Abstract
Unlike upper limb muscles, it remains undocumented as to how motor units in the soleus muscle are organised in terms of recruitment range and discharge rates with respect to their recruitment and de-recruitment thresholds. The possible influence of neuromodulation, such as persistent inward currents (PICs) on lower limb motor unit recruitment and discharge rates has also yet to be reported. To address these issues, electromyographic (EMG) activities from the soleus muscle were recorded using selective branched-wire intramuscular electrodes during ramp-and-hold contractions with intensities up to maximal voluntary contraction (MVC). The multiple single motor unit activities were then derived using a decomposition technique. The onset–offset hysteresis of motor unit discharge, i.e. a difference between recruitment and de-recruitment thresholds, as well as PIC magnitude calculated by a paired motor unit analysis were used to examine the neuromodulatory effects on discharge behaviours, such as minimum firing rate, peak firing rate and degree of increase in firing rate. Forty-two clearly identified motor units from five subjects revealed that soleus motor units are recruited progressively from rest to contraction strengths close to 95% of MVC, with low-threshold motor units discharging action potentials slower at their recruitment and with a lower peak rate than later recruited high-threshold units. This observation is in contrast to the ‘onion skin phenomenon’ often reported for the upper limb muscles. Based on positive correlations of the peak discharge rates, initial rates and recruitment order of the units with the magnitude of the onset–offset hysteresis and not PIC contribution, we conclude that discharge behaviours among motor units appear to be related to a variation in an intrinsic property other than PICs.
In order to vary force in a skeletal muscle, a motor unit pool is activated in two ways: by changing the number of motor units that come into play, i.e. recruitment, and by modulating the discharge frequency of action potentials of the active motor units, i.e. rate coding. The relative contribution of these two processes is known to vary depending upon the muscle and its function. For example, the hand muscles, for which the precise control of incremental force is essential, predominantly employ the rate coding strategy with the upper limit of recruitment (the highest threshold at which new units are still recruited) being 50–75% of MVC (Kukulka & Clamann, 1981; Thomas et al. 1986; Moritz et al. 2005). In contrast, the elbow flexor and shoulder muscles, which require crude and forceful contractions, rely more on the recruitment strategy, with the upper limit of the recruitment being at 75–88% of MVC (Kukulka & Clamann, 1981; De Luca et al. 1982). Despite extensive investigations of muscle of the lower limb, the organisation of recruitment and rate coding over the entire range of voluntary contraction is largely unknown with only tibialis anterior being previously reported to have its upper limit of recruitment at 90% of MVC (Van Custem et al. 1997). It is also an open question as to how the discharge behaviour of motor units is organised with respect to the recruitment threshold, i.e. whether the low-threshold motor units discharge faster or slower than high-threshold units. Previously some literature reported that the peak discharge rate of low-threshold units are greater than that of the high-threshold units (often described as the ‘onion skin phenomenon’, Person & Kudina, 1972; Freund et al. 1975; Monster & Chan, 1977; Freund, 1983; Erim et al. 1996), whereas others have stated the opposite (Tokizane & Shimazu, 1964; Gydikov & Kosarov, 1974; Grimby et al. 1979; Moritz et al. 2005).
New insights into motoneurone discharge behaviour have stemmed from recent observations of sustained discharge of action potentials (plateau potentials) following withdrawal of an excitatory input. These plateau potentials are likely to be a result of persistent inward currents (PICs) mediated by voltage-dependent channels (Hounsgaard et al. 1988; Kiehn & Eken, 1997; for review, see Heckman et al. 2005, 2008). As well as causing the sustained discharge of action potentials, the PIC has an ‘amplifying’ effect on the increase in discharge rate of the motoneurone to a given input and also limits the firing rate (Bennett et al. 1998; Heckman et al. 2008). A number of associations with respect to the PIC effect have been established between the motoneurone in animal preparations and human motor units, including an onset–offset hysteresis of discharge rate at recruitment and de-recruitment, i.e. the lower discharge rate at de-recruitment compared with that at recruitment. (Gorassini et al. 2002). Recently it was proposed that the extent of such a PIC effect can be quantified indirectly using paired motor units analysis (ΔF method). In the analysis, the firing rate of the lower-threshold motor unit of the pair (reporter unit) is used to estimate the synaptic drive common to the motoneurone pool, including the drive to the other higher-threshold (test) motor unit of the pair. The degree to which a PIC helps to sustain the discharge of the test unit is determined from the difference in reporter unit firing rate at recruitment and de-recruitment of the test unit (ΔF). This ΔF value can be assumed to be the relative magnitude of the PIC contribution to the total net excitatory drive to the test unit (Gorassini et al. 2002; Powers et al. 2008). However, it is yet to be observed experimentally in humans how the PICs might influence the recruitment threshold and discharge characteristics of units across the entire motoneurone pool.
This lack of a detailed or decisive study on the organisation of recruitment and rate coding within the entire range of muscle contraction is likely to stem from the technical limitations in analysing electromyographic (EMG) signals, where with an increasing force, a number of motor unit action potentials (MUAPs) are superimposed, making it difficult to identify each of them individually.
We sought to solve these technical problems using two techniques; one is an EMG decomposition technique by which one can identify and discriminate the MUAPs from the EMG signal using interactive software that has been developed by McGill and colleagues (Lateva et al. 2002; McGill et al. 2005). The other is to perform a selective intramuscular EMG recording using branched wire electrodes (Gydikov et al. 1986; Enoka et al. 1988; Mottram et al. 2005) to achieve reasonable decomposition precision by minimising the number of MUAPs interfering with each other. By improving the selectivity and precision of decomposition, i.e. making the pick-up area of the electrodes smaller than originally developed, as well as achieving dual channel recording from nearby sites, it appeared that the technical issues could be tractable.
Thus, the purpose of the present study was to elucidate the upper limit of recruitment (discharge behaviour in relation to the recruitment threshold) and possible influence of PIC on the organisation of recruitment and rate coding in the soleus motor unit pool. To this end, we obtained motor unit recordings during ramp-and-hold contractions up to maximal voluntary contraction (MVC), using selective intramuscular EMG electrodes and analysed these using the decomposition method. Also, the decomposed units were then analysed using the onset–offset hysteresis as well as paired motor unit analysis.
Methods
Subjects
Four healthy male subjects (mean ±s.d. for age, height and weight were 31.8 ± 9.4 yr, 1.73 ± 0.30 m and 73.2 ± 4.7 kg, respectively) participated in the present study. All subjects gave their written informed consent after explanation of the experiment and the risks involved. The procedures were approved by the local university ethics committee and performed according to the Declaration of Helsinki.
Experimental set-up
Subjects were instructed to lie prone on an experimental bench with their right foot firmly strapped to a rigid foot-plate that was connected to a torque transducer (Maywood Instruments, Basingstoke, UK). The knee was maintained close to full extension to allow the triceps surae to fully participate in producing plantar flexion torque (Cresswell et al. 1995). The foot-plate positioned such that the ankle angle was kept at 90 deg. The plantar flexion torque signal was amplified (BK 1-5, Nobel Elektronik, Karlskoga, Sweden), sampled at 100 Hz using a 16-bit Micro 1401 mk-II and Spike2 software (Cambridge Electronic Design, Cambridge, UK), and displayed on a monitor as visual feedback of the torque they produced.
Motor unit recordings
To achieve selective recordings of motor unit action potentials in soleus (SOL), branched bipolar electrodes were used (see Gydikov et al. 1986; Enoka et al. 1988; Mottram et al. 2005). An electrode comprised one or two pairs of wires (stainless steel, 50 μm diameter; California Fine Wire, Grover Beach, CA, USA) where three ∼200 μm lengths of insulation were removed to expose the wire: two on one wire (branched) approximately 600 μm apart, and one on the other directly across from the midpoint between these two (monopolar). The wires were then fastened together with non-toxic glue, inserted into the barrel of a 23-gauge (32 mm) disposable needle and autoclaved for sterilisation. The needle was used to insert the wires, using sterile procedures, into the lateral aspect of SOL, penetrating the muscle fascia. The recording site within the muscle was sometimes adjusted by lightly pulling on the wires until a good signal-to-noise ratio was observed during a brief, weak contraction (5–10% maximal voluntary contraction (MVC)). A single Ag–AgCl reference electrode (diameter 10 mm, Tyco Healthcare Group LP, Hampshire, UK) was placed over the lateral condyle of the tibia. The EMG signals were amplified 1000 times (NL844 Pre-Amplifier, Digitimer Ltd, Welwyn Garden City, UK) and band-pass filtered between 6 and 5 kHz (NL 900L, Digitimer). The EMG signals were analog–digitally converted at a sampling rate of 20 kHz using the same data collection system and software described earlier.
Protocol
The experiment involved the performance of voluntary isometric contractions of the plantar flexors over their entire range of activation. To determine the maximal amount of plantar flexor torque around the ankle each subject could produce, the subjects performed three maximal contractions of the plantar flexors with strong verbal encouragement by the experimenters. The highest torque produced out of the three contractions was used as the MVC torque for the following testing.
The subjects were thereafter asked to perform isometric ramp-and-hold contractions controlled using visual feedback of their voluntary torque displayed on a computer monitor. A horizontal and four vertical lines were presented on the monitor, the horizontal line representing the target torque and the vertical lines indicating timing events of start to increase torque, hold, begin to decrease torque and relax. By the experimenter positioning the lines, the subjects were increasing their plantar flexor torque to MVC in approximately 10 s, holding it at this level for 1 s, and then declining their torque to rest in 10 s (Fig. 1). Several training trials were given until the subject felt accustomed to the contractions and the experimenter was satisfied that the ramp-and-hold pattern was adequately followed. Thereafter the ramp contractions were repeated five times during which EMG and torque data were collected. The absolute values for rates of change in torque during the ramp-up (9.7 ± 0.7%MVC s−1) and ramp-down (8.8 ± 0.3%MVC s−1) phases of the plantar flexion were not significantly different (paired t test, P= 0.052). To minimise the effect of fatigue, a minimum of 90 s of rest was given between contractions.
Figure 1. A representative intramuscular EMG recording obtained from different proximal sites and recruitment de-recruitment thresholds of a motor unit.
As illustrated by raw EMG traces in the middle, the same timings of discharges of motor units from the soleus muscle were observed. During ramp-and-hold isometric plantar flexion in 10 s, the motor units were recruited continuously up to more than 90% of MVC. Also one motor unit commences discharge at 40.9% of MVC with a frequency at 8.3 Hz, and ceases the discharge at 39.9% of MVC with a rate at 2.7 Hz.
Data analysis
Decomposition
The obtained EMG signals were analysed to identify trains of motor unit action potentials (MUAPs), so called ‘spikes’. This decomposition process was performed using an interactive computer program, ‘EMGLAB’, developed by McGill et al. (2005) based in a MATLAB environment (The Mathworks, Natick, MA, USA).
The decomposition procedures were performed manually using the graphical user interface of EMGLAB. The decomposition procedure involved (1) creating templates, (2) classifying MUAPs using template matching, and (3) resolving superimpositions of multiple MUAPs. The EMG signal was processed in 0.5 s segments that were additionally high-pass filtered at 1 kHz. New templates were formed with visual inspection by selecting single MUAPs (spikes with a high degree of similarity in shape that discharged at least three times) from the signal panel and subsequently averaging them. The subsequent MUAPs were then classified using template matching. For template matching, two techniques were employed using the residual signal after the candidate template was removed, and the expected discharge time range. First, a small residual indicates that the candidate template matches very well to a spike and therefore the examined spike belongs to the MUAP with the candidate template. Second, motor units can be assumed to discharge with a relatively regular timing, such that the timing of the next firing can be predicted using the mean interspike intervals (ISIs) of preceding MUAP discharges.
When there was substantial residual, two possibilities were considered. One possibility is that other units discharged coincidentally causing MUAP superimpositions. To resolve these superimpositions, different sets of templates were selected and adjusted to find the best fit, also using the predicted discharge timing of MUAPs. EMGLAB is capable of finding the best-fit alignment of multiple MUAPs (McGill, 2005), such that the residual is minimal. Otherwise, if the residual spikes had a high-degree of similarity and appeared continuously, the spikes were classified into a new MUAP template. In this way, spikes were sorted into MUAP templates and every time they were classified into the existing template, the templates were re-averaged. If there seemed to be no conspicuous activity in the EMG signal in the working 0.5 s segment, the analysis proceeded to the next segment with the existing templates, and was repeated until the last segment.
As the decomposition procedures involve examiner's heuristics, the procedures are inevitably subjective. To validate the decomposition procedures we examined (cross-checked) MUAPs that were recorded from a close-by site (1–2 mm apart) using the second pair of wires. In two subjects, the same MUAPs were recorded from another pair of wires, and verified that the identified units discharged at the same time with a fixed offset, as shown in Channel 1 and Channel 2 in Fig. 1. For the cross-check, the MUAP was recognised only when it exhibited a coincident spike in both channels.
Subsequently the recruitment threshold of each motor unit was determined by moving a 500 ms window forward in steps of 1 ms until the coefficient of variation (CV) of ISIs within the window was less than 0.5 (Moritz et al. 2005). De-recruitment threshold was determined in the same manner but by moving the window backward from the last segment of the signal in 1 ms steps. For the unit whose MUAPs were not followed to the MVC, the discharges with the CV less than 0.5 were taken for later analysis. The toques corresponding to the times of first and last discharges in the window were taken as the recruitment and de-recruitment thresholds, respectively. The difference in torque and discharge rate at recruitment and de-recruitment were then computed as values at recruitment minus those at de-recruitment. As such, positive values represent lower rates at de-recruitment compared to recruitment threshold. The slope of the discharge rate as a function of the plantar flexion torque was also calculated by dividing the amount of increase in the discharge rate by the amount of increase in the torque from the recruitment threshold to MVC.
Paired motor units analysis
To derive the magnitude of contribution of PICs, it was necessary to dissociate the amount of synaptic input and the intrinsic PIC effect. To achieve this, Gorassini et al. (2002) proposed that the difference (ΔF) in discharge rate of one unit (reporter) at recruitment and de-recruitment of the other unit (test) can be an indirect measure of the extent of PIC contribution. This was on the assumption that the rate of the reporter unit should represent the synaptic drive common to both units as long as the rates of the both units are well-correlated and sustained firing of the test unit due to PIC will result in lower discharge rate of the reporter unit.
For this analysis, we performed a rate–rate plot analysis between selected reporter and test units. For each recording, the reporter unit was determined as (1) being simultaneously recorded with the test unit; (2) being as low-threshold as possible; and (3) being trackable at the recruitment and de-recruitment thresholds of the test unit. Among the well-correlated units from the rate–rate analysis (r2 > 0.5), ΔF was calculated as the difference in the reporter rate at recruitment and de-recruitment (recruitment – de-recruitment) of the test unit, and thereafter the magnitude of PIC was further computed as follows:
 |
Statistics
Histograms of the number of motor units recruited and de-recruited with respect to plantar flexion torque were constructed to examine motor unit recruitment and de-recruitment ranges. The differences in the torque and discharge rate between recruitment and de-recruitment thresholds were examined using Student's t test for paired data. Correlations were performed among the following variables: the torque at recruitment, the discharge rate at recruitment, the peak discharge rate, differences in torque and discharge rate at recruitment and de-recruitment, slope of increase in discharge rate as a function of the torque, and the extent of PIC contribution calculated using the paired motor unit analysis. Statistical analysis was performed using MATLAB v7.0 software. The significance level was set at P < 0.05.
Results
Forty-two SOL motor units were clearly identified. Most of those units (37 in total) could be discriminated at plantar flexor torque levels continuing to MVC. The five units that were either lost or stopped firing during the increasing torque or hold phase were re-identified during the decreasing torque phase of the contraction. De-recruitment thresholds for these re-identified units could therefore be obtained.
As shown for a representative recording in Fig. 1, SOL motor units were recruited across the full range of plantar flexion torques. In this particular recording, units were recruited progressively. A common observation, and illustrated in the discharge trace in Fig. 1 for the unit recruited at 39% of MVC, was that motor units generally showed a lower discharge rate at de-recruitment threshold than at recruitment threshold. This was not the case for torque thresholds, which were generally similar for both recruitment and de-recruitment (Fig. 1, bottom trace).
The histograms in Fig. 2 summarise the number of motor units recruited and de-recruited with respect to plantar flexion torque, and shows that the number of motor units with varying thresholds is widely distributed.
Figure 2. The histogram for distribution of recruitment (filled bars) and de-recruitment (open bars) of motor units of the soleus muscle with regard to the plantar flexion torque.
Both recruitment and de-recruitment ranges were widely spread out among the torque.
Figure 3A shows the recruitment threshold and discharge rate of all units discriminated during the ramp-up and -down phase of the plantar flexion contraction. Generally speaking, those motor units recruited at lower torques (low-threshold units) discharged action potentials at lower frequencies at recruitment threshold and reached lower peak discharge rates, as represented by a recording in Fig. 3B. The units recruited at higher torque levels (high-threshold units) exhibited higher firing rates at recruitment threshold, as well as higher peak discharge rates. Interestingly, however, the motor units recruited above 89% of MVC (n= 5) showed relatively low discharge frequencies at recruitment, as well as at their peak. The discharge rates of these highest-threshold units were as low as those units recruited at or below 30% of MVC. In order to see the influence of these highest threshold units on the relationship between recruitment threshold and other discharge properties, correlation analyses were made with and without those highest-threshold units included in the analysis.
Figure 3. Discharge behaviours of SOL motor units as a function of plantar flexion torque.
A, the instantaneous discharge rate (low-pass filtered at 0.5 Hz) of 42 motor units during isometric ramp-and-hold plantar flexion. The present experimental data illustrates that the recruitment of the motor units range from 0.2% to 96.4% of MVC. Also, asymmetry was found during ramp-up and ramp-down phases of the contractions. B, the observation at odds with the often described ‘onion skin phenomenon’, where later-recruited units show higher discharge rate than those recruited earlier.
Recruitment and de-recruitment thresholds
On average, the discharge rate at de-recruitment threshold was significantly lower (nearly half) that at recruitment threshold (P < 0.001; mean ±s.d. for recruitment and de-recruitment were 8.5 ± 2.9 and 4.8 ± 1.9, respectively). This difference, between recruitment and de-recruitment, was not evident for the respective torque thresholds (Fig. 5B), with mean ±s.d. normalised torque values for recruitment and de-recruitment being 47.0 ± 31.6 and 45.6 ± 34.3% of MVC, respectively (P= 0.50).
Figure 4. Relations between the torque at recruitment threshold and the differences in the torque and discharge rate at recruitment and de-recruitment thresholds.
A, while the correlation was insignificant between the toque at recruitment threshold and the difference in discharge rate between recruitment and de-recruitment thresholds (r= 0.05, P= 0.72, n= 42), the correlation was evident when the highest threshold units (open circle) were left out (r= 0.40, P= 0.012, n= 37). B, no correlation was found between the torque at recruitment threshold and the difference in the torque between the recruitment and de-recruitment thresholds, with or without the highest-threshold units (r= 0.05 and −0.02, respectively, P= 0.47 and 0.86, n= 42 and 37, respectively; *P < 0.05).
Differences between recruitment and de-recruitment for discharge rates and torques in relation to recruitment threshold
The difference in discharge rate or torque between recruitment and de-recruitment thresholds is shown in Fig. 4A and B for all motor units. Neither difference in discharge rate nor difference in torque showed a significant correlation with recruitment threshold (r=−0.02 and 0.05, P= 0.47 and 0.72, respectively, Fig. 4A and B grey lines) when all motor units were included in the analysis. However, a significant correlation was found between the difference in discharge rate and recruitment threshold if motor units recruited above 89% of MVC were removed from the analysis (r= 0.40, P= 0.012, Fig. 4A, black line).
Discharge rate at recruitment
The discharge rate at recruitment threshold was found to be significantly correlated with recruitment threshold (r= 0.35, P= 0.017, Fig. 5A, thin line). Furthermore, once the units recruited above 89% were removed form the analysis the correlation was stronger (r= 0.61, P < 0.001, Fig. 5A, thick line). A high degree of correlation was also observed between the discharge rate at recruitment and the difference in discharge rate at recruitment and de-recruitment with or without the units recruited above 89% (r= 0.75 and 0.78, P < 0.001, Fig. 5B, thin and thick lines, respectively).
Figure 5. Relations between the initial discharge rate and the torque at recruitment (A), and between the initial discharge rate and the difference in discharge rate at recruitment and de-recruitment (B).
A, the discharge rate at recruitment was correlated with the recruitment torque (r= 0.35, P= 0.017, n= 42). If the highest units were excluded (open diamonds), the initial discharge rate exhibited a greater correlation coefficient (r= 0.61, P < 0.001, n= 37). B, the initial discharge rate indicated a high correlation with the difference in discharge rate at recruitment and de-recruitment thresholds with or without the highest-threshold units (r= 0.78 and 0.75, respectively, P < 0.001 for both, n= 42 and 37, respectively; *P < 0.05).
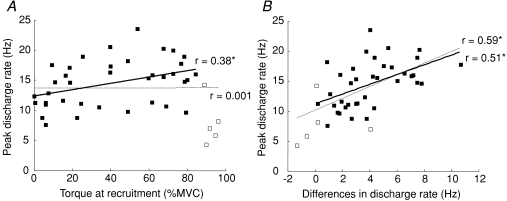
Peak discharge rate
Although the peak discharge rate was not correlated with recruitment threshold for all motor units (r= 0.001, P= 0.99, Fig. 6A, thin line) when the units recruited above 89% were excluded, a significant correlation was found (r= 0.38, P= 0.016, Fig. 6B, thick line). In addition, a significant correlation was found between the peak discharge rate and the difference in discharge rate at recruitment and de-recruitment with or without the units recruited above 89% (r= 0.59 and 0.51, P < 0.001, Fig. 6B, thin and thick lines, respectively).
Figure 6. Relations between the peak discharge rate and the torque at recruitment (A), and the peak discharge rate and the difference in discharge rate at recruitment and de-recruitment (B).
A, while the peak discharge rate had no correlation with the torque at recruitment (r= 0.001, P= 0.99, n= 42), a significant correlation was found in the case where the highest-threshold units (open rectangle) were taken out (r= 0.38, P= 0.017, n= 37). In addition, the peak discharge rate had a good correlation with the difference in discharge rate between the recruitment and de-recruitment thresholds whether the highest-threshold units were included or not (r= 0.59 and 0.51, respectively, P < 0.001 for both, n= 42 and 37, respectively; *P < 0.05).
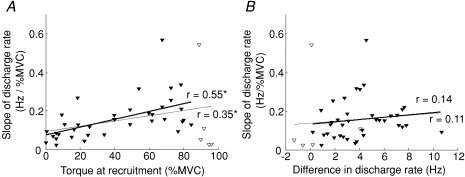
The rate of increase in firing rate as a function of plantar flexion torque
The proportion of increase in discharge rate with respect to the toque (slope) was significantly correlated with the recruitment threshold when either all units were included (r= 0.35, P= 0.017, Fig. 7A, thin line) or when the units recruited above 89% of MVC were not considered (r= 0.55, P < 0.001, Fig. 7A, thick line). On the other hand, no correlation was found between the discharge rate increase and difference in discharge rate between the recruitment and de-recruitment thresholds with or without the units recruited above 89% (r= 0.11 and 0.14 P= 0.47 and 0.40, Fig. 7B, thin and thick lines, respectively).
Figure 7. Relations between the slope of discharge rate with regard to the torque and the torque at recruitment (A), and between the slope and the difference in discharge rate at recruitment and de-recruitment (B).
A, a correlation was observed between the slope of increment in discharge rate and the torque at recruitment (r= 0.35, P= 0.017, n= 42) and furthermore, the removal of the highest-threshold units (open inverted triangle) made the correlation better (r= 0.55, P < 0.001, n= 37). On the other hand, the slope of increment in the discharge rate did not show any correlation with the difference in the discharge rate between recruitment and de-recruitment thresholds no matter whether the highest-threshold units were taken into the analysis (r= 0.11 and 0.14, P= 0.47 and 0.40, n= 42 and 37 respectively; *P < 0.05).
PIC contribution based on paired motor unit analysis to firing behaviours
As shown in Fig. 8A, rate–rate plots of reporter and test units in 31 pairs showed high correlations (r2 > 0.5). As shown in Fig. 8B, among those linearly correlated pairs, a negative correlation was observed between the magnitude of PIC contribution and the torque at recruitment (r=−0.46, P= 0.009). For the 31 pairs, the extent of PIC was not correlated with the onset–offset hysteresis in discharge rate (r=−0.21, P= 0.25), or with any discharge profiles including the initial rate (r=−0.33, P= 0.08), peak rate (r=−0.29, P= 0.10, n= 31), and the rate of increase in firing rate (r=−0.32, P= 0.07).
Figure 8. Results of paired units (ΔF) analysis.
A, a rate–rate plot of 31 pairs of reporter and test units with high correlation coefficients (r2 > 0.5) are shown. Continuous lines represent firing rates during the ramp-up phase whereas dashed lines represent the ramp-down phase. B, relationship between PIC magnitude and torque at recruitment. A correlation was observed between the magnitude of PIC and torque at recruitment for the 31 pairs (r=−0.46, P= 0.009). The removal of the two highest-threshold units (open inverted triangle) made the correlation non-significant (r=−0.34, P= 0.065).
Discussion
The aim of the study was, for the first time, to describe the recruitment threshold and firing properties of soleus motor units across the full range of plantar flexion contraction strength. The main finding was that soleus motor units, unlike motor units previously described in arm muscles, are progressively recruited from rest to contraction strengths close to MVC. An additional observation that is unique to soleus units was that at recruitment, the early recorded low-threshold units had lower firing rates than the later recorded high threshold units. The peak firing rate of the same low threshold units was also found to be less than that of the higher threshold units. Differences between the recruitment and de-recruitment discharge rates (onset–offset hysteresis) were found and were correlated to motor unit recruitment order, initial firing rate and peak firing rate. The extent of PIC contribution was found to be negatively correlated with the recruitment threshold and was not correlated with any measured discharge behaviours.
Recruitment threshold
Previous studies have reported the upper threshold of motor unit recruitment for hand muscles to be approximately 50% of MVC for the adductor pollicis (Kukulka & Clamann, 1981) and between 50 and 75% of MVC for the first dorsal interosseus (De Luca et al. 1982; Thomas et al. 1986; Moritz et al. 2005). For the more proximal and larger upper limb muscle, biceps brachii has been reported to recruit high threshold units up to contraction strengths of 88% of MVC (Kukulka & Clamann, 1981). Despite these latter studies and due to previous technical difficulties in resolving individual units at high contraction strengths, little information has been available on the complete recruitment range of many large muscles. From observations in the current study, it appears that the soleus muscle is somewhat unique in that its high-threshold units are available for recruitment at activation levels close to MVC. Such late recruitment of high threshold units suggests that the soleus muscle utilises both recruitment and rate modulation over its full range of contraction strength rather than solely relying upon rate modulation at high forces, as is the case for many muscles. This behaviour may be related to contractile properties of the muscle such as contractile speed or force production capability as documented by Bellemare et al. (1983). Slow twitch fibres can be tetanised by firing of motor units at low rates, but once the fibres are tetanised any increase in force cannot be achieved by further increases in discharge rate. Fast twitch fibres, on the other hand, require much higher firing rates for fusion. Therefore, to fully activate the soleus muscle, which predominantly consists of slow twitch fibres, motor units need to be recruited progressively up to nearly maximal force level. The relationships found in our results, as well as previous reports (Gydikov & Kosarov, 1974; Moritz et al. 2005), support the postulate that recruitment and rate coding within a motor unit pool are organised to meet the contractile properties of each muscle (Bellemare et al. 1983).
Initial and peak discharge rates and the slope of discharge rate in relation to recruitment threshold
It was shown, with the exception of the units recruited above 89% of MVC, that the initial and peak firing rates were significantly higher for the later recruited higher-threshold motor units than for the earlier recruited low-threshold units. The high-threshold units also showed a greater increase in discharge rate as a function of the torque. These results are consistent with previous experimental investigations in the biceps brachii (Gydikov & Kosarov, 1974) and first dorsal interosseus (Moritz et al. 2005) showing the dependence of the initial and peak rate on the recruitment threshold. However this robust finding is in contrast to the described ‘onion skin phenomenon’ where low-threshold units exhibit higher peak firing rates than high-threshold units (Freund et al. 1975; Erim et al. 1996). It is possible that the limited range of contraction strengths in the studies by Freund et al. (1975) and Erim et al. (1996) may explain their opposite result to what we observed. For example, as seen in Fig. 3 of the present study, if only those motor units recruited up to 60% of MVC were analysed, the low-threshold units would generally show higher discharge rates compared to the high-threshold units within that range. However, the opposite appears true if the torque range is extended to higher contraction levels. The recruitment and rate coding organisation observed in the present study fits the expectation obtained from repetitive discharge properties of motoneurones in the cat preparations (Kernell, 1965). It also fits with the mechanical properties of the motor units since high-threshold motor units require higher discharge frequency to attain a fused force (Grimby et al. 1979). Therefore, as observed in the present experiment, the dependence of the initial and peak discharge on the recruitment threshold is more likely to occur as a general motor unit behaviour to achieve force production across a large or full range of strength.
The difference in discharge rate at recruitment and de-recruitment and its association with persistent inward currents
The finding that the soleus discharge rate at de-recruitment was significantly lower than at recruitment, i.e. an onset–offset hysteresis, is largely consistent with previous studies on upper limb muscles (De Luca et al. 1982; Denier van der Gon et al. 1985; Romaiguère et al. 1993). If PICs are responsible for this onset–offset hysteresis, then a difference between the torque at recruitment and de-recruitment should also be observed. However, our observation of a lack of significant difference in the levels of torque at which recruitment and de-recruitment took place fails to support this idea and is in contrast to previous reports (Person & Kudina, 1972; Denier van der Gon et al. 1985; Romaiguère et al. 1993). A possible explanation for this difference may lie in the variability of the discharge rate of each unit, as the net of both negative and positive torque difference at recruitment and de-recruitment, as reflected by each motor unit discharge, may have lead to the absence of significant hysteresis in the torque. Also, as suggested by Romaiguère et al. (1993) and Gorassini et al. (2002), co-contraction of antagonist muscles may have occurred during the ramp-down phase of the contraction and confounded the plantar flexion torque. An additional possibility is due to the time lag between detection of a MUAP and full expression of motor unit force (see Fuglevand et al. 2006). That is, while the recruitment torque reflects the activities of all previously active units, de-recruitment torque results from the forces of the other active units plus the force contributed by the unit itself.
The observed onset–offset hysteresis in firing rate may be due to the activation of intrinsic conductances in the motoneurone (cf. Gorassini et al. 2002; Heckman et al. 2005, 2008). In animal preparations, motoneurones have been shown to exhibit hysteresis, that is, continuing to discharge at a level of depolarisation that is lower than the level where the initial discharge of the action potentials were initiated, for depolarising synaptic or intracellularly injected inputs (Hounsgaard et al. 1988; Lee & Heckman, 1998; Bennett et al. 1998). Such firing behaviour has been shown to be mediated by the activation of intrinsic voltage-dependent PICs in the dendrites of the motoneurone through voltage-dependent Na+ and Ca2+ channels (Lee & Heckman, 1998; Bennett et al. 1998). Although the occurrence of PICs cannot be substantiated in the present study, on the basis of similar firing behaviours in animal preparations, activation of PICs during the performance of our isometric voluntary contractions may, at least in part, explain the difference in threshold for recruitment compared with de-recruitment.
However, the paired unit F analysis revealed that the PIC magnitude was not related to the observed discharge behaviours, indicating that the PICs were not an underlying factor for determining discharge properties. Furthermore, the magnitude of PIC contribution was considered to be less for high-threshold units, which appears to be in agreement with previous investigations (Lee & Heckman, 1998). Nevertheless, the correlations between the onset–offset hysteresis and the recruitment threshold, as well as initial and peak discharge rates, suggest that some other mechanisms that impact on the discharge behaviours, were in operation but could not be detected by the paired motor unit ΔF analysis. For example, the lowered discharge rate at de-recruitment may also reflect the lowered voltage threshold for repetitive discharges of the spike-generative mechanism at the soma and the initial segment of the axon. This can be mediated by an alteration in the properties of the afterhyperpolarisation (AHP), which can occur as a result of serotonin- or noradrenaline-induced neuromodulation (Rekling et al. 2000; Heckman et al. 2008). To explore such mechanisms, however, further investigation is required.
Discharge characteristics and the association with persistent inward currents
Although the peak discharge rate was not directly correlated with PIC contribution, the observed negative correlation of recruitment threshold with PIC contribution may help explain the observed low peak firing rate in the low-threshold units. While PICs are known to strongly amplify synaptic input, once they are fully activated the efficacy of further input is limited (Lee & Heckman, 2000), which may limit the firing rate of the motoneurone (Bennett et al. 1998; Lee & Heckman, 1998). Alternatively, as suggested by Heckman & Binder (1993), the low peak firing rate for low-threshold units could be accounted for by input organisations that have opposite synaptic excitatory and inhibitory gradients.
It was also observed that the very high-threshold units, which were recruited over 89% of MVC, showed low initial and peak discharge rates, whereas high-threshold units that were recruited earlier exhibited higher initial and high peak discharge rates. The highest units also showed less or even a negative difference in the discharge rate between recruitment and de-recruitment. A possible interpretation for the firing behaviour of these units may be explained in terms of the level of PIC activation. That is, such small or negative differences in recruitment and de-recruitment discharge rate may suggest that PICs for those motoneurones were not activated and correspondingly, due to the lesser amplification of synaptic input, the initial and peak discharge rates were lower. This interpretation seems reasonable, since investigations on cat motoneurones have revealed that PICs are activated in a graded manner, even above their threshold voltage, and depending upon the activation level, the extent of amplification is varied (Hultborn et al. 2004). A negative correlation between estimated PIC magnitude and the recruitment threshold seems to support this view. However, it is yet largely unknown whether the magnitude of PIC activation pertains to the size or recruitment threshold of the motoneurone.
Summary
For the soleus muscle, a successive recruitment of motor units occurs throughout the full contraction range. The discharge behaviour of the motor units is organised such that low-threshold motor units discharge action potentials slower at their recruitment and peak than later recruited high-threshold units. However, an exception was found for the highest-threshold units (>89%), which exhibited discharge rates as low as the low-threshold units (<30%). The variation in discharge behaviours appears to pertain to the difference in the discharge rate at recruitment and de-recruitment, which may be reflected by intrinsic mechanisms other than PICs.
Glossary
Abbreviations
- ISI
interspike interval
- MUAP
motor unit action potential
- MVC
maximal voluntary contraction
- PIC
persistent inward current
Author contributions
T.O. Oya: Conception and design of the study. Completion and analysis of experiments. Drafted and revision of manuscript. S. Riek: Contribution to conception and design of the experiment. Supervision of experiments and critical revision of manuscript. A.G. Cresswell: Contribution to conception and design of the experiment. Supervision of experiments and critical revision of manuscript.
References
- Bellemare F, Woods JJ, Johansson R, Bigland-Ritchie B. Motor-unit discharge rates in maximal voluntary contractions of three human muscles. J Neurophysiol. 1983;50:1380–1392. doi: 10.1152/jn.1983.50.6.1380. [DOI] [PubMed] [Google Scholar]
- Bennett DJ, Hultborn H, Fedirchuk B, Gorassini MA. Synaptic activation of plateaus in hindlimb motoneurons of decerebrate cats. J Neurophysiol. 1998;80:2023–2037. doi: 10.1152/jn.1998.80.4.2023. [DOI] [PubMed] [Google Scholar]
- Cresswell AG, Löscher WN, Thorstensson A. Influence of gastrocnemius muscle length on triceps surae torque development and electromyographic activity in man. Exp Brain Res. 1995;105:283–290. doi: 10.1007/BF00240964. [DOI] [PubMed] [Google Scholar]
- De Luca CJ, LeFever RS, McCue MP, Xenakis AP. Behaviour of human motor units in different muscles during linearly varying contractions. J Physiol. 1982;329:113–128. doi: 10.1113/jphysiol.1982.sp014293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denier van der Gon JJ, ter Haar Romeny BM, van Zuylen EJ. Behaviour of motor units of human arm muscles: differences between slow isometric contraction and relaxation. J Physiol. 1985;359:107–118. doi: 10.1113/jphysiol.1985.sp015577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoka RM, Robinson GA, Kossev AR. A stable, selective electrode for recording single motor-unit potentials in humans. Exp Neurol. 1988;99:761–764. doi: 10.1016/0014-4886(88)90189-6. [DOI] [PubMed] [Google Scholar]
- Erim Z, De Luca CJ, Mineo K, Aoki T. Rank-ordered regulation of motor units. Muscle Nerve. 1996;19:563–573. doi: 10.1002/(SICI)1097-4598(199605)19:5<563::AID-MUS3>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Freund H. Motor unit and muscle activity in voluntary motor control. Physiol Rev. 1983;63:387–436. doi: 10.1152/physrev.1983.63.2.387. [DOI] [PubMed] [Google Scholar]
- Freund H, Büdingen HJ, Dietz V. Activity of single motor units from human forearm muscles during voluntary isometric contractions. J Neurophysiol. 1975;38:933–946. doi: 10.1152/jn.1975.38.4.933. [DOI] [PubMed] [Google Scholar]
- Fuglevand AJ, Dutoit AP, Johns RK, Keen DA. Evaluation of plateau-potential-mediated ‘warm up’ in human motor units. J Physiol. 2006;571:683–693. doi: 10.1113/jphysiol.2005.099705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorassini MA, Yang JF, Siu M, Bennett DJ. Intrinsic activation of human motoneurons: possible contribution to motor unit excitation. J Neurophysiol. 2002;87:1850–1858. doi: 10.1152/jn.00024.2001. [DOI] [PubMed] [Google Scholar]
- Grimby L, Hannerz J, Hedman B. Contraction time and voluntary discharge properties of individual short toe extensor motor units in man. J Physiol. 1979;289:191–201. doi: 10.1113/jphysiol.1979.sp012732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gydikov A, Kosarov D. Some features of different motor units in human biceps brachii. Pflugers Arch. 1974;347:75–88. doi: 10.1007/BF00587056. [DOI] [PubMed] [Google Scholar]
- Gydikov A. Selective recording of motor unit potentials. Electromyogr Clin Neurophysiol. 1986;26:273–281. [PubMed] [Google Scholar]
- Heckman CJ, Gorassini MA, Bennett DJ. Persistent inward currents in motoneuron dendrites: implications for motor output. Muscle Nerve. 2005;31:135–156. doi: 10.1002/mus.20261. [DOI] [PubMed] [Google Scholar]
- Heckman CJ, Binder MD. Computer simulations of motoneuron firing rate modulation. J Neurophysiol. 1993;69:1005–1008. doi: 10.1152/jn.1993.69.4.1005. [DOI] [PubMed] [Google Scholar]
- Heckman CJ, Johnson M, Mottram C, Schuster J. Persistent inward currents in spinal motoneurons and their influence on human motoneuron firing patterns. Neuroscientist. 2008;14:264–274. doi: 10.1177/1073858408314986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hounsgaard J, Hultborn H, Jespersen B, Kiehn O. Bistability of α-motoneurones in the decerebrate cat and in the acute spinal cat after intravenous 5-hydroxytryptophan. J Physiol. 1988;405:345–367. doi: 10.1113/jphysiol.1988.sp017336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn H, Brownstone RB, Toth TI, Gossard J. Key mechanisms for setting the input-output gain across the motoneuron pool. Prog Brain Res. 2004;143:77–95. doi: 10.1016/s0079-6123(03)43008-2. [DOI] [PubMed] [Google Scholar]
- Kernell D. The limits of firing frequency in cat lumbosacral motoneurones possessing different time course of afterhyperpolarization. Acta Physiol Scand. 1965;65:87–100. [Google Scholar]
- Kiehn O, Eken T. Prolonged firing in motor units: evidence of plateau potentials in human motoneurons? J Neurophysiol. 1997;78:3061–3068. doi: 10.1152/jn.1997.78.6.3061. [DOI] [PubMed] [Google Scholar]
- Kukulka CG, Clamann HP. Comparison of the recruitment and discharge properties of motor units in human brachial biceps and adductor pollicis during isometric contractions. Brain Res. 1981;219:45–55. doi: 10.1016/0006-8993(81)90266-3. [DOI] [PubMed] [Google Scholar]
- Lateva ZC, McGill KC, Johanson ME. Electrophysiological evidence of adult human skeletal muscle fibres with multiple endplates and polyneuronal innervation. J Physiol. 2002;544:549–565. doi: 10.1113/jphysiol.2002.023267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Bistability in spinal motoneurons in vivo: systematic variations in persistent inward currents. J Neurophysiol. 1998;80:583–593. doi: 10.1152/jn.1998.80.2.583. [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Adjustable amplification of synaptic input in the dendrites of spinal motoneurons in vivo. J Neurosci. 2000;20:6734–6740. doi: 10.1523/JNEUROSCI.20-17-06734.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill KC, Lateva ZC, Marateb HR. EMGLAB: an interactive EMG decomposition program. J Neurosci Methods. 2005;149:121–133. doi: 10.1016/j.jneumeth.2005.05.015. [DOI] [PubMed] [Google Scholar]
- Monster AW, Chan H. Isometric force production by motor units of extensor digitorum communis muscle in man. J Neurophysiol. 1977;40:1432–1443. doi: 10.1152/jn.1977.40.6.1432. [DOI] [PubMed] [Google Scholar]
- Moritz CT, Barry BK, Pascoe MA, Enoka RM. Discharge rate variability influences the variation in force fluctuations across the working range of a hand muscle. J Neurophysiol. 2005;93:2449–2459. doi: 10.1152/jn.01122.2004. [DOI] [PubMed] [Google Scholar]
- Mottram CJ, Jakobi JM, Semmler JG, Enoka RM. Motor-unit activity differs with load type during a fatiguing contraction. J Neurophysiol. 2005;93:1381–1392. doi: 10.1152/jn.00837.2004. [DOI] [PubMed] [Google Scholar]
- Person RS, Kudina LP. Discharge frequency and discharge pattern of human motor units during voluntary contraction of muscle. Electroencephal Clin Neurophysiol. 1972;32:471–483. doi: 10.1016/0013-4694(72)90058-2. [DOI] [PubMed] [Google Scholar]
- Powers RK, Nardelli P, Cope TC. Estimation of the contribution of intrinsic currents to motoneuron firing based on paired motoneuron discharge records in the decerebrate cat. J Neurophysiol. 2008;100:292–303. doi: 10.1152/jn.90296.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rekling JC, Funk GD, Bayliss DA, Dong XW, Feldman JL. Synaptic control of motoneuronal excitability. Physiol Rev. 2000;80:767–852. doi: 10.1152/physrev.2000.80.2.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romaiguère P, Vedel JP, Pagni S. Comparison of fluctuations of motor unit recruitment and de-recruitment thresholds in man. Exp Brain Res. 1993;95:517–522. doi: 10.1007/BF00227145. [DOI] [PubMed] [Google Scholar]
- Van Custem M, Feiereisen P, Duchateau J, Hainaut K. Mechanical properties and behaviour of motor units in the tibialis anterior during voluntary contractions. Can J Appl Physiol. 1997;22:585–597. doi: 10.1139/h97-038. [DOI] [PubMed] [Google Scholar]
- Thomas CK, Ross BH, Stein RB. Motor-unit recruitment in human first dorsal interosseous muscle for static contractions in three different directions. J Neurophysiol. 1986;55:1017–1029. doi: 10.1152/jn.1986.55.5.1017. [DOI] [PubMed] [Google Scholar]
- Tokizane T, Shimazu H. Functional Differentiation of Human Skeletal Muscle. Corticalization and Spinalizaion of Movement. Springfield, IL: Charles C. Thomas; 1964. [Google Scholar]