Abstract
Glucagon-like peptide-1 (GLP-1) is a neuropeptide released following meal ingestion that, among other effects, decreases gastric tone and motility. The central targets and mechanism of action of GLP-1 on gastric neurocircuits have not, however, been fully investigated. A high density of GLP-1 containing neurones and receptors are present in brainstem vagal circuits, suggesting that the gastroinhibition may be vagally mediated. We aimed to investigate: (1) the response of identified gastric-projecting neurones of the dorsal motor nucleus of the vagus (DMV) to GLP-1 and its analogues; (2) the effects of brainstem application of GLP-1 on gastric tone; and (3) the vagal pathway utilized by GLP-1 to induce gastroinhibition. We conducted our experiments using whole-cell recordings from identified gastric-projecting DMV neurones and microinjection in the dorsal vagal complex (DVC) of anaesthetized rats while monitoring gastric tone. Perfusion with GLP-1 induced a concentration-dependent excitation of a subpopulation of gastric-projecting DMV neurones. The GLP-1 effects were mimicked by exendin-4 and antagonized by exendin-9–39. In an anaesthetized rat preparation, application of exendin-4 to the DVC decreased gastric tone in a concentration-dependent manner. The gastroinhibitory effects of exendin-4 were unaffected by systemic pretreatment with the pro-motility muscarinic agonist bethanechol, but were abolished by systemic administration of the nitric oxide synthase (NOS) inhibitor NG-nitro-l-arginine methyl ester (l-NAME), or by bilateral vagotomy. Our data indicate that GLP-1 activates selective receptors to excite DMV neurones mainly and that the gastroinhibition observed following application of GLP-1 in the DVC is due to the activation of an inhibitory non-adrenergic, non-cholinergic input to the stomach.
Among the many anorexigenic peptides that reduce meal size, the incretin glucagon-like peptide 1 (GLP-1) can also be classified as a neurohumoral agent because of its ability to act as both hormone and transmitter in the periphery as well as the central nervous system (CNS) (Drucker, 2006).
GLP-1 is released into the circulation from intestinal L-cells in response to oral ingestion of a mixed meal, and acts to modulate gastric motility and emptying as well as increasing insulin release via actions at specific GLP-1 receptors on pancreatic β cells (Thorens, 1995; Delgado-Aros et al. 2002; Mayo et al. 2003; Schirra & Goke, 2005; Beglinger & Degen, 2006; Drucker, 2006; Andrews et al. 2007; Holst, 2007). Because of its ease of penetration through the blood–brain barrier (Kastin et al. 2002), GLP-1 also has major effects at the level of the CNS. Centrally acting GLP-1 acts via vagally mediated pathways to induce the release of insulin, decrease food intake and delay gastric emptying, as well as playing a relevant role in interoceptive stress (Rinaman, 1999a,b; Lachey et al. 2005; Baggio & Drucker, 2007; Holst, 2007). In the brainstem, GLP-1 immunoreactive soma are restricted to the nucleus tractus solitarii (NTS), although an extremely high density of GLP-1 binding sites as well as receptor mRNA are found throughout the DVC (i.e. DMV, NTS and the area postrema) (Shimizu et al. 1987; Kanse et al. 1988; Uttenthal et al. 1992; Goke et al. 1995; Larsen et al. 1997). GLP-1 has also been shown to increase the impulse discharge of fibres from the hepatic branch of the vagus nerve, to induce an increase in cFos activation in the NTS and to increase vagal efferent activity, suggesting possible roles in the modulation of all levels of vagal brainstem circuit activity (Nakabayashi et al. 1996; Van Dijk et al. 1996; Nishizawa et al. 2000). Indeed, we have shown recently that GLP-1 excites identified pancreas-projecting DMV neurones via both direct as well as synaptically mediated actions (Wan et al. 2007a,b;).
Vagally mediated gastrointestinal (GI) functions are controlled by two separate pathways both of which originate from the cholinergic preganglionic neurones of the DMV. At the postganglionic level, the parasympathetic vagal fibres comprise two separate and distinct pathways. Activation of the first pathway, a cholinergic excitatory pathway, increases gastric motility, tone and secretion, while its inhibition decreases gastric functions. Activation of the second pathway, a non-adrenergic, non-cholinergic (NANC) pathway, decreases gastric motility and tone (reviewed recently in Travagli et al. 2006). Thus, a vagally mediated decrease in gastric motility, such as that induced by GLP-1, could be obtained either by inhibition or withdrawal of the tonic excitatory cholinergic pathway or by activation of the inhibitory NANC pathway.
Despite the potential clinical relevance of this neurohormone and its stable analogue, exendin-4, neither the actions of GLP-1 at the level of the brainstem vagal circuits nor the actions at postganglionic neurones have been investigated in animal models.
The aims of the present study were to analyse: (1) the response of identified gastric-projecting neurones of the DMV to GLP-1 and its analogues; (2) the effects of brainstem application of GLP-1 on gastric tone; and (3) the vagal pathway utilized by GLP-1 to induce gastroinhibition.
Methods
All procedures, both in vitro and in vivo, were conducted in accordance with the National Institutes for Health guidelines, with the approval of the PBRC Institutional Animal Care and Use Committee and according to the standards on animal experimentation of The Journal of Physiology (Drummond, 2009).
In vitro studies: retrograde tracer application and tissue preparation
The fluorescent tracer DiI was applied along the greater curvature of the stomach of Sprague–Dawley rats as described previously (Browning et al. 1999; Browning & Travagli, 2007). Briefly, rats (12–14 days old) of either sex were anaesthetized deeply (3% isoflurane with air, 600 ml min−1). A deep level of anaesthesia (abolition of the foot pinch withdrawal reflex) was maintained throughout the surgical procedure. The abdominal and thoracic areas were cleaned with alcohol and Novalsan® prior to performing a midline laparotomy. The stomach was exposed, and DiI crystals were apposed to the corpus and fundus areas along the greater curvature of the stomach and embedded in place with fast-hardening epoxy resin that was allowed to dry for 3–5 min. The stomach was replaced in the abdominal cavity, the entire surgical area washed and blotted dry, the wound closed with 5–0 suture and the animal allowed to recover for 10–15 days.
The methods used to prepare the tissue slices has already been described (Travagli et al. 1991; Browning et al. 1999). Briefly, rats were anaesthetized deeply with isofluorane and killed by administration of a bilateral pneumothorax. The brainstem was removed and placed into oxygenated, ice-cold Krebs solution (see below). After being glued to a plastic support, five to six coronal slices (300 μm thick, 1.5–1.8 mm total length) containing the DMV were cut using a vibrating microtome. The slices were incubated and equilibrated for at least 1 h in oxygenated Krebs solution (32 ± 1°C) prior to electrophysiological recording. In each instance, the stomach was examined visually to ensure that the dye had not moved from its site of application and had not diffused into the abdominal milieu. A single slice was then mounted on a custom-made perfusion chamber (volume 500 μl), and kept in place by a nylon web. The slice was maintained at 35 ± 1°C by perfusion with Krebs solution at 2.5 ml min−1.
DMV neurones: identification and recordings
Patch-clamp recordings were made from fluorescently labelled DMV neurones only, visualized with a Nikon E600FN microscope equipped with tetramethylrhodamine isothiocyanate (TRITC) filters. Provided the period of illumination used for neuronal identification is brief (i.e. <5 s), carbocyanine dyes such as DiI do not cause adverse effects (Honig & Hume, 1989; Mendelowitz et al. 1992; Browning et al. 1999). Following labelling of the stomach, an average of five to seven unequivocally labelled neurones were observed in each brainstem slice, the majority of the cells being located in the intermediate DMV.
Patch clamp recordings were conducted using borosilicate patch pipettes with a tip resistance of 3–7 MΩ when filled with a potassium gluconate intracellular solution (see below). Recordings were done using a Axopatch 1D amplifier (Axon Instruments, Union City, CA, USA) and were corrected manually for liquid junction potential. Recordings were made only from neurones having a series resistance < 20 MΩ.
Neurones were recorded in current clamp mode and current was injected to establish a membrane potential of about −60 mV to record changes in firing rate, or about −65 mV to measure changes in membrane potential induced by superfusion with either GLP-1 or its stable analogue exendin-4. Agonists were superfused for a period of time sufficient for the response to reach plateau, usually 1–3 min. The longer perfusion time was necessary when the recorded neurone was deeper in the slice since the agonist was slower in equilibrating at the appropriate concentration. Desensitization to the effects of GLP-1 was not observed. At least 10 min were allowed between successive drug applications. Only one cell per slice was tested.
In vitro data and statistical analysis
Data were acquired at 10 kHz, filtered at 2 kHz, digitized via a Digidata 1320 interface (Axon Instruments) and stored and analysed on a PC utilizing pCLAMP8 software (Axon Instruments). Results are presented as means ±s.e.m. Each neurone served as its own control, i.e. the neurone was assessed before and after drug application and analysed using a paired t-test with significance set at P < 0.05. Neurones were considered as responsive when perfusion with 100 nm GLP-1 or exendin-4 induced a minimum of 3 mV depolarization or induced a 100% increase in action potential firing rate that recovered to baseline levels upon washout. When conducting the concentration–response curve, a minimum of three different concentrations of agonist were tested on the same cell at 10–15 min intervals. The EC50 was calculated using Statistica® software (StatSoft Inc., Tulsa, OK, USA) for each set of responses, the results being expressed as a mean.
Solution composition
The Krebs solution used was (in mm): 120 NaCl, 26 NaHCO3, 3.75 KCl, 1 MgCl2, 2 CaCl2 and 11 dextrose; maintained at pH 7.4 with 95% O2–5% CO2. The potassium gluconate intracellular solution used was (in mm): 128 potassium gluconate, 10 KCl, 0.3 CaCl2, 1 MgCl2, 10 Hepes, 1 EGTA, 2 ATP, 0.25 GTP; adjusted to pH 7.35 with KOH.
In vivo studies: surgical preparations and agonist applications
Experiments were performed on male Sprague–Dawley rats weighing 250–400 g. Animals were fasted overnight (with water ad libitum) before being anaesthetized with an intraperitoneal injection of thiobutabarbital (Inactin®; 120–150 mg kg−1i.p.). An adequate depth of anaesthesia was assessed (absence of the foot pinch withdrawal reflex) throughout the experimental period. Body temperature was monitored by a rectal thermometer and maintained at 37 ± 1°C with a heating pad.
Following complete anaesthesia, rats were intubated with a tracheal catheter and a laparotomy was perfomed. A 6 × 8 mm encapsulated miniature strain gauge (RB Products, Minneapolis, MN, USA) was aligned with the circular smooth muscle fibres and sutured to the anterior gastric corpus. The strain gauge leads were exteriorized, the signal was low pass filtered (0.5 Hz cut off), amplified (QuantaMetrics EXP CLSG-2, Newton, PA, USA), and recorded on a polygraph (Grass model 79, Grass Technologies/Astro-Med Inc., West Warwick, RI, USA) and on a computer using Axotape software (Axon Instruments). Following surgical instrumentation, animals were placed in a stereotaxic frame, and rectal temperature was monitored and maintained at 37 ± 1°C (Physitemp Instruments TCAT 2LV, Clifton, NJ, USA).
The head of the animal was oriented in order to expose the 4th ventricle by midline incision and removal of the overlying neck musculature. The pial membrane above the vagal trigone was dissected and the exposed tissues covered with a warm, saline-infused cotton patch. Following 1 h of stabilization, baseline values of gastric tone were determined as the mean value of the 5 min period immediately preceding drug application.
Drugs were either microinjected (60 nl; n= 11) at the following distance from calamus scriptorius (mm): 0.2–0.3 rostro-caudal, 0.1–0.3 medio-lateral and −0.5 dorso-ventral or applied to the surface of the 4th ventricle at the level of obex (2 μl; n= 25). All drugs were dissolved in isotonic phosphate buffered saline (PBS; in mm: 147.6 NaCl, 83.3 NaH2PO4, 12.9 KH2PO4). Signals of baseline motility and tone were acquired for 30 min after drug application. The drug-induced gastric effects were measured as the average of the 30 s period centred around the peak effect. The basal strain gauge output was monitored for any changes for 10 min following drug infusion.
Separate groups of animals were similarly prepared for control experiments. In one group (n= 8), prior to apposition of the gastric strain gauge, the subdiaphragmatic posterior vagus was sectioned and a ligature of silk suture was gently placed around the left cervical vagus as it passed alongside the internal carotid artery. The ligature was exteriorized through a length of PE-240 tubing for later transection of the vagus nerve. After application of agonist and a 30 min observation period, the ligature was withdrawn, severing the remaining vagal outflow to the stomach. The rat was observed for 30 min prior to a second application of agonist. In another group of animals (n= 7), systemic administration of the muscarinic agonist bethanechol (50 μg kg−1 bolus followed by continuous i.v. infusion 20 μg kg−1 h−1 for 20 min) was used to increase baseline gastric motility. Two minutes after administration of bethanechol, exendin-4 was delivered to the DVC. If exendin-4 reduced gastric tone despite the supramaximal exogenous stimulation of muscarinic cholinergic receptors on the gastric musculature via bethanechol administration, then we could infer that the effects of exendin-4 are not mediated by a postganglionic cholinergic pathway to the stomach but rather by postganglionic NANC pathways.
To confirm the potential involvement of postganglionic NANC pathways, two groups received exendin-4, followed 30 min later by the nitric oxide synthase inhibitor NG-nitro-l-arginine methyl ester (l-NAME; 10 mg kg−1 bolus i.v. injection) (Takahashi & Owyang, 1998). l-NAME was injected alone or in combination with bethanechol. Once the baseline motility and tone were stable, exendin-4 was reapplied. At the conclusion of the experiment, rats were killed by administration of bilateral pneumothorax followed by transcardial perfusion with PBS followed by a solution of 4% paraformaldehyde in PBS. The brainstem was extracted and fixed overnight in 4% paraformaldehyde, 20% sucrose in PBS. Following several rinses in PBS, the brainstem was then frozen and sliced at 40 μm thickness and alternate slices were visualized on a Nikon E400 microscope for identification of the site of injection or stained with cresyl violet for identification of anatomical markers.
In vivo data analysis and statistics
Individual strain gauges were calibrated with a 1 g weight applied externally before and after the experimental procedures, and the drug-induced effects on gastric tone were measured against the averaged value of the repeated 1 g measures. Changes in baseline gastric motor functions were determined by comparing the maximal reduction of the gastric tone signal after drug application relative to the 5 min average of the signal immediately prior to drug application. To avoid mechanical stimulations resulting from use of either an intragastric balloon or from gastric filling with fluid, basal gastric tone was not preset to a fixed value; the data measured are thus the values of the absolute tone displacement. Data were evaluated by comparing the change in response between pre- and post-treatment values within each group by ANOVA or paired t-test (SPSS Inc., Chicago, IL, USA). In all instances, significance was set at P < 0.05.
Chemicals
DiI was purchased from Molecular Probes (Eugene, OR, USA); GLP-1, exendin-4 and exendin-9–39 were purchased from Bachem (Torrance, CA, USA); all other chemicals were purchased from Sigma (St Louis, MO, USA).
Results
In vitro studies
In the current clamp configuration, perfusion with 100 nm GLP-1, or its stable analogue exendin-4, induced a membrane depolarization in 47% of the DMV neurones tested (82 of 172). The remaining 43% of neurones (73 of 172) showed no measurable response. During our analysis, we found 17 neurones that were hyperpolarized by GLP-1, but the limited number of neurones displaying such a response prevented a more complete characterization. The neurones returned to pretreatment baseline values upon wash out of the agonist.
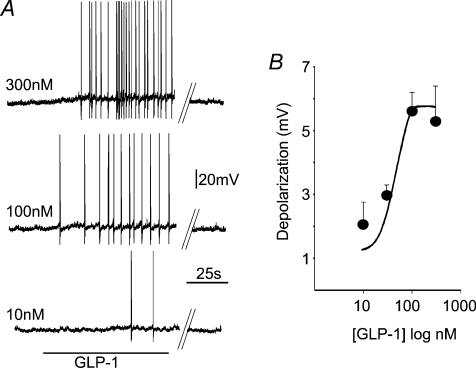
Concentration–response curves to GLP-1 (10–300 nm; n= 3–7 per each concentration) were constructed from cells in which at least three concentrations were tested at 10–15 min intervals. The membrane depolarization induced by GLP-1 was concentration dependent and had an estimated Emax of 5.6 mV at 100 nm and an EC50 of 40 nm (Fig. 1). Similarly, the increase in firing rate induced by GLP-1 was concentration dependent and had a comparable EC50 (not shown).
Figure 1. GLP-1 perfusion depolarizes a subgroup of identified gastric-projecting DMV neurones.
A, representative traces from a DMV neurone illustrating that GLP-1 induces a concentration-dependent increase in action potential firing rate. A recovery period of at least 10–15 min was allowed between successive applications. Parallel lines indicate a 2–3 min break in the recording. Holding potential =−60 mV. B, concentration–response curve for the GLP-1 induced depolarization in neurones current clamped at −65 mV. The EC50 for the GLP-1 response was approximately 40 nm. Each neurone was tested with at least 3 different concentrations of GLP-1.
The response to GLP-1 did not show tachyphylaxis, since 3 min perfusion with 100 nm GLP-1 repeated 10–15 min apart gave similar results. In fact, the first superfusion of GLP-1 depolarized the membrane by 7.6 ± 1.4 mV (P < 0.05 vs. control; n= 5) or increased the frequency of action potential firing from 8.8 ± 3.3 to 32 ± 8 action potentials min−1 (P < 0.05; n= 13) while the second superfusion of GLP-1 depolarized the membrane by 6.4 ± 1.5 mV or increased the firing rate to 23 ± 9 action potentials min−1 (P < 0.05 vs. control; P > 0.05 vs. first application; Fig. 2).
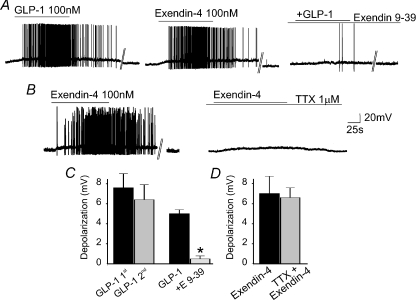
Figure 2. The GLP-1-induced depolarization does not show tachyphylaxys and is mediated by selective GLP-1 receptors.
A, representative traces showing that a similar increase in firing rate is obtained by superfusion of 100 nm GLP-1 (left panel) and its stable analogue exendin-4 (middle panel). The similar increase in firing rate obtained upon perfusion of agonists within a 10 min period also indicates that there is no tachyphylaxis. In the same neurone as above, following a 10 min recovery period in which the neurone was perfused with the selective antagonist exendin-9–39 (100 nm; E 9-39), the action potential firing rate increase induced by perfusion with GLP-1 was attenuated significantly (right panel). Parallel lines indicate a 2–3 min break in the recording. B, representative traces showing that superfusion of 100 nm exendin-4 (left panel) induced a DMV neuronal depolarization resulting in action potential firing. In the same neurone, following 10 min perfusion with tetrodotoxin (TTX), re-application of exendin-4 induced a similar membrane depolarization. Parallel lines indicate a 2–3 min break in the recording. C, summary graph comparing the depolarization induced by successive applications of GLP-1 (n= 5; left panels) and the antagonism of the GLP-1 induced depolarization by exendin-9–39 (n= 4; right panels). *P < 0.05 vs. GLP-1 alone. D, summary graph comparing the depolarization induced by successive applications of exendin-4 in the absence (n= 4; left panels) and in the presence of TTX (n= 4 right panels).
The excitatory response of gastric-projecting DMV neurones induced by GLP-1 was mimicked by perfusion with the receptor selective agonist exendin-4 (100 nm). In fact, in three neurones perfusion with GLP-1 increased the firing rate from 10 ± 5.3 to 28 ± 9.5 action potentials min−1 (P < 0.05). Following wash out and 10 min recovery during which the firing rate returned to baseline values, perfusion with exendin-4 increased the firing rate to 27 ± 12.6 action potentials min−1 (P > 0.05 vs. GLP-1; Fig. 2). Similarly, perfusion with GLP-1 or exendin-4 depolarized the membrane by 5.5 ± 0.5 and 4.5 ± 0.5 mV, respectively (P > 0.05; not shown).
The increase in firing rate and the depolarization induced by 100 nm GLP-1 were antagonized by pretreatment with the receptor selective antagonist exendin-9–39 (100 nm). In four neurones in which GLP-1 increased the firing rate from 14 ± 3.6 to 30 ± 7.2 action potentials min−1, following 10 min pretreatment with exendin-9–39, re-perfusion with GLP-1 in the presence of the antagonist did not change the firing rate (13 ± 11.7% increase in action potentials in exendin-9–39 + GLP-1 vs. exendin-9–39 alone; P < 0.05 vs. GLP-1 alone). Similarly, in three neurones in which exendin-4 (100 nm) increased the firing rate from 8 ± 7.4 to 43 ± 22 action potentials min−1, following 10 min pretreatment with exendin-9–39, and re-perfusion with exendin-4 in the presence of exendin-9–39 the firing rate remained at 7 ± 3.6 action potentials min−1; P < 0.05 vs. exendin-4 alone).
Likewise, in four neurones in which GLP-1 induced a 5 ± 0.4 mV depolarization, following wash-out and 10 min pretreatment with exendin-9–39, re-perfusion with GLP-1 in the presence of the antagonist induced a 0.5 ± 0.3 mV depolarization (P < 0.05 vs. GLP-1 alone; Fig. 2).
In four additional neurones in which exendin-4 induced a 7.1 ± 1.7 mV depolarization, following wash-out and 10 min pretreatment with tetrodotoxin (1 μm), re-perfusion with exendin-4 in the presence of tetrodotoxin induced a 6.6 ± 1.1 mV depolarization (P > 0.05 vs. exendin alone; Fig. 2).
These in vitro data indicate that GLP-1 or exendin-4 depolarizes (i.e. excites) a subpopulation of identified gastric-projecting DMV neurones via activation of selective GLP-1 receptors. These data suggest, therefore, that the gastroinhibition induced by centrally acting GLP-1 is likely to be mediated via activation rather than inhibition of a vagal efferent pathway.
In vivo studies
Microinjection of PBS in the left DVC did not induce any significant effect on gastric tone (n= 3; P > 0.05) nor did application of 2 μl of PBS to the floor of the 4th ventricle (n= 7; P > 0.05).
In 11 rats, microinjection of 450 pmol of exendin-4 in the left DVC induced a −0.20 ± 0.04 g decrease in gastric tone (P < 0.05) that returned to baseline values within 8.56 ± 1.52 min (Fig. 3). Three of these rats received a posterior subdiaphragmatic vagotomy (n.b., the soma of the DMV neurones that form the posterior branches of the vagus nerve are located in the right side of DMV, and conversely, the soma of the neurones projecting through the anterior vagus are located in the left side of DMV). Following a 30 min stabilization period, microinjection of 450 pmol of exendin-4 in the left DVC induced a −0.26 ± 0.13 g decrease in gastric tone. The effects of microinjection of exendin-4 were completely abolished (i.e. −0.01 ± 0.01 g) by a left cervical vagotomy (Fig. 4).
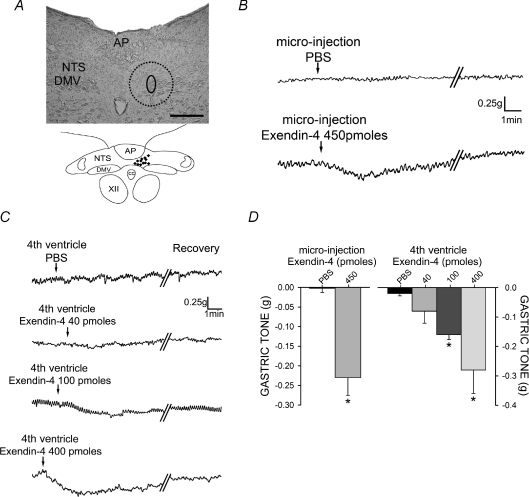
Figure 3. GLP-1 receptor activation in the DVC dose-dependently mediates gastric inhibition.
A, histological verification of DVC microinjection sites. Top, a representative photomicrograph of a 40 μm thick brainstem injection site with the region of the injection circled including the potential region of diffusion (dotted line) into the DMV and adjacent NTS. Bottom, a cumulative schematic representation of injection areas. Scale bar = 200 μm B, representative trace showing that microinjection of exendin-4 produces a rapid gastric inhibition. Parallel oblique lines indicate a 2–4 min break in the recording. C, representative traces of the dose-dependent gastroinhibition following 4th ventricle application of exendin-4. D, summary graph of gastroinhibition following microinjection and 4th ventricle application of exendin-4. *P < 0.05 vs. PBS.
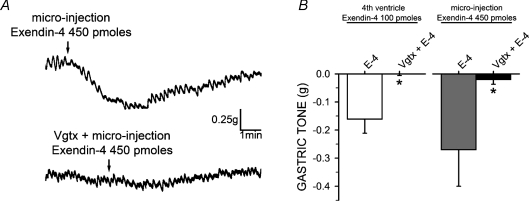
Figure 4. The gastroinhibitory effects of exendin-4 are mediated through vagal efferent fibres.
A, representative gastric motility traces showing the gastroinhibition obtained upon exendin-4 microinjection into the DVC in naive rats (top); following vagotomy reapplication of exendin-4 no longer induced gastroinhibition (bottom). B, summary graph of gastric inhibition following 4th ventricle application and DVC micro-injection of exendin-4 alone and following vagotomy (Vgtx). *P < 0.05 vs. exendin-4 (E-4) alone.
The loss of effects of GLP-1 on gastric tone following complete (i.e. bilateral) vagotomy indicates that the effects of GLP-1 in the DVC are vagally mediated. Furthermore, since the effects of the microinjection of GLP-1 in vagally intact animals are similar to that obtained in animals following a contralateral vagotomy, these data indicate that the decrease in gastric tone induced by microinjection in the DVC is most likely mediated by activation of ipsilaterally projecting DMV neurones rather than a combined effect of GLP-1 on both DMV neurones and contralaterally projecting NTS neurones.
The response to application of exendin-4 was dose dependent since 4th ventricular application of 40 pmol induced a −0.08 ± 0.04 g (n= 6), administration of 100 pmol induced a −0.16 ± 0.02 g (n= 7) and 400 pmol induced a −0.29 ± 0.06 g (n= 7) decrease in gastric tone (all results P < 0.05; Fig. 3).
Five of these rats received a posterior subdiaphragmatic vagotomy. Following a 30 min stabilization period, application of 400 pmol of exendin-4 induced a −0.2 ± 0.06 g decrease in gastric tone that was completely prevented by a subsequent left cervical vagotomy (Fig. 4).
These data indicate that the effects of the application of exendin-4 were vagally mediated even when the agonist was placed on the floor of the 4th ventricle.
Peripheral vagal pathways involved in the GLP-1 mediated gastroinhibition
The following series of experiments were designed to identify the postganglionic pathway involved in the vagally mediated effects of exendin-4. The rationale behind these experimental procedures consisted in stimulating the cholinergic muscarinic pathway maximally by intravenous administration of the non-selective muscarinic agonist bethanechol, prior to application of exendin-4 to the floor of the 4th ventricle. Thus, if exendin-4 were to inhibit gastric functions via inhibition of the postganglionic cholinergic excitatory pathway, its effects would be negated in the presence of bethanechol, due to the maximal activation of the postjunctional cholinergic muscarinic receptors present on the gastric musculature. If, however, exendin-4 were to inhibit gastric functions despite the presence of bethanechol, then we would surmise that the gastroinhibitory effects occur via activation of the inhibitory NANC pathway (Lewis et al. 2002; Rogers et al. 2003; Shi et al. 2005; Holmes et al. 2009).
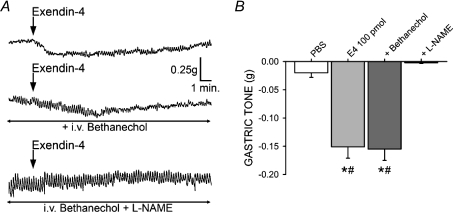
In seven rats, application of 400 pmol of exendin-4 to the floor of the 4th ventricle induced a −0.28 ± 0.08 g decrease in gastric tone (P < 0.05; Fig. 5). The response to exendin-4 was 106 ± 11.3% of control following bethanechol infusion (P > 0.05 vs. exendin-4 alone), but was completely antagonized following administration of the nitric oxide synthase (NOS) inhibitor l-NAME (i.e. 0.85 ± 0.86% of control; P < 0.05).
Figure 5. The gastroinhibitory effects of exendin-4 occur through a NANC mechanism of action.
A, exendin-4 application to the 4th ventricle induces a decrease in gastric tone (top); following systemic administration of bethanechol, reapplication of exendin-4 is still gastroinhibitory (middle); however, following systemic administration of bethanechol +l-NAME, the exendin-4 gastroinhibition was antagonized completely. B, summary graph of the gastroinhibition observed following PBS and sequential application of exendin-4 alone, in the presence of bethanechol, and bethanechol +l-NAME (*P < 0.05 vs. PBS, #P < 0.05 vs. bethanechol +l-NAME).
Since it could be argued that pretreatment with bethanechol induces a large increase in gastric tone and motility, thus placing the stomach in a non-physiological condition, we conducted a further series of experiments in which 400 pmol of exendin-4 was microinjected in the DVC before and after i.v. administration of l-NAME. In three naive animals, microinjection of exendin-4 decreased gastric tone by −0.11 ± 0.03 g. Following l-NAME pretreatment, microinjection of exendin-4 had no effect (i.e. 0 ± 0.0 g; P < 0.05 vs. exendin-4 alone).
These data indicate that the decrease in gastric tone induced by administration of exendin-4 in the DVC is mediated by activation of the gastroinhibitory NANC postganglionic pathway.
Discussion
In the present paper we have shown that (1) a subpopulation of identified gastric-projecting DMV neurones is depolarized by exogenously applied GLP-1; (2) these effects are mediated via interactions with selective GLP-1 receptors located, most likely, on preganglionic DMV neurones; (3) activation of GLP-1 receptor induces a vagally mediated gastroinhibition; and (4) the gastroinhibition occurs through activation of the postganglionic vagal NANC pathway.
Our data suggest that GLP-1, which would be released either following ingestion of a meal (Baggio & Drucker, 2007) or from local GLP-1 immunoreactive neurones (Larsen et al. 1997; Merchenthaler et al. 1999; Rinaman, 1999b; Vrang et al. 2003), acts at the level of brainstem vagal circuits to decrease gastric tone. This effect involves the direct excitation of a subgroup of vagal efferent motoneurones of the DMV controlling the NANC postganglionic pathway. Although the source of GLP-1 in the DVC (neurotransmitter or neurohormone) has not been elucidated, our results support a possible physiologically relevant role of GLP-1 to activate brainstem vagal motoneurones controlling gastric motility. The influence of GLP-1 receptors on NTS terminals apposing DMV neurones cannot, however, be discounted and it is possible that at least some of the effects of GLP-1 occur through the release of glutamate from NTS terminals impinging upon DMV neurones. This scenario, however, is unlikely to explain the dramatic increase in firing rate or the membrane depolarization induced by perfusion with GLP-1 or exendin-4, and the lack of effect of TTX on DMV membrane depolarization. Further support of a mainly direct effect of GLP-1 on DMV neurones can be derived from the observation that vagal motor innervation to the GI tract DMV is ipsilateral, while the innervation from NTS is bilateral (Travagli et al. 2006). If, for example, one microinjects a drug into the left DMV and the gastric response is unaffected by a posterior subdiaphragmatic vagotomy, one can conclude that the majority of the drug effects are via direct actions on DMV neurones. In the present study, contralateral vagotomy (to eliminate any potential NTS-mediated excitation of contralateral DMV neurones) does not affect the gastric response to microinjection of GLP-1 into the DMV. When combined with our electrophysiological data demonstrating that TTX does not affect the response of DMV neurones to GLP-1 and our data demonstrating that bethanecol does not block the GLP-1-induced gastric relaxation, the most likely scenario to account for all of these results is a principal action of GLP-1 to excite DMV neurones directly.
Small amounts of GLP-1 are released during the cephalic phase of digestion, but a larger amount of the peptide is released from intestinal endocrine L-cells following ingestion of a mixed meal, particularly meals rich in carbohydrates and fats (Brubaker, 2006). Once released, GLP-1 has a relatively short plasma half-life, approximately 2 min, due to its degradation by the ubiquitous enzyme dipeptidyl peptidase-4 to the inactive forms GLP-1 (9–37) and GLP-1 (9–36)-NH2 (Baggio & Drucker, 2007; Holst, 2007). Since GLP-1 receptors are located on abdominal vagal afferent fibres and nodose ganglion neurones as well as neurones of the NTS, and the NTS itself contains GLP-1 immunoreactive neurones (Larsen et al. 1997; Merchenthaler et al. 1999; Rinaman, 1999b; Vrang et al. 2003; Baggio & Drucker, 2007; Holst, 2007), the relative importance of the peripheral vs. central GLP-1 site of action still awaits clarification.
In support of a possible direct central site of action of GLP-1, as shown in the present paper, GLP-1 has been shown to cross the blood–brain barrier via a simple diffusion mechanism (Kastin et al. 2002). Furthermore, the vasculature supplying the DVC is composed of fenestrated capillaries capable of allowing passage of large molecules from the circulation (Gross et al. 1990; Cottrell & Ferguson, 2004). It is thus possible, although unlikely given its short half-life, that peripherally released GLP-1 activates vagal brainstem circuits in a hormonal-like manner.
A more likely possibility is that GLP-1 significantly affects brainstem vagal physiology when released in a neurotransmitter-like manner by local NTS neurones that have been activated by GI-related stimuli. In fact, many meal-related and sensory inputs from subdiaphragmatic viscera are conveyed to the brainstem via vagal afferent fibres that impinge on NTS neurones. A subpopulation of NTS neurones synthesise GLP-1 and display an increased c-Fos immunoreactivity following either gastric distention, i.e. an action similar to meal distention (Vrang et al. 2003), or administration of the meal related peptide cholecystokinin (Billig et al. 2001). Further support for a direct centrally mediated, non-paracrine action of GLP-1 comes from studies showing that GLP-1 significantly inhibited centrally induced antral motility and its gastroinhibitory effect persisted after vagal deafferentation (Nagell et al. 2006).
Vagally mediated gastroinhibition is accomplished by either a withdrawal of cholinergic tone or an increase in NANC activity, much of which is mediated by the release of nitric oxide, NO (reviewed recently in Travagli et al. 2006). GLP-1 receptors are functionally coupled to adenylate cyclase via the stimulatory G protein Gs, resulting in an increase of intracellular calcium and an inhibition of potassium conductances (Baggio & Drucker, 2007; Mayo et al. 2003). It is not surprising, therefore, that the few studies available in the literature, along with the present paper, describe neurostimulatory effects of GLP-1 (Kakei et al. 2002; Acuna-Goycolea & van den Pol, 2004; Ma et al. 2007; Wan et al. 2007b).
The excitatory effects of GLP-1 mainly on identified gastric-projecting vagal motoneurones, when combined with the in vivo data herein, reporting the gastroinhibition induced by brainstem application of the stable analogue exendin-4, suggest that they are likely to be due to activation of the NANC vagal pathway, rather than withdrawal of the vagal cholinergic pathway. Indeed, our data show that the GLP-1-induced gastroinhibition was still present during systemic administration of the muscarinic agonist bethanechol. The complete antagonism of the gastric-relaxation induced by brainstem administration of exendin-4 obtained by pretreatment with the NOS inhibitor l-NAME supports our hypothesis that the gastroinhibitory effects of GLP-1 are mediated by activation of a vagal NANC pathway.
Thus, in contrast to previous reports suggesting that GLP-1 delays gastric emptying via an inhibition of vagal efferent activity (Imeryuz et al. 1997; Wettergren et al. 1998; Schirra et al. 2000), the results of the present study suggest that GLP-1 decreases gastric tone, at least in part, via an activation of vagal efferent inhibitory NANC activity. Experimental (Tolessa et al. 2001) as well clinical (Andrews et al. 2007) studies have shown that the effects of systemic administration of GLP-1 are blunted significantly by NOS inhibitors. Despite the strong evidence, provided in the present paper, that the gastroinhibition in response to exendin-4 microinjection is mediated by activation of NANC vagal pathways, one cannot necessarily exclude the possibility that GLP-1 also has paracrine effects mediated via withdrawal of vagal cholinergic tone. Rather, our data suggests that the gastroinhibitory effects induced by activation of GLP-1 receptors in the DVC are mediated by activation of postganglionic neurones that are part of the NANC pathway.
It is possible that, as we have hypothesized for the actions of cholecystokinin (Holmes et al. 2009), the vagally mediated effects of GLP-1 are exerted via two separate mechanisms. The paracrine effect of GLP-1 would occur via activation of vagal afferent fibres and would result in gastroinhibition through withdrawal of cholinergic tone. Conversely, a neurohormonal effect of GLP-1 would occur via the interaction of GLP-1 with motoneurones of vagal brainstem circuits and, as shown clearly in the present paper, would result in gastroinhibition mediated through the activation of postganglionic NANC pathways.
In conclusion, the present experiments are the first to describe the actions of GLP-1 upon the activity of gastric-projecting DMV motoneurones as well as upon basal gastric tone. These observations are extended further to show that medullary GLP-1 induces a gastroinhibition via activation of vagal NANC pathways. These data suggest that, in addition to a possible paracrine mechanism of action, GLP-1 may be involved in a larger, more sustained integration of gastric reflex functions at the level of the brainstem vagal circuits. The well established actions of GLP-1 to activate adenylate cyclase, combined by our previous reports of cAMP–protein kinase A mediated receptor trafficking (Browning & Travagli, 2001, 2006; Browning et al. 2004), suggest that GLP-1, at the level of brainstem, may induce plasticity within the vagal neurocircuitry controlling gastric functions. Furthermore, one may speculate that pathophysiological changes in the responsiveness of vagal neurocircuitry to GLP-1 may be one contributing factor to the post-prandial onset of dyspepsia.
Acknowledgments
We thank Cesare M. Travagli, Zoraide Travagli and W. Nairn Browning for support and encouragement. Technical contribution by Z. L. Zheng. No conflict of interest exists. This work was supported by NIH grants no. DK 55530 (R.A.T.) and NINDS no. 49177 (G.M.H.).
Glossary
Abbreviations
- CNS
central nervous system
- DMV
dorsal motor nucleus of the vagus
- DVC
dorsal vagal complex
- GLP-1
glucagon-like peptide 1
- NANC
non-adrenergic, non-cholinergic
- NOS
nitric oxide synthase
- NTS
nucleus tractus solitarius
Author contributions
All the authors have contributed to the conception, design, analysis and interpretation of the data, the drafting and revising of the article, and the final approval of the version to be published. All the experiments were conducted at the Pennington Biomedical Research Center, Louisiana State University, Baton Rouge.
References
- Acuna-Goycolea C, van den Pol AN. Glucagon-like peptide 1 excites hypocretin/orexin neurons by direct and indirect mechanisms: implication for viscera-mediated arousal. J Neurosci. 2004;24:8141–8152. doi: 10.1523/JNEUROSCI.1607-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews CN, Bharucha AE, Camilleri M, Low PA, Seide BM, Burton D, Baxter K, Zinsmeister AR. Nitrergic contribution to gastric relaxation induced by glucagon-like peptide-1 (GLP-1) in healthy adults. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1359–1365. doi: 10.1152/ajpgi.00403.2006. [DOI] [PubMed] [Google Scholar]
- Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131–2157. doi: 10.1053/j.gastro.2007.03.054. [DOI] [PubMed] [Google Scholar]
- Beglinger C, Degen L. Gastrointestinal satiety signals in humans: physiologic roles for GLP-1 and PYY? Physiol Behav. 2006;89:460–464. doi: 10.1016/j.physbeh.2006.05.048. [DOI] [PubMed] [Google Scholar]
- Billig I, Yates BJ, Rinaman L. Plasma hormone levels and central c-Fos expression in ferrets after systemic administration of cholecystokinin. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1243–R1255. doi: 10.1152/ajpregu.2001.281.4.R1243. [DOI] [PubMed] [Google Scholar]
- Browning KN, Kalyuzhny AE, Travagli RA. μ-Opioid receptor trafficking on inhibitory synapses in the rat brainstem. J Neurosci. 2004;24:7344–7352. doi: 10.1523/JNEUROSCI.1676-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning KN, Renehan WE, Travagli RA. Electrophysiological and morphological heterogeneity of rat dorsal vagal neurones which project to specific areas of the gastrointestinal tract. J Physiol. 1999;517:521–532. doi: 10.1111/j.1469-7793.1999.0521t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning KN, Travagli RA. The peptide TRH uncovers the presence of presynaptic 5-HT1A receptors via activation of a second messenger pathway in the rat dorsal vagal complex. J Physiol. 2001;531:425–435. doi: 10.1111/j.1469-7793.2001.0425i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning KN, Travagli RA. Short-term receptor trafficking in the dorsal vagal complex: An overview. Auton Neurosci. 2006;126–127:2–8. doi: 10.1016/j.autneu.2006.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning KN, Travagli RA. Functional organization of presynaptic metabotropic glutamate receptors in vagal brainstem circuits. J Neurosci. 2007;27:8979–8988. doi: 10.1523/JNEUROSCI.1105-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brubaker PL. The glucagon-like peptides: pleiotropic regulators of nutrient homeostasis. Ann N Y Acad Sci. 2006;1070:10–26. doi: 10.1196/annals.1317.006. [DOI] [PubMed] [Google Scholar]
- Cottrell GT, Ferguson AV. Sensory circumventricular organs: central roles in integrated autonomic regulation. Regul Pept. 2004;117:11–23. doi: 10.1016/j.regpep.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Delgado-Aros S, Kim DY, Burton DD, Thomforde GM, Stephens D, Brinkmann BH, Vella A, Camilleri M. Effect of GLP-1 on gastric volume, emptying, maximum volume ingested, and postprandial symptoms in humans. Am J Physiol Gastrointest Liver Physiol. 2002;282:G424–G431. doi: 10.1152/ajpgi.2002.282.3.G424. [DOI] [PubMed] [Google Scholar]
- Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3:153–165. doi: 10.1016/j.cmet.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters in The Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goke R, Larsen PJ, Mikkelsen JD, Sheikh SP. Distribution of GLP-1 binding sites in the rat brain: evidence that exendin-4 is a ligand of brain GLP-1 binding sites. Eur J Neurosci. 1995;7:2294–2300. doi: 10.1111/j.1460-9568.1995.tb00650.x. [DOI] [PubMed] [Google Scholar]
- Gross PM, Wall KM, Pang JJ, Shaver SW, Wainman DS. Microvascular specializations promoting rapid interstitial solute dispersion in nucleus tractus solitarius. Am J Physiol Regul Integr Comp Physiol. 1990;259:R1131–1138. doi: 10.1152/ajpregu.1990.259.6.R1131. [DOI] [PubMed] [Google Scholar]
- Holmes GM, Tong M, Travagli RA. Effects of brainstem cholecystokinin-8s on gastric tone and esophageal-gastric reflex. Am J Physiol Gastrointest Liver Physiol. 2009;296:G621–631. doi: 10.1152/ajpgi.90567.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87:1409–1439. doi: 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- Honig MG, Hume RI. DiI and DiO: versatile fluorescent dyes for neuronal labelling and pathway tracing. Trends Neurosci. 1989;12:333–341. [PubMed] [Google Scholar]
- Kakei M, Yada T, Nakagawa A, Nakabayashi H. Glucagon-like peptide-1 evokes action potentials and increases cytosolic Ca2+ in rat nodose ganglion neurons. Auton Neurosci. 2002;102:39–44. doi: 10.1016/s1566-0702(02)00182-0. [DOI] [PubMed] [Google Scholar]
- Kanse SM, Kreymann B, Ghatei MA, Bloom SR. Identification and characterization of glucagon-like peptide-1 7–36 amide-binding sites in the rat brain and lung. FEBS Lett. 1988;241:209–212. doi: 10.1016/0014-5793(88)81063-9. [DOI] [PubMed] [Google Scholar]
- Kastin AJ, Akerstrom V, Pan W. Interactions of glucagon-like peptide-1 (GLP-1) with the blood-brain barrier. J Mol Neurosci. 2002;18:7–14. doi: 10.1385/JMN:18:1-2:07. [DOI] [PubMed] [Google Scholar]
- Imeryuz N, Yegen BC, Bozkurt A, Coskun T, Villanueva-Penacarrillo ML, Ulusoy NB. Glucagon-like peptide-1 inhibits gastric emptying via vagal afferent-mediated central mechanisms. Am J Physiol Gastrointest Liver Physiol. 1997;273:G920–G927. doi: 10.1152/ajpgi.1997.273.4.G920. [DOI] [PubMed] [Google Scholar]
- Lachey JL, D'Alessio DA, Rinaman L, Elmquist JK, Drucker DJ, Seeley RJ. The role of central glucagon-like peptide-1 in mediating the effects of visceral illness: differential effects in rats and mice. Endocrinology. 2005;146:458–462. doi: 10.1210/en.2004-0419. [DOI] [PubMed] [Google Scholar]
- Larsen PJ, Tang-Christensen M, Holst JJ, Orskov C. Distribution of glucagon-like peptide-1 and other preproglucagon-derived peptides in the rat hypothalamus and brainstem. Neuroscience. 1997;77:257–270. doi: 10.1016/s0306-4522(96)00434-4. [DOI] [PubMed] [Google Scholar]
- Lewis MW, Hermann GE, Rogers RC, Travagli RA. In vitro and in vivo analysis of the effects of corticotropin releasing factor on rat dorsal vagal complex. J Physiol. 2002;543:135–146. doi: 10.1113/jphysiol.2002.019281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Bruning J, Ashcroft FM. Glucagon-like peptide 1 stimulates hypothalamic proopiomelanocortin neurons. J Neurosci. 2007;27:7125–7129. doi: 10.1523/JNEUROSCI.1025-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Mayo KE, Miller LJ, Bataille D, Dalle S, Goke B, Thorens B, Drucker DJ. International Union of Pharmacology. XXXV. The glucagon receptor family. Pharmacol Rev. 2003;55:167–194. doi: 10.1124/pr.55.1.6. [DOI] [PubMed] [Google Scholar]
- Mendelowitz D, Yang M, Andresen MC, Kunze DL. Localization and retention in vitro of fluorescently labelled aortic baroreceptor terminal on neurons from the nucleus tractus solitarius. Brain Res. 1992;581:339–343. doi: 10.1016/0006-8993(92)90729-s. [DOI] [PubMed] [Google Scholar]
- Merchenthaler I, Lane M, Shughrue P. Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. J Comp Neurol. 1999;403:261–280. doi: 10.1002/(sici)1096-9861(19990111)403:2<261::aid-cne8>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Nagell CF, Wettergren A, Orskov C, Holst JJ. Inhibitory effect of GLP-1 on gastric motility persists after vagal deafferentation in pigs. Scand J Gastroenterol. 2006;41:667–672. doi: 10.1080/00365520500408253. [DOI] [PubMed] [Google Scholar]
- Nakabayashi H, Nishizawa M, Nakagawa A, Takeda R, Niijima A. Vagal hepatopancreatic reflex effect evoked by intraportal appearance of tGLP-1. Am J Physiol Endocrinol Metab. 1996;271:E808–E813. doi: 10.1152/ajpendo.1996.271.5.E808. [DOI] [PubMed] [Google Scholar]
- Nishizawa M, Nakabayashi H, Kawai K, Ito T, Kawakami S, Nakagawa A, Niijima A, Uchida K. The hepatic vagal reception of intraportal GLP-1 is via receptor different from the pancreatic GLP-1 receptor. J Auton Nerv Syst. 2000;80:14–21. doi: 10.1016/s0165-1838(99)00086-7. [DOI] [PubMed] [Google Scholar]
- Rinaman L. A functional role for central glucagon-like peptide-1 receptors in lithium chloride-induced anorexia. Am J Physiol Regul Integr Comp Physiol. 1999a;277:R1537–R1540. doi: 10.1152/ajpregu.1999.277.5.R1537. [DOI] [PubMed] [Google Scholar]
- Rinaman L. Interoceptive stress activates glucagon-like peptide-1 neurons that project to the hypothalamus. Am J Physiol Regul Integr Comp Physiol. 1999b;277:R582–R590. doi: 10.1152/ajpregu.1999.277.2.R582. [DOI] [PubMed] [Google Scholar]
- Rogers RC, Travagli RA, Hermann GE. Noradrenergic neurons in the rat solitary nucleus participate in the esophageal-gastric relaxation reflex. Am J Physiol Regul Integr Comp Physiol. 2003;285:R479–R489. doi: 10.1152/ajpregu.00155.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirra J, Houch P, Wank U, Arnold R, Goke B, Katschinski M. Effects of glucagon-like peptide-1 (7–36) amide on antro-pyloro-duodenal motility in the interdigestive state and with duodenal lipid perfusion in humans. Gut. 2000;46:622–631. doi: 10.1136/gut.46.5.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirra J, Goke B. The physiological role of GLP-1 in human: incretin, ileal brake or more? Regul Pept. 2005;128:109–115. doi: 10.1016/j.regpep.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Shi M, Jones AR, Ferreira M, Jr, Sahibzada N, Gillis RA, Verbalis JG. Glucose does not activate non-adrenergic, non-cholinergic (NANC) inhibitory neurons in the rat stomach. Am J Physiol Regul Integr Comp Physiol. 2005;288:R742–750. doi: 10.1152/ajpregu.00561.2004. [DOI] [PubMed] [Google Scholar]
- Shimizu I, Hirota M, Ohboshi C, Shima K. Identification and localization of glucagon-like peptide-1 and its receptor in rat brain. Endocrinology. 1987;121:1076–1082. doi: 10.1210/endo-121-3-1076. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Owyang C. Regional differences in the nitrergic innervation between the proximal and the distal colon in rats. Gastroenterology. 1998;115:1504–1512. doi: 10.1016/s0016-5085(98)70029-0. [DOI] [PubMed] [Google Scholar]
- Thorens B. Glucagon-like peptide-1 and control of insulin secretion. Diabete Metab. 1995;21:311–318. [PubMed] [Google Scholar]
- Tolessa T, Naslund E, Hellstrom PM. The inhibitory mechanism of GLP-1, but not glucagon, on fasted gut motility is dependent on the L-arginine/nitric oxide pathway. Regul Pept. 2001;98:33–40. doi: 10.1016/s0167-0115(00)00220-2. [DOI] [PubMed] [Google Scholar]
- Travagli RA, Gillis RA, Rossiter CD, Vicini S. Glutamate and GABA-mediated synaptic currents in neurons of the rat dorsal motor nucleus of the vagus. Am J Physiol Gastrointest Liver Physiol. 1991;260:G531–G536. doi: 10.1152/ajpgi.1991.260.3.G531. [DOI] [PubMed] [Google Scholar]
- Travagli RA, Hermann GE, Browning KN, Rogers RC. Brainstem circuits regulating gastric function. Annu Rev Physiol. 2006;68:279–305. doi: 10.1146/annurev.physiol.68.040504.094635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uttenthal LO, Toledano A, Blazquez E. Autoradiographic localization of receptors for glucagon-like peptide-1 (7–36) amide in rat brain. Neuropeptides. 1992;21:143–146. doi: 10.1016/0143-4179(92)90036-v. [DOI] [PubMed] [Google Scholar]
- Van Dijk G, Thiele TE, Donahey JC, Campfield LA, Smith FJ, Burn P, Bernstein IL, Woods SC, Seeley RJ. Central infusions of leptin and GLP-1-(7–36) amide differentially stimulate c-FLI in the rat brain. Am J Physiol Regul Integr Comp Physiol. 1996;271:R1096–R1100. doi: 10.1152/ajpregu.1996.271.4.R1096. [DOI] [PubMed] [Google Scholar]
- Vrang N, Phifer CB, Corkern MM, Berthoud HR. Gastric distension induces c-Fos in medullary GLP-1/2-containing neurons. Am J Physiol Regul Integr Comp Physiol. 2003;285:R470–R478. doi: 10.1152/ajpregu.00732.2002. [DOI] [PubMed] [Google Scholar]
- Wan S, Browning KN, Travagli RA. Glucagon-like peptide-1 modulates synaptic transmission to identified pancreas-projecting vagal motoneurons. Peptides. 2007a;28:2184–2191. doi: 10.1016/j.peptides.2007.08.016. [DOI] [PubMed] [Google Scholar]
- Wan S, Coleman FH, Travagli RA. Glucagon-like peptide-1 (GLP-1) excites pancreas-projecting preganglionic vagal motoneurons. Am J Physiol Gastrointest Liver Physiol. 2007b;292:G1474–1482. doi: 10.1152/ajpgi.00562.2006. [DOI] [PubMed] [Google Scholar]
- Wettergren A, Wojdemann M, Holst JJ. Glucagon-like peptide-1 inhibits gastropancreatic function by inhibiting central parasympathetic outflow. Am J Physiol Gastrointest Liver Physiol. 1998;275:G984–G992. doi: 10.1152/ajpgi.1998.275.5.G984. [DOI] [PubMed] [Google Scholar]