Abstract
Background
Using diffusion tensor imaging (DTI), we previously reported abnormalities in two critical white matter tracts in schizophrenia, the uncinate fasciculus (UF) and the cingulum bundle (CB), both related to fronto–temporal connectivity. Here, we investigate these two bundles in unmedicated subjects with schizotypal personality disorder (SPD).
Methods
Fifteen male SPD subjects and 15 male control subjects were scanned with line-scan DTI. Fractional anisotropy (FA) and mean diffusivity (Dm) were used to quantify water diffusion, and cross-sectional area was defined with a directional threshold method. Exploratory correlation analyses were evaluated with Spearman’s rho, followed by post hoc hierarchical regression analyses.
Results
We found bilaterally reduced FA in the UF of SPD subjects. For CB, there was no significant group difference for FA or Dm measures. Additionally, in SPD, reduced FA in the right UF was correlated with clinical symptoms, including ideas of reference, suspiciousness, restricted affect, and social anxiety. In contrast, left UF area was correlated with measures of cognitive function, including general intelligence, verbal and visual memory, and executive performance.
Conclusions
These findings in SPD suggest altered fronto–temporal connectivity through the UF, similar to findings in schizophrenia, and intact neocortical–limbic connectivity through the CB, in marked contrast with what has been reported in schizophrenia.
Keywords: Diffusion tensor imaging, schizotypal personality disorder, uncinate fasciculus, cingulum bundle, fronto-temporal connectivity, fractional anisotropy
Schizotypal personality disorder (SPD) and schizophrenia share a common genetic diathesis, and many of the same phenomenological features, including cognitive distortion, social deficits, and restricted affectivity. They differ, however, in that both do not evince psychotic symptoms (see reviews in Dickey et al 2002a; Siever and Davis 2004). The study of SPD is thus of interest because it makes possible the clarification of biological commonalities and distinctions between SPD and schizophrenia, as well as providing a clearer picture of schizophrenia spectrum disorders because patients diagnosed with SPD do not suffer from confounding features of schizophrenia, such as chronic severe illness, the need for long-term medication, and recurrent hospitalization.
With respect to structural neuroimaging studies, several studies show similarities between SPD and schizophrenia, including gray matter volume reductions in superior temporal gyrus and Heschl’s gyrus (Dickey et al 1999, 2002b, 2003; Downhill et al 2001; Hirayasu et al 2000a, 2000b). These findings suggest a common endophenotype in these brain regions, which further suggest a predisposition toward neurodevelopmental aberrations.
With respect to frontal lobe regions, there are reports of volume reduction in schizophrenia (see review in Shenton et al 2001); however, with the exception of one study showing a lack of normal right > left asymmetry in the anterior cingulate gyrus of female SPD subjects (Takahashi et al 2002), compatible with findings in schizophrenia (Goldstein et al 1999), frontal lobe abnormalities have not been reported in SPD (Buchsbaum et al 2002).
Also of note, fronto–temporal connectivity abnormalities in schizophrenia have long been of interest (Akbarian et al 1996; Benes 2000; Deakin and Simpson 1997; Friston and Frith 1995; Kraepelin 1919/1971; McGuire and Frith 1996; Meyer-Lindenberg et al 2001; Weinberger et al 1992; Wernicke 1906), though their role in SPD has not been thoroughly investigated. With the advent of diffusion tensor imaging (DTI), however, neural connectivity can be assessed, because subtle white matter abnormalities can be evaluated, including organization and coherence (Basser 1995; Basser et al 1994; see also Kubicki et al 2002b for review).
Indeed, impaired white matter integrity within prefrontal (Buchsbaum et al 1998) and temporal lobes (Ardekani et al 2003), as well as abnormalities within the fiber bundles connecting these regions, including the uncinate fasciculus (UF) (Burns et al 2003; Kubicki et al 2002a), cingulum bundle (CB) (Kubicki et al 2003; Sun et al 2003; Wang et al 2004), and arcuate fasciculus (Burns et al 2003), are the most frequent findings in recent DTI studies in schizophrenia. In addition, diffusion abnormalities in the genu of the corpus callosum (Agartz et al 2001; Ardekani et al 2003; Foong et al 2000b, 2002), internal capsule (Buchsbaum et al 1998), and in whole white matter (Lim et al 1999; Minami et al 2003) have been reported in schizophrenia.
In the present study, we evaluated white matter integrity of the UF and CB, using anisotropy (fractional anisotropy; FA) and diffusivity (mean diffusivity; Dm) o f water molecule movement (Papadakis et al 1999) in SPD and control subjects. We also evaluated the cross-sectional area, using a directional threshold method (Kubicki et al 2002a, 2003). We selected these two fiber tracts because our group has shown them to be abnormal in schizophrenia (Kubicki et al 2002a, 2003). We also note that the UF interconnects with the anterior temporal and inferior frontal cortices (Ebeling and von Cramon 1992; Kier et al 2004; Klingler and Gloor 1960; Kondo et al 2003; Petrides and Pandya 1988; Ungerleider et al 1989) and that the CB connects the cingulate gyri with prefrontal, temporal, and parietal areas (Goldman-Rakic 1988; Vogt et al 1979), regions likely important in schizophrenia and by extension SPD. We hypothesize that UF and CB white matter integrity might also be altered in an unmedicated SPD population. To our knowledge, this is the first study applying DTI techniques to SPD.
Methods and Materials
Subjects
Subjects were 15 neuroleptic-naïve male SPD subjects (SPDs) and 15 male normal control subjects (NCs), all right-handed. Demographic data are summarized in Table 1. The SPDs and NCs were recruited from advertisements. Inclusion criteria were 1) age 18–55 years; 2) English as the primary language; 3) no history of neurological disorders; 4) no history of substance dependence ever or abuse in the last year; 5) no lifetime use of typical or atypical antipsychotic medications; and 6) no use of medications that might affect magnetic resonance imaging (MRI). The Structured Clinical Interview for DSM-IV and the Structured Clinical Interview for DSM-IV Personality Disorders were conducted by a licensed neuropsychologist or psychiatrist. Anyone meeting criteria for an Axis I psychotic or bipolar disorder in either group was excluded, and any Axis I o r II diagnosis in an NC resulted in exclusion. Interviews were videotaped, and half were reviewed by a second licensed psychologist for consensus of diagnosis and to test interrater reliability for SPD diagnosis, which was high (κ = .89, n = 25) (Dickey et al 1999). This study was approved by the institutional review boards of the VA Boston Healthcare System and Harvard Medical School. Written informed consent was obtained from all subjects before study participation.
Table 1.
Demographic Data for Schizotypal Personality Disorder (SPD) and Normal Control (NC) Subjects
| Student t Test (Two-Tailed) |
|||||
|---|---|---|---|---|---|
| SPD subjects (n = 15) | NC Subjects (n = 15) | t | df | p | |
| Sex Ratio (% Male) | 100 | 100 | |||
| Age (y) | 37.7 ± 12.4 | 32.7 ± 12.4 | 1.09 | 28 | .28 |
| Socioeconomic Status (SES)a | 3.1 ± 1.5 | 2.6 ± 1.1 | .99 | 28 | .33 |
| Parental SES | 1.7 ± 0.9 | 2.3 ± 1.3 | −1.29 | 28 | .21 |
| Education (y) | 15.5 ± 2.6 | 17.1 ± 3.0 | −1.48 | 28 | .15 |
| Total Intelligence Quotient | 111.6 ± 10.9 | 116.3 ± 12.0 | −1.1 | 27 | .28 |
Higher numbers represent lower SES.
Clinical and Neuropsychological Measures
Handedness was evaluated with the Edinburgh inventory (Oldfield 1971). Socioeconomic status (SES) of SPDs and NCs and their parents was evaluated with the Hollingshead two-factor index (Hollingshead 1965). For SPDs only, clinical symptoms were measured with the Structured Interview for Schizotypy (SIS; Kendler et al 1989, 1995), Scale for the Assessment of Positive Symptoms (SAPS; Andreasen 1984), and Scale for the Assessment of Negative Symptoms (SANS; Andreasen 1981). For self-report clinical measures, we used the Schizotypal Personality Questionnaire (SPQ; Raine 1991), the Beck Depression Inventory (BDI; Beck 1978), and the State-Trait Anxiety Inventory (STAI; Spiel-berger 1983).
As part of a comprehensive neuropsychological battery, subjects from both groups were evaluated with the Wechsler Adult Intelligence Scale (WAIS-III; Wechsler 1997a); the Boston Naming Test (Kaplan et al 1983), a test related to language function; the California Verbal Learning Test (CVLT) (semantic cluster score and the semantic cluster ratio; Delis et al 1987), a word-list learning test that requires semantic clustering for more efficient performance; the Wechsler memory scale (WMS-III; Wechsler 1997b), including immediate and delayed measures of verbal and visual memory; the Rey-Osterrieth complex figure test (Lezak 1995), a visual memory task; the Wisconsin Card Sorting Test (WCST; Heaton 1981), a measure requiring concept formation, abstraction, and mental flexibility; and the Trail Making Test (Reitan 1958), a measure of visuomotor processing speed.
Data for subjects recruited before the introduction of some of the tests, most notably the WMS-III, were not available. Moreover, a few subjects elected not to participate in some measures.
MRI Acquisition and Data Processing
Subjects were scanned with line scan diffusion tensor imaging (LSDI) (Gudbjartsson et al 1996; Maier et al 1998), a DTI technique based on sequential acquisition of parallel columns lying in the image plane. This information is described elsewhere (Kubicki et al 2004). Briefly, MR scans were performed with a quadrature head coil on a 1.5-Tesla GE Echospeed system (General Electric Medical Systems, Milwaukee, Wisconsin), which permits maximum gradient amplitudes of 40 mT/m. We began with three orthogonal T1-weighted images used as localizers (sagittal, axial oblique aligned to the anterior commissure–posterior commissure [AC-PC] line, and another sagittal oblique aligned to the interhemispheric fissure). From the last sagittal oblique T1-weighted image, the LSDI sequence in coronal orientation was then aligned perpendicular to the AC-PC line. For each slice, six images with high (1000 sec/mm2) diffusion weighting along six noncollinear and noncoplanar directions and two images with low (5 sec/mm2) diffusion weighting were collected. For low diffusion weighting, we collected only two images because the diffusion-related signal changes are minimal. The following scan parameters were used: field of view 220 × 165 mm; 128 × 96 scan matrix (256 × 192 image matrix); slice thickness = 4 mm; interslice distance = 1 mm; receiver bandwidth ±4 kHz; echo time = 64 msec; effective repetition time = 2592 msec; scan time = 60 sec per slice section. We acquired 31–35 coronal slices covering the entire brain, depending on brain size. Total scan time was 31–35 min. After reconstruction, diffusion-weighted images were transferred to a SUN workstation (Sun Microsystems, Santa Clara, California), on which eigenvalues (λ1, λ2, and λ3), eigenvectors, Dm, and FA were calculated. Fractional anisotropy, a measure of the fraction of the magnitude of the tensor that can be ascribed to the anisotropic diffusion, and Dm, the average of three eigenvalues of the tensor, were calculated with the following formula (Papadakis et al 1999):
Definition of Bundles
The UF and CB regions of interest (ROIs) were automatically defined by thresholding the out-of-plane principal diffusion component (λ1z), which was calculated with this formula:
where λ1 is the largest eigenvalue, and e1z is the out-of-plane component of the eigenvector associated with the largest eigen-value (λ1) (Figure 1). We used 1.1 μm2/msec as the common threshold for both bundles for all subjects. This directional threshold was a little higher than 1.0 μm2/msec of our previous study (Kubicki et al 2002a, 2003).
Figure 1.
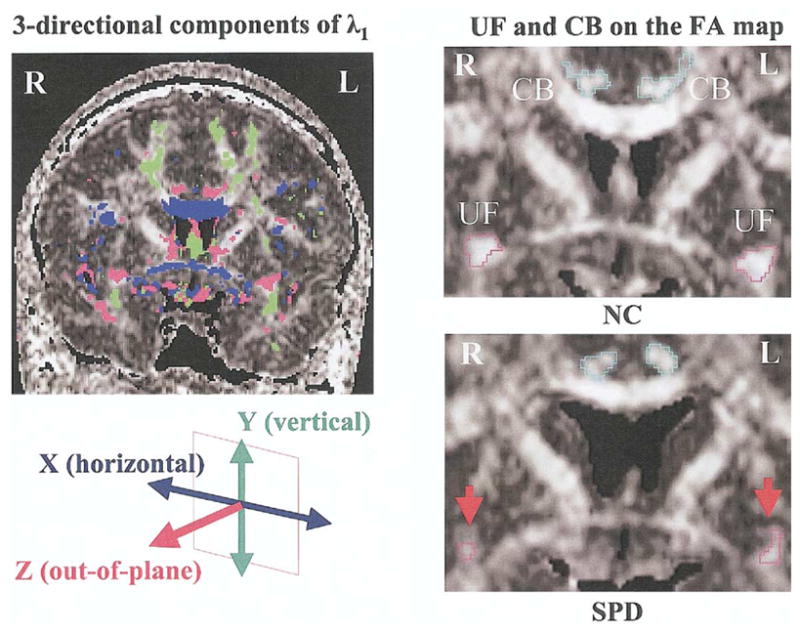
Definition of fiber bundles. (Left panel) Three-directional principal diffusion components having the largest eigenvalues (λ1) o n the coronal plane of the fractional anisotropy (FA) map. We used the out-of-plane principal diffusion component of maximum diffusivity (λ1z) t o define both bundles of uncinate fasciculus (UF) and cingulum bundle (CB) semi-automatically. The common threshold was 1.1 μm2/msec for both bundles in all cases for both groups. (Right panel) Uncinate fasciculus (red) and CB (blue) on FA maps of a normal control subject (NC) and a schizotypal personality disorder subject (SPD). Note the smaller area and decreased intensity in UF of the SPD subject (indicated by red arrows). R, right; L, left.
For UF, we selected one coronal slice, perpendicular to the AC-PC line, which intersects UF in the anterior temporal stem. This is the densest portion of the UF fiber tract, but sometimes there were two possible slices in which the UF seemed to be distributed densely. In that case, we compared the mean λ1z magnitude above the threshold of 1.1 μm2/msec and then selected the one slice that showed the larger λ1z value to focus on the out-of-plane component of the UF. Subsequent manual exclusion of uncertain voxels from the ROI was not needed because we used a higher threshold.
For the CB ROI, we excluded ROI outside of the anterior and posterior boundaries defined by the genu and splenium of corpus callosum and included ROI inside these regions to focus on the out-of-plane component of CB. That is, the most anterior coronal slice in which the corpus callosum was separately seen above and below was the first slice of CB, and the most posterior coronal slice in which the corpus callosum was separately seen above and below was the last slice of CB. There were 9 –12 coronal slices that met this criterion for CB slice selection.
For UF DTI measures, FA and Dm were averaged within an ROI, and cross-sectional area of the ROI was calculated according to the number of voxels within the ROI, for subsequent group comparison. For CB DTI measures, the average values of FA, Dm, and cross-sectional area within an ROI were summed over all CB slices, and then these three DTI measures were averaged by the total number of serial CB slices for subsequent group comparison.
Statistical Analysis
Student t tests were performed to assess group differences in age, SES, parental SES, years of education, and total intelligence quotient (IQ). For diffusion measures, repeated-measures analysis of variance (ANOVA) was applied to mean FA, Dm, and cross-sectional areas for both UF and CB. We used left and right hemispheres as the within-subject factor and diagnostic group as the between-subjects factor. To evaluate the likely importance of these findings, we also computed Cohen’s effect size (Cohen 1988).
Clinical/cognitive correlations with DTI measures (i.e., mean FA, Dm, and cross-sectional area of UF and CB) were evaluated with Spearman’s rho (ρ). Correlations were evaluated on an exploratory basis, with p <.05 as the cut-off for reporting statistical significance, rather than by using a correction for multiple correlations. We selected Spearman ρ correlations because we used small sample sizes (Siegel and Castellan 1988) and because some measures were not normally distributed, as demonstrated by a significant Shapiro-Wilk statistic (p <.05 for both groups: BDI, WCST [percent perseverative errors and number of trials to complete first category]; for SPDs: left UF area, visual reproduction [immediate recall] of WMS-III, Rey-Osterrieth complex figure test [delayed recall]; for NCs: right CB area, estimated total IQ of WAIS-III, total correct score of Boston naming test, and visual reproduction [delayed recall] of WMS-III). Finally, following the Nestor et al (2004) study of schizophrenia and following our correlational findings between FA measures and clinical/cognitive measures, we next used hierarchical regression to explore further the pattern of correlations. The Student t test was used to evaluate group difference between SPD and NC groups in cognitive measurements.
Results
Demographic Data
There were no significant differences between groups in terms of age, SES, parental SES, length of education, or total IQ (see Table 1).
DTI Measures of Uncinate Fasciculus
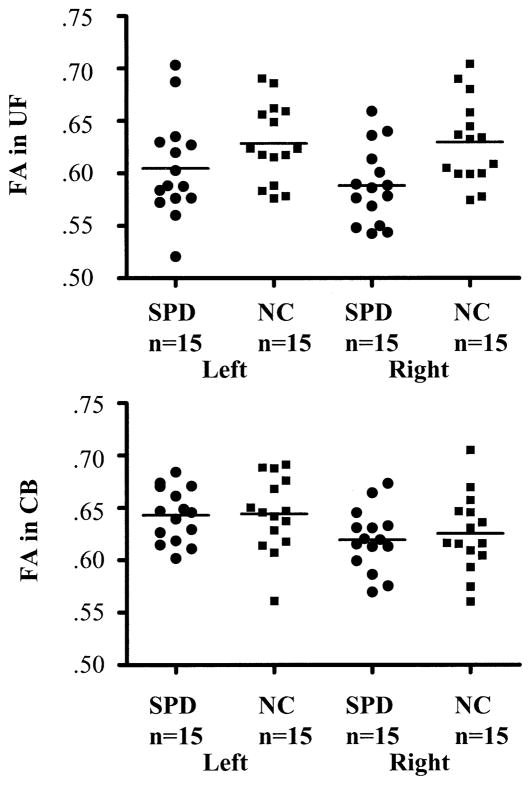
Descriptive statistics of DTI measures and results of repeated-measures ANOVA are summarized in Table 2. Repeated-measures ANOVA revealed that SPDs, compared with NCs, showed lower mean FA [F(1,28) = 7.50, p = .01] bilaterally for UF (see Figure 2). Neither group × side interaction [F(1,28) = 1.02, p = .32] nor main effect of side [F(1,28) = .74, p = .40] was significant for mean UF FA. Subjects with SPD, compared with NCs, had higher Dm [F (1,28) = 3.53, p = .07], with a trend-level difference bilaterally for UF. For cross-sectional area of UF, there was no significant group difference [F(1,28) = .17, p = .68]. The effect sizes of Cohen’s d were medium to large for mean FA (left UF = .55, right UF = 1.10) and Dm (left UF = .57, right UF = .63) but small for mean area (left UF = .16, right UF = .10).
Table 2.
Descriptive Statistics of DTI Measures and Repeated-Measures ANOVA
| SPD (n = 15) |
NC (n = 15) |
Main Effect of Group |
Main Effect of Side |
||||||
|---|---|---|---|---|---|---|---|---|---|
| DTI Measures | Side | Mean | SD | Mean | SD | F (1,28) | p | F (1,28) | p |
| UF FA | Left | .60 | .05 | .63 | .04 | 7.50 | .01 | .74 | .40 |
| Right | .59 | .04 | .63 | .04 | |||||
| UF Dm (μm2/msec) | Left | .78 | .04 | .77 | .02 | 3.53 | .07 | 3.01 | .99 |
| Right | .78 | .02 | .77 | .02 | |||||
| UF Area (mm2) | Left | 23.5 | 11.4 | 22.0 | 6.8 | .17 | .68 | .07 | .79 |
| Right | 22.7 | 10.3 | 21.8 | 7.4 | |||||
| CB FA | Left | .64 | .03 | .64 | .04 | .11 | .75 | 39.17 | 3.001 |
| Right | .62 | .03 | .63 | .04 | |||||
| CB Dm (μm2/msec) | Left | .75 | .03 | .75 | .02 | .31 | .59 | 7.08 | .01 |
| Right | .75 | .03 | .74 | .02 | |||||
| CB Area (mm2) | Left | 24.5 | 6.0 | 23.6 | 4.9 | .41 | .53 | 12.17 | .002 |
| Right | 22.8 | 5.1 | 21.4 | 4.2 | |||||
DTI, diffusion tensor imaging; ANOVA, analysis of variance; SPD, schizotypal personality disorder subjects; NC, normal control subjects; UF, uncinate fasciculus; FA, fractional anistropy; Dm, mean diffusivity; Area, cross-sectional area; CB, cingulum bundle.
Figure 2.
Group comparison of fractional anisotropy (FA). Scatterplots of FA indices in the left and right uncinate fasciculus (UF) and cingulum bundle (CB) of schizotypal personality disorder (SPD) and normal control (NC) groups are shown. The minimum value of FA is 0, indicating isotropic water diffusion, and the maximum value is 1, indicating completely unidirected anisotropic diffusion. The SPD subjects showed lower mean FA [F(1,28) = 7.50, p = .01] in bilateral UF, compared with NC subjects. In contrast, there were no significant group differences in FA in CB, but there was significant left > right asymmetry [F(1,28) = 39.17, p <.001].
DTI Measures of CB
For CB, there were no significant group differences in mean FA [F(1,28) = .11, p = .75] (see Figure 2), Dm [F (1,28) = .31, p = .59], or in cross-sectional area [F(1,28) = .41, p = .53]. There were, however, significant left > right asymmetries for CB in mean FA [F(1,28) = 39.17, p <.001], Dm [F (1,28) = 7.08, p = .01], and cross-sectional area [F(1,28) = 12.17, p = .002], compatible with previous findings in schizophrenia (Kubicki et al 2003; Wang et al 2004). For number of slices selected for CB, there was no significant difference [t (28) = .21, p = .84] between SPDs (10.5 ±.92 slices) and NCs (10.5 ±.83 slices). All DTI measures of CB showed small effect sizes of Cohen’s d (e.g., effect size of left CB FA = .03), suggesting that it would be difficult to obtain sufficient power to detect group differences even if the sample size was greatly increased.
DTI Correlations with Clinical and Cognitive Measures
Correlations with UF
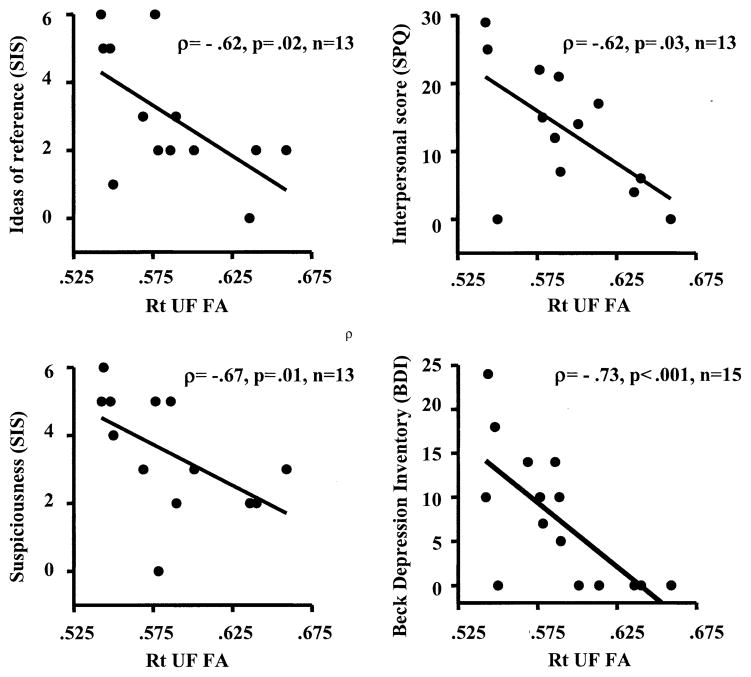
In the correlation analyses, summarized in Table 3, reduced right UF FA for SPDs was negatively correlated with the SIS for “ideas of reference” (ρ = −.62, p = .02) and “suspiciousness” (ρ = −.67, p = .01) and on the SPQ for interpersonal score (ρ = −.62, p = .03), which consists of four subscales of excessive social anxiety, no close friends, constricted affect, and suspiciousness (see Figure 3). Also of note, reduced right UF FA was negatively correlated with total score on the BDI (ρ = −.73, p = .002) (see Figure 3) and trait anxiety from the STAI (ρ = −.68, p = .02) for SPD subjects only.
Table 3.
Summary of Exploratory Correlations between DTI and Clinical/Cognitive Measures
| SPD Group |
NC Group |
||||||
|---|---|---|---|---|---|---|---|
| DTI Measures | Clinical/Cognitive Measures | ρ | p | n | ρ | p | n |
| Right UF FA | Ideas of reference (SIS) | −.62 | .02 | 13 | N/A | ||
| Suspiciousness (SIS) | −.67 | .01 | 13 | N/A | |||
| Interpersonal score (SPQ) | −.62 | .03 | 13 | N/A | |||
| Beck Depression Inventory (BDI) | −.73 | 3.001 | 15 | .21 | .53 | 11 | |
| Trait Anxiety (STAI) | −.68 | .02 | 11 | .31 | .35 | 11 | |
| Left UF Area | WAIS-III: estimated total IQ | .68 | .01 | 14 | .35 | .21 | 15 |
| WAIS-III: vocabulary (aged scaled score) | .56 | .04 | 14 | .54 | .04 | 15 | |
| Boston Naming Test: total correct score | .65 | .02 | 13 | −.31 | .38 | 10 | |
| CVLT: total words learned (aged scaled score) | .69 | .006 | 14 | −.17 | .60 | 13 | |
| CVLT: total semantic clusters | .63 | .02 | 14 | −.19 | .56 | 12 | |
| CVLT: total semantic cluster ratio | .58 | .03 | 14 | −.20 | .53 | 12 | |
| CVLT: free recall (short delay) | .71 | .005 | 14 | −.26 | .42 | 12 | |
| CVLT: free recall (long delay) | .67 | .009 | 14 | −.24 | .46 | 12 | |
| WMS-III: visual reproduction (immediate recall) | .70 | .006 | 14 | .02 | .97 | 7 | |
| WMS-III: visual reproduction (delayed recall) | .61 | .02 | 14 | .18 | .70 | 7 | |
| WMS-III: logical memory (immediate recall of story B) | .57 | .04 | 13 | −.39 | .34 | 8 | |
| WMS-III: memory for faces (immediate recall) | .86 | .001 | 11 | .22 | .61 | 8 | |
| WCST: perseverative errors (percent) | −.64 | .03 | 12 | −.14 | .66 | 14 | |
| WCST: trials to complete the first category | −.65 | .02 | 12 | −.22 | .50 | 14 | |
| Trail Making Test: time to complete trail B | −.64 | .01 | 14 | −.41 | .16 | 13 | |
| Bilateral UF Area | Rey-Osterrieth complex figure test (immediate recall) | ||||||
| Left | .54 | .04 | 14 | −.04 | .91 | 12 | |
| Right | .70 | .005 | 14 | −.09 | .78 | 12 | |
| Rey-Osterrieth complex figure test (delayed recall) | |||||||
| Left | .57 | .03 | 14 | .01 | .98 | 12 | |
| Right | .72 | .004 | 14 | .02 | .96 | 12 | |
| Bilateral CB Area | Subjects Age | ||||||
| Left | −.67 | .006 | 15 | .03 | .92 | 15 | |
| Right | −.53 | .04 | 15 | .15 | .60 | 15 | |
| CVLT: recall errors intrusions (free recall) | |||||||
| Left | −.73 | .003 | 14 | .24 | .45 | 12 | |
| Right | −.58 | .03 | 14 | .03 | .93 | 12 | |
| CVLT: recall errors intrusions (cued recall) | |||||||
| Left | −.89 | <.001 | 14 | .58 | .05 | 12 | |
| Right | −.65 | .01 | 14 | .59 | .05 | 12 | |
| WMS-III: memory for faces (delayed recall) | |||||||
| Left | .78 | .005 | 11 | −.37 | .37 | 8 | |
| Right | .75 | .008 | 11 | −.48 | .23 | 8 | |
| Left CB Area | SANS: total score | −.58 | .03 | 14 | N/A | ||
| WAIS-III: block design (aged scaled score) | .60 | .03 | 14 | −.07 | .82 | 15 | |
DTI, diffusion tensor imaging; SPD, schizotypal personality disorder; NC, normal control; UF, uncinate fasciculus; FA, fractional anisotropy; SI S, structured Interview for Schizotypy; SPQ, Schizotypal Personality Questionnaire; BDI, Beck Depression Inventory; N/A, not applicable; WAIS-III, Wechsler A dult Intelligence Scale–Third Edition; IQ, intelligence quotient; CVLT, California Verbal Learning Test; WMS-III, Wechsler Memory Test–Third Edition; WCST, Wisconsin Card Sorting Test; CB, Cingulum bundle; SANS, Scale for the Assessment of Negative Symptoms.
Figure 3.
Correlations between right uncinate fasciculus fractional anisotropy (Rt UF FA) and clinical symptoms and personality traits in the schizotypal personality disorder group. SIS, Structured Interview for Schizotypy; SPQ, Schizotypal Personality Questionnaire.
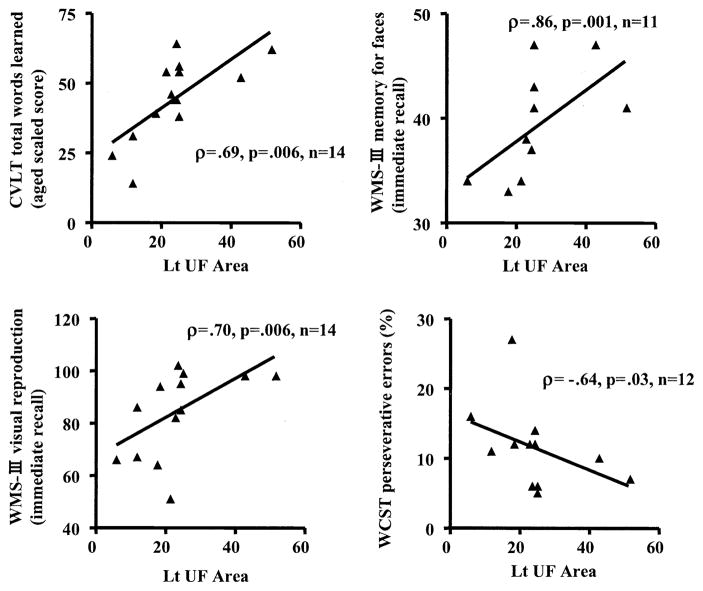
In contrast to right UF FA, which demonstrated significant correlations with clinical symptoms, the left UF area was correlated with general intelligence, language, verbal and visual memory, and executive functions in SPDs, with the exception of visual memory in the Rey-Osterrieth complex figure test, which correlated with bilateral UF areas (see Table 3 and Figure 4). Despite multiple correlations with cognitive measures, cross-sectional area of UF did not show any group differences and had small effect sizes (Cohen’s d values: left UF = .16, right UF = .10). Moreover, for all correlations with left UF area, only WAIS-III vocabulary aged scaled score showed the same correlation in SPDs as in NCs (see Table 3). Finally, time to complete trails B in the Trail Making Test was longer [t (25) = 2.27, p = .03] in SPDs than in NCs.
Figure 4.
Correlations between left uncinate fasciculus cross-sectional area (Lt UF Area) and cognitive functions in the schizotypal personality disorder group. CVLT, California Verbal Learning Test; WMS-III, Wechsler Memory Scale–Third Edition; WCST, Wisconsin Card Sorting Test.
Correlations with CB
For CB, which did not exhibit any significant SPD–NC difference for the DTI measures, bilateral CB area was negatively correlated with SPDs’ age (left: ρ = −.67, p = .006; right: ρ = −.53, p = .04), CVLT recall errors intrusions in free recall (left: ρ = −.73, p = .003; right: ρ = −.58, p = .03) and cued recall (left: ρ = −.89, p = .001; right: ρ = −.65, p = .01), and positively correlated with WMS-III memory for faces delayed recall (left: ρ = .78, p = .005; right: ρ = .75, p = .008). Left CB area was correlated negatively with total score of SANS (ρ = −.58, p = .03) and positively with block design aged scaled score in WAIS-III (ρ = .60, p = .03) within SPDs. The same or similar correlations were not observed in NCs.
Post Hoc Hierarchical Regression Analyses
As noted above, left and right UF correlated with different functional variables. For SPDs, left UF area correlated with lower scores on tests of verbal memory and language, whereas reduced right UF FA correlated with symptom/personality ratings. These univariate correlations thus revealed evidence of dissociation between verbal abilities and left UF on one hand and clinical symptoms and right UF on the other. To control for inflation of type I error in calculating multiple univariate correlations, we explored and tested this structure–function dissociation with hierarchical regression. We first regressed left UF and right UF over verbal memory, because our prior studies of patients with schizophrenia showed that left UF uniquely accounted for performance on verbal memory (see Nestor et al 2004). Specifically, we entered both brain regions (left UF, right UF) as predictors in a hierarchical regression, first with the CVLT as the dependent variable and then with SPQ (interpersonal score) as the dependent variable.
Findings showed that for the CVLT, left UF area revealed a significant R2 change of .51 [F(1,12) = 12.55, p < .01], in contrast to the nonsignificant R2 change of .03 [F(1,11) = .63, p = .44] accounted for by the right UF area. Left UF area and CVLT total words learned aged scaled score revealed a partial correlation value of .73 and a semipartial correlation value of .72, as compared with values of −.23 and −.16 for right UF area and CVLT total words learned aged scaled score. These values indicate that left UF area uniquely accounted for 53.3% and 51.8% of the variance in CVLT total words learned aged scaled score, whereas the right UF area accounted for 5.3% and 2.6% of the same variance. Further analyses demonstrated that only left UF area [β = .79, t (11) = 3.51, p < .01] contributed significantly to the CVLT score.
We then regressed right UF and left UF over symptoms, selecting overall level of SPQ traits as the dependent variable most likely to describe the clinical phenomenology of our sample. For SPQ (interpersonal score), right UF FA produced a significant R2 change of .36 [F(1,11) = 6.22, p =.03], as compared with the nonsignificant R2 change of .03 [F(1,10) = .41, p = .54] accounted for by the left UF FA. Right UF FA and SPQ (interpersonal score) revealed a partial correlation value of −.60 and a semipartial correlation value of −.59, as compared with values of −.20 and −.16 for left UF FA and SPQ (interpersonal score). These values indicate that the right UF FA uniquely accounted for 36.0% and 34.8% of variance in SPQ (interpersonal score), whereas left UF FA accounted for 4.0% and 2.6% of the same variance. Likewise, the right UF FA [β = −.59, t (10) = −2.38, p = .04] but not the left UF FA contributed significantly to SPQ (interpersonal score).
Discussion
Diffusion tensor imaging findings from the present study strongly suggest that fronto–temporal connectivity through the UF, one of the most prominent fiber bundles connecting the frontal and temporal lobes, is altered in SPD, and that this alteration shows an interesting dissociation between the left UF area and verbal deficits on one hand and reduced right UF anisotropy and increased clinical symptoms on the other. Moreover, the observed DTI correlations with clinical and cognitive measures suggest that a disturbance in connectivity between different brain regions, rather than abnormalities within the separate regions themselves, might be responsible for some of the observed clinical symptoms and cognitive dysfunctions in SPD.
Compared with previous DTI findings on schizophrenia, findings of bilaterally abnormal UF integrity in male subjects with SPD are similar to findings reported in schizophrenia, although two previous DTI studies (Burns et al 2003; Kubicki et al 2002a) in schizophrenia showed reduced anisotropy in left UF; more specifically, reduced normal left > right asymmetries in UF anisotropy (Kubicki et al 2002a) and a trend-level reduction in left UF anisotropy (Burns et al 2003). In contrast, there were no significant differences in CB between SPDs and NCs, whereas we previously reported CB integrity disruption in schizophrenia (Kubicki et al 2003), and two other studies (Sun et al 2003; Wang et al 2004) also demonstrated CB abnormalities in schizophrenia.
The fact that there is some overlap between findings in SPD and schizophrenia suggests that SPD might be a “forme fruste” or “milder” presentation of pathology among the schizophrenia spectrum disorders and supports a dimensional conceptualization of schizophrenia spectrum disorders, rather than a categorical classification, such as in DSM-IV. Accordingly, the similarity in findings for UF anisotropy in SPD and schizophrenia suggests that UF integrity disruption might be along a continuum of dysfunction. This lack of UF cohesion in the schizophrenia spectrum is reminiscent of classic disconnection syndromes (Mesulam 1985), in that a lack of integration between two brain regions results in specific deficits. In schizophrenia and SPD, but unlike isolated lesions found in the classic disconnection syndromes, the deficits are more widespread and result in a larger range of abnormalities, as exemplified by the range of correlations described in this report.
One potential pathogenic explanation for this more widespread abnormality might come from postmortem data. On the basis of histochemical studies of postmortem brains of schizophrenic subjects, Deakin and Simpson (1997) proposed that an abnormal amount of glutamatergic innervation from orbitofrontal cortex to anterior temporal cortex through UF might be an essential neurodevelopmental abnormality for schizophrenia. This glutamatergic excitotoxicity might result in reduced UF anisotropy in both schizophrenia and SPD populations.
In contrast, areas of dissimilarity between SPD and schizophrenia, such as observed for CB anisotropy, suggest that there might be certain protective factors operating in SPD that might contribute to more limited clinical symptoms compared with schizophrenia. More specifically, Siever and Davis (2004) speculate that there is a “greater frontal capacity in SPD than schizophrenia.” Because CB projects disproportionately to the frontal lobe, the finding of disrupted CB in schizophrenia, but not in SPD, supports Siever and Davis’ hypothesis that the prefrontal cortex in SPD acts to “buffer” other brain regions against the decimating effects of an abnormal temporal lobe (Siever and Davis 2004). Intact CB in SPD might therefore represent part of that prefrontal “buffer.”
Also noteworthy were the clinical correlations with DTI measures in SPDs, suggesting that altered temporal–frontal connectivity through the UF might play an important role in the phenomenology of SPD. Specifically, reduced FA in right UF was correlated with clinical symptoms, including ideas of reference (SIS), suspiciousness (SIS, SPQ), restricted affect (SPQ, BDI), anxiety (SPQ, STAI), and no close friends (SPQ). In contrast, cognitive correlations with DTI in SPDs showed that the left UF area was correlated with measures of general intelligence, verbal and visual memory, and executive functions. These latter correlations are consistent with previous neuropsychological findings in SPD, which have shown decrements in general intelligence (Mitropoulou et al 2002; Voglmaier et al 2000), in performance on the CVLT (Voglmaier et al 1997), and in visual reproduction on the WMS-III (Mitropoulou et al 2002), as well as poor performance on executive functions, such as the WCST and the Trail Making Test (Mitropoulou et al 2002; Trestman et al 1995), all of which provide further evidence that fronto–temporal disconnectivity through the left UF might play a crucial role in cognitive distortion in SPD. The Rey-Osterrieth Complex Figure test, however, demonstrated right-dominant correlations, possibly reflecting contributions of visual memory and visuo-spatial planning, which are required in this test.
Of interest to the present study, a recent DTI study of schizophrenia by Nestor et al (2004) reported reduced left UF FA in schizophrenic subjects compared with control subjects, which was correlated with lower declarative–episodic verbal memory, whereas reduced left CB cross-sectional area correlated with errors in executive functions related to performance monitoring. The association between verbal memory and left UF is similar to that reported here for SPD. Moreover, results from the hierarchical multiple regression analyses in the present study provide evidence of dissociation between disruptions of left UF and reduced verbal memory on the one hand and right UF and SPQ traits on the other.
Anatomically, UF interconnects with anterior temporal and inferior frontal regions. More specific links include connecting amygdala and uncus with subcallosal regions (Ebeling and von Cramon 1992; Kier et al 2004; Klingler and Gloor 1960). A human histochemical study has shown that UF also carries cholinergic fibers from the basal nucleus of Meynert, as a part of cholinergic pathway that supplies frontal, parietal, and temporal neocortices, and perisylvian division of frontoparietal operculum, insula, and superior temporal gyrus (Selden et al 1998). Also of note, UF is closely related to the ventral visual pathway (Ungerleider et al 1989), although dissection studies with monkeys reveal that UF dissection itself did not yield visual memory disturbances alone (Gaffan and Eacott 1995a, 1995b). Results from the present study, in light of these anatomical findings, suggest that UF-mediated neural circuits involving the amygdala might be attributed to emotional aspects of SPD pathophysiology. Altered cholinergic innervation through the UF with decreased anisotropy might also be relevant to cognitive distortion in SPD. In the present study, some visual memory tests, including visual reproduction and memory for faces in WMS-III and immediate and delayed recall in the Rey-Osterrieth complex figure test, demonstrated significant correlation with UF area, suggesting that the ventral visual pathway involved with UF might also be affected in SPD.
The present findings of significant reduction of anisotropy with a trend-level increase of mean diffusivity in UF could be attributed to 1) decreased density of axons; 2) decreased degree of myelination of axons; and/or 3) impaired axonal membranes. A recent postmortem study of UF in schizophrenia demonstrated that there were no differences in the number and density of fibers in UF between schizophrenic and control subjects (Highley et al 2002). Additionally, there is growing evidence suggesting that glial cells, particularly oligodendrocytes, which form myelin sheaths around axons, are abnormal in schizophrenia (Davis et al 2003; Hakak et al 2001; Uranova et al 2001a, 2001b, 2004). Hakak and coworkers, in fact, reported abnormal expression of myelin-related genes in schizophrenia, which suggests a disruption in oligodendrocyte function (Hakak et al 2001). Furthermore, Uranova and coworkers showed both qualitative and quantitative abnormalities in postmortem brains of schizophrenic subjects in the oligodendroglia of the prefrontal cortex and caudate nucleus (Uranova et al 2001a, 2001b). Also, a magnetization transfer imaging study revealed that decreased magnetization transfer ratio, a putative index of myelin or axonal density, was observed in frontal and temporal regions of schizophrenia (Foong et al 2000a, 2001). Taken together, these findings suggest that UF anisotropy reduction might be attributed to oligodendrocyte dysfunction within the fronto–temporal circuitry. Because SPDs in our study also showed disturbed UF anisotropy, it is possible that a similar process might be occurring in SPD, although future studies focused on glial cells are needed to confirm such speculations.
We note several methodological issues in our study. One limitation is that DTI is more prone to artifact, including partial volume effects, chemical shift artifacts, bulk motion, and spatial distortion, compared with standard MRI acquisition protocols used for most volumetric studies, and thus such artifacts might have influenced our findings. The resolution of DTI is also low compared with most recent volumetric studies, and low spatial resolution can increase partial volume effects, which could decrease mean anisotropy, particularly within small ROIs; however, the LSDI (Gudbjartsson et al 1996; Maier et al 1998) protocol we used is less prone to chemical shift artifacts, bulk motion, and spatial distortions than is the conventional single-shot diffusion-weighted echo-planar protocol (Turner et al 1990), although the LSDI sequence is four to six times slower (Kubicki et al 2004). Moreover, with LSDI, motion artifact can be monitored for each voxel, and we demonstrated that motion artifact did not differ significantly between groups. Furthermore, to reduce partial volume effects, we took advantage of coronal image acquisitions that were perpendicular to both the densest portion of the UF at the temporal stem and the largest portion of the CB. We are thus confident that, given the current state-of-the-art technology, we controlled for the influence of possible artifacts due to the method we used.
Also of note, our group developed a directional threshold method for cross-sectional ROI definition of fiber bundles, such as UF and CB. The greatest advantage of this method is that it is based on directional information obtained only from the diffusion tensor, whereas conventional fixed ROI methods and exploratory voxel-based approaches discard this important information. There are limitations to this method, however. First, it works only for the unidirectional portion of fiber bundles and not for their dispersive portions or for fiber crossings. Therefore, we were limited to just one slice that included the densest portion at the anterior temporal stem to evaluate UF, and we excluded CB portions outside of the anterior and posterior boundaries defined by the genu and splenium of corpus callosum. Second, torque of the brains or any shape asymmetries that might exist between groups might affect the main direction of the bundle or threshold and thus might affect the anisotropy results, although all the DTI images were aligned to the AC-PC line with T1-weighted images to minimize these possibilities. Third, this method produces an additional variable, the cross-sectional area within the ROI defined by thresholding λ1z magnitude; however, this cross-sectional area has not been well interpreted physiologically, although it is speculated that it might also yield a quantitative measure of connectivity between different brain regions because it includes information about fiber directionality (λ1z) as well as bundle volume. In fact, many meaningful correlations were observed between the cross-sectional areas and cognitive measures in the present study in SPDs and in our previous study in schizophrenia (Nestor et al 2004).
In summary, the present DTI study of SPD demonstrates fronto–temporal disconnectivity through the UF in association with cognitive distortion, social deficit, and restricted affectivity, and intact neocortical–limbic connection through CB, with the latter in marked contrast with what has been reported in schizophrenia. Also, the present study offers biological commonality and distinction between SPD and schizophrenia in terms of neural connectivity. More specifically, neural circuitry through the UF might be affected in both clinical entities, whereas CB integrity might be preserved in SPD. This method thus has the potential of elucidating further the neuropathology that underlies altered neural connectivity in SPD.
Acknowledgments
This study was supported in part by the Department of Veterans Affairs Merit Awards (MES, RWM), a Research Enhancement Award Program (REAP) and a Middleton Award (RWM) from the Department of Veterans Affairs, grants from the National Institute of Health (K02 MH 01110 and R01 MH 50747 to MES, R01 MH 40799 to RWM, RO3 MH068464-01 to MK, and an NIH Roadmap for Medical Research Grant U54 EB005149 to RK) and the National Alliance for Research on Schizophrenia and Depression (MK), a V A Advanced Career Development Award (CCD), the Wodercroft Foundation (MK), and the Welfide Medicinal Research Foundation, Japan (MN).
We thank Marie Fairbanks for administrative support; Noriomi Kuroki, M.D., for helpful suggestions; Paul G. Nestor, Ph.D., for statistical consultation; Hae-Jeong Park, Ph.D., and Sylvain Bouix, Ph.D., for technical support; and Sunnie Kim, B.A., Erin Connor, B.A., and Lisa Lucia, B.A., for their support as the research assistants.
References
- Agartz I, Andersson JL, Skare S. Abnormal brain white matter in schizophrenia: A diffusion tensor imaging study. Neuroreport. 2001;12:2251–2254. doi: 10.1097/00001756-200107200-00041. [DOI] [PubMed] [Google Scholar]
- Akbarian S, Kim JJ, Potkin SG, Hetrick WP, Bunney WE, Jr, Jones EG. Maldistribution of interstitial neurons in prefrontal white matter of the brains of schizophrenic patients. Arch Gen Psychiatry. 1996;53:425– 436. doi: 10.1001/archpsyc.1996.01830050061010. [DOI] [PubMed] [Google Scholar]
- Andreasen NC. Scale for the Assessment of Negative Symptoms (SANS) Iowa City, Iowa: University of Iowa College of Medicine, Department of Psychiatry; 1981. [Google Scholar]
- Andreasen NC. Scale for the Assessment of Positive Symptoms (SAPS) Iowa City, Iowa: University of Iowa College of Medicine, Department of Psychiatry; 1984. [Google Scholar]
- Ardekani BA, Nierenberg J, Hoptman MJ, Javitt DC, Lim KO. MRI study of white matter diffusion anisotropy in schizophrenia. Neuroreport. 2003;14:2025–2029. doi: 10.1097/00001756-200311140-00004. [DOI] [PubMed] [Google Scholar]
- Basser PJ. Inferring microstructural features and the physiological state of tissues from diffusion-weighted images. NMR Biomed. 1995;8:333–344. doi: 10.1002/nbm.1940080707. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Mattiello J, LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophys J. 1994;66:259 –267. doi: 10.1016/S0006-3495(94)80775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT. The Beck Depression Inventory. Philadelphia: Center for Cognitive Therapy; 1978. [Google Scholar]
- Benes FM. Emerging principles of altered neural circuitry in schizophrenia. Brain Res Brain Res Rev. 2000;31:251–269. doi: 10.1016/s0165-0173(99)00041-7. [DOI] [PubMed] [Google Scholar]
- Buchsbaum MS, Nenadic I, Hazlett EA, Spiegel-Cohen J, Fleischman MB, Akhavan A, et al. Differential metabolic rates in prefrontal and temporal Brodmann areas in schizophrenia and schizotypal personality disorder. Schizophr Res. 2002;54:141–150. doi: 10.1016/s0920-9964(01)00361-9. [DOI] [PubMed] [Google Scholar]
- Buchsbaum MS, Tang CY, Peled S, Gudbjartsson H, Lu D, Hazlett EA, et al. MRI white matter diffusion anisotropy and PET metabolic rate in schizophrenia. Neuroreport. 1998;9:425– 430. doi: 10.1097/00001756-199802160-00013. [DOI] [PubMed] [Google Scholar]
- Burns J, Job D, Bastin ME, Whalley H, Macgillivray T, Johnstone EC, et al. Structural disconnectivity in schizophrenia: A diffusion tensor magnetic resonance imaging study. Br J Psychiatry. 2003;182:439 – 443. [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2. Hillsdale, New Jersey: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- Davis KL, Stewart DG, Friedman JI, Buchsbaum M, Harvey PD, Hof PR, et al. White matter changes in schizophrenia: Evidence for myelin-related dysfunction. Arch Gen Psychiatry. 2003;60:443– 456. doi: 10.1001/archpsyc.60.5.443. [DOI] [PubMed] [Google Scholar]
- Deakin JF, Simpson MD. A two-process theory of schizophrenia: Evidence from studies in post-mortem brain. J Psychiatr Res. 1997;31:277–295. doi: 10.1016/s0022-3956(96)00042-8. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test Manual-Research Edition. San Diego, California: The Psychological Corporation; 1987. [Google Scholar]
- Dickey CC, McCarley RW, Shenton ME. The brain in schizotypal personality disorder: A review of structural MRI and CT findings. Harv Rev Psychiatry. 2002a;10:1–15. doi: 10.1080/10673220216201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickey CC, McCarley RW, Voglmaier MM, Frumin M, Niznikiewicz MA, Hirayasu Y, et al. Smaller left Heschl’s gyrus volume in patients with schizotypal personality disorder. Am J Psychiatry. 2002b;159:1521–1527. doi: 10.1176/appi.ajp.159.9.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickey CC, McCarley RW, Voglmaier MM, Niznikiewicz MA, Seidman LJ, Demeo S, et al. An MRI study of superior temporal gyrus volume in women with schizotypal personality disorder. Am J Psychiatry. 2003;160:2198 –2201. doi: 10.1176/appi.ajp.160.12.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickey CC, McCarley RW, Voglmaier MM, Niznikiewicz MA, Seidman LJ, Hirayasu Y, et al. Schizotypal personality disorder and MRI abnormalities of temporal lobe gray matter. Biol Psychiatry. 1999;45:1393–1402. doi: 10.1016/s0006-3223(99)00030-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downhill JE, Jr, Buchsbaum MS, Hazlett EA, Barth S, Lees Roitman S, Nunn M, et al. Temporal lobe volume determined by magnetic resonance imaging in schizotypal personality disorder and schizophrenia. Schizophr Res. 2001;48:187–199. doi: 10.1016/s0920-9964(00)00131-6. [DOI] [PubMed] [Google Scholar]
- Ebeling U, von Cramon D. Topography of the uncinate fascicle and adjacent temporal fiber tracts. Acta Neurochir (Wien) 1992;115:143–148. doi: 10.1007/BF01406373. [DOI] [PubMed] [Google Scholar]
- Foong J, Maier M, Barker GJ, Brocklehurst S, Miller DH, Ron MA. In vivo investigation of white matter pathology in schizophrenia with magnetisation transfer imaging. J Neurol Neurosurg Psychiatry. 2000a;68:70 –74. doi: 10.1136/jnnp.68.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foong J, Maier M, Clark CA, Barker GJ, Miller DH, Ron MA. Neuropathological abnormalities of the corpus callosum in schizophrenia: A diffusion tensor imaging study. J Neurol Neurosurg Psychiatry. 2000b;68:242–244. doi: 10.1136/jnnp.68.2.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foong J, Symms MR, Barker GJ, Maier M, Miller DH, Ron MA. Investigating regional white matter in schizophrenia using diffusion tensor imaging. Neuroreport. 2002;13:333–336. doi: 10.1097/00001756-200203040-00017. [DOI] [PubMed] [Google Scholar]
- Foong J, Symms MR, Barker GJ, Maier M, Woermann FG, Miller DH, et al. Neuropathological abnormalities in schizophrenia: Evidence from magnetization transfer imaging. Brain. 2001;124:882– 892. doi: 10.1093/brain/124.5.882. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Frith CD. Schizophrenia: A disconnection syndrome? Clin Neurosci. 1995;3:89 –97. [PubMed] [Google Scholar]
- Gaffan D, Eacott MJ. Uncinate fascicle section leaves delayed matching-to-sample intact, with both large and small stimulus sets. Exp Brain Res. 1995a;105:175–180. doi: 10.1007/BF00242192. [DOI] [PubMed] [Google Scholar]
- Gaffan D, Eacott MJ. Visual learning for an auditory secondary reinforcer by macaques is intact after uncinate fascicle section: Indirect evidence for the involvement of the corpus striatum. Eur J Neurosci. 1995b;7:1866 –1871. doi: 10.1111/j.1460-9568.1995.tb00707.x. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Topography of cognition: Parallel distributed networks in primate association cortex. Annu Rev Neurosci. 1988;11:137–156. doi: 10.1146/annurev.ne.11.030188.001033. [DOI] [PubMed] [Google Scholar]
- Goldstein JM, Goodman JM, Seidman LJ, Kennedy DN, Makris N, Lee H, et al. Cortical abnormalities in schizophrenia identified by structural magnetic resonance imaging. Arch Gen Psychiatry. 1999;56:537–547. doi: 10.1001/archpsyc.56.6.537. [DOI] [PubMed] [Google Scholar]
- Gudbjartsson H, Maier SE, Mulkern RV, Morocz IA, Patz S, Jolesz FA. Line scan diffusion imaging. Magn Reson Med. 1996;36:509 –519. doi: 10.1002/mrm.1910360403. [DOI] [PubMed] [Google Scholar]
- Hakak Y, Walker JR, Li C, Wong WH, Davis KL, Buxbaum JD, et al. Genome-wide expression analysis reveals dysregulation of myelination-related genes in chronic schizophrenia. Proc Natl Acad Sci U S A. 2001;98:4746 –4751. doi: 10.1073/pnas.081071198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK. Wisconsin Card Sorting Test, Manual. Odessa, Florida: Psychological Assessment Resources; 1981. [Google Scholar]
- Highley JR, Walker MA, Esiri MM, Crow TJ, Harrison PJ. Asymmetry of the uncinate fasciculus: A post-mortem study of normal subjects and patients with schizophrenia. Cereb Cortex. 2002;12:1218 –1224. doi: 10.1093/cercor/12.11.1218. [DOI] [PubMed] [Google Scholar]
- Hirayasu Y, McCarley RW, Salisbury DF, Tanaka S, Kwon JS, Frumin M, et al. Planum temporale and Heschl gyrus volume reduction in schizophrenia: A magnetic resonance imaging study of first-episode patients. Arch Gen Psychiatry. 2000a;57:692– 699. doi: 10.1001/archpsyc.57.7.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirayasu Y, Shenton ME, Salisbury DF, McCarley RW. Hippocampal and superior temporal gyrus volume in first-episode schizophrenia. Arch Gen Psychiatry. 2000b;57:618 – 619. doi: 10.1001/archpsyc.57.6.618. [DOI] [PubMed] [Google Scholar]
- Hollingshead A. Two Factor Index of Social Position. New Haven, Connecticut: Yale University Press; 1965. [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S, Segal O. Boston Naming Test. Philadelphia: Lea & Febiger; 1983. [Google Scholar]
- Kendler KS, Lieberman JA, Walsh D. The Structured Interview for Schizotypy (SIS): A preliminary report. Schizophr Bull. 1989;15:559 –571. doi: 10.1093/schbul/15.4.559. [DOI] [PubMed] [Google Scholar]
- Kendler KS, McGuire M, Gruenberg AM, Walsh D. Schizotypal symptoms and signs in the Roscommon Family Study. Their factor structure and familial relationship with psychotic and affective disorders. Arch Gen Psychiatry. 1995;52:296 –303. doi: 10.1001/archpsyc.1995.03950160046009. [DOI] [PubMed] [Google Scholar]
- Kier EL, Staib LH, Davis LM, Bronen RA. MR imaging of the temporal stem: Anatomic dissection tractography of the uncinate fasciculus, inferior occipitofrontal fasciculus, and Meyer’s loop of the optic radiation. AJNR Am J Neuroradiol. 2004;25:677– 691. [PMC free article] [PubMed] [Google Scholar]
- Klingler J, Gloor P. The connections of the amygdala and of the anterior temporal cortex in the human brain. J Comp Neurol. 1960;115:333–369. doi: 10.1002/cne.901150305. [DOI] [PubMed] [Google Scholar]
- Kondo H, Saleem KS, Price JL. Differential connections of the temporal pole with the orbital and medial prefrontal networks in macaque monkeys. J Comp Neurol. 2003;465:499 –523. doi: 10.1002/cne.10842. [DOI] [PubMed] [Google Scholar]
- Kraepelin E. Dementia Praecox. New York: Churchill Living-stone; 19191971. [Google Scholar]
- Kubicki M, Maier SE, Westin CF, Mamata H, Ersner-Hershfield H, Estepar R, et al. Comparison of single-shot echo-planar and line scan protocols for diffusion tensor imaging. Acad Radiol. 2004;11:224 –232. doi: 10.1016/s1076-6332(03)00563-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicki M, Westin CF, Maier SE, Frumin M, Nestor PG, Salisbury DF, et al. Uncinate fasciculus findings in schizophrenia: A magnetic resonance diffusion tensor imaging study. Am J Psychiatry. 2002a;159:813– 820. doi: 10.1176/appi.ajp.159.5.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicki M, Westin CF, Maier SE, Mamata H, Frumin M, Ersner-Hershfield H, et al. Diffusion tensor imaging and its application to neuropsychiatric disorders. Harv Rev Psychiatry. 2002b;10:324 –336. doi: 10.1080/10673220216231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicki M, Westin CF, Nestor PG, Wible CG, Frumin M, Maier SE, et al. Cingulate fasciculus integrity disruption in schizophrenia: A magnetic resonance diffusion tensor imaging study. Biol Psychiatry. 2003;54:1171–1180. doi: 10.1016/s0006-3223(03)00419-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lezak M. Neuropsychological Assessment. 3. New York: Oxford University Press; 1995. [Google Scholar]
- Lim KO, Hedehus M, Moseley M, de Crespigny A, Sullivan EV, Pfefferbaum A. Compromised white matter tract integrity in schizophrenia inferred from diffusion tensor imaging. Arch Gen Psychiatry. 1999;56:367–374. doi: 10.1001/archpsyc.56.4.367. [DOI] [PubMed] [Google Scholar]
- Maier SE, Gudbjartsson H, Patz S, Hsu L, Lovblad KO, Edelman RR, et al. Line scan diffusion imaging: Characterization in healthy subjects and stroke patients. AJR Am J Roentgenol. 1998;171:85–93. doi: 10.2214/ajr.171.1.9648769. [DOI] [PubMed] [Google Scholar]
- McGuire PK, Frith CD. Disordered functional connectivity in schizophrenia. Psychol Med. 1996;26:663– 667. doi: 10.1017/s0033291700037673. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Principles of Behavioral and Cognitive Neurology. Philadelphia: F.A. Davis; 1985. [Google Scholar]
- Meyer-Lindenberg A, Poline JB, Kohn PD, Holt JL, Egan MF, Weinberger DR, et al. Evidence for abnormal cortical functional connectivity during working memory in schizophrenia. Am J Psychiatry. 2001;158:1809 –1817. doi: 10.1176/appi.ajp.158.11.1809. [DOI] [PubMed] [Google Scholar]
- Minami T, Nobuhara K, Okugawa G, Takase K, Yoshida T, Sawada S, et al. Diffusion tensor magnetic resonance imaging of disruption of regional white matter in schizophrenia. Neuropsychobiology. 2003;47:141–145. doi: 10.1159/000070583. [DOI] [PubMed] [Google Scholar]
- Mitropoulou V, Harvey PD, Maldari LA, Moriarty PJ, New AS, Silverman JM, et al. Neuropsychological performance in schizotypal personality disorder: Evidence regarding diagnostic specificity. Biol Psychiatry. 2002;52:1175–1182. doi: 10.1016/s0006-3223(02)01426-9. [DOI] [PubMed] [Google Scholar]
- Nestor PG, Kubicki M, Gurrera RJ, Niznikiewicz M, Frumin M, McCarley RW, et al. Neuropsychological correlates of diffusion tensor imaging in schizophrenia. Neuropsychology. 2004;18:629 – 637. doi: 10.1037/0894-4105.18.4.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Papadakis NG, Xing D, Houston GC, Smith JM, Smith MI, James MF, et al. A study of rotationally invariant and symmetric indices of diffusion anisotropy. Magn Reson Imaging. 1999;17:881– 892. doi: 10.1016/s0730-725x(99)00029-6. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Association fiber pathways to the frontal cortex from the superior temporal region in the rhesus monkey. J Comp Neurol. 1988;273:52– 66. doi: 10.1002/cne.902730106. [DOI] [PubMed] [Google Scholar]
- Raine A. The SPQ: A scale for the assessment of schizotypal personality based on DSM-III-R criteria. Schizophr Bull. 1991;17:555–564. doi: 10.1093/schbul/17.4.555. [DOI] [PubMed] [Google Scholar]
- Reitan RM. Validity of the Trail Making Test as an indication of organic brain damage. Percept Mot Skills. 1958;8:271. [Google Scholar]
- Selden NR, Gitelman DR, Salamon-Murayama N, Parrish TB, Mesulam MM. Trajectories of cholinergic pathways within the cerebral hemispheres of the human brain. Brain. 1998;121:2249 –2257. doi: 10.1093/brain/121.12.2249. [DOI] [PubMed] [Google Scholar]
- Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophr Res. 2001;49:1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel S, Castellan NJ. Nonparametric Statistics for the Behavioral Sciences. 2. New York: McGraw-Hill; 1988. [Google Scholar]
- Siever LJ, Davis KL. The pathophysiology of schizophrenia disorders: Perspectives from the spectrum. Am J Psychiatry. 2004;161:398 – 413. doi: 10.1176/appi.ajp.161.3.398. [DOI] [PubMed] [Google Scholar]
- Spielberger CD. The State-Trait Anxiety Inventory. Palo Alto, California: Consulting Psychologists Press; 1983. [Google Scholar]
- Sun Z, Wang F, Cui L, Breeze J, Du X, Wang X, et al. Abnormal anterior cingulum in patients with schizophrenia: A diffusion tensor imaging study. Neuroreport. 2003;14:1833–1836. doi: 10.1097/00001756-200310060-00015. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Suzuki M, Kawasaki Y, Kurokawa K, Hagino H, Yamashita I, et al. Volumetric magnetic resonance imaging study of the anterior cingulate gyrus in schizotypal disorder. Eur Arch Psychiatry Clin Neurosci. 2002;252:268 –277. doi: 10.1007/s00406-002-0392-3. [DOI] [PubMed] [Google Scholar]
- Trestman RL, Keefe RS, Mitropoulou V, Harvey PD, deVegvar ML, Lees-Roitman S, et al. Cognitive function and biological correlates of cognitive performance in schizotypal personality disorder. Psychiatry Res. 1995;59:127–136. doi: 10.1016/0165-1781(95)02709-2. [DOI] [PubMed] [Google Scholar]
- Turner R, Le Bihan D, Maier J, Vavrek R, Hedges LK, Pekar J. Echo-planar imaging of intravoxel incoherent motion. Radiology. 1990;177:407–414. doi: 10.1148/radiology.177.2.2217777. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Gaffan D, Pelak VS. Projections from inferior temporal cortex to prefrontal cortex via the uncinate fascicle in rhesus monkeys. Exp Brain Res. 1989;76:473– 484. doi: 10.1007/BF00248903. [DOI] [PubMed] [Google Scholar]
- Uranova N, Orlovskaya D, Vikhreva O, Zimina I, Kolomeets N, Vostrikov V, et al. Electron microscopy of oligodendroglia in severe mental illness. Brain Res Bull. 2001a;55:597– 610. doi: 10.1016/s0361-9230(01)00528-7. [DOI] [PubMed] [Google Scholar]
- Uranova NA, Orlovskaia DD, Vikhreva OV, Zimina IS, Rakhmanova VI. [Morphometric study of ultrastructural changes in oligodendroglial cells in the postmortem brain in endogenous psychoses] Vestn Ross Akad Med Nauk. 2001b;(76):42–48. [PubMed] [Google Scholar]
- Uranova NA, Vostrikov VM, Orlovskaya DD, Rachmanova VI. Oligo-dendroglial density in the prefrontal cortex in schizophrenia and mood disorders: A study from the Stanley Neuropathology Consortium. Schizophr Res. 2004;67:269 –275. doi: 10.1016/S0920-9964(03)00181-6. [DOI] [PubMed] [Google Scholar]
- Voglmaier MM, Seidman LJ, Niznikiewicz MA, Dickey CC, Shenton ME, McCarley RW. Verbal and nonverbal neuropsychological test performance in subjects with schizotypal personality disorder. Am J Psychiatry. 2000;157:787–793. doi: 10.1176/appi.ajp.157.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voglmaier MM, Seidman LJ, Salisbury D, McCarley RW. Neuropsychological dysfunction in schizotypal personality disorder: A profile analysis. Biol Psychiatry. 1997;41:530 –540. doi: 10.1016/s0006-3223(96)00056-x. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Rosene DL, Pandya DN. Thalamic and cortical afferents differentiate anterior from posterior cingulate cortex in the monkey. Science. 1979;204:205–207. doi: 10.1126/science.107587. [DOI] [PubMed] [Google Scholar]
- Wang F, Sun Z, Cui L, Du X, Wang X, Zhang H, et al. Anterior cingulum abnormalities in male patients with schizophrenia determined through diffusion tensor imaging. Am J Psychiatry. 2004;161:573–575. doi: 10.1176/appi.ajp.161.3.573. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale. 3. San Antonio, Texas: Psychological Corporation, Harcourt Brace Jovanovich; 1997a. [Google Scholar]
- Wechsler D. The Wechsler Memory Scale. 3. San Antonio, Texas: Psychological Corporation, Harcourt Brace Jovanovich; 1997b. [Google Scholar]
- Weinberger DR, Berman KF, Suddath R, Torrey EF. Evidence of dysfunction of a prefrontal-limbic network in schizophrenia: A magnetic resonance imaging and regional cerebral blood flow study of discordant monozygotic twins. Am J Psychiatry. 1992;149:890 – 897. doi: 10.1176/ajp.149.7.890. [DOI] [PubMed] [Google Scholar]
- Wernicke C. Grundrisse der Psychiatrie. Leipzig, Germany: Thieme; 1906. [Google Scholar]