Abstract
Study Objectives
To describe trends in adverse drug reaction (ADR) scores as blood pressure (BP) came under control during a study of physician-pharmacist collaboration in the management of hypertension.
Design
Secondary analysis from a randomized controlled clinical trial.
Setting
Five primary care clinics affiliated with the University of Iowa, Iowa City, IA, USA.
Subjects
One hundred seventy-nine patients with uncontrolled primary hypertension aged 21-85 years taking 0-3 antihypertensive medications at baseline.
Intervention
Patient-reported symptoms suggestive of ADRs were recorded at each study visit using a structured ADR questionnaire. Social support (SS) and self-efficacy (SE) questionnaires were also administered at each study visit.
Measurements and Main Results
ADR scores decreased significantly from baseline to the end of the study (p<0.0001) in both the control (26.5 to 18.4) and intervention (29.9 to 22.7) groups although there were no differences between groups. Antihypertensive medication use increased for both intervention (1.5±1.0 to 2.4±0.9) and control (1.4±1.0 to 1.9±1.0) groups. We performed additional analyses on SE and SS to determine a potential reason for the reduction in adverse symptom scores despite an increase in medication use. Improvements in SE and SS scores were significantly and independently associated with improvement in ADR score (p<0.05).
Conclusions
ADR scores improved despite an increase in antihypertensive medication use. Improvements in SS and, to a lesser extent, SE are associated with improvements in ADR scores. Patients should not expect an increase in distressful symptoms as their BP becomes controlled with antihypertensive medications, especially when adequate social support is available.
Keywords: Hypertension, Adverse reactions, Symptoms, Social support, Self efficacy
Introduction
Antihypertensives are frequently associated with adverse drug reactions (ADRs) that may limit treatment options and reduce patient adherence which may hinder blood pressure (BP) control. It is commonly believed that antihypertensive agents cause ADRs or adverse symptoms that make patients feel worse than they did before beginning medication for their “asymptomatic” disease. It is thought that different discontinuation rates for various classes of antihypertensive agents are probably due to different rates of adverse symptoms.1-4
It is clear that more aggressive treatment of hypertension and treating to goal BP can reduce cardiovascular events.5, 6 It is also known that achieving BP goals usually requires two or more antihypertensives.7 Increasing numbers of antihypertensives in a regimen may lead to even more side effects.
In contrast, several studies have found that quality of life (QOL) or symptoms actually improved once BP became controlled.8-10 It is known that distressful symptoms (e.g. fatigue, dizziness) are more common in patients with hypertension than in those without hypertension.8 It has also been found that there are more distressful symptoms in patients with uncontrolled hypertension than in those with controlled hypertension.8 These distressful symptoms reduce QOL and may be caused by hypertension, antihypertensives, other conditions or unknown causes.11-14
Psychosocial factors may have an impact on symptoms. Some believe poor social support (SS), defined as a person’s network of family and friends that provide them with encouragement and assistance when needed, may be associated with poor treatment adherence and may negatively impact morbidity and mortality.15-18 Self-efficacy (SE), the focus of Bandura’s social cognitive theory, describes a person’s belief in their capacity to achieve goals and meet expectations.19-23 High SE has been associated with improved treatment adherence.24 The effect of high SS or SE on adverse reactions to antihypertensive medications has not been previously reported.
Recently a randomized, controlled study of physician-pharmacist collaboration to improve BP control was completed.25 During this study, patient-reported symptoms suggestive of ADRs were recorded by a research nurse at each study visit. SS and SE questionnaires were also completed at each study visit. The purpose of the present study is to characterize trends of distressful symptoms once BP came under control. A secondary purpose is to determine if ADRs are influenced by SS and SE.
Methods
The main results of this study have been previously reported but will briefly be reviewed here.25 Patients with uncontrolled hypertension from five Iowa City-area clinics were recruited for the study. Patients were eligible for the study if they were males or females aged 21-85 years, and receiving zero to three antihypertensive agents with no changes to their regimen within the past four weeks. To qualify, non-diabetic patients had a clinic BP value (average of the last three readings) between 145-179 mmHg systolic or 95-109mmHg diastolic BP, and diabetic patients had clinic BP readings of 135-179 mm Hg systolic BP or 85-109 mm Hg diastolic BP.
Patients were excluded if they had previously been seen by the 24-hr BP monitoring consult service, had a BP >180/110 mmHg or any evidence of hypertensive urgency or emergency, had a recent myocardial infarction or stroke (within the past six months prior to enrollment), had New York Heart Association Class III or IV congestive heart failure, unstable angina, serious renal or hepatic disease, were pregnant, had poor prognosis with a life expectancy estimated at less than three years, or had dementia or cognitive impairment. The project was approved by the University of Iowa Institutional Review Board.
Patients were randomized to either the intervention or the control group. Patients in both groups had scheduled study visits with a research nurse at baseline, 2, 4, 6, 8, and 9 months. Several measurements were collected at baseline including patient demographics, clinic BP, ADR26, SE23, and SS16, 17 questionnaires. The research nurses verbally administered all questionnaires and were available to help patients interpret the questions if necessary. At each data collection visit the nurse measured the subjects’ BP three times with a mercury sphygmomanometer using standardized techniques from BP clinical trials.27, 28 The second and third values were averaged and used as the clinic BP. The research nurses underwent certification every three months to demonstrate correct measurement techniques. The clinic BP values were provided to the physician and/or clinical pharmacist for patients in both the control and intervention groups. Goal BP was defined as <140/90mmHg for non-diabetic patients and <130/80mmHg for diabetic patients which was based on current U.S. guidelines.6 For patients in the intervention group, the clinical pharmacist conducted a baseline interview. Patients in both the control and intervention groups saw their physicians at the baseline visit.
Patients in the control group received usual care from their physicians, in addition to structured data collection visits with the research nurse. For patients in the intervention group, pharmacists who were practitioners in the clinic reviewed patient demographic data, risk factors, co-existing diseases, smoking status and adherence information obtained by the research nurse before interviewing the patients. During the interview, the pharmacists evaluated patient factors that might impede achieving goal BP and the patients’ current treatment strategies as compared to clinical guidelines. The pharmacists then formulated recommendations to share with the physicians before physicians met with patients. Recommendations were usually made face-to-face. The physicians could choose to accept or reject the pharmacists’ recommendations. At subsequent visits, if BP was not controlled, the pharmacists made further recommendations to the physicians and any changes were recorded in the study case report forms. If goal BP was achieved, the pharmacists and physicians reinforced strategies to maintain control.
Patient responses to the ADR, SE, or SS questionnaires were not shared with clinicians in either group. In the intervention arm, patients were typically seen by the pharmacist at each visit and the pharmacist routinely asked patients if they were experiencing any problems with their medications or any new symptoms. When patients mentioned an adverse effect the pharmacist either gave the patient strategies to overcome the problem or made an alteration in the patient’s medication regimen in collaboration with the physician. A summary of pharmacist recommendations has been previously reported.29 Medication regimens in both the control and intervention groups could be adjusted at any time by the patient’s clinician for any reason including but not limited to cost, lack of efficacy, or adverse effects.
For this analysis, we specifically analyzed clinic BP, number of medications, ADR scores, and SS and SE scores. The adverse reaction questionnaire was developed for another study and included 47 questions of typical medication side effects (Appendix 1).26 To reveal common adverse symptoms, the questions were formulated to review several systems including gastrointestinal, musculoskeletal, genitourinary, pulmonary, cardiovascular, cutaneous, central nervous system, and ENT (ear, nose, and throat). For each potential reaction the patient was asked: “in the past 4 weeks how much have you been bothered by….” The patient could rate the potential reaction: zero (not at all), 1 (a little bit), 2 (somewhat), 3 (quite a bit) or 4 (very much). The responses for each patient were summed (potential range from 0-188). The questionnaire included questions for side effects commonly attributed to antihypertensive medications (e.g. cough, swelling in feet or legs, fatigue).
For this study, the SS questionnaire focused on patients’ family lives. The questionnaire consisted of nine true-false questions. SS scores could range from 0 to 9, with a high score indicating high SS. The SE questionnaire for this study focused on a patient’s confidence in their ability to take their medications as prescribed in different situations such as at home or at a party. The SE questionnaire had 27 questions each answered with a 5-point (1 being “not confident,” 5 being “totally confident”) Likert scale. SE scores could range from 27 to 135, with higher numbers indicating higher SE.
For all descriptive statistics, means, standard deviations and ranges were calculated. For the ADR Questionnaire, the total score and system scores were summed. ADR scores were treated as continuous variables and SAS Proc Mixed models with random patient effects were fit to incorporate all available data from baseline through 9 months in an intention-to-treat analysis. Systolic BP was fit with a similar Mixed model as ADR scores. We evaluated the SS and SE scores using paired and unpaired t-tests. Proc Mixed was used to perform multivariate analysis to assess predictor variables for the change in ADR score when controlling for baseline demographics, number of coexisting conditions, baseline ADR score, number of antihypertensive medications, systolic BP, study group assignment, SE and SS. Number of medications between the two groups was analyzed using the Mantel-Haenszel chi-square test and the classes of medications were analyzed using Pearson chi-square tests. The analyses were performed using SAS (SAS Institute Inc., SAS 9.1.3, Cary, NC).
Results
A total of 179 patients were enrolled in the study. Patient demographic data are summarized in Table 1. Nineteen patients withdrew from the study; 10 from the control group and 9 from the intervention group. Tables 2 and 3 display the number and types of medications used in the control and intervention groups at baseline and at the end of the study. The number of antihypertensive medications increased for both intervention (1.5±1.0 to 2.4±0.9) and control (1.4±1.0 to 1.9±1.0) groups. The groups were not significantly different at baseline, but at the end of the study the intervention group was on a greater number of antihypertensive medications than the control group (p=0.003). The most commonly used medication classes (% of patients in control group and % of patients in intervention group) at the end of the study were diuretics (58.8% and 87.0%), beta-adrenergic blockers (45.6% and 39.1%), angiotensin-converting enzyme inhibitors (39.7% and 64.1%) and calcium channel blockers (29.4% and 33.7%).
Table 1.
Baseline Patient Demographic Data
| Intervention (n=101) | Control (n=78) | |
|---|---|---|
| Number (%) | Number (%) | |
| Gender | ||
| Male | 42 (41.6%) | 36 (46.2%) |
| Female | 59 (58.4%) | 42 (53.8%) |
| Race | ||
| Caucasian | 89 (88.1%) | 74 (94.9%) |
| Non-Caucasian | 12 (11.9%) | 4 (5.1%) |
| Mean age (Std.Dev.) | 59.6 (±13.7) | 61.9 (±11.3) |
| Mean Body Mass Index (Std.Dev.) | 32.3 (±7.7) | 31.8 (±14.7) |
| Co-morbid Conditions | ||
| Diabetes | 25 (24.8%) | 19 (24.4%) |
| Stroke or TIA | 9 (8.9%) | 2 (2.6%) a |
| Myocardial Infarction | 4 (4.0%) | 5 (6.4%) |
| Peripheral arterial disease | 3 (3.0%) | 2 (2.6%) |
| Angina | 2 (2.0%) | 0 |
| Heart failure | 2 (2.0%) | 0 |
| Coronary artery bypass | 1 (1.0%) | 6 (7.7%) b |
| Nephropathy | 1 (1.0%) | 0 |
| Systolic Blood Pressure | 153.1±10.0 | 150.3±9.0 |
| Diastolic Blood Pressure | 84.9±12.0 | 85.4±11.0 |
p=0.117
p=0.044, Fisher’s exact test. Unless otherwise indicated, groups were not significantly different.
Table 2.
Antihypertensive Medication Use
| Percent of Patients | ||||
|---|---|---|---|---|
| Baseline | End-of-study | |||
| Control (n=78) |
Intervention (n=101) |
Control (n=68) |
Intervention (n=92) |
|
| Number of Medications | ||||
| 0 | 21.8% | 16.8% | 7.4% | 0 |
| 1 | 26.9% | 33.7% | 23.5% | 15.2% |
| 2 | 33.3% | 25.7% | 42.7% | 37.0% |
| 3 | 18.0% | 23.8% | 16.2% | 32.6% |
| 4 | 0 | 0 | 8.8% | 14.1% |
| 5 | 0 | 0 | 1.5% | 1.1% |
There is a significant difference in the number of medications in the intervention group compared to the control group at the end of the study (p=0.0033, Mantel-Haenszel chi-square test).
Table 3.
Antihypertensive Medication Summary by Drug Class
| Percent of Patients | ||||
|---|---|---|---|---|
| Baseline | End-of-study | |||
| Control (n=78) |
Intervention (n=101) |
Control (n=68) |
Intervention (n=92) |
|
| Medication Class | ||||
| Diuretics | 42.3% | 48.5% | 58.8% | 87.0% a |
| Beta-adrenergic Blockers | 38.5% | 36.6% | 45.6% | 39.1% |
| ACE Inhibitor | 30.8% | 41.6% | 39.7% | 64.1% b |
| Angiotensin Receptor Blockers | 16.7% | 9.9% | 19.1% | 13.0% |
| Calcium Channel Blockers | 15.4% | 17.8% | 29.4% | 33.7% |
| Alpha-adrenergic Blockers | 2.6% | 1.0% | 1.5% | 2.2% |
| Central Alpha-adrenergic Blockers | 0 | 1.0% | 0 | 1.1% |
| Vasodilators | 1.3% | 0 | 2.9% | 1.1% |
| Aldosterone Blockers | 0 | 0 | 0 | 4.4% |
p<0.0001
p=0.0022, Pearson chi-square tests (all compared to control group). Unless otherwise indicated, groups were not significantly different.
Total ADR scores improved from baseline in both the intervention (29.9 to 22.7) and control (26.5 to 18.4) groups (p<0.0001), with higher numbers indicating higher ADRs (Figure 1). The ADR scores between control and intervention groups were not significantly different at any time point. For patients on no antihypertensive medication at baseline, ADR scores improved similarly in both the intervention (25.4 to 20.8) and control (18.9 to 14.4) groups. There was no significant effect of the number of antihypertensive medications on the ADR scores in the multivariate analysis.
Figure 1.
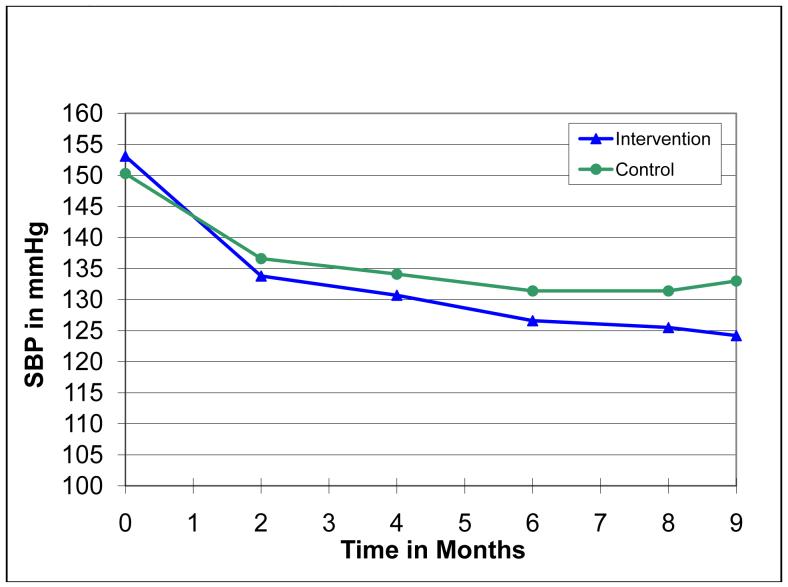
The change in scores for reported ADR symptoms in various organ system subcategories is displayed in Figure 2. ADR scores for all system categories declined significantly (indicating lower ADRs) from baseline to the end of the study (p<0.05). However, there were no significant differences between control and intervention groups for any system category, with the exception of the gastrointestinal category in which the intervention group had a slightly higher score throughout the study (p=0.027). ADRs in the CNS category account for approximately one-third of the total ADR score at both baseline and 9 month visits. CNS symptoms included dizziness, fatigue, difficulty concentrating, etc. (see Appendix 1, Section I for a complete listing). As we previously reported, average systolic BP in the intervention group dropped from 153.1±10.0 mmHg at baseline to 124.2±9.7 mmHg at 9 months.25 The control group’s average systolic BP dropped from 150.3±9.0 mmHg at baseline to 133.0±14.2 mmHg at 9 months (Figure 3).
Figure 2.
ADR Scores by Symptom Category
Figure 3.
Systolic Blood Pressure by Visit
The intervention group showed improvement from baseline in SE scores (121.01 to 124.35; p=0.0005); however, SE scores did not change in the control group nor were they different between groups at either time point (Table 4). Average SS scores did not change for either group.
Table 4.
Social Support and Self-Efficacy Scores
| Intervention (n=101) | Control (n=78) | |
|---|---|---|
| Social Support | ||
| Baseline | 7.71 | 7.82 |
| End-of-Study | 7.93 | 7.79 |
| Self-Efficacy | ||
| Baseline | 121.01 | 123.49 |
| End-of-Study | 124.36 a | 124.21 |
p=0.0005, paired t-test (compared to baseline). Unless otherwise indicated, groups were not significantly different.
Results of the multivariate analysis are shown in Table 5. The regression coefficients give both the magnitude and direction of adjusted relationships between predictor factors and ADR score. Variables significantly and independently associated with ADR score (p<0.05) were baseline ADR score, SE score, SS score, and time in the study. ADR score was 0.82 points lower for every one-point higher SS score (p<0.018) after controlling all other predictors in the model. Similarly, ADR score was 0.1 point lower for every one point greater SE score (p=0.034). ADR score was 0.86 lower for every one month the patient stayed in the study (p<.0001). A one point higher ADR score at baseline was associated with a 0.75 point lower ADR score at 9 months (p<0.0001). The number of antihypertensive medications trended towards increasing ADR score, but did not reach significance (p=0.067). Systolic BP did not significantly effect ADR score (p=0.897).
Table 5.
Multivariate Analysis of Predictor Variables of Change in ADR Scores
| Variable | Coefficient | Standard Error | p-value |
|---|---|---|---|
| Age | 0.01243 | 0.04576 | 0.7863 |
| Gender | 0.4393 | 1.1367 | 0.6997 |
| Education (>highschool) | 0.1184 | 1.1320 | 0.9169 |
| Income (≥$25,000) | 1.2589 | 1.4841 | 0.3976 |
| Married vs. unmarried | -0.8585 | 1.1965 | 0.4741 |
| Insured vs. uninsured | 1.1212 | 3.1443 | 0.7219 |
| Number of coexisting conditions | 0.6763 | 0.7529 | 0.3705 |
| Baseline ADR score | 0.7501 | 0.03257 | <0.0001 |
| Number of antihypertensives | 1.0341 | 0.5604 | 0.0669 |
| Systolic BP | -0.00623 | 0.04806 | 0.8971 |
| SE score | -0.09970 | 0.04655 | 0.0338 |
| SS Score | -0.8208 | 0.3438 | 0.0182 |
| Study time period (months) | -0.8587 | 0.1770 | <0.0001 |
| Control vs. Intervention | -1.4183 | 1.0866 | 0.1938 |
Discussion
We found that distressful symptoms suggestive of ADRs improved from baseline in both intervention and control groups even though medication use increased. The decline in ADR scores paralleled the reduction in BP. SS and SE scores independently lead to improvements in ADR scores. The number of antihypertensive medications used did not significantly impact ADR score. Our findings seem to contradict prevailing wisdom that increasing medications cause patients to experience side effects, which leads to nonadherence and ultimately poor outcomes. Other studies support our finding.
Materson et al conducted a large study of single agent antihypertensive therapy for males with hypertension.10 They observed lower ADRs with a diuretic (hydrochlorothiazide, 1.1%), beta blocker (atenolol, 2.2%) or angiotensin converting enzyme (ACE) inhibitor (captopril, 4.8%) when compared to placebo (6.4%), a calcium channel blocker (diltiazem, 6.5%), prazosin (13.8%) or clonidine (10.1%). The fact that the ADRs were more frequent with placebo than with the diuretic, beta blocker and ACE inhibitor suggested that hypertension, in fact, might not be completely asymptomatic and that select treatments may reduce ADRs for some patients.
Erickson et al compared the prevalence and intensity of symptoms and the health-related QOL of hypertensive patients prescribed an antihypertensive.8 The hypertensive patients had no other symptomatic diagnoses or medications. They compared these scores to those in patients without chronic disease and who were taking no medication. They found that hypertensive patients taking antihypertensives reported more symptoms (8.8±7.8) than the control group (4.7±4.8) (p=0.0001) and they reported more symptom distress (32.2±46.2) than the control group (12.0±18.2) (p=0.0001). Hypertensive patients also had lower scores or reduced health-related QOL on most domains of the Short-Form 36 than the control group. Additionally, their analysis discovered that hypertensive patients with BP ≥140/90 mm Hg reported more symptoms, related distress and lower health-related QOL than non-hypertensive patients. Patients with controlled BP had fewer distressful symptoms than those with uncontrolled BP but more than those without hypertension. Their findings suggest that distressful symptoms might be reduced if BP becomes controlled. Their data also suggest that even when BP is controlled, symptoms may still remain higher than in patients without hypertension. The reduced number of reported ADRs could be interpreted as an improvement in QOL as patients are no longer experiencing negative symptoms. This theory is supported by another study by Erickson in which symptoms had a negative influence on QOL in patients with hypertension.14
In the current study ADRs in the CNS category account for one-third of the total ADR score. Although many antihypertensive medications are thought to cause side effects in the CNS category such as dizziness and fatigue, these symptom scores improved over the course of the study as blood pressure improved. CNS side effects could be attributed to high blood pressure and would be expected to improve with appropriate therapy.
The results of our multivariate analysis suggest that BP lowering alone does not significantly improve adverse reactions. While our results do not confirm the theory that BP reduction independently improves symptoms, for our study population as a whole ADRs improved as BP came under control despite increased use of antihypertensives. The previously mentioned studies did not measure SS and SE so ours may be the first to evaluate the influence of these important predictors of medication adherence when blood pressure becomes better controlled..
One may hypothesize that a factor behind the reduction in ADR scores over the course of the current study was due to therapeutic selection of newer antihypertensives touted to have fewer side effects. However, the most commonly used classes of medications in both intervention and control groups at the beginning and end of the study were diuretics, beta blockers, and ACE inhibitors. In fact, 87.0% of patients in the intervention group and 58.8% of patients in the control group were utilizing a diuretic at the end of the study compared to 48.5% and 42.3% respectively at the beginning of the study. Despite the increased use of diuretics, beta blockers and ACE inhibitors, patients in both treatment groups reported fewer ADRs over the course of the study.
Another possible theory for improved ADRs over the course of the study is that the decline in reported distressful symptoms is a learning effect or desensitization due to the repetition of the questionnaire at each study visit such that ADR responses diminish despite persisting symptoms. This theory could be supported by the multivariate analysis results displaying a 0.86 point lower ADR score for every one month the patient stayed in the study (p<0.0001). However, the learning effect is only one of many possible factors influencing ADR results.
SE, the focus of Bandura’s social cognitive theory, describes a person’s belief in their capacity to achieve goals and meet expectations.19-23 If a person exhibits high SE, meaning they believe they will be successful in a task, they are more likely to put forth the effort and time necessary to achieve their goals and to have a more positive attitude about the undertaking. It is also possible that patients with high SE may have a more positive perception of their quality of life. To our knowledge, SE has not previously been shown to reduce adverse reactions. High SE is associated with improved medication adherence in patients with hyperlipidemia, HIV/AIDS, and patients undergoing transplant.30 Curtin et al conducted an observational study of SE and self-management behavior in patients with chronic kidney disease and found higher SE to be consistently correlated with better self-management behaviors.31 The mechanism by which SE improves medication adherence and self-management behavior is unclear, but could be partially mediated by improved capacity to manage adverse reactions. Our findings of improved ADRs with higher SE are consistent in theory with the benefits of high SE seen in medication adherence and general self-management behavior.
SS is a person’s network of family and friends that provide them with encouragement and assistance when needed.16, 17 It is thought that patients with strong SS will have the necessary encouragement and assistance to be adherent to a sometimes complex medication regimen and lifestyle modifications to gain control of a chronic disease like hypertension. It is also possible that patients with strong SS have a better quality of life than those with weak SS.
High SS has long been linked to improved health outcomes, including lower morbidity and mortality.18, 32-35 Rodriguez-Artalejo et al found poor SS is a significant predictor of hospital readmission after myocardial infarction.36 Our results are consistent with the theory that improved SS is associated with improved symptoms. In our study improving SS lead to a reduction in adverse reactions.
Interventions aimed at promoting SS and SE may encourage treatment adherence in part by increasing tolerability to adverse reactions. Suggested interventions include support groups, cognitive behavioral therapy, and patient education.18 In our study SE improved significantly in the pharmacist intervention group, but there was no difference between control and intervention groups at any time for SE or SS. One could argue that SE improved as a result of the pharmacist intervention. However, as there is no significant difference between groups, perhaps simply meeting regularly with a research nurse has a positive impact on SS and SE.
In summary, we theorized that higher SS or SE would lead to lower symptoms and ADRs if patients became more comfortable with their medications and were supported by family. When controlling for other variables our data suggest that the decline in ADR scores was correlated with improvement in SS and SE. Systolic BP and number of antihypertensive medications did not influence ADR scores. These findings are important since clinicians and patients frequently attribute symptoms to medications. Improving SS and SE may improve a patient’s ability to manage or overcome ADRs. Obviously, new ADRs need to be investigated but patients should not expect to feel worse during successful treatment of their hypertensive condition, especially when SS and SE are high. The results of our study and that of Materson suggest that this finding is true even if the added medications are diuretics, beta blockers and ACE inhibitors.10
Limitations
This study did not attempt to attribute specific symptoms to a given antihypertensive. Therefore, the symptoms could be caused by a variety of factors including other medications, hypertension or other conditions. We did not collect data on non-antihypertensive medications. Patients in both groups were taking other medications in addition to their antihypertensive therapy. However, antihypertensive medications were the only group systematically changed over the course of the study. Additionally, our structured approach to measure symptoms was similar to other studies that have used global approaches to measure distressful symptoms in patients with hypertension.
Our ADR questionnaire was administered every 2 months and therefore it may not have picked up ADRs that occurred immediately after starting a new antihypertensive and either remitted over time or with a change in therapy occurring during the interval between scheduled study visits.
Literacy was not evaluated in this study. However, the research nurse read the questionnaires to patients and answered any questions the patients may have had so we do not believe literacy influenced the results.
Finally, the generalizability of our results may be limited because our study population is largely Caucasian with moderately elevated systolic BP. Future research should include minority patients and those with more significantly elevated BP readings.
Conclusion
ADR scores improved despite an increase in antihypertensive medication use. Higher SS and SE led to a decrease in reported adverse reactions. However, there is the possibility that patients became more comfortable with the survey instrument and/or research nurse leading to lower reported symptoms over time. Even so, patients should not expect an increase in distressful symptoms as their BP becomes controlled with antihypertensive medications, especially when SS and SE is high. Interventions by pharmacists that successfully at promote SS and SE will likely yield improved health outcomes and may minimize the potential adverse effects of additional antihypertensive medications .
Acknowledgments
The authors would like to acknowledge the assistance of Janyce Stewart, RN and Gail Ardery, PhD (project managers); George Bergus, MD, Jeffrey Dawson, ScD, William Doucette, PhD, Elizabeth Chrischilles, PhD, and Arthur Hartz, MD, PhD, (study steering committee); Michael Ernst, PharmD, Jessica Milchak, PharmD, Jennifer Steffensmeier, PharmD, Michael Kelly, PharmD (intervention pharmacists); Karen Kluesner, RN and Sheryl Eastin, RN (research nurses); Paul James, MD, Christopher Goerdt, MD and David Katz, MD (data and safety monitoring board).
Funding for this project was supported by the National Heart, Lung, and Blood Institute, 1 R01 HL069801-01A1. Dr. Carter is also supported by the CRIISP Center, Iowa City Department of Veterans Affairs.
References
- 1.Ross SD, Akhras KS, Zhang S, Rozinsky M, Nalysnyk L.Discontinuation of antihypertensive drugs due to adverse events: a systematic review and meta-analysis Pharmacotherapy Aug 2001218940–953. [DOI] [PubMed] [Google Scholar]
- 2.Monane M, Bohn RL, Gurwitz JH, Glynn RJ, Levin R, Avorn J.The effects of initial drug choice and comorbidity on antihypertensive therapy compliance: results from a population-based study in the elderly Am J Hypertens Jul 1997107 Pt 1697–704. [DOI] [PubMed] [Google Scholar]
- 3.Esposti E Degli, Sturani A, Di Martino M, et al. Long-term persistence with antihypertensive drugs in new patients J Hum Hypertens Jun 2002166439–444. [DOI] [PubMed] [Google Scholar]
- 4.Esposti L Degli, Esposti E Degli, Valpiani G, et al. A retrospective, population-based analysis of persistence with antihypertensive drug therapy in primary care practice in Italy Clin Ther Aug 20022481347–1357.; discussion 1346.
- 5.Julius S, Kjeldsen SE, Weber M, et al. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trial Lancet Jun 19 200436394262022–2031. [DOI] [PubMed] [Google Scholar]
- 6.Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report Jama May 21 2003289192560–2572. [DOI] [PubMed] [Google Scholar]
- 7.Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) Jama Dec 18 2002288232981–2997. [DOI] [PubMed] [Google Scholar]
- 8.Erickson SR, Williams BC, Gruppen LD.Perceived symptoms and health-related quality of life reported by uncomplicated hypertensive patients compared to normal controls J Hum Hypertens Aug 2001158539–548. [DOI] [PubMed] [Google Scholar]
- 9.Neaton JD, Grimm RH, Jr., Prineas RJ, et al. Treatment of Mild Hypertension Study. Final results. Treatment of Mild Hypertension Study Research Group Jama Aug 11 19932706713–724. [PubMed] [Google Scholar]
- 10.Materson BJ, Reda DJ, Cushman WC, et al. Single-drug therapy for hypertension in men. A comparison of six antihypertensive agents with placebo. The Department of Veterans Affairs Cooperative Study Group on Antihypertensive Agents N Engl J Med Apr 1 199332813914–921. [DOI] [PubMed] [Google Scholar]
- 11.Anderson RT, Hogan P, Appel L, Rosen R, Shumaker SA.Baseline correlates with quality of life among men and women with medication-controlled hypertension. The trial of nonpharmacologic interventions in the elderly (TONE) J Am Geriatr Soc Sep 19974591080–1085. [DOI] [PubMed] [Google Scholar]
- 12.Bulpitt CJ, Dollery CT, Carne S.Change in symptoms of hypertensive patients after referral to hospital clinic Br Heart J Feb 1976382121–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hollenberg NK, Williams GH, Anderson R.Medical therapy, symptoms, and the distress the cause: relation to quality of life in patients with angina pectoris and/or hypertension Arch Intern Med May 22 2000160101477–1483. [DOI] [PubMed] [Google Scholar]
- 14.Erickson SR, Williams BC, Gruppen LD.Relationship between symptoms and health-related quality of life in patients treated for hypertension Pharmacotherapy Mar 2004243344–350. [DOI] [PubMed] [Google Scholar]
- 15.Sarason IG, Sarason BR, Shearin EN. Social support as an individual difference variable: Its stability, origins, and relational aspects. J Personality and Social Psychology. 1982;50:845–855. [Google Scholar]
- 16.Sarason IG, Sarason BR.Concomitants of social support: attitudes, personality characteristics, and life experiences J Pers Sep 1982503331–344. [DOI] [PubMed] [Google Scholar]
- 17.Doherty WJ, Schrott HG, Metcalf L, Iasiello-Vailas L.Effect of spouse support and health beliefs on medication adherence J Fam Pract Nov 1983175837–841. [PubMed] [Google Scholar]
- 18.Reblin M, Uchino BN.Social and emotional support and its implication for health Curr Opin Psychiatry Mar 2008212201–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bandura A. Self Efficacy Changing Societies. Cambridge University Press; 1995. [Google Scholar]
- 20.Bandura A, Jeffery RW, Wright CL.Efficacy of participant modeling as a function of response induction aids J Abnorm Psychol Feb 197483156–64. [DOI] [PubMed] [Google Scholar]
- 21.Bandura A, Jourden EJ. Self-regulatory mechanisms governing the impact of social comparison on complex decision making. J Pers Soc Psychol. 1991;60:941–951. [Google Scholar]
- 22.Bandura A, Reese L, Adams NE.Microanalysis of action and fear arousal as a function of differential levels of perceived self-efficacy J Pers Soc Psychol Jul 19824315–21. [DOI] [PubMed] [Google Scholar]
- 23.De Geest S, Abraham I, Gemoets H, Evers G.Development of the long-term medication behaviour self-efficacy scale: qualitative study for item development J Adv Nurs Feb 1994192233–238. [DOI] [PubMed] [Google Scholar]
- 24.Fuertes JN, Mislowack A, Bennett J, et al. The physician-patient working alliance Patient Educ Couns Apr 200766129–36. [DOI] [PubMed] [Google Scholar]
- 25.Carter B, Bergus G, Dawson J, et al. A cluster-randomized trial to evaluate physician/pharmacist collaboration to improve blood pressure control. J Clin Hypertens. 2008 doi: 10.1111/j.1751-7176.2008.07434.x.;In Press.
- 26.Kaboli P, Hoth A, Carter B. The VA Enhanced Pharmacy Outpatient Clinic (EPOC) Study: A randomized-controlled pharmacist-physician intervention trial. J Gen Intern Med. 2004;19:227. [Google Scholar]
- 27.Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans and experimental animals: Part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research Hypertension Jan 2005451142–161. [DOI] [PubMed] [Google Scholar]
- 28.Wright JT, Jr., Bakris G, Greene T, et al. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial Jama Nov 20 2002288192421–2431. [DOI] [PubMed] [Google Scholar]
- 29.Von Muenster SJ, Carter BL, Weber CA, et al. Description of pharmacist interventions during physician-pharmacist co-management of hypertension Pharm World Sci Jan 2008301128–135. [DOI] [PubMed] [Google Scholar]
- 30.Denhaerynck K, Abraham I, Gourley G, et al. Validity testing of the Long-Term Medication Behavior Self-Efficacy Scale J Nurs Meas Winter 2003113267–282. [DOI] [PubMed] [Google Scholar]
- 31.Curtin RB, Walters BA, Schatell D, Pennell P, Wise M, Klicko K.Self-efficacy and self-management behaviors in patients with chronic kidney disease Adv Chronic Kidney Dis Apr 2008152191–205. [DOI] [PubMed] [Google Scholar]
- 32.Seeman TE.Social ties and health: the benefits of social integration Ann Epidemiol Sep 199665442–451. [DOI] [PubMed] [Google Scholar]
- 33.Hanson BS, Isacsson SO, Janzon L, Lindell SE.Social network and social support influence mortality in elderly men. The prospective population study of “Men born in 1914,” Malmo, Sweden Am J Epidemiol Jul 19891301100–111. [DOI] [PubMed] [Google Scholar]
- 34.House JS, Landis KR, Umberson D.Social relationships and health Science Jul 29 19882414865540–545. [DOI] [PubMed] [Google Scholar]
- 35.Berkman LF, Syme SL.Social networks, host resistance, and mortality: a nine-year follow-up study of Alameda County residents Am J Epidemiol Feb 19791092186–204. [DOI] [PubMed] [Google Scholar]
- 36.Rodriguez-Artalejo F, Guallar-Castillon P, Herrera MC, et al. Social network as a predictor of hospital readmission and mortality among older patients with heart failure J Card Fail Oct 2006128621–627. [DOI] [PubMed] [Google Scholar]