from experiments with exposure to 10 μm H2O2 or t-BOOH show no change in 100 Hz tetanic force as compared to before peroxide application (6.4 ± 3.1%, n= 10, P > 0.05). Furthermore, the rate of contraction and relaxation was not changed by peroxide application: half-contraction time −0.5 ± 5.3% and half-relaxation time −4.9 ± 6.2% of that before peroxide application (P > 0.05). We measured [Ca2+]i in the experiment depicted in Fig. 1 and in one more experiment and observed no consistent effect of peroxide application on the indo-1 R during 100 Hz tetani (8% increase in one fibre and 8% decrease in the other as compared to before peroxide application). [Ca2+]i was not measured in the other experiments where peroxide was applied.
In accordance with the results obtained in soleus fibres, FDB fibres showed no temperature-dependent difference in the force produced during 100 Hz tetani at 31°C (347 ± 38 kPa, n= 10) and 37°C (346 ± 32 kPa, n= 11).
Force and [Ca2+]i during fatigue at normal and elevated temperature
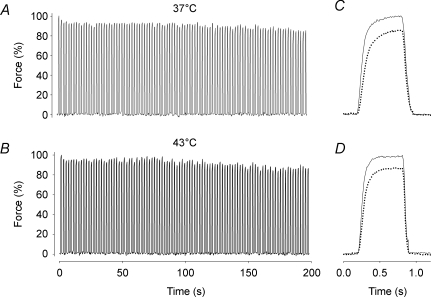
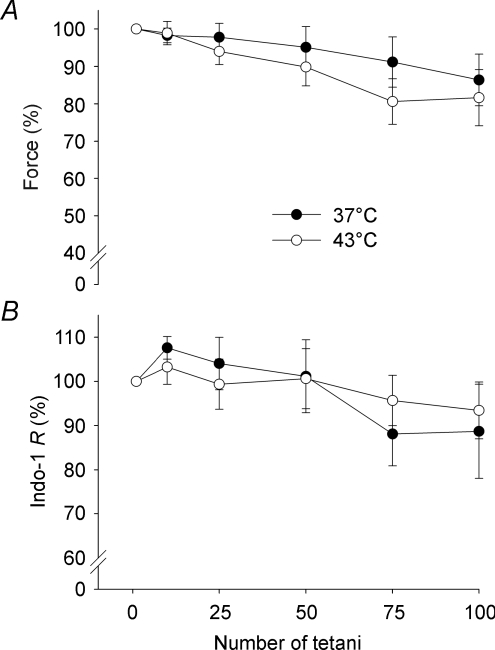
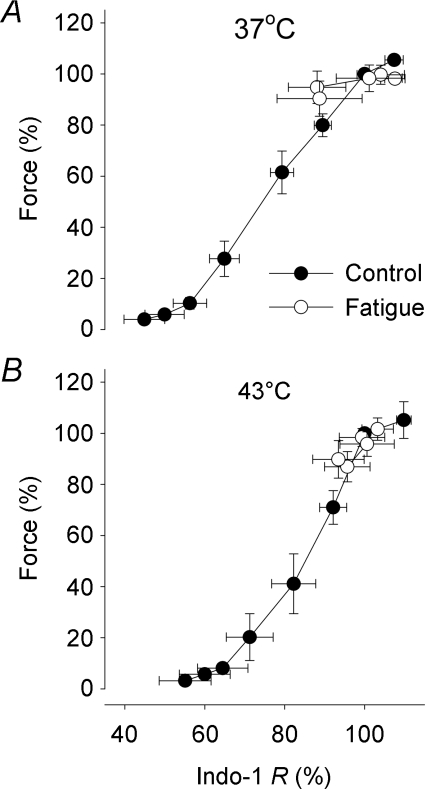
Figure 3 shows representative continuous force records obtained during the series of 100 tetani. There was only a minor force decrease during the series of contractions both at 37°C (Fig. 3A) and 43°C (Fig. 3B). Typical records for the first and last tetani in the series of 100 tetani at 37°C and 43°C are superimposed in Fig. 3C and D, respectively. At both temperatures the last tetanus showed a minor (∼15%) force decrease and no change in the time course of contraction and relaxation. Figure 4A shows that the decrease in force over the series of 100 tetani was similar at 37°C (14 ± 7%) and 43°C (18 ± 8%). The mean indo-1 R was also slightly decreased at the end of fatiguing stimulation, but again there was no significant difference between 37°C (11 ± 10%) and 43°C (7 ± 6%) (Fig. 4B). Force vs. indo-1 R plots were constructed to investigate the relation between force and [Ca2+]i during fatigue (Fig. 5). The results show a similar relationship between tetanic force and indo-1 R under control conditions and during fatigue both at 37 and 43°C.
Figure 3. Tetanic force was well maintained in intact soleus fibres during fatiguing stimulation at 37 and 43°C.
Typical continuous force records from intact single soleus fibres fatigued by 100 Hz, 600 ms tetanic contractions repeated every 2 s at 37°C (A) and 43°C (B). Superimposed force records on an expanded time axis from the first (continuous line) and last (dotted line) tetani of the fatigue run at 37°C (C) and 43°C (D). Force in the first tetanus set to 100%.
Figure 4. Force and [Ca2+]i showed little change during fatiguing stimulation at both 37°C and 43°C.
Mean (±s.e.m.) data of relative force (A; n= 7–9) and indo-1 R (reflecting [Ca2+]i) (B; n= 5–6) measured during the 1st, 10th, 25th, 50th, 75th and 100th contraction of the fatigue protocol. •, 37°C; ○, 43°C. Data are expressed relative to the first tetanus, which was set to 100% in each muscle fibre.
Figure 5. Force–[Ca2+]i relationship during fatiguing stimulation of soleus fibres was similar to that under control conditions at both 37°C and 43°C.
Mean (±s.e.m.) data of relative force plotted against relative indo-1 R obtained under control conditions (•) and during fatigue (○) at 37°C (A) and 43°C (B). Data are expressed relative to the first tetanus, which was set to 100% in each muscle fibre (n≥ 5).
Additional experiments were performed on the more easily fatigued FDB fibres and again no accelerated fatigue development was observed at increased temperature. Thus, the number of tetani required to decrease force to 40% of initial was 70 ± 25 at 31°C (n= 18) and 94 ± 23 at 37°C (n= 21) (P > 0.05).
Force and [Ca2+]i during fatigue of soleus fibres at 43°C in the presence of peroxides
Some soleus fibres were fatigued at 43°C under increased oxidative stress, i.e. with 10 μm peroxide added to the Tyrode solution (H2O2 for four fibres and t-BOOH for five fibres). The changes in force during fatigue runs in the presence of 10 μm peroxide were similar to those observed at 37°C and 43°C in standard Tyrode solution (Fig. 6). [Ca2+]i was measured in one soleus fibre exposed to 10 μm H2O2 during fatigue and the indo-1 R was decreased by 8% after 100 tetani, which is very similar to the decrease observed in fibres fatigued at 37°C and at 43°C without peroxide (see Fig. 4B). Interestingly, all fibres exposed to 10 μmt-BOOH went into a contracture and died about 10 min after fatiguing stimulation, even when t-BOOH was washed out immediately after the series of 100 tetani. This was never seen in fibres exposed to the standard Tyrode solution. In 10 μm H2O2, fibres could still contract 30 min after fatiguing stimulation. When the H2O2 concentration was increased to 100 μm, fibres developed a contracture and died within 3–6 min.
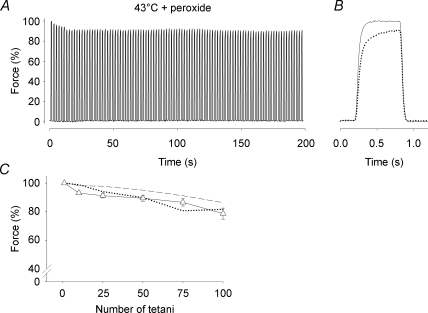
Figure 6. Tetanic force was well maintained in intact soleus fibres during fatiguing stimulation at 43°C in the presence of peroxide.
A, typical continuous force records from a soleus fibre fatigued by 100 Hz, 600 ms tetanic contractions repeated every 2 s at 43°C in the presence of 10 μm H2O2. Force is expressed relative to the first tetanus, which was set to 100%. B, superimposed force records on an expanded time axis from the first (continuous line) and last (dotted line) tetani of the fatigue run. C, mean (±s.e.m.) data of relative force measured during the 1st, 10th, 25th, 50th, 75th and 100th fatiguing tetani at 43°C in the presence of 10 μm H2O2 or t-BOOH (Δ, n= 9). For comparison, mean data from soleus fibres fatigued at 37°C (dashed line) and 43°C (dotted line) in the absence of peroxide are also shown (data from Fig. 2A).
Discussion
The main purpose of the present study was to test whether fatigue-resistant soleus fibres display premature fatigue development when stimulated at the highest temperature they might experience in vivo. Our major novel result shows no difference in fatigability between the normal and the high temperature, and premature fatigue at the high temperature did not occur even after application of 10 μm peroxide.
In the present study, we used intact single fibres or small fibre bundles to investigate the mechanisms of fatigue at temperatures measured during exercise in vivo (Brooks et al. 1971; Saltin et al. 1972; Gonzalez-Alonso et al. 1999). A reason for using these preparations rather than isolated whole muscle is that the latter preparation has been shown to be problematic as hypoxia speeds up fatigue development (Zhang et al. 2006b), especially at temperatures above 25°C (Segal & Faulkner, 1985; Barclay, 2005). Before fatigue, the absolute force was not different between normal (37°C) and high physiological temperatures (43°C), which allowed a direct comparison between the fatigability at the two temperatures. The soleus fibres showed no signs of premature fatigue at either 37 or 43°C, with a decrease in force and [Ca2+]i (i.e. indo-1 R) of only ∼10–15% at both temperatures, which is similar to previous results at 24–26°C (Bruton et al. 2003; Zhang et al. 2006b). Thus, we observed no signs of premature fatigue at high temperatures in fatigue-resistant soleus muscle fibres.
A numbers of studies have shown increased ROS production in skeletal muscle between room and physiological temperatures or between normal and high physiological temperature (37–43°C) (Zuo et al. 2000; van der Poel & Stephenson, 2002; Arbogast & Reid, 2004; Edwards et al. 2007), although there is at least one study where this was not observed (Kolbeck et al. 1997). In fast-twitch extensor digitorum longus fibres the increased ROS production at high temperature was accompanied by a reduction in tetanic force, which was counteracted by application of antioxidant or reducing agents (van der Poel & Stephenson, 2002; Edwards et al. 2007). Conversely, a force depression at high temperature, which was not prevented by antioxidants or reversed by reducing agents, has been described in slow-twitch peroneus longus fibres and strips of diaphragm muscles (van der Poel & Stephenson, 2002; Oliver et al. 2008). In the present study, we observed no decrease in tetanic force in soleus fibres when the temperature was increased from 37 to 43°C. Furthermore, tetanic force was not decreased in FDB when the temperature was increased from 31 to 37°C. Thus, intact soleus and FDB fibres can tolerate a temperature ∼6°C above the in situ temperature without displaying any decrease in tetanic force production.
We also increased the level of oxidative stress at 43°C by adding 10 μm peroxide to the bath solution. Peroxides were used because they readily pass through the cell membrane and they were added in the naturally occurring form (H2O2) and in a form that cannot (t-BOOH) be metabolized by cells. While addition of peroxides results in an increased amount of oxidants in the muscle cells, it differs from the endogenous increase in ROS production during exercise where mainly superoxide ions are formed (Powers & Jackson, 2008). A major portion of the superoxide ions are subsequently converted to H2O2 via superoxide dismutases or spontaneously, but the highly reactive molecules hydroxyl radical and peroxynitrite may also be formed. Our results show no change in tetanic force or contractile speed of soleus fibres during 5 min of exposure to peroxides at 43°C in the unfatigued state. Moreover, when fatigued at 43°C in the presence of 10 μm peroxide, soleus fibres displayed only a minor force decrease which was very similar to that observed at 37 and 43°C in the absence of added peroxides. Thus, the increase in oxidative stress induced by acute exposure to peroxides does not adversely affect the contractile function of soleus fibres either in the unfatigued state or during fatiguing stimulation. Nevertheless, we observed deleterious effects of peroxides but these only occurred after prolonged exposure and fatiguing stimulation: 10 μmt-BOOH induced cell death and increasing the H2O2 to 100 μm also caused cell death. Furthermore, while it might be suggested that an effect of peroxides on sarcoplasmic reticulum (SR) Ca2+ handling was counteracted by an effect on the myofibrils that resulted in unchanged force production before and during fatigue, the results of our experiments with [Ca2+]i measurements during peroxide exposure (n= 2) indicate that this is unlikely to be the case.
Studies mainly performed on easily fatigued muscle have shown three principal components underlying the decrease in force during fatigue: (i) decreased ability of cross-bridges to generate force, (ii) decreased myofibrillar Ca2+ sensitivity, and (iii) decreased Ca2+ release from the SR (Allen et al. 1995). Force–[Ca2+]i plots can be used to distinguish between these factors since (i) and (ii) shift the force–[Ca2+]i relationship downwards and to the right, respectively. In the present study we analysed the force decrease in soleus fibres during fatigue at 37 and 43°C in this respect and found that data points obtained during fatigue fell along the force–[Ca2+]i relationship of unfatigued fibres. Thus, there were no signs of impaired myofibrillar function, i.e. factors (i) and (ii) above. By exclusion the force decrease was then due to decreased SR Ca2+ release. This might in turn be due to: impaired action potential transmission along the surface membrane or in the t-tubules; impaired function of the t-tubular voltage sensors, the dihydropyridine receptors, or the SR Ca2+ release channels, the ryanodine receptors, or the coupling between these two proteins (Allen et al. 2008a). In the present study we did not attempt to distinguish between these possible mechanisms.
We also performed experiments on easily fatigued FDB fibres and observed no accelerated fatigue development at high (37°C) temperature, which is in accordance with recent results from other laboratories (Cifelli et al. 2007; Roots et al. 2009). However, it is in contrast to results obtained in mouse FDB fibres with an experimental design similar to that of the present study, where rapid fatigue development was observed at 37°C (Moopanar & Allen, 2005, 2006). However, a very recent study from that same laboratory shows that the early fatigue development in FDB fibres at 37°C was due to iron ions leaking out of a stainless steel heat exchanger and did not occur when an aluminium heat exchanger was used (Reardon & Allen, 2009). Thus, recent studies do not support the idea of accelerated fatigue development at high temperature in isolated fast-twitch, easily fatigued fibres.
Conclusion and implications for human exercise
Several studies have reported that hyperthermia impairs performance of prolonged isolated (Clarke et al. 1958; Nybo & Nielsen, 2001; Todd et al. 2005) or global (Gonzalez-Alonso et al. 1999; Drust et al. 2005) exercise in humans. Our results show that the function of isolated intact muscle fibres is preserved at high temperatures. Therefore, in our opinion the reduction in performance during exercise performed at high temperature in vivo is not due to factors intrinsic to the muscle fibres. Instead, altered function of the central nervous system appears to have a central role in the impairment of exercise performance at high temperature (Nybo & Nielsen, 2001; Todd et al. 2005; Nybo, 2008).
Acknowledgments
This work was supported by the Swedish Research Council, the Swedish National Center for Sports Research, and funds from the Karolinska Institute.
Glossary
Abbreviations
- [Ca2+]i
free cytosolic Ca2+ concentration
- FDB
flexor digitorum brevis
- ROS
reactive oxygen and nitrogen species
- SR
sarcoplasmic reticulum
- t-BOOH
tert-butyl hydroperoxide
Author contributions
N.P., H.W. and J.D.B. contributed to the conception and design of the study. All authors were involved in the analysis and interpretation of data and in drafting and revising the manuscript. All authors approved the published version of the manuscript. Experiments were performed at the Karolinska Institute.
References
- Allen DG, Duty S, Westerblad H. Metabolic changes in muscle during exercise; their effects on muscle function. Proc Aust Physiol Pharmacol Soc. 1993;24:65–75. [Google Scholar]
- Allen DG, Lamb GD, Westerblad H. Impaired calcium release during fatigue. J Appl Physiol. 2008a;104:296–305. doi: 10.1152/japplphysiol.00908.2007. [DOI] [PubMed] [Google Scholar]
- Allen DG, Lamb GD, Westerblad H. Skeletal muscle fatigue: cellular mechanisms. Physiol Rev. 2008b;88:287–332. doi: 10.1152/physrev.00015.2007. [DOI] [PubMed] [Google Scholar]
- Allen DG, Lännergren J, Westerblad H. Muscle cell function during prolonged activity: cellular mechanisms of fatigue. Exp Physiol. 1995;80:497–527. doi: 10.1113/expphysiol.1995.sp003864. [DOI] [PubMed] [Google Scholar]
- Andrade FH, Reid MB, Allen DG, Westerblad H. Effect of hydrogen peroxide and dithiothreitol on contractile function of single skeletal muscle fibres from the mouse. J Physiol. 1998;509:565–575. doi: 10.1111/j.1469-7793.1998.565bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade FH, Reid MB, Westerblad H. Contractile response of skeletal muscle to low peroxide concentrations: myofibrillar calcium sensitivity as a likely target for redox-modulation. FASEB J. 2001;15:309–311. doi: 10.1096/fj.00-0507fje. [DOI] [PubMed] [Google Scholar]
- Arbogast S, Reid MB. Oxidant activity in skeletal muscle fibres is influenced by temperature, CO2 level, and muscle-derived nitric oxide. Am J Physiol Regul Integr Comp Physiol. 2004;287:R698–R705. doi: 10.1152/ajpregu.00072.2004. [DOI] [PubMed] [Google Scholar]
- Barclay CJ. Modelling diffusive O2 supply of isolated preparations of mammalian skeletal and cardiac muscle. J Muscle Res Cell Motil. 2005;26:225–235. doi: 10.1007/s10974-005-9013-x. [DOI] [PubMed] [Google Scholar]
- Brooks GA, Hittelman KJ, Faulkner JA, Beyer RE. Tissue temperatures and whole-animal oxygen consumption after exercise. Am J Physiol. 1971;221:427–431. doi: 10.1152/ajplegacy.1971.221.2.427. [DOI] [PubMed] [Google Scholar]
- Brooks SV, Vasilaki A, Larkin LM, McArdle A, Jackson MJ. Repeated bouts of aerobic exercise lead to reductions in skeletal muscle free radical generation and nuclear factor κB activation. J Physiol. 2008;586:3979–3990. doi: 10.1113/jphysiol.2008.155382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruton JD, Lännergren J, Westerblad H. Effects of CO2-induced acidification on the fatigue resistance of single mouse muscle fibres at 28°C. J Appl Physiol. 1998;85:478–483. doi: 10.1152/jappl.1998.85.2.478. [DOI] [PubMed] [Google Scholar]
- Bruton JD, Tavi P, Aydin J, Westerblad H, Lännergren J. Mitochondrial and myoplasmic Ca2+ in single fibres from mouse limb muscles during repeated tetanic contractions. J Physiol. 2003;551:179–190. doi: 10.1113/jphysiol.2003.043927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cifelli C, Bourassa F, Gariepy L, Banas K, Benkhalti M, Renaud JM. KATP channel deficiency in mouse flexor digitorum brevis causes fibre damage and impairs Ca2+ release and force development during fatigue in vitro. J Physiol. 2007;582:843–857. doi: 10.1113/jphysiol.2007.130955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke R, Hellon R, Lind AR. The duration of sustained contractions of the human forearm at different muscle temperatures. J Physiol. 1958;143:454–473. doi: 10.1113/jphysiol.1958.sp006071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coupland ME, Puchert E, Ranatunga KW. Temperature dependence of active tension in mammalian (rabbit psoas) muscle fibres: effect of inorganic phosphate. J Physiol. 2001;536:879–891. doi: 10.1111/j.1469-7793.2001.00879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drust B, Rasmussen P, Mohr M, Nielsen B, Nybo L. Elevations in core and muscle temperature impairs repeated sprint performance. Acta Physiol Scand. 2005;183:181–190. doi: 10.1111/j.1365-201X.2004.01390.x. [DOI] [PubMed] [Google Scholar]
- Edwards JN, Macdonald WA, van der Poel C, Stephenson DG. O2*− production at 37°C plays a critical role in depressing tetanic force of isolated rat and mouse skeletal muscle. Am J Physiol Cell Physiol. 2007;293:C650–C660. doi: 10.1152/ajpcell.00037.2007. [DOI] [PubMed] [Google Scholar]
- Gonzalez E, Messi ML, Zheng Z, Delbono O. Insulin-like growth factor-1 prevents age-related decrease in specific force and intracellular Ca2+ in single intact muscle fibres from transgenic mice. J Physiol. 2003;552:833–844. doi: 10.1113/jphysiol.2003.048165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Alonso J, Teller C, Andersen SL, Jensen FB, Hyldig T, Nielsen B. Influence of body temperature on the development of fatigue during prolonged exercise in the heat. J Appl Physiol. 1999;86:1032–1039. doi: 10.1152/jappl.1999.86.3.1032. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Hennig R, Lømo T. Firing patterns of motor units in normal rats. Nature. 1985;314:164–166. doi: 10.1038/314164a0. [DOI] [PubMed] [Google Scholar]
- Kolbeck RC, She ZW, Callahan LA, Nosek TM. Increased superoxide production during fatigue in the perfused rat diaphragm. Am J Respir Crit Care Med. 1997;156:140–145. doi: 10.1164/ajrccm.156.1.9610041. [DOI] [PubMed] [Google Scholar]
- Marechal G, Beckers-Bleukx G. Force-velocity relation and isomyosins in soleus muscles from two strains of mice (C57 and NMRI) Pflügers Arch. 1993;424:478–487. doi: 10.1007/BF00374911. [DOI] [PubMed] [Google Scholar]
- Moopanar TR, Allen DG. Reactive oxygen species reduce myofibrillar Ca2+ sensitivity in fatiguing mouse skeletal muscle at 37°C. J Physiol. 2005;564:189–199. doi: 10.1113/jphysiol.2005.083519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moopanar TR, Allen DG. The activity-induced reduction of myofibrillar Ca2+ sensitivity in mouse skeletal muscle is reversed by dithiothreitol. J Physiol. 2006;571:191–200. doi: 10.1113/jphysiol.2005.101105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nybo L. Hyperthermia and fatigue. J Appl Physiol. 2008;104:871–878. doi: 10.1152/japplphysiol.00910.2007. [DOI] [PubMed] [Google Scholar]
- Nybo L, Nielsen B. Hyperthermia and central fatigue during prolonged exercise in humans. J Appl Physiol. 2001;91:1055–1060. doi: 10.1152/jappl.2001.91.3.1055. [DOI] [PubMed] [Google Scholar]
- Oliver SR, Wright VP, Parinandi N, Clanton TL. Thermal tolerance of contractile function in oxidative skeletal muscle: no protection by antioxidants and reduced tolerance with eicosanoid enzyme inhibition. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1695–R1705. doi: 10.1152/ajpregu.90429.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pate E, Bhimani M, Franks-Skiba K, Cooke R. Reduced effect of pH on skinned rabbit psoas muscle mechanics at high temperatures: implications for fatigue. J Physiol. 1995;486:689–694. doi: 10.1113/jphysiol.1995.sp020844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers SK, Jackson MJ. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol Rev. 2008;88:1243–1276. doi: 10.1152/physrev.00031.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racinais S, Girard O, Micallef JP, Perrey S. Failed excitability of spinal motoneurons induced by prolonged running exercise. J Neurophysiol. 2007;97:596–603. doi: 10.1152/jn.00903.2006. [DOI] [PubMed] [Google Scholar]
- Ranatunga KW. Effects of acidosis on tension development in mammalian skeletal muscle. Muscle Nerve. 1987;10:439–445. doi: 10.1002/mus.880100510. [DOI] [PubMed] [Google Scholar]
- Reardon TF, Allen DG. Time to fatigue is increased in mouse muscle at 37°C; the role of iron and reactive oxygen species. J Physiol. 2009;587:4705–4716. doi: 10.1113/jphysiol.2009.173005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristow M, Zarse K, Oberbach A, Klöting N, Birringer M, Kiehntopf M, Stumvoll M, Kahn CR, Blüher M. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci U S A. 2009;106:8665–8670. doi: 10.1073/pnas.0903485106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roots H, Ball G, Talbot-Ponsonby J, King M, McBeath K, Ranatunga KW. Muscle fatigue examined at different temperatures in experiments on intact mammalian (rat) muscle fibres. J Appl Physiol. 2009;106:378–384. doi: 10.1152/japplphysiol.90883.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltin B, Gagge AP, Bergh U, Stolwijk JA. Body temperatures and sweating during exhaustive exercise. J Appl Physiol. 1972;32:635–643. doi: 10.1152/jappl.1972.32.5.635. [DOI] [PubMed] [Google Scholar]
- Segal SS, Faulkner JA. Temperature-dependent physiological stability of rat skeletal muscle in vitro. Am J Physiol Cell Physiol. 1985;248:C265–C270. doi: 10.1152/ajpcell.1985.248.3.C265. [DOI] [PubMed] [Google Scholar]
- Todd G, Butler JE, Taylor JL, Gandevia SC. Hyperthermia: a failure of the motor cortex and the muscle. J Physiol. 2005;563:621–631. doi: 10.1113/jphysiol.2004.077115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Poel C, Stephenson DG. Reversible changes in Ca2+-activation properties of rat skeletal muscle exposed to elevated physiological temperatures. J Physiol. 2002;544:765–776. doi: 10.1113/jphysiol.2002.024968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerblad H, Bruton JD, Lännergren J. The effect of intracellular pH on contractile function of intact, single fibres of mouse muscle declines with increasing temperature. J Physiol. 1997;500:193–204. doi: 10.1113/jphysiol.1997.sp022009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SJ, Andersson DC, Sandström ME, Westerblad H, Katz A. Cross bridges account for only 20% of total ATP consumption during submaximal isometric contraction in mouse fast-twitch skeletal muscle. Am J Physiol Cell Physiol. 2006a;291:C147–C154. doi: 10.1152/ajpcell.00578.2005. [DOI] [PubMed] [Google Scholar]
- Zhang SJ, Bruton JD, Katz A, Westerblad H. Limited oxygen diffusion accelerates fatigue development in mouse skeletal muscle. J Physiol. 2006b;572:551–559. doi: 10.1113/jphysiol.2005.104521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo L, Christofi FL, Wright VP, Liu CY, Merola AJ, Berliner LJ, Clanton TL. Intra- and extracellular measurement of reactive oxygen species produced during heat stress in diaphragm muscle. Am J Physiol Cell Physiol. 2000;279:C1058–C1066. doi: 10.1152/ajpcell.2000.279.4.C1058. [DOI] [PubMed] [Google Scholar]