Abstract
Microcalcifications are an important diagnostic marker for breast cancer on mammograms, yet the mechanism of their formation is poorly understood. Indeed, there is presently no short-latency, high-yield, syngeneic rodent model of the process. Bone morphogenetic protein-2 (BMP-2) is a key mediator of physiological bone formation and pathological vasculature calcification, but its role in breast cancer microcalcification is unknown. In this study, R3230 rat breast tumors were adapted to cell culture, transduced with adenoviral BMP-2, and inoculated into a syngeneic host. Tumor growth and calcium salt deposition were quantified in living animals over time using micro-computed tomography, and probed chemically using near-infrared fluorescence. Plasma BMP-2 levels were quantified over time by ELISA. Within three weeks, 100% of breast tumors developed microcalcifications, which were absent from all normal tissues. Importantly, when two tumors were initiated in a single host, the ipsilateral tumor expressing BMP-2 was able to induce microcalcification in the contralateral tumor that was not expressing BMP-2, suggesting that BMP-2 can act humorally. Taken together, we describe the first reproducible rodent model of breast cancer microcalcification, prove that BMP-2 expression is sufficient for initiating the process, and lay the foundation for a new generation of targeted diagnostic agents.
Keywords: Bone Morphogenetic Protein-2, Breast Cancer, Microcalcification, Mammography, Animal Models
INTRODUCTION
Breast cancer is a leading cause of cancer-related deaths, with 182,460 women in the United States expected to be diagnosed with the disease in 2008 [1]. Despite its deficiencies, diagnostic x-ray mammography [2] remains the most widely available screening tool for the early detection of breast cancer, and reduces breast cancer mortality rates by 20% to 35% in women aged 50 to 69 years, and slightly less in women aged 40 to 49 years [3].
A hallmark of breast cancer on diagnostic mammography is microcalcification, which occurs in 30% to 50% of patients [4]. Although mammography cannot distinguish the chemical form of the calcium salts present, and therefore relies on the pattern of crystal deposition [5], microcalcification is actually of two major types. Type I crystals, found more frequently in benign ductal cysts, are birefringent and colorless, and are composed of calcium oxalate [4]. Type II crystals, most often seen in proliferative lesions and associated with breast cancer cells, are non-birefringent and basophilic, and are composed of calcium hydroxyapatite (HA) [6]. HA itself appears to have potent bioactivity, inducing mitogenesis and increased enzymatic activity of matrix metalloproteinases (MMPs) MMP-2 and MMP-9 [7]; these responses can be blocked by inhibiting calcium phosphate crystallization with phosphocitrate [8].
Despite its prevalence and importance in breast cancer, the mechanism of HA deposition is poorly understood. In fact, there is presently no reproducible, high-penetrance animal model available for studying the molecular pathways of breast cancer microcalcification. Although multiple rodent models of breast carcinogenesis have been developed with the use of MMTV vectors or BRCA1/BRCA2 knockouts [9–12], microcalcification of these tumors is an extremely rare and unpredictable event. A straightforward, reproducible, syngeneic, short-latency rodent model of breast cancer microcalcification could potentially have a major impact on the development of improved diagnostic imaging for the disease.
Since many physiological processes are conserved in malignancies, a signal transduction pathway of particular interest in breast cancer microcalcification is that mediated by bone morphogenetic protein-2 (BMP-2). Synthesized as a 60-kDa precursor, BMP-2 is processed intracellularly to a small 18-kDa monomer that is secreted [13,14]. BMP-2 is a member of the transforming growth factor-β (TGF-β) superfamily and is involved in embryogenesis and morphogenesis of various tissues and organs [15–17]. It is also a key mediator of calcium salt mineralization. Recombinant adenoviruses expressing BMP-2 can effectively induce orthotopic ossification when either adenovirus-transduced osteoblast progenitors or the viral vectors themselves are injected into the quadriceps of athymic mice [18,19]. Preclinical studies have also shown that ectopic BMP-2 expression can be utilized in various therapeutic interventions such as bone defects, non-union fractures, spinal fusion, osteoporosis, and root canal surgery [20–23]. Locally overexpressed BMP-2 can also induce calcification in MCF-7 xenograft breast tumors [24], although the kinetics, efficiency, chemical form, and reproducibility of this model have not been described.
The present study was designed to determine if BMP-2 could be playing a role in breast cancer microcalcification, to determine whether it exerted its biological activity systemically or only locally, and to develop a reproducible, robust animal model for the testing of new diagnostic agents for breast cancer.
MATERIALS AND METHODS
Adaptation of the R3230 Mammary Adenocarcinoma to Cell Culture
Serially-passaged R3230 mammary tumors [25] were the generous gift of Dr. Ralph Weissleder (Center for Molecular Imaging Research, Massachusetts General Hospital, Boston, MA). To adapt the cells to cell culture, fresh tumor (measuring approximately 1 cm in diameter) was harvested from a Fischer 344 female rat host. Within 30 min of tumor resection, the tumor was homogenized with a tissue grinder (Model 23, Kontes Glass Co., Vineland, NJ) using aseptic technique and suspended in 7 mL of RPMI 1640 (INC Biomedicals, Aurora, IL) in a sterile 50-mL conical centrifuge tube. The tumor cell suspension was centrifuged at 700g for 10 min and washed twice with 20 mL of PBS. Cell pellets were suspended in 4 mL of 0.25% w/v Trypsin-Versene (Cambrex, Walkersville, MD) solution and incubated at 37°C with 5% CO2 for 30 min. The trysin-versene digestion was stopped by adding 30 mL of complete RPMI 1640 media containing 10% FBS (Gemini Bio-products, Calabasas, CA) and antibiotics (penicillin [100 U] and streptomycin [100 mg]). The cell suspension was transferred to a sterile 15-mL conical centrifuge tube and the cells were centrifuged at 700g for 10 min at room temperature (RT). The supernatant was discarded carefully, so as not to disturb the cell pellet. Cell pellets were resuspended in 5 mL of RPMI + 10% FBS and 100 μL were used for Coulter counting of cell number. Cells (2 × 106) were seeded onto a T-25 flask with 10 mL of RPMI 1640 media supplemented with 10% FBS. Cells were fed twice weekly, during which time R3230 tumor cells began to outgrow other contaminating cells such as fat cells, blood cells, endothelial cells, and primary cells. Cells were split weekly at a 1:3 ratio into new flasks, and within 2–3 weeks a uniform monolayer of R3230 cells, with their distinctive morphology, was obtained. Cells were further expanded and stocks stored in 90% FBS + 10% dimethylsulfoxide (DMSO) in LN2.
PCR Amplification and Isolation of the hBMP-2 Coding Sequence
The cDNA for human BMP-2A (originally cloned from osteosarcoma cell line U-2 OS) was purchased from the ATCC (Rockville, MD) as a lambda gt10 phage. Host strain Escherichia coli c600 (ATCC) was used for phage plaque formation, and the hBMP-2 coding sequence was directly PCR-amplified from phage plaques. Briefly, a plaque was touched with a sterile tip and transferred into 10 μL of 10 μM MgSO4 in H2O. Each 50 μL PCR reaction contained 1 μM of each primer, 0.5 mM each dNTP, 1 μL (5U/μL) of Deep Vent DNA polymerase and 2 μL of phage plaque suspension. PCR primers were 5′-GGGTTAACCTCGAGATGGTGGC CGGGACCCGCTG-3′ and 5′-GGGTTAACTCTAGAGCGACACCCACAACCCTCCA–3′. XhoI and XbaI sites, respectively, are shown underlined within the sequences. PCR was performed as follows: 95°C for 2 min, 28 cycles of PCR (94°C for 20 sec, 55°C for 30 sec, and 72°C for 90 sec), extension at 72°C for 10 min, and stop at 4°C. PCR products were purified using a Qiaquick PCR purification kit (Qiagen, Valencia, CA) for DNA sequencing, restriction enzymes digestion, and subsequent cloning.
Production of AdGFP/BMP-2 Adenoviruses and Cell Transduction
To facilitate the expression of BMP-2 in breast cancer cells, hBMP-2 was first cloned into a shuttle vector, pAdTrack-CMV [26]. pAdTrack-CMV also co-expresses the enhanced Aequorea victoria green fluorescent protein (GFP), which can be used as a convenient surrogate for adenovirus infection and gene transduction. The resultant plasmid (pAdTrackCMV-BMP2) was verified by bidirectional DNA sequencing, linearized by digestion with restriction endonuclease PmeI, and co-transfected with the adenoviral backbone vector pAdEasy-1 [26] into E. coli BJ5183 competent cells. Recombinants were selected for kanamycin resistance, and recombination confirmed by PCR and multiple restriction endonuclease analysis.
The resultant adenoviral vector pAdETC/BMP2 was linearized with PacI and transfected into 293 cells for adenovirus production. Two weeks after initial transfection, cells were harvested, pelleted, suspended in PBS (4 ml for up to ten 10-cm plates of HEK293 cells), and lysed by three freeze/thaw cycles. The lysate was then used to re-infect HEK293 cells for virus amplification. For large-scale production, final lysates were placed in pre-prepared dialysis tubes (Spectra/Por 12–14 kDa MWCO, Spectrum Rancho Dominguez, CA), which were placed in dry PEG 8000 powder on ice for several hours until the desired volume (2 ml) was reached. This method concentrated the virus without loss of titer. Virus titration was performed as described previously [27]. Typical titers of final adenovirus lysate preparations used for R3230 cell infection were 1.4 × 1011 PFU/ml. For long-term storage, lysates were adjusted to 10% final glycerol, flash frozen in LN2, and stored at −80°C.
R3230 cells grown at 70% to 80% confluence on 10-cm diameter tissue culture plates were washed once with DMEM, incubated with adenovirus at a multiplicity of infection (MOI) of 500:1 for 2 hours, then returned to DMEM containing 10% fetal bovine serum (FBS; Gemini Bio-products, Calabasas, CA) for 48 hours.
Breast Tumor Induction in Syngeneic Hosts
All animals were used under the supervision of an approved institutional protocol. Anesthesia was induced using intraperitoneal (IP) injection of a mixture of 50 mg/kg ketamine hydrochloride (Ketaject; Phoenix Pharmaceutical, St. Joseph, MO) and 5 mg/kg xylazine hydrochloride (Bayer, Shawnee Mission, KS). Booster anesthesia injections at 1/10th the dose were administered every 30–60 min IP as needed.
After harvesting adenovirus-transduced cells with trypsin/EDTA and washing with phosphate buffered saline, pH 7.4 (PBS), 2×107 adenovirus-transduced R3230 cells suspended in 350 μL of DMEM were slowly implanted subcutaneously into the mammary fat pad of syngeneic Fischer 344 female rats (Taconic Farms, Germantown, NY) using a 1 cc syringe and 1 1/2″ 21-gauge needle. Tumor growth was initially assessed by palpation every 3 days, with tumors forming a raised hard nodule over the otherwise smooth breast plate, and by microCT and fluorescence imaging as described throughout. At the time of injection, rats averaged 7 to 9 weeks of age and weighed 150 ± 20 g. For comparison of microcalcification formation in AdGFP/BMP-2 versus AdGFP transduced tumors, N = 8 rats in each group were used. N = 4 rats were used for bilateral tumor formation. N = 8 rats per group were used for the time course study of GFP expression. Resected tumors were fixed in formalin, processed for histology, and stained using hematoxylin and eosin (H&E) or the method of von Kossa [28].
Immunofluorescence Analysis of BMP-2 and GFP Expression in R3230 Cells
For immunofluorescence analysis of cellular BMP-2 expression, R3230 cells were seeded at 3×104 into a 12-well plate onto glass cover slips and cultured for 24 hr in RPMI-1640 containing 10% FBS and penicillin (100 U/mL) and streptomycin (100 μg/mL). Cells were then infected with AdGFP/BMP-2 or AdGFP at an MOI of 106 and 0 (mock) for 24 hr, fixed with 2% paraformaldehyde in PBS for 15 min, washed with PBS, then incubated with 5 μg/mL of an affinity-purified goat polyclonal antibody specific for human BMP-2/4 (Fitzgerald Industries Intl., Concord, MA), in PBS supplemented with 1% non-fat dried milk, for 1 hr at room temperature. Cells were then thoroughly washed with PBS and stained with 5 μg/mL of a Cy3-conjugated donkey anti-goat IgG (Jackson ImmunoResearch Laboratories Inc., West Grove, PA) for 1 hr in the dark, washed with PBS-T and DAPI for 5 min, and mounted with Fluoromount-G for observation under fluorescence microscopy.
Immunoblotting Analysis of BMP-2 Expression in R3230 Cells
For immunoblot analysis of cellular BMP-2 expression, R3230 cells were seeded onto 6-well plates until 60–80% confluent, and infected as described above. Within 24 hr of infection, GFP signal could be seen in ≈ 80% of cells. Cells were then washed once with PBS, refed with 750 μL of PBS per well, and incubated at 37°C for an additional 24 hr (i.e., 48 hr post-infection). 750 μL of cell culture supernatant was collected, desalted, and concentrated 5-fold in PBS with a 10 kDa MWCO Vivaspin filter (VivaScience Sartorius Group, Hannover, Germany). For measurement of BMP-2 levels, the equivalent of 75 μL of supernatant mixed with 4X sample loading buffer (PAGEgel Inc, San Diego, CA), heated at 90°C for 10 min, and separated by 16% Tris-Tricine SDS-PAGE. Comparison was made to recombinant human BMP-2 produced in bacteria (Liu et al., manuscript in preparation). After electrophoretic transfer (100 V for 1 hr), PVDF membranes were dried at 37°C for 1 hr, washed in methanol for 2 sec, and blocked with 2% non-fat dry milk in PBS containing 0.05% Tween-20 (PBS-T) overnight at 4°C. After washing the membrane with PBS-T, 2 μg/mL of mouse monoclonal anti-human BMP-2 antibody (R&D Systems, Minneapolis, MN) in PBS-T containing 1% NFD milk was added and incubated for 1 hr at RT. Bound antibodies were detected using horseradish peroxidase (HRP)-conjugated donkey anti-mouse IgG secondary antibody (Jackson ImmunoResearch Laboratories Inc., West Grove, PA) followed by ECL detection system (Pierce, Rockford, IL) according to the manufacturer’s instructions. Band density was quantitated by densitometric analysis. Known amounts of pure recombinant hBMP-2 (R&D Systems) were used in parallel lanes to create a calibration curve for quantitation of supernatant protein concentration.
Micro-Computed Tomography
MicroCT scans of the entire animal (snout to tail), at 90 μm resolution, and microCT scans of breast tumors at 45 μm resolution, were performed weekly for 5 weeks using an eXplore Locus microCT scanner (GE Healthcare, Milwaukee, WI). General imaging system parameters included: 80 kV, 450 mA, bin mode 2×2, field-of-view 8.0 × 4.5 cm. For 90 μm resolution scans, pixel size was set to 95 μm, the angle of increment was 0.9 mm, scan time was 20 min, and reconstruction time was 10 min. For 45 μm resolution scans, pixel size was set to 48 μm, the angle of increment was 0.5 mm, scan time was 45 min, and reconstruction time was 45 min. Visualization and quantification were performed using MicroView (GE Healthcare) software.
NIR Fluorescence Imaging using Pam 800
The HA-specific, targeted NIR fluorophore Pam800 has been described in detail previously [29]. Four rats (2 rats with AdGFP/BMP-2 R3230 tumor, 2 rats with control R3230 tumor) were administered Pam800, diluted into 100 μL of PBS, pH 7.4, intravenously at a dose of 20 nmol per rat. Five hr post-injection, resected tumors were imaged with our previously described NIR fluorescence imaging system [30].
Visible Wavelength Fluorescence Imaging for GFP
All visible fluorescence data were collected using a Maestro™ (CRI, Woburn, MA) in vivo imaging system. Hyperspectral imaging was performed daily for the first 13 days post-tumor inoculation, weekly for 5 weeks, after animal sacrifice, and after tumor resection. The hair of rats was removed either by shaving or using a depilatory cream (Nair). Multispectral imaging data sets (cubes) were acquired with images typically spaced every 10 nm throughout the desired spectral range. For GFP fluorescence, animals were imaged for fluorescence emission from 500 to 720 nm using a blue excitation filter (445 to 490 nm) and a 515-nm long-pass secondary emission filter.
Quantitation of Serum BMP-2 Levels
Serum levels of human BMP-2 were measured on days 0, 7, 14, 21, 28, and 35 using an enzyme-linked immunosorbent assay (ELISA) specific for human BMP-2 (Antigenix America, Huntington Station, NY), which does not cross-react with rat BMP-2. Briefly, the plates were coated with anti-BMP-2 antibody (1 μg/mL, 100 μL/well) in carbonate buffer, pH 9.6, and blocked with ELISA Coating Stabilizer (200 μL/well). The collected sera with serial dilutions were added to the coated plates (100 μL/well) in triplicate and incubated at RT for 2 hr. After 4 washes of PBS-T, biotinylated anti-BMP-2 antibody (1:1000 dilution [1 μg/mL], 100 μL/well) was added and incubated for 2 hr. After four washes of PBS-T, streptavidin-HRP (1:1000 dilution, 100 μL/well) was added and incubated for 30 min. After 5 washes with PBS-T, TMB substrate (100 μL/well) was added and incubated at RT for 10 min. Reactions were stopped using 2N sulfuric acid (100 μL/well) and absorbance at 450 nm was measured with a VersaMax Tunable Microplate Reader (Molecular Devices, Sunnyvale, CA).
RT-PCR of Excised Breast Tumors
Bilateral tumors were excised from anesthetized rats, flash frozen in LN2, and stored at −80°C. Total RNA was extracted from tumors using Trizol® reagent (GIBCO/BRL, Grand Island, NY) according to the manufacturer’s instructions. Sample RNA (5 μg) was treated with amplification grade DNase I (Invitrogen, Carlsbad, CA) as described by the manufacturer’s protocol before reverse transcription. First-strand cDNA was synthesized from RNA by reverse transcription (RT) using a Qiagen OneStep RT-PCT kit (Qiagen GmbH, Hilden, Germany) and the primers listed below. In a parallel control reaction, RT was performed without specific primers and processed for PCR to exclude DNA contamination. The polymerase chain reaction (PCR) mixture, in a total volume of 25 μL, contained 50 μM deoxynucleoside triphosphates, 0.5 U Taq DNA polymerase, 1.5 mM MgCL2, 1X PCR buffer, and the primer pair at 1 μM. The primers used for amplification are as follows, with product sizes in parentheses:
| hBMP-2 (377 bp) | sense: 5′-TCATCCCAGCCCTCTGACGA-3′ |
| anti-sense: 5′-GAAACTGCTATTGTTTCCTA-3′ | |
| EGFP (312 bp) | sense: 5′-TTCAAGGACGACGGCAACTA-3′ |
| anti-sense: 5′-GTGCTCAGGTAGTGGTTGT-3′ | |
| AdBMP-2/SV40pA (280 bp) | sense: 5′-GCTATTGCTTTATTTGTAACCATT-3′ |
| anti-sense: 5′-AAGAACTATC AGGACATGGT-3′ | |
| Rat BMP-2 (374 bp) | sense: 5′-TTATCCCGGCCTTCGGAGGAC-3′ |
| anti-sense: 5′-GAAACTACTATTTCCCAAAGCTTC-3′ | |
| Rat GAPDH (270 bp) | sense: 5′-GTGAACGGATTTGGCCGTAT-3′ |
| anti-sense: 5′-ACTCCACGACATACTCAGCA-3′ |
For PCR, the reaction mixture was initially denatured at 95°C for 2 min, then subjected to 28 cycles of: 94°C for 30 sec; 54°C at 60 sec; 72°C for 60 sec. After final extension at 72°C for 7 min, products were separated on a 2.5% agarose gel along with base pair markers, visualized with ethidium bromide, and quantified for band intensity. The GAPDH gene was used as an appropriate reference in RT-PCR studies of R3230 tumor cells with or without viral infection.
Statistical Analyses
Two-tailed Student t-tests were used for significance testing. Means are presented along with standard errors.
RESULTS
Adaptation of R3230 Rat Breast Tumors to Cell Culture
R3230 breast adenocarcinomas are typically passaged serially in syngeneic Fischer 344 female rats, which precludes genetic manipulation. As described in Materials and Methods, we adapted R3230 cells to tissue culture while maintaining their high (43 out of 46 inoculations; >93%) tumor induction rate after inoculation subcutaneously into the mammary fat pad.
Adenoviral Co-Expression of BMP-2 and GFP
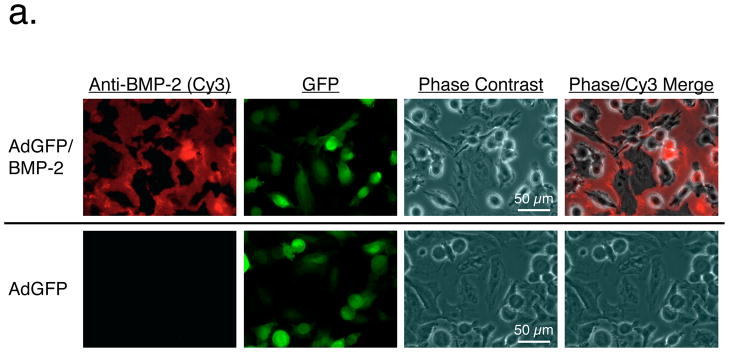
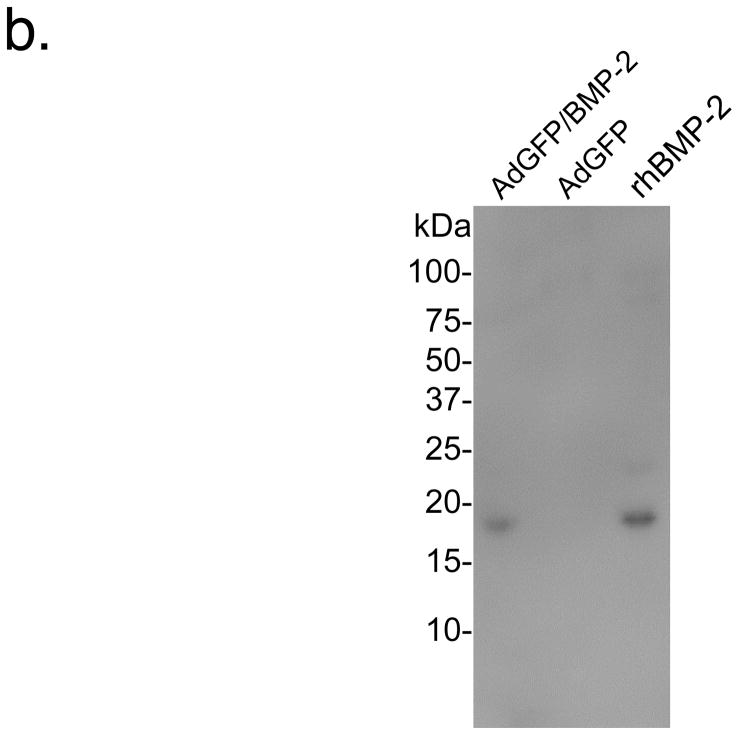
R3230 cells were infected at a MOI of 500:1 with adenoviruses expressing enhanced GFP alone (controls) or co-expressing GFP and human BMP-2. To confirm that BMP-2 was being secreted extracellularly as a mature protein, immunofluorescence and immunoblotting were performed. As shown in Figure 1a, BMP-2 deposited extracellularly within 48 hours after adenoviral transduction, even when cells were co-expressing high levels of GFP. Immunoblotting (Figure 1b) confirmed that BMP-2 was indeed secreted, showing the expected molecular weight of 18 kDa. Quantitative immunoblotting using recombinant BMP-2 as a calibration standard revealed levels of 0.2 μg/ml in the cell supernatant secreted between 24 to 48 hours after transduction.
Figure 1. Adenoviral co-expression of BMP-2 and GFP.
a. Immunofluorescence detection of BMP-2 and GFP in adenovirus transduced R3230 cells. R3230 cells infected with AdGFP/BMP-2 virus (top) and control AdGFP viruses (bottom) 48 hours previously were assessed for expression of BMP-2 (left column) and GFP (2nd column). A phase contrast (3rd column) and merge of BMP-2 and phase contrast images (right column) are also shown.
b. Secretion of BMP-2 by adenovirus transduced R3230 cells. The equivalent of 75 μL of cell culture supernatant collected between 24 and 48 hours from R3230 cells transduced with AdGFP/BMP-2 (left lane) or control GFP viruses (middle lane), and 50 ng of recombinant human BMP-2 (rhBMP-2; right lane), were resolved on a 16% Tris-Tricine SDS-PAGE gel and subjected to immunoblot analysis using a BMP-2-specific antibody.
Breast Cancer Microcalcifications Induced by BMP-2 Expression
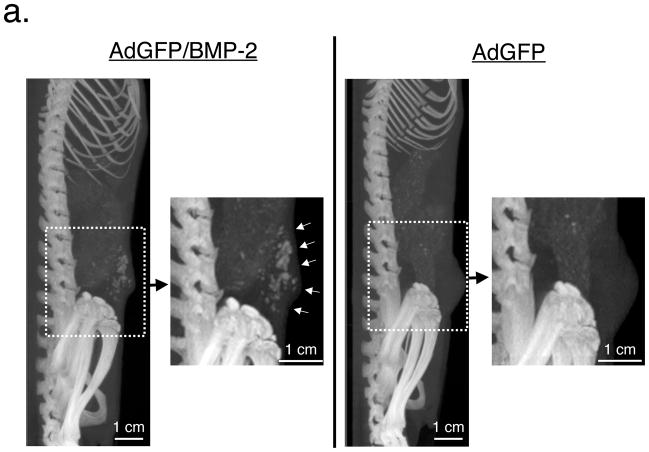
R3230 cells inoculated into the mammary pad of Fischer 344 rats formed large, histology-consistent, breast tumors over the course of 5 weeks. Interestingly, cells infected with AdGFP/BMP-2 for 48 hours prior to inoculation formed tumors that produced microcalcifications in 100% (8 out of 8 tumors) of cases (Figure 2a). In contrast, small volume (<1%) microcalcifications occurred in only a single control (AdGFP) tumor out of 8 inoculated (Figure 2a), and this was in an area of apparent tumor necrosis. Importantly, full body microCT scanning failed to demonstrate microcalcifications in any normal tissue, even as microcalcifications in tumors approached high levels.
Figure 2. Molecular imaging of breast cancer microcalcifications.
a. Longitudinal microCT imaging of Fischer 344 rats with R3230 breast tumors. Representative microCT scans (maximal intensity projections of 90 μm resolution scan) of week 4 animals having a single tumor comprised of R3230 cells transduced pre-injection with either AdGFP/BMP-2 (left) or AdGFP (right). Insets show high-resolution (45 μm) maximal intensity projection of the tumors. White arrows indicate microcalcifications.
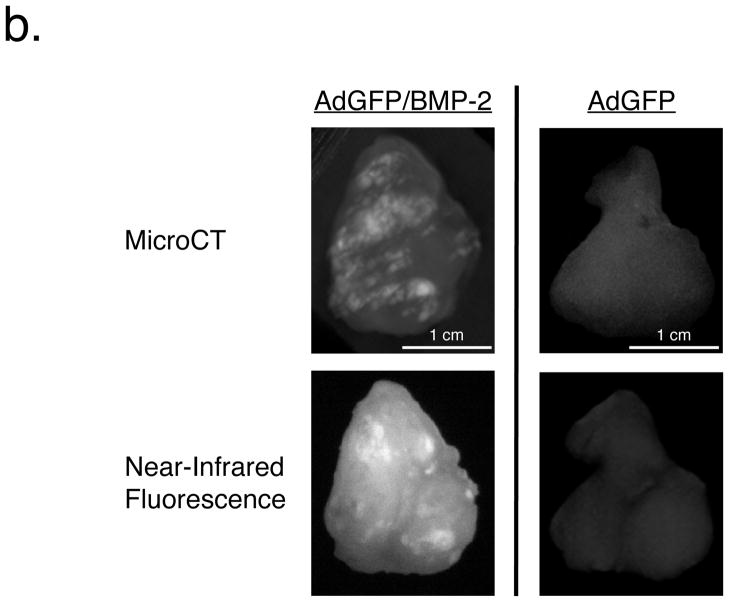
b. Hydroxyapatite content of breast tumor microcalcifications. Five hours after intravenous injection of 20 nmol of HA-targeted NIR fluorophore Pam800, AdGFP/BMP-2 tumors (left) or control AdGFP tumors (right) were excised and subjected to 45 μm microCT (top; maximal intensity projections) and planar reflectance NIR fluorescence (bottom).
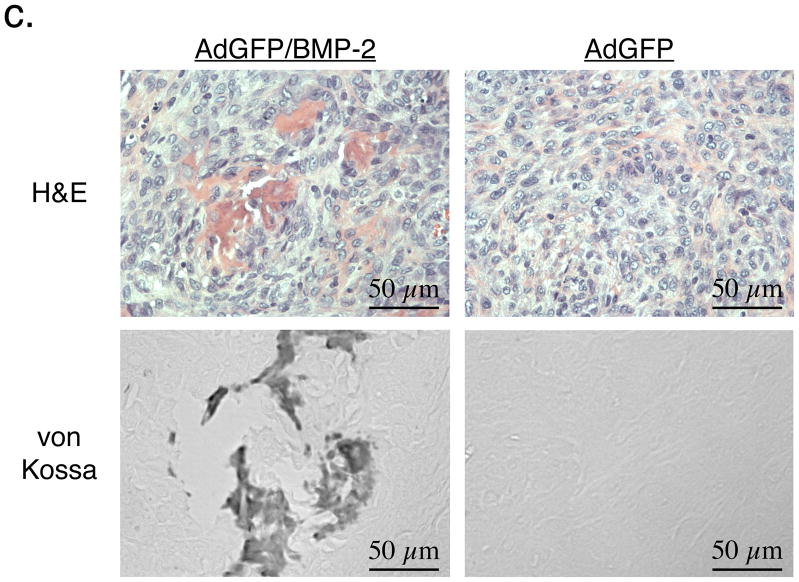
c. Tumor histology. Shown are representative sections from AdGFP/BMP-2 tumors (left) or control AdGFP tumors (right) stained using H&E (top) or the method of von Kossa (bottom).
To determine whether the calcium salt HA was a component of the microcalcification seen by microCT, a cohort of tumor-bearing animals were injected intravenously with a NIR fluorophore specific for HA [29,31–33]. As shown in Figure 2b, intense NIR fluorescence signal (signal to background ratios greater than 5:1) in AdGFP/BMP-2 tumors, but not in AdGFP tumors, suggesting that HA is, indeed, a major component of BMP-2 induced microcalcification.
As shown in Figure 2c, H&E histology of AdGFP/BMP-2 tumors revealed an intense osteoblast-like reaction. Von Kossa staining confirmed the presence of abundant calcium salts in AdGFP/BMP-2 tumors but not control AdGFP tumors.
Kinetics of Tumor Growth, Microcalcification Formation, and Serum BMP-2 Levels
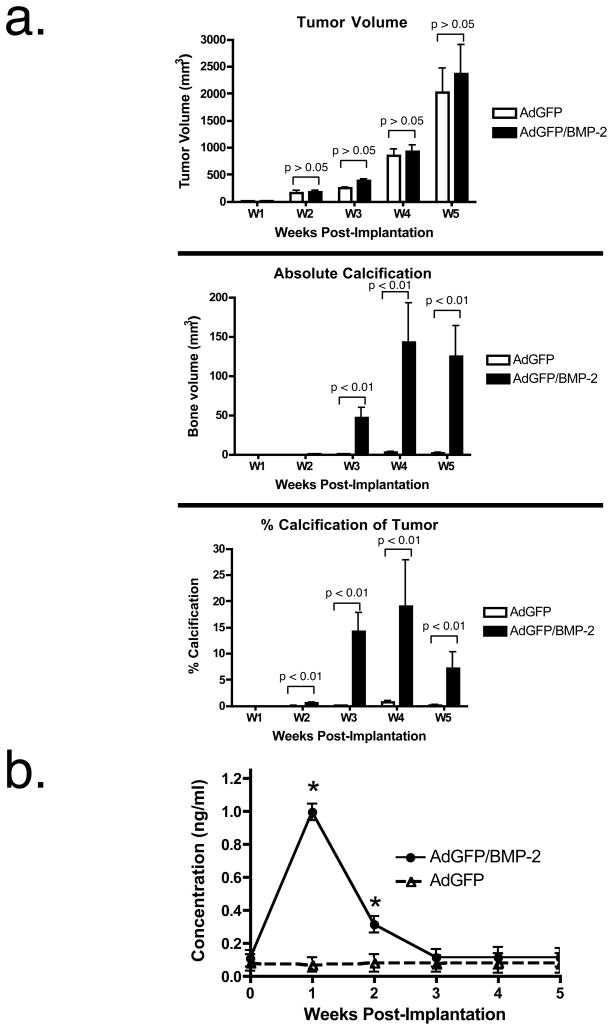
After a lag period of approximately 2 weeks, R3230 breast tumors exhibit exponential growth in vivo (Figure 3a). There was no statistical difference between tumors transduced prior to inoculation with AdGFP or AdGFP/BMP-2. Beginning at day 21 after R3230 cell inoculation, however, microcalcifications were easily detectable by microCT, accounting for up to 15% of tumor volume (Figure 3a). Absolute levels of microcalcification stabilized by week 4. Percent tumor calcification actually decreased during week 5 as tumor volume increased exponentially, suggesting that the process of calcification is a complex function of cell proliferation and local environment. Intriguingly, serum levels of human BMP-2 in rats inoculated with AdGFP/BMP-2 peaked at week 1, and became undetectable by week 3 (Figure 3b).
Figure 3. Kinetics of breast tumor growth, microcalcification formation, and serum BMP-2 levels.
a. Rats with AdGFP transduced breast tumors (white bars) or AdGFP/BMP-2-transduced breast tumors (black bars) were scanned weekly by microCT and breast tumor volume (top), microcalcification volume (middle), and percentage of microcalcification per tumor (bottom) were quantified. Shown are means ± SEM for N = 8 rats per condition.
b. BMP-2 serum ELISA from rats bearing AdGFP/BMP-2 tumors. Shown are mean ± SEM (N = 3 rats per condition). Asterisks denote statistically significant differences from the control (p < 0.01) as measured using a two-tailed Student’s t-test.
BMP-2 as a Humoral Mediator of Breast Cancer Microcalcification
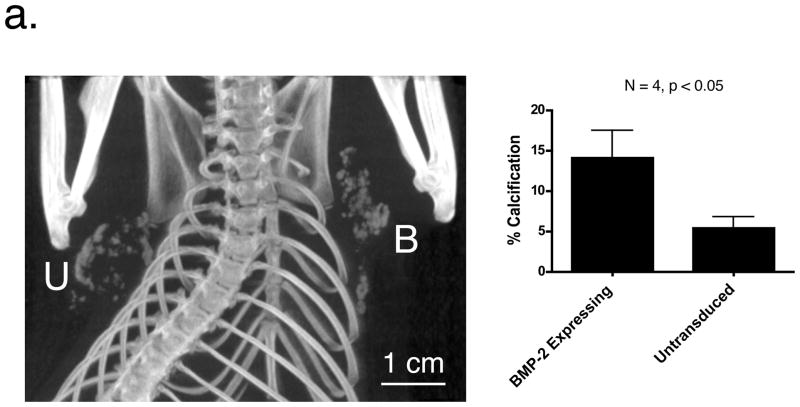
The above experiments confirmed that local secretion of BMP-2 was sufficient to produce microcalcification of breast cancers. To determine whether BMP-2 could also act as a humor mediator of the process, bilateral tumors were grown in the same animal. The ipsilateral fat pad was inoculated with R3230 cells transduced with AdGFP/BMP-2 and the contralateral fat pad with mock-infected (untransduced) R3230 cells. Kinetics of tumor growth and microcalcification in the BMP-2 expressing tumor followed the pattern described for single tumors (data not shown). However, in 100% (4 out of 4) of animals having bilateral tumors, microcalcifications were also found in the contralateral, untransduced tumor, albeit with statistically significant lower percentages of calcification (Figure 4a).
Figure 4. Microcalcification formation in untransduced, contralateral tumors.
a. MicroCT imaging of bilateral tumors. Rats inoculated with bilateral tumors were scanned using microCT at 4 weeks. U = contralateral tumor whose R3230 cells were untransduced prior to inoculation. B = ipsilateral tumor whose R3230 cells were transduced with AdGFP/BMP-2 prior to inoculation. Percentage of microcalcifications per tumor (mean ± SEM) is shown for N = 4 animals.
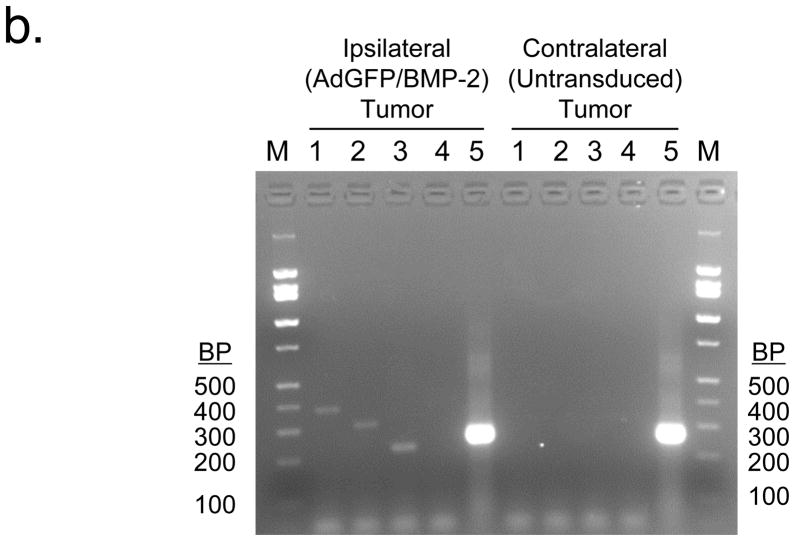
b. RT-PCR analysis of bilateral tumors. Tumors excised 4 weeks after inoculation were subjected to RT-PCR analysis on a 2.5% agarose gel. Lane 1 = hBMP-2 (377 bp); Lane 2 = GFP (312 bp); Lane 3 = partial of BMP-2 sequence and SV40 polyA sequence from adenovirus (280 bp); Lane 4 = rat BMP-2 (374 bp); rat GAPDH (270 bp).
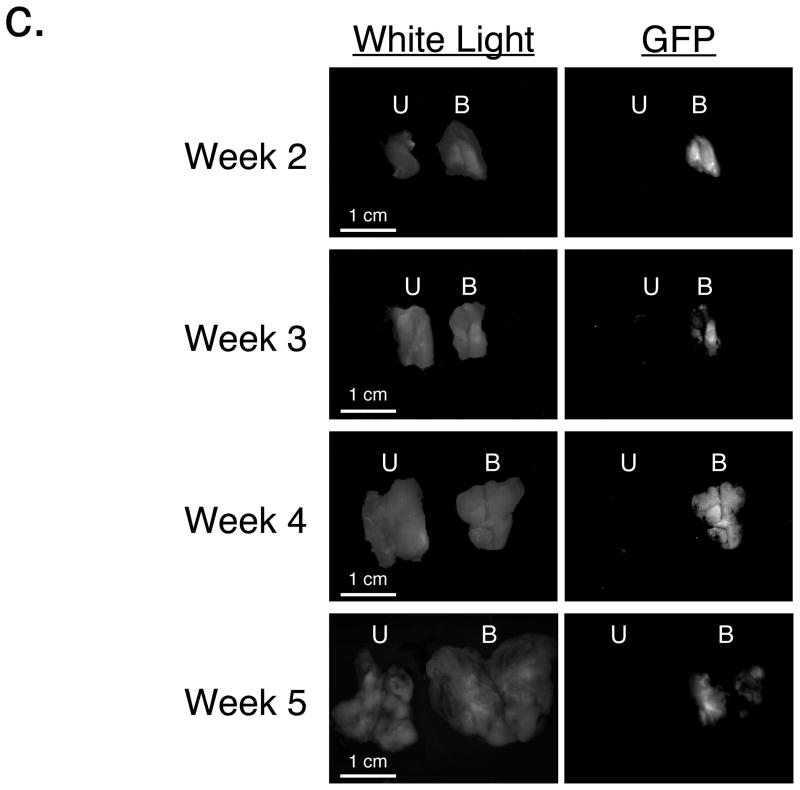
c. Expression of GFP in ipsilateral and contralateral tumors. Cohorts of N = 8 Fischer 344 rats with bilateral R3230 tumors as described in Figure 4a were sacrificed weekly. Tumors were bisected and imaged using white light (left) or GFP fluorescence (right). U = contralateral tumor whose R3230 cells were untransduced prior to inoculation. B = ipsilateral tumor whose R3230 cells were transduced with AdGFP/BMP-2 prior to inoculation. GFP fluorescence images have identical exposure times and normalizations.
To rule out infection of the contralateral tumor by adenovirus, RT-PCR was employed. No human BMP-2, GFP, or adenovirus transcripts could be amplified from untransduced, contralateral tumors, whereas ipsilateral BMP-2 expressing tumors had easily detectable levels of all three (Figure 4b). Importantly, neither tumor had detectable levels of the rat homolog of BMP-2. To ensure that viral replication was not a confounding variable, R3230 cells were infected in tissue culture with AdGFP/BMP-2 and AdGFP at a MOI of 500:1. Although all cells were highly fluorescent within 24 hours, supernatant harvested at 48 hours post-infection did not transfer GFP expression to any other cell line tested (data not shown). Moreover, in cohorts of rats bearing bilateral tumors (as described above) that were sacrificed weekly after R3230 cell inoculation, GFP fluorescence could only be found in the ipsilateral, BMP-2 expressing tumors and not in the contralateral untransduced tumor (Figure 4c). Unlike serum BMP-2 levels, which peaked by week 1, GFP levels were relatively constant, peaked at week 4, then decreased. Taken together, these data suggest that BMP-2 is capable of acting as a humoral factor to produce microcalcifications in breast cancer.
DISCUSSION
Although microcalcification is an important diagnostic sign for breast cancer on mammography, very little is known about the mechanism of calcium salt deposition. Our data suggest that, at least in a syngeneic model system, BMP-2 is sufficient for inducing high levels of microcalcification in breast adenocarcinoma, while sparing normal tissues. While these results are consistent with the fact that BMP-2 mRNA is expressed in normal human mammary epithelial cells and normal mammary glands, and in up to 85% of human breast cancers [34], an unanswered question is whether BMP-2 is exerting its effect on microcalcification through the breast cancer cells themselves, rare osteoblast-like cells within the tumor, or through the surrounding stroma. For example, the kinetics of BMP-2-induced microcalcification, and the presence of HA as a major component, are highly analogous to ectopic bone formation [23], and it is possible that pericyte-derived, osteoblast-like cells, contribute to ossification, as they do in a model of BMP-stimulated skull wound repair [35].
Even under normal circumstances, the relationship among BMP-2, the vasculature, and osteoprogenitor cells is complex. Biologically active BMP-2 is a disulfide-linked homodimer that binds to heterodimeric type 1 and type 2 BMP receptors (BMPRs), which themselves have serine/threonine kinase activity [36]. Upon BMP-2 binding, BMPR2 phosphorylates and activates BMPR1, which, in turn, phosphorylates the regulatory Smads and modulates target gene expression [37,38]. BMPR activation promotes physiological vascularization, and appears to also be involved in tumor angiogenesis by stimulating the Id1 and p38 MAPK pathway [39,40]. There is also feedback built into the system, since it has been recently demonstrated that PI-3 kinase contributes to lovastatin-induced MAPK activation, which together with Akt kinase, regulates BMP-2 expression [41]. Using our animal model, it should now be possible to identify the signal transduction pathways that mediate breast cancer microcalcification under our particular experimental conditions, thus laying a foundation for future studies aimed at understanding human breast cancer calcification.
Although multiple prior studies [22,42] in bone healing and remodeling have utilized adenovirus-transduced BMP-2, ours is the first to use it for breast cancer cells. Previously, BMP-2 has been expressed stably in human MCF-7 breast cancer cells using a plasmid and cell selection [24,40], and the BMP-2 receptor has been expressed both transiently and stably using plasmids [43]. The former studies provided important insight into the role of BMP-2 in tumorigenicity and angiogenesis, and suggested that BMP-2 was also playing a role in calcification. Our study utilized adenovirus-based transduction of BMP-2 so that protein levels, and the timing of its expression, could be controlled precisely, and without the need for long-term aminoglycoside selection.
The kinetics of tumor growth, BMP-2 expression, and microcalcification (Figure 3) are extremely interesting. Tumor growth is exponential over the 5 weeks studied, with no statistical difference in tumor volume or visual difference in in vivo pattern of growth over this time. This latter result is in contrast to prior, contradictory studies, where BMP-2 expression has improved tumorigenicity [24] and angiogenesis [40] of xenograft tumors, but inhibited proliferation of breast cancer cells in culture [44]. It is likely that in tumors, such as our syngeneic model, where tumor take rate is close to 100%, the overall contribution of BMP-2 is negligible. With respect to calcification, however, BMP-2’s effects are profound. At the time of tumor cell inoculation, BMP-2 levels are at their highest, and decline rapidly to baseline by week 3. Microcalcification, however, is only measurable starting in week 3, increases rapidly, and plateaus by week 4. Our data prove that even transient exposure to BMP-2 is sufficient to trigger molecular changes in the calcification process that persist long after the exposure. Consistent with our kinetic data is the possibility that this event occurs during angiogenesis, at which time pericytes are active. Our data are also suggest that calcification in human breast cancer, which occurs in only 30 to 50% of women, might be an epiphenomenon of a similar transient event.
Independent of molecular mechanism, our study provides the first straightforward, reproducible, syngeneic, and short latency rodent model of breast cancer microcalcification. Although by week 3, the percentage of tumor volume harboring calcifications is much higher (≈15%) than that seen in human breast cancer, earlier time points provide clinically realistic levels of ≈1% (week 2), and any other desired level selectable based on time. Our animal model is of paramount importance for the development of novel contrast agents that could provide improved sensitivity and specificity for breast cancer screening. For example, our group has previously shown that the bisphosphonate pamidronate can be used as a modular ligand to create targeted contrast agents specific for HA [29,32,33]. Using this new animal model, one could develop microcalcification-targeted contrast agents for magnetic resonance imaging (MRI) and positron emission tomography (PET), two modalities that may play an increasing role in breast cancer screening over the coming years. One could also use it to optimize NIR fluorescent bisphosphonates for tomographic optical imaging of breast cancer, with microCT serving as the ground truth.
Acknowledgments
We thank Ralph Weissleder, M.D., Ph.D. (Massachusetts General Hospital) for the generous gift of the initial R3230 tumors, Barbara L. Clough for editing, and Eugenia Trabucchi and Alice Gugelmann for administrative assistance. We gratefully acknowledge funding from the following sources: National Institutes of Health (R01-CA-115296 to J.V.F., R01-EB-005805 to J.V.F., R01-EB-004582 to R.E.L); GE Medical Systems to R.E.L; Lewis Family Trust to R.E.L. and J.V.F.
ABBREVIATIONS
- BMP-2
bone morphogenetic protein-2
- BMPRs
bone morphogenetic protein receptors
- GFP
green fluorescent protein
- HA
hydroxyapatite
- MMPs
matrix metalloproteinases
- MOI
multiplicity of infection
- NIR
near-infrared
References
- 1.Cancer Facts and Figures. American Cancer Society; 2008. http://www.cancer.org/downloads/STT/2008CAFFfinalsecured.pdf. [Google Scholar]
- 2.Van Ongeval C, Bosmans H, Van Steen A. Current status of digital mammography for screening and diagnosis of breast cancer. Curr Opin Oncol. 2006;18:547–54. doi: 10.1097/01.cco.0000245306.21403.88. [DOI] [PubMed] [Google Scholar]
- 3.Elmore JG, Armstrong K, Lehman CD, Fletcher SW. Screening for breast cancer. JAMA. 2005;293:1245–56. doi: 10.1001/jama.293.10.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morgan MP, Cooke MM, McCarthy GM. Microcalcifications associated with breast cancer: an epiphenomenon or biologically significant feature of selected tumors? J Mammary Gland Biol Neoplasia. 2005;10:181–7. doi: 10.1007/s10911-005-5400-6. [DOI] [PubMed] [Google Scholar]
- 5.Stomper PC, Geradts J, Edge SB, Levine EG. Mammographic predictors of the presence and size of invasive carcinomas associated with malignant microcalcification lesions without a mass. AJR Am J Roentgenol. 2003;181:1679–84. doi: 10.2214/ajr.181.6.1811679. [DOI] [PubMed] [Google Scholar]
- 6.Haka AS, Shafer-Peltier KE, Fitzmaurice M, Crowe J, Dasari RR, Feld MS. Identifying microcalcifications in benign and malignant breast lesions by probing differences in their chemical composition using Raman spectroscopy. Cancer Res. 2002;62:5375–80. [PubMed] [Google Scholar]
- 7.Morgan MP, Cooke MM, Christopherson PA, Westfall PR, McCarthy GM. Calcium hydroxyapatite promotes mitogenesis and matrix metalloproteinase expression in human breast cancer cell lines. Mol Carcinog. 2001;32:111–7. doi: 10.1002/mc.1070. [DOI] [PubMed] [Google Scholar]
- 8.Cooke MM, McCarthy GM, Sallis JD, Morgan MP. Phosphocitrate inhibits calcium hydroxyapatite induced mitogenesis and upregulation of matrix metalloproteinase-1, interleukin-1beta and cyclooxygenase-2 mRNA in human breast cancer cell lines. Breast Cancer Res Treat. 2003;79:253–63. doi: 10.1023/a:1023908307108. [DOI] [PubMed] [Google Scholar]
- 9.Kim JB, O’Hare MJ, Stein R. Models of breast cancer: is merging human and animal models the future? Breast Cancer Res. 2004;6:22–30. doi: 10.1186/bcr645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pasini B, Casalis Cavalchini GC, Genovese T, Manoukian S. Evaluating breast cancer risk: available models to assess individual breast cancer risk and probability to be a BRCA mutation carrier. J Exp Clin Cancer Res. 2002;21:23–9. [PubMed] [Google Scholar]
- 11.Tsukamoto AS, Grosschedl R, Guzman RC, Parslow T, Varmus HE. Expression of the int-1 gene in transgenic mice is associated with mammary gland hyperplasia and adenocarcinomas in male and female mice. Cell. 1988;55:619–25. doi: 10.1016/0092-8674(88)90220-6. [DOI] [PubMed] [Google Scholar]
- 12.Tyrer J, Duffy SW, Cuzick J. A breast cancer prediction model incorporating familial and personal risk factors. Stat Med. 2004;23:1111–30. doi: 10.1002/sim.1668. [DOI] [PubMed] [Google Scholar]
- 13.Urist MR, Sato K, Brownell AG, Malinin TI, Lietze A, Huo YK, Prolo DJ, Oklund S, Finerman GA, DeLange RJ. Human bone morphogenetic protein (hBMP) Proc Soc Exp Biol Med. 1983;173:194–9. doi: 10.3181/00379727-173-41630. [DOI] [PubMed] [Google Scholar]
- 14.Wang EA, Rosen V, Cordes P, Hewick RM, Kriz MJ, Luxenberg DP, Sibley BS, Wozney JM. Purification and characterization of other distinct bone-inducing factors. Proc Natl Acad Sci U S A. 1988;85:9484–8. doi: 10.1073/pnas.85.24.9484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vainio S, Karavanova I, Jowett A, Thesleff I. Identification of BMP-4 as a signal mediating secondary induction between epithelial and mesenchymal tissues during early tooth development. Cell. 1993;75:45–58. [PubMed] [Google Scholar]
- 16.Wozney JM, Rosen V, Celeste AJ, Mitsock LM, Whitters MJ, Kriz RW, Hewick RM, Wang EA. Novel regulators of bone formation: molecular clones and activities. Science. 1988;242:1528–34. doi: 10.1126/science.3201241. [DOI] [PubMed] [Google Scholar]
- 17.Yamaguchi A, Katagiri T, Ikeda T, Wozney JM, Rosen V, Wang EA, Kahn AJ, Suda T, Yoshiki S. Recombinant human bone morphogenetic protein-2 stimulates osteoblastic maturation and inhibits myogenic differentiation in vitro. J Cell Biol. 1991;113:681–7. doi: 10.1083/jcb.113.3.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li JZ, Li H, Sasaki T, Holman D, Beres B, Dumont RJ, Pittman DD, Hankins GR, Helm GA. Osteogenic potential of five different recombinant human bone morphogenetic protein adenoviral vectors in the rat. Gene Ther. 2003;10:1735–43. doi: 10.1038/sj.gt.3302075. [DOI] [PubMed] [Google Scholar]
- 19.Kang Q, Sun MH, Cheng H, Peng Y, Montag AG, Deyrup AT, Jiang W, Luu HH, Luo J, Szatkowski JP, Vanichakarn P, Park JY, Li Y, Haydon RC, He TC. Characterization of the distinct orthotopic bone-forming activity of 14 BMPs using recombinant adenovirus-mediated gene delivery. Gene Ther. 2004;11:1312–20. doi: 10.1038/sj.gt.3302298. [DOI] [PubMed] [Google Scholar]
- 20.Feeley BT, Conduah AH, Sugiyama O, Krenek L, Chen IS, Lieberman JR. In vivo molecular imaging of adenoviral versus lentiviral gene therapy in two bone formation models. J Orthop Res. 2006;24:1709–21. doi: 10.1002/jor.20229. [DOI] [PubMed] [Google Scholar]
- 21.Helm GA, Sheehan JM, Sheehan JP, Jane JA, Jr, diPierro CG, Simmons NE, Gillies GT, Kallmes DF, Sweeney TM. Utilization of type I collagen gel, demineralized bone matrix, and bone morphogenetic protein-2 to enhance autologous bone lumbar spinal fusion. J Neurosurg. 1997;86:93–100. doi: 10.3171/jns.1997.86.1.0093. [DOI] [PubMed] [Google Scholar]
- 22.Olmsted-Davis EA, Gugala Z, Gannon FH, Yotnda P, McAlhany RE, Lindsey RW, Davis AR. Use of a chimeric adenovirus vector enhances BMP2 production and bone formation. Hum Gene Ther. 2002;13:1337–47. doi: 10.1089/104303402760128568. [DOI] [PubMed] [Google Scholar]
- 23.Varady P, Li JZ, Alden TD, Kallmes DF, Williams MB, Helm GA. CT and radionuclide study of BMP-2 gene therapy-induced bone formation. Acad Radiol. 2002;9:632–7. doi: 10.1016/s1076-6332(03)80307-0. [DOI] [PubMed] [Google Scholar]
- 24.Clement JH, Raida M, Sanger J, Bicknell R, Liu J, Naumann A, Geyer A, Waldau A, Hortschansky P, Schmidt A, Hoffken K, Wolft S, Harris AL. Bone morphogenetic protein 2 (BMP-2) induces in vitro invasion and in vivo hormone independent growth of breast carcinoma cells. Int J Oncol. 2005;27:401–7. [PubMed] [Google Scholar]
- 25.Goldberg SN, Girnan GD, Lukyanov AN, Ahmed M, Monsky WL, Gazelle GS, Huertas JC, Stuart KE, Jacobs T, Torchillin VP, Halpern EF, Kruskal JB. Percutaneous tumor ablation: increased necrosis with combined radio-frequency ablation and intravenous liposomal doxorubicin in a rat breast tumor model. Radiology. 2002;222:797–804. doi: 10.1148/radiol.2223010861. [DOI] [PubMed] [Google Scholar]
- 26.He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci U S A. 1998;95:2509–14. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anderson RD, Haskell RE, Xia H, Roessler BJ, Davidson BL. A simple method for the rapid generation of recombinant adenovirus vectors. Gene Ther. 2000;7:1034–8. doi: 10.1038/sj.gt.3301197. [DOI] [PubMed] [Google Scholar]
- 28.Thompson SW, Hunt RD. Selected histochemical and histopathological methods. Charles C. Thomas, Inc; Springfield, IL: 1966. [Google Scholar]
- 29.Bhushan KR, Tanaka E, Frangioni JV. Synthesis of conjugatable bisphosphonates for molecular imaging of large animals. Angew Chem Int Ed Engl. 2007;46:7969–71. doi: 10.1002/anie.200701216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakayama A, del Monte F, Hajjar RJ, Frangioni JV. Functional near-infrared fluorescence imaging for cardiac surgery and targeted gene therapy. Mol Imaging. 2002;1:365–77. doi: 10.1162/15353500200221333. [DOI] [PubMed] [Google Scholar]
- 31.Lenkinski RE, Ahmed M, Zaheer A, Frangioni JV, Goldberg SN. Near-infrared fluorescence imaging of microcalcification in an animal model of breast cancer. Acad Radiol. 2003;10:1159–64. doi: 10.1016/s1076-6332(03)00253-8. [DOI] [PubMed] [Google Scholar]
- 32.Zaheer A, Lenkinski RE, Mahmood A, Jones AG, Cantley LC, Frangioni JV. In vivo near-infrared fluorescence imaging of osteoblastic activity. Nat Biotechnol. 2001;19:1148–54. doi: 10.1038/nbt1201-1148. [DOI] [PubMed] [Google Scholar]
- 33.Zaheer A, Murshed M, De Grand AM, Morgan TG, Karsenty G, Frangioni JV. Optical imaging of hydroxyapatite in the calcified vasculature of transgenic animals. Arterioscler Thromb Vasc Biol. 2006;26:1132–6. doi: 10.1161/01.ATV.0000210016.89991.2a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alarmo EL, Kuukasjarvi T, Karhu R, Kallioniemi A. A comprehensive expression survey of bone morphogenetic proteins in breast cancer highlights the importance of BMP4 and BMP7. Breast Cancer Res Treat. 2006;103:239–46. doi: 10.1007/s10549-006-9362-1. [DOI] [PubMed] [Google Scholar]
- 35.Sato K, Urist MR. Induced regeneration of calvaria by bone morphogenetic protein (BMP) in dogs. Clin Orthop Relat Res. 1985:301–11. [PubMed] [Google Scholar]
- 36.Hoodless PA, Haerry T, Abdollah S, Stapleton M, O’Connor MB, Attisano L, Wrana JL. MADR1, a MAD-related protein that functions in BMP2 signaling pathways. Cell. 1996;85:489–500. doi: 10.1016/s0092-8674(00)81250-7. [DOI] [PubMed] [Google Scholar]
- 37.Liu X, Yue J, Frey RS, Zhu Q, Mulder KM. Transforming growth factor beta signaling through Smad1 in human breast cancer cells. Cancer Res. 1998;58:4752–7. [PubMed] [Google Scholar]
- 38.Wang Q, Wei X, Zhu T, Zhang M, Shen R, Xing L, O’Keefe RJ, Chen D. BMP-2 activates Smad6 gene transcription through bone-specific transcription factor Runx2. J Biol Chem. 2007;282:10742–8. doi: 10.1074/jbc.M610997200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kimura N, Matsuo R, Shibuya H, Nakashima K, Taga T. BMP2-induced apoptosis is mediated by activation of the TAK1-p38 kinase pathway that is negatively regulated by Smad6. J Biol Chem. 2000;275:17647–52. doi: 10.1074/jbc.M908622199. [DOI] [PubMed] [Google Scholar]
- 40.Raida M, Clement JH, Leek RD, Ameri K, Bicknell R, Niederwieser D, Harris AL. Bone morphogenetic protein 2 (BMP-2) and induction of tumor angiogenesis. J Cancer Res Clin Oncol. 2005;131:741–50. doi: 10.1007/s00432-005-0024-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ghosh-Choudhury N, Mandal CC, Ghosh Choudhury G. Statin-induced Ras activation integrates PI 3 kinase signal to AKT and MAPK for BMP-2 expression in osteoblast differentiation. J Biol Chem. 2006;282:4983–93. doi: 10.1074/jbc.M606706200. [DOI] [PubMed] [Google Scholar]
- 42.Ishihara A, Shields KM, Litsky AS, Mattoon JS, Weisbrode SE, Bartlett JS, Bertone AL. Osteogenic gene regulation and relative acceleration of healing by adenoviral-mediated transfer of human BMP-2 or -6 in equine osteotomy and ostectomy models. J Orthop Res. 2008 doi: 10.1002/jor.20585. [DOI] [PubMed] [Google Scholar]
- 43.Pouliot F, Blais A, Labrie C. Overexpression of a dominant negative type II bone morphogenetic protein receptor inhibits the growth of human breast cancer cells. Cancer Res. 2003;63:277–81. [PubMed] [Google Scholar]
- 44.Arnold SF, Tims E, McGrath BE. Identification of bone morphogenetic proteins and their receptors in human breast cancer cell lines: importance of BMP2. Cytokine. 1999;11:1031–7. doi: 10.1006/cyto.1999.0508. [DOI] [PubMed] [Google Scholar]