Abstract
Regular physical activity is beneficial in preventing the risk of cardiovascular complications of diabetes. Recent studies showed a cardioprotective role of oxytocin (OT) to induce natriuretic peptides (NPs) and nitric oxide (NO) release. It is not known if the diabetic state is associated with a reduced OT–NPs–NO system and if exercise training improves this system. To address this, we investigated the effects of treadmill running using the db/db mouse model of type 2 diabetes. Eight-week-old db/db mice were subjected to running 5 days per week for a period of 8 weeks. The lean db/+ littermates were used as controls. Sedentary db/db mice were obese and hyperglycaemic, and exercise training was not effective in reducing body weight and the hyperglycaemic state. Compared to control mice, db/db mice had lower heart weight and heart-to-body weight ratios. In these mice, this was associated with augmented cardiac apoptosis, cardiomyocyte enlargement and collagen deposits. In addition, db/db mice displayed significant downregulation in gene expression of OT (76%), OT receptors (65%), atrial NP (ANP; 43%), brain NP (BNP; 87%) and endothelial nitric oxide synthase (eNOS) (54%) in the heart (P < 0.05). Exercise training had no effect on expression of these genes which were stimulated in control mice. In response to exercise training, the significant increment of anti-apoptotic Bcl-2 gene expression was observed only in control mice (P < 0.05). In conclusion, downregulation of the OT–NPs–NO system occurs in the heart of the young db/db mouse. Exercise training was not effective in reversing the defect, suggesting impairment of this cardiac protective system in diabetes.
The role of physical activity is clearly warranted in type 2 diabetes, especially in industrialized countries where the prevalence is increased (Tuomilehto et al. 2001). An active lifestyle reduces obesity, impaired glucose tolerance and hyperlipidaemia (Hawley & Lessard, 2008), and is frequently recommended as a primary treatment for type 2 diabetes (Boule et al. 2001) to decrease blood glucose and the risk of cardiovascular disease (Syvanne & Taskinen, 1997).
Exercise decreases visceral adiposity in overweight patients who presumably possess some degree of leptin resistance (Hawley & Lessard, 2008). The obese db/db mouse model, which exhibits a mutation in the leptin receptor, displays a phenotype that is similar to human type 2 diabetes. To date, using the db/db mouse model, the role of exercise in alleviating the consequences of diabetes has focused mainly on the vasculature (Esser et al. 2007; Moien-Afshari et al. 2008), although the initial study by Tang & Reed (2001) reported that swimming training improved hyperglycaemia. Voluntary exercise in the db/db mouse does not improve obesity and hyperglycaemia, but reduces cardiovascular risk factors, such as plasma lipids, oxidative stress and endothelial dysfunction (Esser et al. 2007). Similarly, in the db/db mice subjected to forced treadmill running, a lack of beneficial effect of exercise on obesity and hyperglycaemia was also reported (Sennott et al. 2008).
The health effects of exercise are attributed to complex and still not explained mechanisms. Nevertheless, it is clear that endurance exercise induces phenotypic changes in cardiac muscle. For instance, we have recently documented that chronic exercise training in rats markedly activates factors important in cardioprotection such as oxytocin (OT), OT receptors (OTR) and atrial natriuretic peptide (ANP) as well as nitric oxide synthases (NOS) (Gutkowska et al. 2007). Indeed, levels of OT, OTR and ANP were all increased in rat left ventricular tissue following 8 weeks of treadmill running. Although a study by Bakos et al. (2007) failed to report an increase in OT levels in cardiac tissue in rats after 3 weeks of voluntary running, the reason possibly relating to the type and volume of training, we nonetheless hypothesize that positive effects of exercise training on the heart are associated with enhancement of these systems. In the previous studies, the presence and synthesis of OT and OTR have been demonstrated in all heart compartments and in the vasculature (Gutkowska et al. 1997; Jankowski et al. 2004). The functionality of this system has been established by the ability of OT to induce ANP (Gutkowska et al. 1997) release and NO (Mukaddam-Daher et al. 2001) from isolated and perfused heart. Cardiovascular effects of OT include natriuresis and reduction in blood pressure, negative inotropy and chronotropy, parasympathetic neuromodulation as well as vasodilatation triggered by the NO pathway (Gutkowska & Jankowski, 2008; Ondrejcakova et al. 2009). OT is also known to mimic many of the effects of insulin in adipocytes, stimulating glucose oxidation, lipogenesis and glycogen synthesis, and these insulin-like activities are due to OT binding to the OTR (Gimpl & Fahrenholz, 2001). Recent evidence indicates that OT enhances glucose uptake in rat cardiomyocytes even in ischaemic conditions (Coderre et al. 2005) and this function is also attributed to ANP (Sosa et al. 2007). The ANP and BNP have been shown recently to stimulate lipolysis in human fat cells through the cGMP-dependent protein kinase (Lafontan et al. 2008).
With the observation that exercise training improves cardiovascular risk factors despite obesity and hyperglycaemia (Esser et al. 2007; Sennott et al. 2008) we were interested in determining how regular exercise training affects the cardioprotective OT–NPs–NO system in an obese and diabetic animal model. For this reason, we evaluated first the cardiac OT–NPs–NO expression in hearts of db/db mice, and second, investigated the responsiveness of these factors in db/db mice subjected to treadmill running.
Methods
Animals
The Midwestern University Research and Animal Care Committee approved this study. All animals used in this study were cared for in accordance with the recommendations in The Guide for the Care and Use of Laboratory Animals, National Institute of Health, Publ. No. 85-23, 1986. Eight-week-old male diabetic-prone mice of the C57BL/KsJ strain were obtained from Jackson Laboratories (Bar Harbor, ME, USA). The C57BL/KsJ-leptdb-leptdb strain displays many of the metabolic perturbations associated with type 2 diabetes. The onset of diabetes in the db/db mouse is gradual and characterized by the obese phenotype with hyperglycaemia and subsequent hyperinsulinaemia as a result of two mutant copies of the leptin receptor gene. The lean littermates, which possess one mutant and one normal copy of the leptin receptor (db/+), were used as controls.
Exercise training protocol
After 1 week of acclimatization, lean control and db/db mice were randomly assigned to the following groups: sedentary (DS) and treadmill running (DT) group. Treadmill training consisted of moderate intensity exercise 5 days per week on an electrically driven treadmill (Columbus Instr., Columbus, OH, USA) for a period of 8 weeks. The training regimen consisted of a 3 week graded increase in exercise duration and intensity as follows: week 1, 10 min at 10 m min−1; week 2, 20 min at 10 m min−1; week 3, 30 min at 12 m min−1 and weeks 4 to 8, 30 min at 15 m min−1. This protocol was selected because it was associated with improved cardiac function in the CREBa133 murine model of dilated cardiomyopathy (Spencer et al. 2000). Mice were provided with food and water ad libidum, maintained in a room with alternating 12 h light–dark cycle and kept at 22°C.
Blood and tissue sampling
At the end of the exercise training protocol and 48 h after the last exercise session, overnight-fasted mice were killed in the morning between 8.00 and 11.00. This 48 h period was selected to eliminate the effect of the last exercise bout on insulin sensitivity (James et al. 1983). Non-anaesthetized mice were kept warm on a heating pad for a period of 30 min before blood was obtained from the submandibular vein. Blood was collected, centrifuged (3000 rpm at 4°C, for 5 min), and plasma was kept at −80°C for later measurement of plasma glucose. Mice were then immediately killed by cervical dislocation and hearts were removed and frozen with clamps pre-cooled to the temperature of liquid N2 for bio-molecular analysis for the expression of genes and proteins.
Histology
Hearts were removed and placed in 100 ml of isopentane cooled to the temperature of dry ice and kept at −80°C. For histology, the hearts were fixed by perfusion in Tissuefix solution containing neutral buffered formaldehyde solution (Laboratory Gilles Chaput Inc., Montreal, Quebec, Canada), embedded in wax, and cut into 5 μm sections. The ventricles were cross-sectioned midway between the apex and the coronary groove. The sections were stained with haematoxylin–eosin and scanned, and the images of surfaces of ventricular cross-sections were measured with help of Image-J software (National Institutes of Health, Bethesda, MD, USA; http://www.nih). To measure myocytes size, the surface of 50 cells was recorded manually, at least in seven photographs, and calculated in μm2. The red collagen deposits in the picro-sirius-stained sections (double refraction) allowed specific separation of red collagen fluorescence in the black background. Quantification of collagen was performed using Image-J software and threshold function. For detection of apoptosis in the cells the commercial Signal Stain Cleaved Caspase-3 kit has been used (Asp175) (Cat. No: 8120 Cell Signalling Technology; http://www.cellsignal.com). The slides were counter-stained with Mayer's haematoxylin. DNA apoptosis was also investigated by DeadEnd Fluorometric TUNEL System (Cat. No: G3250, Promega Corporation, Madison, WI, USA). Photographs were taken with the inverted microscope Nikon Eclipse model T square 2000-S (Nikon, Tokyo, Japan; http://www.nikon.com) equipped with a Q Imaging QICAM-IR Fast Digital 1394 CCD camera and QCapture acquisition software. Immunocytochemistry was performed according to a previously published protocol (Jankowski et al. 2004).
RT-PCR
Gene expression levels were identified by relative quantitative RT-PCR as recently reported (Gutkowska et al. 2007). The oligonucleotide primers used for real-time quantitative PCR are listed in Table 1. The relative expression of the RT-PCR products was determined according to the ΔΔCt method (Gutkowska et al. 2007). Each sample was run in duplicate. GAPDH was chosen for normalization because we examined the stability of its expression between the experimental groups in relation to the amount of total cardiac RNA and results indicate that GAPDH was not affected by diabetes or exercise. As a control, melting point analysis revealed that each of the primer pairs amplified a single predominant product, and agarose electrophoresis demonstrated PCR products of single bands of predicted size.
Table 1.
PCR primer sequences
| Gene | Sense primer (5′–3′) | Antisense primer (5′–3′) | Accession No. |
|---|---|---|---|
| OT | CCTACAGCGGATCTCAGACTGA | TCAGAGCCAGTAAGCCAAGCA | NM_011025 |
| OTR | CGACTCAGGACGAAGGTGGAGGA | AAGATGACCTTCATCATTGTTC | NM-001081147 |
| ANP | CCTGTGTACAGTGCGGTGTC | CCTAGAAGCACTGCCGTCTC | NM_008725 |
| BNP | CTGAAGGTGCTGTCCCAGAT | GTTCTTTTGTGAGGCCTTGG | NM_008726 |
| NPR-A | AGTACGCCAACAACCTGGAG | AAGAGCTGTAAAGCCCACGA | NM_008727 |
| NPR-B | TCATGACAGCCCATGGTAAA | GGTGACAATGCAGATGTTGG | NM_173788 |
| NPR-C | TGACACCATTCGGAGAATCA | TTTCACGGTCCTCAGTAGGG | NM_010933 |
| eNOS | AACCAGCGTCCTGCAAAC | AACCAGCGTCCTGCAAAC | NM_008713 |
| Ba | AAAGTGCCCGAGCTGATCA | AGCCACAAAGATGGTCACTGTCT | NM_007527 |
| BCL2 | AGTTCGGTGGGGTCATGTGTG | CCAGGTATGCACCCAGAGTG | NM_009741 |
| GAPDH | TTCACCACCATGGAGAAGGC | GGCATGGACTGTGGTCATGA | NM_008084 |
OT, oxytocin; OTR, oxytocin receptor; ANP, atrial natriuretic peptide; BNP, brain natriuretic peptide; NPR, natriuretic peptide receptor; eNOS, endothelial nitric oxide synthase; Bax, BCL2-associated X protein; BCL2, B-cell leukemia/lymphoma 2; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Western analysis
The method has been described previously (Gutkowska et al. 2007). Protein concentrations were determined by modified Bradford assay. Twenty micrograms of total protein was separated on 8% SDS polyacrylamide gel and electrotransferred onto PVDF membranes (Hybond-C; Amersham-Pharmacia, Canada). Immunoreactivity was assessed with primary antibodies obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA): goat anti-OTR (1: 5000, sc-8102), rabbit anti-eNOS (1: 4000, sc-654) diluted in blocking buffer. As an internal control, the blots were reprobed with an anti-β-actin antibody (sc-1616, Santa Cruz). The blots were visualized in a chemiluminescence detection system (RPN2132, Amersham-Pharmacia, Canada) and exposed to Kodak X-Omat film at different time points. Band density was measured by SCION software.
Statistical analysis
The analysis was performed using the statistical software package of Prism 3.00 (GraphPad Software, San Diego, CA, USA). Data were evaluated by t test or with two-factors (mouse strain and exercise training) as well as one-way analysis of variance (ANOVA) as appropriate. Detailed analysis of treatments in two-way ANOVA was carried out only if the results revealed significant differences among main effects or interactions (P < 0.05). In two-way ANOVA, the post hoc analysis using the Bonferroni procedure was carried out only if significant interaction was obtained. For data where no interactions were present, the one-way ANOVA tool has been used to test for differences in a single factor. All values are expressed as mean ±s.e.m. with significance defined as P≤ 0.05.
Results
Heart structure of 16-week-old db/db mice
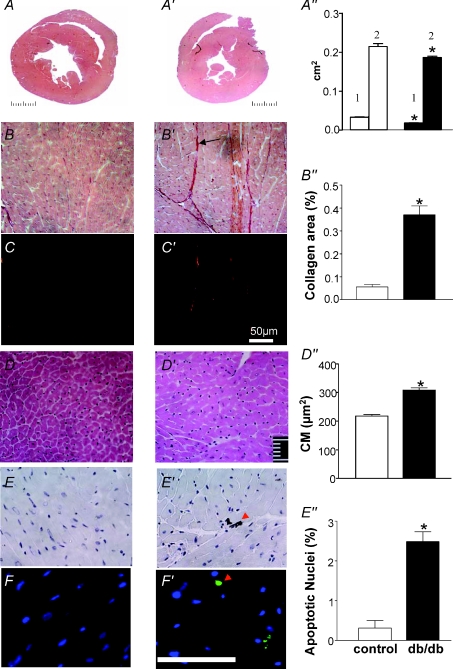
The analysis of surfaces of ventricular cross-sections with the help of Image-J software demonstrated higher ventricular surface and cavities in the control mice (Fig. 1A) than in the db/db mice (Fig. 1A′). The picro-sirius staining of the sections showed small amounts of collagen fibres (red colour) in the samples collected from control mice (Fig. 1B and C) and abundant collagen deposits in specimens from db/db mice (Fig. 1B′ and C′). These observations were confirmed by quantitative analysis of collagen-positive areas using polarized light (Fig. 1B″). In addition, the analysis of control cardiac sections (Fig. 1D) and the sections of db/db mice (Fig. 1D′) revealed marked enlargement of cardiomyocytes (Fig. 1D″). Cell apoptosis examined by both caspase-3 staining (Fig. 1E and E′) and the TUNEL assay (Fig. 1F and F′) indicated few apoptotic cell nuclei in control sections (0.2%) and significantly higher number of apoptotic nuclei in sections of db/db mice (2.5%, Fig. 1E″).
Figure 1. The representative histopathological images of heart from control and db/db mice at the age of 16 weeks.
The image of ventricular cross-sections from control (A) and db/db mice (A′) and graph bars (A″) illustrating quantitatively the surface of ventricular cavities (1) and total ventricular surface (2). Sirius red staining shows low collagen deposit (red colour) in the sections of heart of control mice (B and C) and abundant collagen in the sections from heart of db/db (B′ and C′). Quantitative analysis of collagen deposits calculated under double refraction allowing specific separation of collagen fluorescence in black background indicates significant elevation of cardiac collagen deposits in db/db vs. control mice (B″). HE (haematoxylin–eosin) staining shows that in comparison to control mice (D), the cardiomyocyte (CM) surface is elevated in db/db mice (D′). Caspase−3 immunocytochemistry (E) and Tunel analysis (F) of apoptotic nuclei in cardiac sections revealed low number or the absence of positive nuclei in sections from control mice, and the presence of apoptotic nuclei in cardiac sections from db/db mice (E′ and F′, respectively). *P < 0.05; scale bars, 50 μm.
Effect of exercise training on the physical characteristics of db/db mice
Table 2 shows the physical characteristics of diabetic mice following exercise training. At the beginning of the study, body weight was greater in diabetic mice compared to control mice and following 8 weeks remained unchanged. However, body weight was increased after 8 weeks in control mice and after exercise training. As expected, plasma glucose was significantly (P < 0.05) elevated in the diabetic animals, compared to control mice. At the end of exercise training, DT mice remained hyperglycaemic. Heart weight was significantly lower in DT mice compared to CT mice after training and the heart-to-body weight ratios in DS and DT mice were significantly lower (P < 0.05) compared to CS and CT mice.
Table 2.
Physical characteristics of control and diabetic db/db mice after exercise training
| Parameter | Group | Initial time (week 0) | Final time (week 8) |
|---|---|---|---|
| Body weight (g) | CS | 23.7 ± 0.7 | 26.8 ± 1.0*† |
| CT | 23.8 ± 0.4 | 26.3 ± 0.4*† | |
| DS | 35.1 ± 0.7* | 34.1 ± 2.0* | |
| DT | 33.7 ± 1.3* | 33.8 ± 1.4* | |
| Glucose (mmol l−1) | CS | 6.4 ± 0.4 | 5.1 ± 0.9 |
| CT | 7.3 ± 0.2 | 7.2 ± 0.3 | |
| DS | 18.8 ± 1.8* | 19.6 ± 1.3* | |
| DT | 17.8 ± 1.6* | 20.6 ± 2.2* | |
| Heart weight (mg) | CS | — | 145 ± 5 |
| CT | — | 149 ± 5 | |
| DS | — | 125 ± 8* | |
| DT | — | 121 ± 7* | |
| Heart/body weight ratio (×10−3) | CS | — | 5.5 ± 0.3 |
| CT | — | 5.6 ± 0.1 | |
| DS | — | 3.7 ± 0.2* | |
| DT | — | 3.5 ± 0.2* |
Values are presented as mean ±s.e.m. for 10–12 animals per group. *P < 0.05 compared to control mice; †P < 0.05 compared to respective control at initial week 0. CS, control sedentary mice; CT, control trained mice; DS, diabetic sedentary; DT, diabetic trained.
Effect of diabetes and exercise training on the cardiac OT system and eNOS
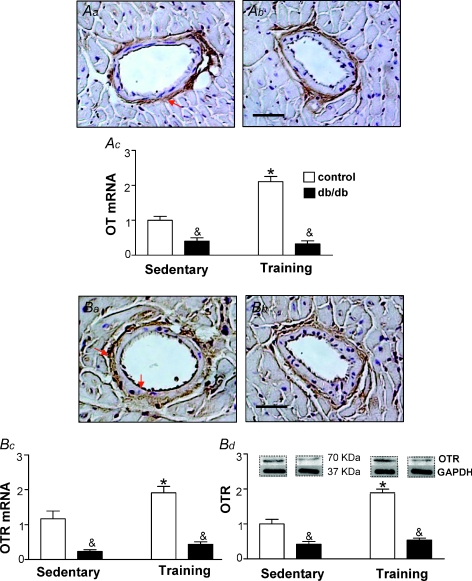
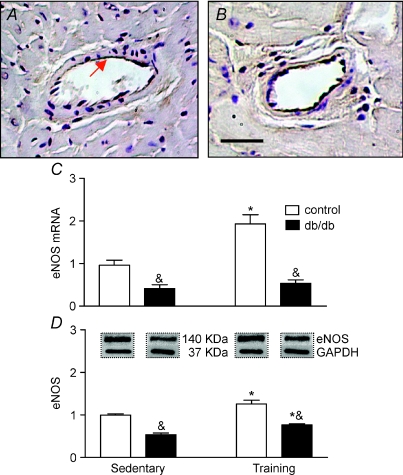
As db/db mice develop cardiac pathology it was logical to expect that a defect is associated with genes involved in cardioprotection. As presented in Fig. 2, the OT was mainly localized in tunica adventitia, whereas OTR was predominantly expressed in tunica intima and adventitia. As expected, eNOS was highly expressed in tunica intima of coronary vessels (Fig. 3). No differences were found in immunolocalization of the OT system and eNOS between control lean mice and db/db phenotype. The two-way ANOVA analysis indicated that the main effects, namely the db/db phenotype and training exercise, significantly modified gene expression and protein level for these factors in the heart. The interaction between mouse phenotype and exercise training was observed for OT mRNA (F1,19= 39.63, P < 0.0001), OTR mRNA (F1,18= 16.56, P= 0.0001), OTR protein levels (F1,18= 23.96, P= 0.0001) and eNOS mRNA (F1,180, P= 0.0012). No significant mouse strain and training interaction (F1,18= 0.18, P= 0.67) was observed for eNOS protein. Indeed, as illustrated in Fig. 2, the expression of OT and its cognate receptors were downregulated in hearts of db/db mice as determined by RT-PCR and confirmed by Western blot (both at P < 0.001). Furthermore, we observed that eNOS mRNA and protein were lower in diabetic animals comparing to control animals (Fig. 3, both at P < 0.001). Further analysis of diabetic mice revealed a non-significant effect on eNOS mRNA (P= 0.20) and a significant effect of training on eNOS protein (P < 0.05). Training had no effect on the changes in OTR protein (P= 0.25) and OTR mRNA (P= 0.07) in diabetic mice, while a significant effect of training was observed in control hearts (P= 0.005 and P= 0.002, respectively).
Figure 2. Expression of OT system in the heart of control and db/db mice.
Immunostaining of OT is predominantly detected in tunica adventitia of coronary vessels both in lean control (Aa) and db/db mice (Ab). db/db mice were kept in sedentary conditions or subjected to exercise training and the lean littermates (db/+) were used as controls. Gene expression determined by real-time PCR is presented for OT mRNA in Ac. OTR was predominantly detected both in tunica adventitia and tunica intima in lean control (Ba) and db/db mice (Bb). The expression of OTR in animals kept in sedentary conditions and subjected to exercise training is presented in Bc (OTR mRNA), and OTR protein by Western blot in Bd. Bars show the mean ±s.e.m., of 2–3 independent experiments testing 5–6 animals in each group. Significance of the training effect: *P < 0.05. Significance of the db/db phenotype: &P < 0.05.
Figure 3. Expression of endothelial NO synthase (eNOS) in the heart of control and db/db mice.
The predominant immunostaining of eNOS was detected in endothelial cell layer of lean control (A) and db/db mice (B). The eNOS expression was investigated in hearts of sedentary db/db mice, db/db mice subjected to exercise training and in the lean littermate controls. Expression of eNOS mRNA is shown in C, and eNOS protein by Western blot in D. Bars show the mean ±s.e.m., of 2–3 independent experiments testing 5–6 animals in each group. Significance of the training effect: *P < 0.05. Significance of the db/db phenotype: &P < 0.05.
Effect of diabetes and exercise training on the transcript of cardiac natriuretic peptides
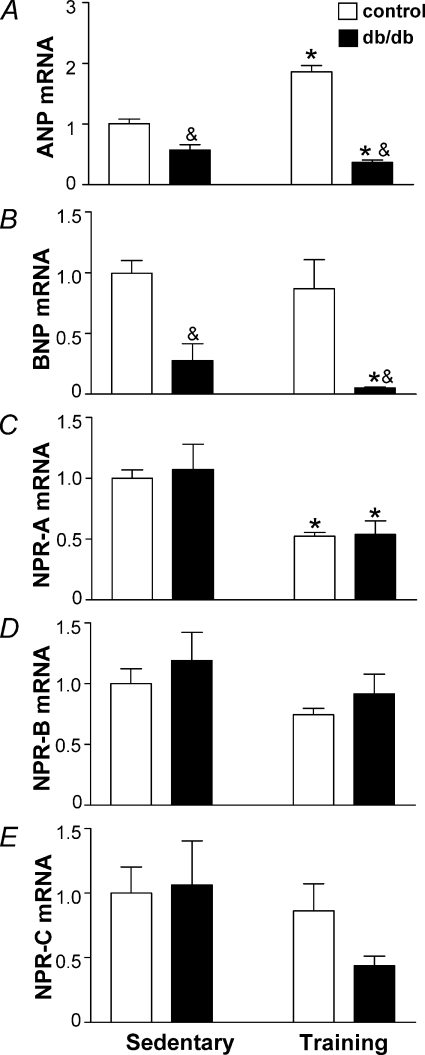
The RT-PCR analysis indicates a 6.6-fold lower BNP mRNA expression in db/db mice compared to control mice (Fig. 4B, P < 0.001). No significant strain and exercise training interaction (F1,19, P= 0.31) was observed using two-way ANOVA analysis. Following exercise training, BNP mRNA expression was not changed in the control group (Fig. 4B); however, diabetic mice had lowered BNP mRNA (P < 0.001). While diabetes had a very profound lowering effect on BNP expression, only a 2-fold downregulation of ANP mRNA was observed (P < 0.05; Fig. 4A). Following exercise training, ANP mRNA was increased in the control mice (P < 0.001) whereas diabetic mice had reduced ANP mRNA (P= 0.049). In support of this, the two-way ANOVA analysis indicated significant interactions of db/db phenotype and exercise training on ANP mRNA expression (F1,18= 58.74, P < 0.0001). No significant differences in expression of cardiac natriuretic peptide receptors (NPRs) between db/db mice and control mice were observed. Analysis of NPRs mRNAs indicated a decrease of NPR-A expression in response to the exercise training, without effects on NPR-B mRNA and NPR-C mRNA (Fig. 4C, D and E).
Figure 4. Expression of natriuretic peptide system in the heart of sedentary db/db mice, db/db mice subjected to exercise training and in the lean littermate controls.
Expression of atrial natriuretic peptide (ANP) mRNA is presented in A, brain natriuretic peptide (BNP) mRNA in B, natriuretic peptide receptor A (NPR-A) mRNA in C, NPR-B mRNA in D, and for NPR-C mRNA in E. Bars show the mean ±s.e.m., n= 5–7, of 2–3 independent experiments. Significance of the training effect: *P < 0.05. Significance of the db/db phenotype: &P < 0.05.
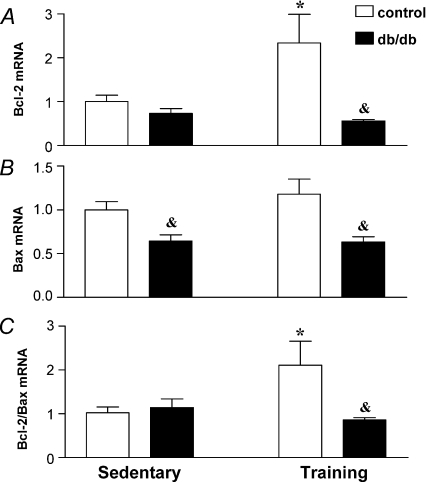
Reverse transcription polymerase chain reaction was performed to quantify the mRNA levels of Bax, the pro-apoptotic gene, and the anti-apoptotic gene, Bcl-2. As shown in Fig. 5, the db/db phenotype induced different effects on the level of gene expression of Bcl-2 and Bax. The interaction between mouse phenotype and exercise training was observed for Bcl-2 mRNA (F1,20= 4.87, P= 0.0391) but not for Bax mRNA (F1,20= 0.65, P= 0.43). The decrease of Bax gene expression in db/db mice was statistically significant compared with control mice (P < 0.01). In response to exercise training, the significant increment of anti-apoptotic Bcl-2 gene expression was restricted to control mice (P < 0.05). Two-way ANOVA analysis revealed interaction of mouse strain and exercise training on the values of Bcl-2 to Bax mRNA ratios (F1,20= 14.27, P= 0.0012). Post hoc analysis indicated a significant reduction of the Bcl-2 to Bax mRNA ratios in control mice but no effect on this ratio in db/db mice (P < 0.05).
Figure 5.
RNA expression for Bcl-2 (A), Bax (B) and Bcl-2 to Bax ratio (C) in the heart of sedentary db/db mice, db/db mice subjected to exercise training and in the lean littermates controls. Bars show the mean ±s.e.m., n= 6, of 2–3 independent experiments. Significance of the training effect: *P < 0.05. Significance of the db/db phenotype: &P < 0.05.
Discussion
This study was designed to determine the effects of the diabetic state using a model representative of type 2 diabetes on gene and protein expression of the OT-associated cardioprotective factors and the consequences of treadmill exercise training on their expression. The main findings of this study are: (i) changed cardiac structure in db/db mice identified by altered cardiac geometry, augmented cardiac apoptosis, cardiomyocyte enlargement and collagen deposits; (ii) 8 weeks of exercise training of db/db mice did not have any effect on body weight, blood glucose and the exercise-stimulated anti-apoptotic marker, Bcl-2/Bax mRNA ratio; (iii) the cardiac OT system, ANP, BNP and eNOS genes are down-regulated in the db/db mice in comparison to lean control mice; (iv) in db/db mice, exercise training had no effect on OT and OTR expression, but decreased the expression of cardiac NPs and their functional NPR-A receptor; and (v) the increase of eNOS protein in the heart of db/db mice was the only positive effect of exercise training.
In the db/db mouse model, the Ob-R receptor encoded by the mouse diabetic (db) gene is mutated leading to obesity, hyperleptinaemia, hyperinsulinaemia and insulin resistance (Chen et al. 1996). Although in humans the dysfunctional leptin receptor is rarely a cause of diabetes, the db/db mouse provides an animal model of type 2 diabetes characterized by initial insulin resistance followed by the defect in insulin secretion (Leibel et al. 1997), features that are characteristic of human type 2 diabetes. Nonetheless, it is well known that leptin regulates cardiovascular function and energy substrate metabolism, and in obesity, perturbations in leptin signalling are reported (Bray & York, 1997).
The onset of diabetes in db/db mice is gradual and 4-week-old mice are normoglycaemic but hyperinsulinaemic and it is therefore likely that hyperinsulinaemia alters cardiac gene expression, cardiac structure and function, the cardiac substrate energy profile and efficiency (Buchanan et al. 2005). As observed in this study, 16-week-old db/db mice have altered cardiac structure based on caspase-3 staining and the Tunel method to demonstrate DNA fragmentation. Using these methods, we observed augmented apoptosis only in the hearts from db/db mice and the cell loss in db/db mice can explain the decreased heart weight seen in the young db/db mice. We also observed further evidence of altered cardiac structure by the presence of enlarged cardiomyocytes and the accumulation of collagen deposits, although the magnitude of these changes was not sufficient to elevate the cardiac mass. This early onset of apoptosis is consistent with previous recent studies in the heart of db/db mice that apoptosis is increased in young (2–3 months old) and is more accentuated in old animals (12–14 months old) (Barouch et al. 2006). Cardiac myocyte apoptosis has been implicated in the transition from compensated to decompensated hypertensive hypertrophy (Li et al. 1997), and recently it has been demonstrated that chronic levels of cardiac myocyte apoptosis are a causal component in the pathogenesis of heart failure (Wencker et al. 2003).
To date, no attention has been given to the OT status in diabetes, a linked system involving NPs and NO that is associated with metabolism and heart function. We investigated these cardiac systems in db/db mice because of the involvement of the OT system in cardioprotection on ischaemia-reperfusion-induced myocardial damage via its negative chronotropic effect (Ondrejcakova et al. 2009), potential benefits of positive partner relationships on resting heart rate and blood pressure (Grewen et al. 2005), and our recent finding that downregulation of the cardiac OT–NPs–NO system induced by oestrogen deficiency is reversed by chronic exercise training (Gutkowska et al. 2007). We previously reported that the OTR is present on cardiac myocytes secreting ANP (Gutkowska et al. 1997) as well as on endothelial cells (Jankowski et al. 2004) and in intrinsic cardiac neurons regulating contractility via the NO pathway (Mukaddam-Daher et al. 2001). It is unclear why the OT system-linked genes are downregulated in the heart of db/db mice, although the following mechanisms and consequences on heart structure are possible. First, the hyperglycaemic state of db/db mice has been recently associated with depletion of GATA4 protein expression in the heart, the transcription factor involved in the regulation of NP synthesis (Kobayashi et al. 2007). GATA4-depleted hearts also show an upregulation in the expression of the pro-apoptotic factor caspase-12 (Oka et al. 2006). GATA4 is involved in OT-mediated cardiomyogenesis in embryonic (Danalache et al. 2007; Matsuura et al. 2004) mouse stem cells and decreased function of the eNOS gene (Danalache et al. 2007). Second, altered gene expression can develop as a response to a chronic and sustained over-supply of fatty acids, which is characteristic of diabetic hearts (Finck & Kelly, 2007).
In agreement with this, we observed decreased mRNA expression of ANP and BNP in hearts of db/db mice with accompanying reduced heart weight. This deficiency in ANP and BNP can impair several physiological functions, such as diuresis, natriuresis and vasodilatation, as well as the suppression of the renin–angiotensin–aldosterone, vasopressin, endothelin and sympathetic nervous system. Recent reports indicate that plasma NPs levels are reduced in obese patients (Mehra et al. 2004) and this could provoke the increased sodium retention and volume expansion characteristic of them. Further, NPs have cytoprotective and anti-hypertrophic actions in cultured cardiomyocytes and inhibit both DNA and collagen synthesis in cardiac fibroblasts (Woods, 2004), and a reduction in NPs would probably be associated with hypertrophy of cardiomyocytes and increased collagen deposits in heart. In db/db hearts, we show the reduction in NPs is associated with increased apoptosis, cardiomyocyte hypertrophy and increased collagen.
The function of the NP system in the hearts of models of diabetes has received little attention. Data from Yue et al. (2007) suggest that ANP transcript elevation in the db/db hearts is correlated with an increased cardiac mass and occurs not earlier than at the age of 15–21 weeks. However, the high variability of transcript measurements in the individual hearts of db/db mice questions the significance of this finding and hampers the conclusion drawn on the dynamics of ANP expression during the progression of cardiac pathology in this model. Alteration in the cardiovascular system, such as atrophy of the arterial walls in the offspring of NO-defective rats, has been shown (Kristek & Gerova, 2004), suggesting the contribution of NO deficiency in cardiac atrophy. This is in agreement with the lower expression of NOS in db/db mice and indicates that endothelial dysfunction is involved in cardiovascular complications of diabetes (Forstermann & Munzel, 2006).
Our results showing the lack of benefit of chronic treadmill exercise training on the obese state and fasting glucose are consistent with earlier reports for voluntary wheel- and forced wheel-running in the db/db model of diabetes (Esser et al. 2007), and cannot be attributed to an insufficient training stimulus. The observation that lean control mice responded to exercise training with an upregulation of the OTR system and NOS expression is consistent with the fact that the training stimulus was sufficient to investigate the genes of concern. However, what is intriguing is that BNP expression was not increased in lean control mice when training was effective at upregulating the OT system. In our recent paper (Gutkowska et al. 2007), we also observed that training exercise increased the mRNA of OTR whereas the expression of BNP mRNA was not changed in the left ventricle. However, in hearts from ovariectomized rats, we observed the parallel upregulation of OTR mRNA and BNP mRNA expression following exercise training. We concluded that BNP gene expression in ovariectomized rats subjected to exercise training was largely dependent on the oestrogen status. In agreement with the data reported in the current study, it has already been shown that ANP increased in physiological hypertrophy induced by exercise training in the rat whereas mRNA BNP level was not changed (Kong et al. 2005). Moreover, because these two genes show very distinctive change patterns during the development of hypertrophy and heart failure, the ANP-to-BNP ratio in addition to the absolute levels are proposed in diagnosis and therapeutic strategy in decompensated heart failure.
It is possible that defective leptin signalling in hearts from dbdb mice can lead to an OT system unresponsive to exercise training. However, exercise training decreased the NPs mRNA level in db/db hearts without significant changes in OT/OTR expression. Gene expression of NPs is regulated by a variety of patho-physiological stimuli and the impacts of diabetes per se on cardiac NPs gene expression have been addressed in several studies. In the genetically obese Zucker rat, the myocardial signal transduction cascade PKC–MAPK–ANP mRNA seems to be markedly impaired and this abnormal cardiac cell signalling in obese rats reflects an early phase in the cardiac pathogenesis accompanying obesity (Morabito et al. 2001). On the other hand, rats with streptozotocin-induced diabetes display increased heart ANP mRNA (and BNP mRNA) levels (Feng et al. 2008), whereas non-obese diabetic (NOD) mice have reduced ANP mRNA levels in the heart (Mifune et al. 1992). In parallel, streptozotocin-treated pigs display increased atrial but not ventricular BNP mRNA expression (Christoffersen et al. 2002). It is clear from these studies that the diabetic status alters cardiac NP mRNA and this is also accompanied by chamber-specific differences; what contributes to these observations is unknown. However, the role of GATA4 should be considered based on the observation that hearts from db/db mice display a reduction in GATA4 content (Kobayashi et al. 2007). In mice with cardiac-restricted GATA4 deletion, ANP mRNA is significantly reduced and animals display the blunted hypertrophic response and gene expression when exposed to exercise training (Oka et al. 2006). In addition, db/db mice exhibit an increase sympathetic tone with parasympathetic inhibition (Goncalves et al. 2009). Lowering of cardiac NPs expression in response to training exercise can indicate some beneficial effect because physical training in patients with heart failure are associated with blunting of adrenergic overactivity and NPs overexpression (Passino et al. 2006).
In conclusion, we demonstrate for the first time that alterations in the OT–NPs–NO system occur in hearts from db/db mice. In hearts from this model of type 2 diabetes, gene expression for OT, ANP, BNP and eNOS are downregulated and exercise training had limited protection in preserving cardiac levels of these peptides. The exception was an improvement in eNOS protein expression following exercise training and this occurred without effects on obesity and hyperglycaemia. Clearly, further work is warranted to determine the physiological importance of impaired expression of cardioprotective genes in diabetes. Human genomic studies suggest that BNP may protect against type 2 diabetes (Meirhaeghe et al. 2007). Accumulating evidence indicates a role for OT in the regulation of food balance and in leptin control (Blevins et al. 2004), as well as its stimulation of glucose uptake in rat skeletal muscles (Lee et al. 2008) and cardiomyocytes (Coderre et al. 2005). Therefore, strategies aimed at increasing the activity of the cardioprotective genes in diabetes may be effective in preventing cardiac dysfunction.
Acknowledgments
The authors would like to thank Ms Jennifer Lelièvre for her secretarial help. This work was supported by the Canadian Institute of Health Research (CIHR, MOP-53217, NET Program) and the Canadian Heart and Stroke Foundation (CHSF, NET Program) as grants to J.G. and M.J., and by the Office of Research and Sponsored Programs of Midwestern University and the Diabetes Action and Research Education Foundation to T.L.B.
Glossary
Abbreviations
- ANP
atrial natriuretic peptide
- Bax
Bcl-2-associated protein
- Bcl2
B-cell CLL/lymphoma 2
- BNP
brain natriuretic peptide
- cGMP
guanosine 3′,5′-(cyclic)phosphate
- eNOS
endothelial nitric oxide synthase
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- MAPK
mitogen-activated protein (MAP) kinase
- NO
nitric oxide
- NOS
nitric oxide synthase
- NPs
natriuretic peptides
- NPRs
natriuretic peptide receptors
Author's contribution
J.G. (conception, design, data analysis, writing), T.L.B. (conception, data analysis, design, writing), D.B. (design, data analysis), D.W. (design, data analysis), J.-M.L. (design, data analysis) and M.J. (conception, design, data analysis, writing). All authors contributed in drafting the article and revision as well as approved final form of the manuscript.
References
- Bakos J, Hlavacova N, Makatsori A, Tybitanclova K, Zorad S, Hinghofer-Szalkay H, Johansson BB, Jezova D. Oxytocin levels in the posterior pituitary and in the heart are modified by voluntary wheel running. Regul Pept. 2007;139:96–101. doi: 10.1016/j.regpep.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Barouch LA, Gao D, Chen L, Miller KL, Xu W, Phan AC, Kittleson MM, Minhas KM, Berkowitz DE, Wei C, Hare JM. Cardiac myocyte apoptosis is associated with increased DNA damage and decreased survival in murine models of obesity. Circ Res. 2006;98:119–124. doi: 10.1161/01.RES.0000199348.10580.1d. [DOI] [PubMed] [Google Scholar]
- Blevins JE, Schwartz MW, Baskin DG. Evidence that paraventricular nucleus oxytocin neurons link hypothalamic leptin action to caudal brain stem nuclei controlling meal size. Am J Physiol Regul Integr Comp Physiol. 2004;287:R87–R96. doi: 10.1152/ajpregu.00604.2003. [DOI] [PubMed] [Google Scholar]
- Boule NG, Haddad E, Kenny GP, Wells GA, Sigal RJ. Effects of exercise on glycemic control and body mass in type 2 diabetes mellitus: a meta-analysis of controlled clinical trials. JAMA. 2001;286:1218–1227. doi: 10.1001/jama.286.10.1218. [DOI] [PubMed] [Google Scholar]
- Bray GA, York DA. Clinical review 90: Leptin and clinical medicine: a new piece in the puzzle of obesity. J Clin Endocrinol Metab. 1997;82:2771–2776. doi: 10.1210/jcem.82.9.4224. [DOI] [PubMed] [Google Scholar]
- Buchanan J, Mazumder PK, Hu P, Chakrabarti G, Roberts MW, Yun UJ, Cooksey RC, Litwin SE, Abel ED. Reduced cardiac efficiency and altered substrate metabolism precedes the onset of hyperglycemia and contractile dysfunction in two mouse models of insulin resistance and obesity. Endocrinology. 2005;146:5341–5349. doi: 10.1210/en.2005-0938. [DOI] [PubMed] [Google Scholar]
- Chen H, Charlat O, Tartaglia LA, Woolf EA, Weng X, Ellis SJ, Lakey ND, Culpepper J, Moore KJ, Breitbart RE, Duyk GM, Tepper RI, Morgenstern JP. Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell. 1996;84:491–495. doi: 10.1016/s0092-8674(00)81294-5. [DOI] [PubMed] [Google Scholar]
- Christoffersen C, Goetze JP, Bartels ED, Larsen MO, Ribel U, Rehfeld JF, Rolin B, Nielsen LB. Chamber-dependent expression of brain natriuretic peptide and its mRNA in normal and diabetic pig heart. Hypertension. 2002;40:54–60. doi: 10.1161/01.hyp.0000021780.21830.dd. [DOI] [PubMed] [Google Scholar]
- Coderre L, Jankowski M, Gutkowska J. Oxytocin: a new hormone implicated in the regulation of glucose uptake in heart. Hypertension. 2005;46:851. [Google Scholar]
- Danalache BA, Paquin J, Donghao W, Grygorczyk R, Moore JC, Mummery CL, Gutkowska J, Jankowski M. Nitric oxide signalling in oxytocin-mediated cardiomyogenesis. Stem Cells. 2007;25:679–688. doi: 10.1634/stemcells.2005-0610. [DOI] [PubMed] [Google Scholar]
- Esser KA, Su W, Matveev S, Wong V, Zeng L, McCarthy JJ, Smart EJ, Guo Z, Gong MC. Voluntary wheel running ameliorates vascular smooth muscle hyper-contractility in type 2 diabetic db/db mice. Appl Physiol Nutr Metab. 2007;32:711–720. doi: 10.1139/H07-058. [DOI] [PubMed] [Google Scholar]
- Feng B, Chen S, Chiu J, George B, Chakrabarti S. Regulation of cardiomyocyte hypertrophy in diabetes at the transcriptional level. Am J Physiol Endocrinol Metab. 2008;294:E1119–E1126. doi: 10.1152/ajpendo.00029.2008. [DOI] [PubMed] [Google Scholar]
- Finck BN, Kelly DP. Peroxisome proliferator-activated receptor γ coactivator-1 (PGC-1) regulatory cascade in cardiac physiology and disease. Circulation. 2007;115:2540–2548. doi: 10.1161/CIRCULATIONAHA.107.670588. [DOI] [PubMed] [Google Scholar]
- Forstermann U, Munzel T. Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation. 2006;113:1708–1714. doi: 10.1161/CIRCULATIONAHA.105.602532. [DOI] [PubMed] [Google Scholar]
- Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiol Rev. 2001;81:629–683. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- Goncalves AC, Tank J, Diedrich A, Hilzendeger A, Plehm R, Bader M, Luft FC, Jordan J, Gross V. Diabetic hypertensive leptin receptor-deficient db/db mice develop cardioregulatory autonomic dysfunction. Hypertension. 2009;53:387–392. doi: 10.1161/HYPERTENSIONAHA.108.124776. [DOI] [PubMed] [Google Scholar]
- Grewen KM, Girdler SS, Amico J, Light KC. Effects of partner support on resting oxytocin, cortisol, norepinephrine, and blood pressure before and after warm partner contact. Psychosom Med. 2005;67:531–538. doi: 10.1097/01.psy.0000170341.88395.47. [DOI] [PubMed] [Google Scholar]
- Gutkowska J, Jankowski M. Oxytocin revisited: it is also a cardiovascular hormone. J Am Soc Hypertens. 2008;2:318–325. doi: 10.1016/j.jash.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Gutkowska J, Jankowski M, Lambert C, Mukaddam-Daher S, Zingg HH, McCann SM. Oxytocin releases atrial natriuretic peptide by combining with oxytocin receptors in the heart. Proc Natl Acad Sci U S A. 1997;94:11704–11709. doi: 10.1073/pnas.94.21.11704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutkowska J, Paquette A, Wang D, Lavoie JM, Jankowski M. Effect of exercise training on cardiac oxytocin and natriuretic peptide systems in ovariectomized rats. Am J Physiol Regul Integr Comp Physiol. 2007;293:R267–R275. doi: 10.1152/ajpregu.00071.2007. [DOI] [PubMed] [Google Scholar]
- Hawley JA, Lessard SJ. Exercise training-induced improvements in insulin action. Acta Physiol (Oxf) 2008;192:127–135. doi: 10.1111/j.1748-1716.2007.01783.x. [DOI] [PubMed] [Google Scholar]
- James DE, Burleigh KM, Kraegen EW, Chisholm DJ. Effect of acute exercise and prolonged training on insulin response to intravenous glucose in vivo in rat 1. J Appl Physiol. 1983;55:1660–1664. doi: 10.1152/jappl.1983.55.6.1660. [DOI] [PubMed] [Google Scholar]
- Jankowski M, Danalache B, Wang D, Bhat P, Hajjar F, Marcinkiewicz M, Paquin J, McCann SM, Gutkowska J. Oxytocin in cardiac ontogeny. Proc Natl Acad Sci U S A. 2004;101:13074–13079. doi: 10.1073/pnas.0405324101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S, Mao K, Zheng H, Wang X, Patterson C, O’Connell TD, Liang Q. Diminished GATA4 protein levels contribute to hyperglycemia-induced cardiomyocyte injury. J Biol Chem. 2007;282:21945–21952. doi: 10.1074/jbc.M703048200. [DOI] [PubMed] [Google Scholar]
- Kong SW, Bodyak N, Yue P, Liu Z, Brown J, Izumo S, Kang PM. Genetic expression profiles during physiological and pathological cardiac hypertrophy and heart failure in rats. Physiol Genomics. 2005;21:34–42. doi: 10.1152/physiolgenomics.00226.2004. [DOI] [PubMed] [Google Scholar]
- Kristek F, Gerova M. Hypotrophy of conduit artery walls of the offspring of nitric oxide-defective rats. Braz J Med Biol Res. 2004;37:601–606. doi: 10.1590/s0100-879x2004000400018. [DOI] [PubMed] [Google Scholar]
- Lafontan M, Moro C, Berlan B, Crampes F, Sengenes C, Galitzky G. Control of lipolysis by natriuretic peptides and cyclic GMP. Trends Endocrinol Metab. 2008;19:130–137. doi: 10.1016/j.tem.2007.11.006. [DOI] [PubMed] [Google Scholar]
- Lee ES, Uhm KO, Lee YM, Kwon J, Park SH, Soo KH. Oxytocin stimulates glucose uptake in skeletal muscle cells through the calcium-CaMKK-AMPK pathway. Regul Pept. 2008;151:71–74. doi: 10.1016/j.regpep.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Leibel RL, Chung WK, Chua SC., Jr The molecular genetics of rodent single gene obesities. J Biol Chem. 1997;272:31937–31940. doi: 10.1074/jbc.272.51.31937. [DOI] [PubMed] [Google Scholar]
- Li Z, Bing OH, Long X, Robinson KG, Lakatta EG. Increased cardiomyocyte apoptosis during the transition to heart failure in the spontaneously hypertensive rat. Am J Physiol Heart Circ Physiol. 1997;272:H2313–H2319. doi: 10.1152/ajpheart.1997.272.5.H2313. [DOI] [PubMed] [Google Scholar]
- Matsuura K, Nagai T, Nishigaki N, Oyama T, Nishi J, Wada H, Sano M, Toko H, Akazawa H, Sato T, Nakaya H, Kasanuki H, Komuro I. Adult cardiac Sca-1-positive cells differentiate into beating cardiomyocytes. J Biol Chem. 2004;279:11384–11391. doi: 10.1074/jbc.M310822200. [DOI] [PubMed] [Google Scholar]
- Mehra MR, Uber PA, Park MH, Scott RL, Ventura HO, Harris BC, Frohlich ED. Obesity and suppressed B-type natriuretic peptide levels in heart failure. J Am Coll Cardiol. 2004;43:1590–1595. doi: 10.1016/j.jacc.2003.10.066. [DOI] [PubMed] [Google Scholar]
- Meirhaeghe A, Sandhu MS, McCarthy MI, de Groote P, Cottel D, Arveiler D, Ferrieres J, Groves CJ, Hattersley AT, Hitman GA, Walker M, Wareham NJ, Amouyel P. Association between the T-381C polymorphism of the brain natriuretic peptide gene and risk of type 2 diabetes in human populations. Hum Mol Genet. 2007;16:1343–1350. doi: 10.1093/hmg/ddm084. [DOI] [PubMed] [Google Scholar]
- Mifune H, Suzuki S, Honda J, Kobayashi Y, Noda Y, Hayashi Y, Mochizuki K. Atrial natriuretic peptide (ANP): a study of ANP and its mRNA in cardiocytes, and of plasma ANP levels in non-obese diabetic mice. Cell Tissue Res. 1992;267:267–272. doi: 10.1007/BF00302964. [DOI] [PubMed] [Google Scholar]
- Moien-Afshari F, Ghosh S, Elmi S, Rahman MM, Sallam N, Khazaei M, Kieffer TJ, Brownsey RW, Laher I. Exercise restores endothelial function independently of weight loss or hyperglycaemic status in db/db mice. Diabetologia. 2008;51:1327–1337. doi: 10.1007/s00125-008-0996-x. [DOI] [PubMed] [Google Scholar]
- Morabito D, Vallotton MB, Lang U. Obesity is associated with impaired ventricular protein kinase C-MAP kinase signalling and altered ANP mRNA expression in the heart of adult Zucker rats. J Investig Med. 2001;49:310–318. doi: 10.2310/6650.2001.33895. [DOI] [PubMed] [Google Scholar]
- Mukaddam-Daher S, Lin YL, Gutkowska J, Cardinal R. Negative inotropic and chronotropic effects of oxytocin. Hypertension. 2001;38:292–296. doi: 10.1161/01.hyp.38.2.292. [DOI] [PubMed] [Google Scholar]
- Oka T, Maillet M, Watt AJ, Schwartz RJ, Aronow BJ, Duncan SA, Molkentin JD. Cardiac-specific deletion of Gata4 reveals its requirement for hypertrophy, compensation, and myocyte viability. Circ Res. 2006;98:837–845. doi: 10.1161/01.RES.0000215985.18538.c4. [DOI] [PubMed] [Google Scholar]
- Ondrejcakova M, Ravingerova T, Bakos J, Pancza D, Jezova D. Oxytocin exerts protective effects on in vitro myocardial injury induced by ischemia and reperfusion. Can J Physiol Pharmacol. 2009;87:137–142. doi: 10.1139/Y08-108. [DOI] [PubMed] [Google Scholar]
- Passino C, Severino S, Poletti R, Piepoli MF, Mammini C, Clerico A, Gabutti A, Nassi G, Emdin M. Aerobic training decreases B-type natriuretic peptide expression and adrenergic activation in patients with heart failure. J Am Coll Cardiol. 2006;47:1835–1839. doi: 10.1016/j.jacc.2005.12.050. [DOI] [PubMed] [Google Scholar]
- Sennott J, Morrissey J, Standley PR, Broderick T. Treadmill exercise training fails to restore defects in glucose, insulin and muscle glut4 content in the db/db mouse model of diabetes. Pathophysiology. 2008;15:173–179. doi: 10.1016/j.pathophys.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Sosa V, Carbo R, Guarner V. Participation of glucose transporters on atrial natriuretic peptide-induced glucose uptake by adult and neonatal cardiomyocytes under oxygenation and hypoxia. Eur J Pharmacol. 2007;568:83–88. doi: 10.1016/j.ejphar.2007.04.040. [DOI] [PubMed] [Google Scholar]
- Spencer KT, Collins K, Korcarz C, Fentzke R, Lang RM, Leiden JM. Effects of exercise training on LV performance and mortality in a murine model of dilated cardiomyopathy. Am J Physiol Heart Circ Physiol. 2000;279:H210–H215. doi: 10.1152/ajpheart.2000.279.1.H210. [DOI] [PubMed] [Google Scholar]
- Syvanne M, Taskinen MR. Lipids and lipoproteins as coronary risk factors in non-insulin-dependent diabetes mellitus. Lancet. 1997;350:SI20–SI23. doi: 10.1016/s0140-6736(97)90024-6. [DOI] [PubMed] [Google Scholar]
- Tang T, Reed MJ. Exercise adds to metformin and acarbose efficacy in db/db mice. Metab Clin Exp. 2001;50:1049–1053. doi: 10.1053/meta.2001.25596. [DOI] [PubMed] [Google Scholar]
- Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P, Keinanen-Kiukaanniemi S, Laakso M, Louheranta A, Rastas M, Salminen V, Uusitupa M. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- Wencker D, Chandra M, Nguyen K, Miao W, Garantziotis S, Factor SM, Shirani J, Armstrong RC, Kitsis RN. A mechanistic role for cardiac myocyte apoptosis in heart failure. J Clin Invest. 2003;111:1497–1504. doi: 10.1172/JCI17664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods RL. Cardioprotective functions of atrial natriuretic peptide and B-type natriuretic peptide: a brief review. Clin Exp Pharmacol Physiol. 2004;31:791–794. doi: 10.1111/j.0305-1870.2004.04073.x. [DOI] [PubMed] [Google Scholar]
- Yue P, Arai T, Terashima M, Sheikh AY, Cao F, Charo D, Hoyt G, Robbins RC, Ashley EA, Wu J, Yang PC, Tsao PS. Magnetic resonance imaging of progressive cardiomyopathic changes in the db/db mouse. Am J Physiol Heart Circ Physiol. 2007;292:H2106–H2118. doi: 10.1152/ajpheart.00856.2006. [DOI] [PubMed] [Google Scholar]