Summary
Glycoprotein B (gB) homologues within the herpesvirus family display high sequence conservation, and a number of gB homologues contain a cleavage motif R-X-K/R-R recognized by the cellular protease furin. Epstein-Barr virus (EBV) gB contains this motif and cleaved gB is found in EBV virions. To determine the functional significance of this cleavage motif in EBV gB a deletion mutant (gB Δfurin) was created lacking the motif. This cleavage mutant was expressed well in cell culture but not cleaved. Experiments examining gB Δfurin in a cell fusion assay revealed that fusion was reduced by 52% in epithelial and 28% in B cells when compared with wild type EBV gB. This decrease in cell:cell fusion is similar to that observed with multiple α-herpesvirus gB cleavage mutants and supports a conserved function for cleaved gB. Interestingly, cell-to-cell spread of EBV may be more efficient in epithelial cells than B cells.
Epstein-Barr virus (EBV) is an orally transmitted human γ-herpesvirus that establishes a persistent infection in greater than 90% of the world’s adult population. EBV is spread through saliva, and following initial infection the virus establishes life-long latency in B cells of its human host (Rickinson, 2007). EBV is recognized to infect epithelial cells as well as B lymphocytes during its normal cycle of persistence (Hutt-Fletcher, 2007). EBV is the causative agent of infectious mononucleosis, and has also been associated with a number of human malignancies of epithelial and B cell origin, including Burkitt’s lymphoma and of nasopharyngeal carcinoma (Rickinson, 2007). Similar to other herpesvirus family members, EBV encodes a number of membrane glycoproteins. Membrane glycoproteins are important in a variety of viral processes including entry of herpesviruses into target cells. Along with membrane glycoproteins gH and gL, herpesvirus glycoprotein B (gB) has been shown to be essential for herpesvirus fusion, and together they form the core virus fusion machinery (Pereira, 1994; Spear & Longnecker, 2003).
Herpesvirus gB homologues are highly conserved, and a number of gB homologues across all three of the herpesvirus subfamilies (alpha, beta, and gamma) possess a known cleavage motif R-X-K/R-R recognized by the cellular protease furin and are cleaved (Backovic et al., 2007; Baghian et al., 2000; Britt & Vugler, 1989; Fleckenstein et al., 1982; Hampl et al., 1984; Johannsen et al., 2004; Loh, 1991; Meredith et al., 1989; Okazaki, 2007; Ross et al., 1989; Sullivan et al., 1989; van Drunen Littel-van den Hurk & Babiuk, 1986; Vey et al., 1995; Whealy et al., 1990; Wolfer et al., 1990). Epstein-Barr virus has been shown to possess the defined cleavage motif and is cleaved at this defined site by a cellular protease (Backovic et al., 2007). In EBV virions, most gB present is in the cleaved form with only a fraction of total gB present in the uncleaved form (Johannsen et al., 2004). The physiological relevance of this proteolytic processing of gB to its function in infection is not well understood and loss of cleavage has no effect on viral growth of bovine herpes virus 1 (BoHV-1), pseudorabies virus (PRV), or human cytomegalovirus (HCMV) gB (Kopp et al., 1994; Okazaki, 2007; Strive et al., 2002). However, loss of cleavage in BoHV-1 and PVR gB decreases viral cell-to-cell spread, suggesting that these cleaved herpesvirus gBs may function differently between virus-cell and cell-cell fusion (Kopp et al., 1994; Okazaki, 2007). Previous studies have shown that there is not sufficient homology between the different subfamily gBs to allow complementation in other members of the herpesvirus family (Lee et al., 1997). Unlike alpha and betaherpesvirus gB homologs, EBV gB is present predominantly in the membranes of the nucleus and endoplasmic reticulum (ER) and only in small amounts on the plasma membrane (Emini et al., 1987; Gong & Kieff, 1990; Gong et al., 1987; Qualtiere & Pearson, 1979).
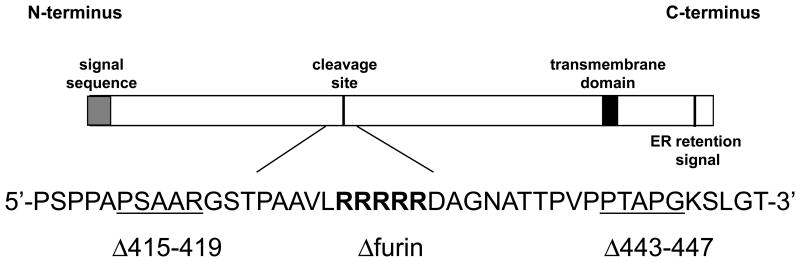
While the function of gB cleavage has been investigated for a number of herpesviruses, the function of EBV gB cleavage has not previously been examined. To investigate the importance of gB cleavage for fusion activity of EBV gB, a group of mutants were constructed. Mutants were generated using a QuikChange site-directed mutagenesis kit (Stratagene), with the plasmid encoding wild type EBV gB in the Stratagene pSG5 vector used as a template (Haan et al., 2001). Positive clones were sequenced, grown in large quantities, isolated using an EndoFree Plasmid Maxi Kit (Qiagen), and sequenced again. Three mutants were constructed; one in which the specific cleavage motif (RRRRR) was deleted and two in which five amino acid stretches near each side of the cleavage motif (Fig. 1) were deleted to serve as controls.
Fig. 1.
Schematic representation of EBV gB including cleavage motif and surrounding sequence. The relative locations of other known functional domains are also indicated. Residues in bold are deleted in the gB Δfurin mutant and underlined residues are deleted in the gB Δ415–419 mutant and gB Δ443–447 mutant respectively.
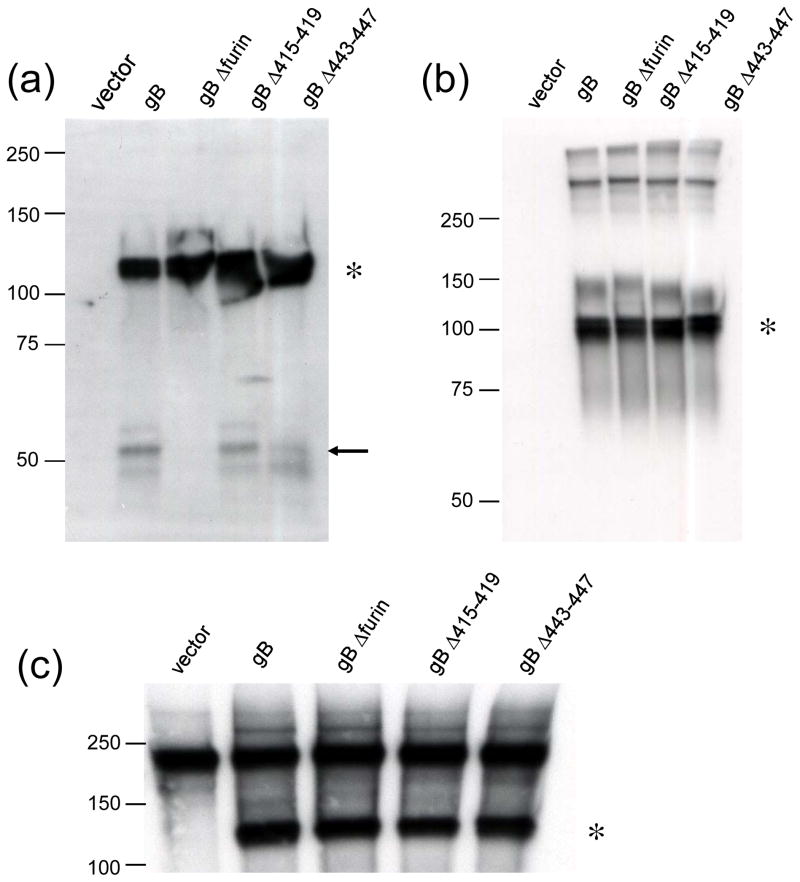
To investigate the expression of wild type gB and the gB mutants described in Fig. 1, immunoprecipitation followed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (Laemmli, 1970) and Western blotting (Backovic et al., 2007; Burnette, 1981) was performed on proteins expressed by Chinese hamster ovary (CHO)-K1-transfected cells (Fig. 2). Briefly, following lysis of transfected CHO-K1 cells with 1% Triton-X lysis buffer, EBV gB was immunoprecipitated with protein G-Sepharose beads (GE healthcare) bound to monoclonal anti-gB antibody CL55 (Backovic et al., 2007; McShane & Longnecker, 2004) before SDS-PAGE and Western blotting. Immunoprecipitates were run on 10% Tris-HCl Ready Gel precast gels (Bio-Rad) in sodium dodecyl sulfate (SDS) running buffer at 100 V for 100 min. Proteins were transferred to Immobilon-P membranes in transfer buffer at 100 V for 90 min with cooling. Blots were blocked in Tris-buffered saline with Tween (TBST) with 5% milk for an hour at room temperature (RT) or overnight at 4°C and then incubated for an hour at RT with a rabbit polyclonal anti-gB antibody (Backovic et al., 2007; McShane & Longnecker, 2004) diluted 1:1000 in blocking solution. Blots were washed, and a horseradish peroxidase (HRP)-conjugated anti-rabbit IgG (Cell Signaling) was applied for 45 min at RT. Blots were washed again and then mixed in equal volumes of ECL solutions and exposed to hyperfilm (Amersham Biosciences).
Fig. 2.
Cleavage, intracellular expression, and cell surface expression of wild type and mutant EBV gB. (a–c) CHO-K1 cells were transiently transfected to express wild type or mutant gB. Transfected cells were lysed and gB protein was precipitated by the gB-specific monoclonal antibody CL55. The immunoprecipitates were analyzed by SDS-PAGE and Western blotting under reducing (a) or non-reducing (b-c) conditions. Reducing conditions indicate the presence of β-mercaptoethanol in the SDS sample buffer utilized for SDS-PAGE. The full-length gB monomer is indicated by an asterisk. (a) The gB C-terminal cleavage product is indicated by an arrow. (c) To examine cell surface expression of gB transfected cells were biotinylated prior to lysis and immunoprecipitation with gB-specific monoclonal antibody CL55. Biotinylated gB was visualized by SDS-PAGE and Western blotting with avidin conjugated to horseradish peroxidase (Bio-Rad) under non-reducing conditions. Size markers in kDa are noted to the left of blots. Differences in the apparent size of gB (compare gB indicated by an asterisk in panels b with a and c) in reducing and non-reducing conditions is likely due to the presence of disulfide bonds in non-reduced samples compared to reduced samples.
Under reducing conditions (Fig. 2a), all three gB mutants were expressed similarly to wild type gB, indicating that the described mutations did not affect production of the protein. However, the C-terminal cleavage product of gB, indicated by an arrow, was not present in the lane of gB Δfurin indicating that this mutant was not cleaved as wild-type gB was. The mutants containing the deletions on either side of the cleavage motif were cleaved similar to wild-type gB, indicating that loss of gB cleavage was due to loss of the specific cleavage motif and was not a non-specific effect of deletion in the region. Under non-reducing conditions (Fig. 2b) it was shown that all three gB mutants were glycosylated and were able to oligomerize similar to wild-type gB, indicating that these processes were not significantly affected by the described deletions. The gB cleavage product was not visible under these conditions.
To examine cell surface localization of wild type gB and the gB mutants, transfected CHO-K1 cell surface proteins were biotinylated with the membrane-impermeable biotinylation agent sulfosuccinimidyl-6-(biotin-amido) hexanoate (Pierce) prior to lysis and SDS-PAGE and Western blot analysis. This established approach (Daniels & Amara, 1998) has been used successfully for detection of surface-expressed EBV gB (Backovic et al., 2007). Following biotinylation, surface-expressed gB was immunoprecipitated with anti-gB antibody as described, immunoprecipitates were run on a 7.5% Tris-HCl Ready Gel precast gel (Bio-Rad) in SDS running buffer, proteins were transferred to Immobilon-P membranes as described, and biotinylated gB was detected by avidin conjugated to horseradish peroxidase (Bio-Rad) (Fig. 2c). All three gB mutants showed comparable surface expression to wild type gB (Fig. 2c), indicating that the deletions did not affect trafficking of the protein to the cell surface. A background band was apparent in all samples including the vector control just under the 250 kDa marker (Fig. 2c).
To determine the ability of gB variants to mediate fusion with the two cell types that EBV infects in vivo, epithelial and B cells, a virus-free cell-based fusion assay was utilized (Backovic et al., 2007; Kirschner et al., 2006; McShane & Longnecker, 2004; 2005). Mammalian B cells were Daudi cells (Daudi B lymphocytes; American Type Culture Collection) stably selected with G418 to express T7 RNA polymerase (Silva et al., 2004). Mammalian epithelial cells were human embryonic kidney 293T14 cells that express simian virus 40 large T antigen (293T; American Type Culture Collection) and have been modified to stably express T7 RNA polymerase under selection of 100μg/ml zeocin (Omerovic et al., 2005). The cells were maintained in culture as previously described (McShane & Longnecker, 2004). Briefly, effector CHO-K1 cells were transfected (Kirschner et al., 2006; McShane & Longnecker, 2004) with plasmids encoding the glycoproteins required for fusion (gB, gH, gL, and gp42 transfected for fusion with both epithelial and B cells), as well as a plasmid encoding luciferase under the control of T7 polymerase. Six hours following transfection cells were washed and returned to Ham’s F-12 complete media, and 24 h post transfection cells were washed with PBS and detached with Versene. All cells (CHO-K1, 293T14, and Daudi-T7) were counted with a Beckman Coulter Z1 particle counter, then the effector and the target cells were mixed in equal amounts (0.25 × 106 cells per sample) and plated in duplicate into a 24-well plate in Ham’s F-12 medium. Twenty-four hours later, the cells were washed with PBS and lysed, and luciferase was quantified by using the Promega Reporter Assay system. Relative luciferase activity was measured on a Perkin-Elmer Victor plate reader.
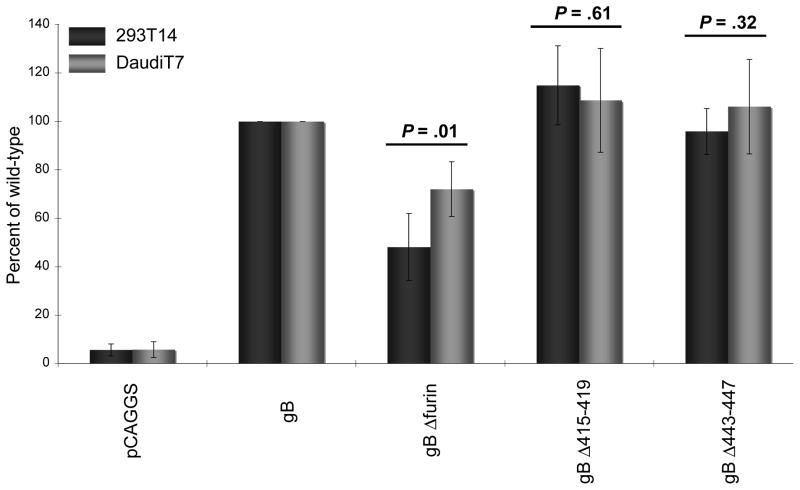
The ability of each of the three gB mutants to mediate fusion with epithelial and B cells is summarized in Fig. 3. The gB Δ415–419 and gB Δ443–447 mutants showed no significant difference in ability to mediate fusion similar to wild type gB, with epithelial or B cells. This similar ability indicated that deletions made to this region of the EBV gB protein near the furin cleavage motif had no significant effect on ability of gB to mediate fusion. A significant decrease in fusion ability was seen, however, with the gB Δfurin mutant, which exhibited 48% and 72% of the wild-type gB fusion activity with epithelial and B cells, respectively. This decrease in ability to mediate fusion indicated that loss of gB cleavage had an inhibitory effect on EBV-induced cell-cell fusion, with both epithelial and B cells.
Fig. 3.
Effect of gB cleavage site mutation on membrane fusion. Relative luciferase activity was measured in a cell fusion assay using CHO-K1 cells transfected with gH, gL, gp42, and wild-type or mutant gB and overlaid with epithelial cells (293T14) or B cells (DaudiT7). The data shown were averaged from five independent experiments and luciferase activity was normalized to wild-type levels, which was set to 100% for both epithelial and B cells. Error bars represent standard deviation. Difference in fusion levels between epithelial and B cells analyzed by Student’s t test, P values indicated above each gB mutant.
A role for proteolytic cleavage of gB has been previously indicated in cell-to-cell spread of both BoHV-1 and PRV (Kopp et al., 1994; Okazaki, 2007). While this function of gB cleavage in these α-herpesviruses is well characterized, the function of cleavage of other herpesvirus gB homologues containing the identified cleavage motif was unknown. We have shown that similar to BoHV-1 and PRV gB, cleavage of EBV gB plays a role in cell:cell fusion, indicating a conserved function of cleavage across gB homologues from different herpesvirus subfamilies. While a general decrease in cell-to-cell spread has been previously observed with cleavage-deficient BoHV-1 and PRV gB mutants, our study is the first to attempt to quantify cell fusion, allowing greater insight into the functional significance of gB cleavage. Interestingly, a greater decrease was seen in EBV-induced fusion with epithelial cells than fusion with B cells. This difference, while small, was statistically significant (P < 0.05) and may indicate a difference in gB function during fusion with each cell type. This hypothesis is supported by a previous study showing that epithelial cell fusion, but not B cell fusion, is mediated with gB mutants with enhanced cell-surface expression, independent of other viral proteins (McShane & Longnecker, 2004).
It is possible that cell-to-cell spread of EBV is more efficient with epithelial cells than with B cells. Fusion levels with transiently transfected HEK-293-P (epithelial) cells are increased in relation to similarly transfected Daudi B cells in a virus-free cell fusion assay (McShane & Longnecker, 2004), and this result was duplicated when the stably-transfected cell lines 293T14 (epithelial) and Daudi T7 (B) were substituted. Reactivation of EBV from latency is quickly controlled by the immune system in immunocompetent individuals. Efficient cell-to-cell spread of EBV between oral epithelial cells may improve viral spread to uninfected individuals by allowing production of larger amounts of infectious virions during the small window of virus reactivation before host immune system detection and elimination. EBV infection of some epithelial cell lines is known to occur more efficiently by cell-cell contact (Imai et al., 1998; Speck & Longnecker, 2000). Efficient cell-to-cell spread of EBV between B lymphocytes may not significantly affect viral spread after EBV reactivation since virions released from infected B lymphocytes efficiently infect epithelial cells, the cell type from which a majority of viruses shed in saliva are produced (Hutt-Fletcher, 2007). Efficient cell-to-cell spread of EBV between epithelial cells may confer a survival advantage to EBV. This advantage could help explain both the general difference in quantity of cell-cell fusion observed between epithelial and B cells and the specific difference in effect of gB cleavage motif mutation. Further studies beyond our in vitro fusion assay will be necessary to conclusively determine the efficiency or relevance of cell-to-cell spread of EBV between epithelial and B cells. The varying levels of cell-cell fusion inhibition caused by eliminating gB cleavage and the previously suggested difference in the function of EBV gB between virus entry and cell:cell fusion highlight the critical and complicated role gB plays in EBV infection and spread.
Acknowledgments
We thank Jessica Reimer and Marija Backovic for helpful advice and technical assistance with the biotinylation assay. Dr. Lindsey Hutt-Fletcher kindly provided the gB monoclonal antibody CL55. We thank the members of the Longnecker and Spear laboratories for help and support. This research was supported by Public Health Service grants RO1 AI067048 (R.L) and T32 AI060523 (J.S.) from the National Institute of Allergy and Infectious Diseases (R.L.).
References
- Backovic M, Leser GP, Lamb RA, Longnecker R, Jardetzky TS. Characterization of EBV gB indicates properties of both class I and class II viral fusion proteins. Virology. 2007;368:102–113. doi: 10.1016/j.virol.2007.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baghian A, Luftig M, Black JB, Meng YX, Pau CP, Voss T, Pellett PE, Kousoulas KG. Glycoprotein B of human herpesvirus 8 is a component of the virion in a cleaved form composed of amino- and carboxyl-terminal fragments. Virology. 2000;269:18–25. doi: 10.1006/viro.2000.0198. [DOI] [PubMed] [Google Scholar]
- Britt WJ, Vugler LG. Processing of the gp55–116 envelope glycoprotein complex (gB) of human cytomegalovirus. Journal of Virology. 1989;63:403–410. doi: 10.1128/jvi.63.1.403-410.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette WN. “Western blotting”: electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Analytical Biochemistry. 1981;112:195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Daniels GM, Amara SG. Selective labeling of neurotransmitter transporters at the cell surface. Methods in Enzymology. 1998;296:307–318. doi: 10.1016/s0076-6879(98)96023-2. [DOI] [PubMed] [Google Scholar]
- Emini EA, Luka J, Armstrong ME, Keller PM, Ellis RW, Pearson GR. Identification of an Epstein-Barr virus glycoprotein which is antigenically homologous to the varicella-zoster virus glycoprotein II and the herpes simplex virus glycoprotein B. Virology. 1987;157:552–555. doi: 10.1016/0042-6822(87)90300-x. [DOI] [PubMed] [Google Scholar]
- Fleckenstein B, Muller I, Collins J. Cloning of the complete human cytomegalovirus genome in cosmids. Gene. 1982;18:39–46. doi: 10.1016/0378-1119(82)90054-3. [DOI] [PubMed] [Google Scholar]
- Gong M, Kieff E. Intracellular trafficking of two major Epstein-Barr virus glycoproteins, gp350/220 and gp110. J Virol. 1990;64:1507–1516. doi: 10.1128/jvi.64.4.1507-1516.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong M, Ooka T, Matsuo T, Kieff E. Epstein-Barr virus glycoprotein homologous to herpes simplex virus gB. J Virol. 1987;61:499–508. doi: 10.1128/jvi.61.2.499-508.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haan KM, Lee SK, Longnecker R. Different functional domains in the cytoplasmic tail of glycoprotein B are involved in Epstein-Barr virus-induced membrane fusion. Virology. 2001;290:106–114. doi: 10.1006/viro.2001.1141. [DOI] [PubMed] [Google Scholar]
- Hampl H, Ben-Porat T, Ehrlicher L, Habermehl KO, Kaplan AS. Characterization of the envelope proteins of pseudorabies virus. Journal of Virology. 1984;52:583–590. doi: 10.1128/jvi.52.2.583-590.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutt-Fletcher LM. Epstein-Barr virus entry. Journal of Virology. 2007;81:7825–7832. doi: 10.1128/JVI.00445-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai S, Nishikawa J, Takada K. Cell-to-cell contact as an efficient mode of Epstein-Barr virus infection of diverse human epithelial cells. Journal of Virology. 1998;72:4371–4378. doi: 10.1128/jvi.72.5.4371-4378.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannsen E, Luftig M, Chase MR, Weicksel S, Cahir-McFarland E, Illanes D, Sarracino D, Kieff E. Proteins of purified Epstein-Barr virus. Proc Natl Acad Sci U S A. 2004;101:16286–16291. doi: 10.1073/pnas.0407320101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschner AN, Omerovic J, Popov B, Longnecker R, Jardetzky TS. Soluble Epstein-Barr virus glycoproteins gH, gL, and gp42 form a 1:1:1 stable complex that acts like soluble gp42 in B-cell fusion but not in epithelial cell fusion. Journal of Virology. 2006;80:9444–9454. doi: 10.1128/JVI.00572-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp A, Blewett E, Misra V, Mettenleiter TC. Proteolytic cleavage of bovine herpesvirus 1 (BHV-1) glycoprotein gB is not necessary for its function in BHV-1 or pseudorabies virus. Journal of Virology. 1994;68:1667–1674. doi: 10.1128/jvi.68.3.1667-1674.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee SK, Compton T, Longnecker R. Failure to complement infectivity of EBV and HSV-1 glycoprotein B (gB) deletion mutants with gBs from different human herpesvirus subfamilies. Virology. 1997;237:170–181. doi: 10.1006/viro.1997.8765. [DOI] [PubMed] [Google Scholar]
- Loh LC. Synthesis and processing of the major envelope glycoprotein of murine cytomegalovirus. Virology. 1991;180:239–250. doi: 10.1016/0042-6822(91)90028-a. [DOI] [PubMed] [Google Scholar]
- McShane MP, Longnecker R. Cell-surface expression of a mutated Epstein-Barr virus glycoprotein B allows fusion independent of other viral proteins. Proc Natl Acad Sci U S A. 2004;101:17474–17479. doi: 10.1073/pnas.0404535101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McShane MP, Longnecker R. Analysis of fusion using a virus-free cell fusion assay. Methods Mol Biol. 2005;292:187–196. doi: 10.1385/1-59259-848-x:187. [DOI] [PubMed] [Google Scholar]
- Meredith DM, Stocks JM, Whittaker GR, Halliburton IW, Snowden BW, Killington RA. Identification of the gB homologues of equine herpesvirus types 1 and 4 as disulphide-linked heterodimers and their characterization using monoclonal antibodies. Journal of General Virology. 1989;70:1161–1172. doi: 10.1099/0022-1317-70-5-1161. [DOI] [PubMed] [Google Scholar]
- Okazaki K. Proteolytic cleavage of glycoprotein B is dispensable for in vitro replication, but required for syncytium formation of pseudorabies virus. Journal of General Virology. 2007;88:1859–1865. doi: 10.1099/vir.0.82610-0. [DOI] [PubMed] [Google Scholar]
- Omerovic J, Lev L, Longnecker R. The amino terminus of Epstein-Barr virus glycoprotein gH is important for fusion with epithelial and B cells. J Virol. 2005;79:12408–12415. doi: 10.1128/JVI.79.19.12408-12415.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira L. Function of glycoprotein B homologues of the family herpesviridae. Infect Agents Dis. 1994;3:9–28. [PubMed] [Google Scholar]
- Qualtiere LF, Pearson GR. Epstein-Barr virus-induced membrane antigens: immunochemical characterization of Triton X-100 solubilized viral membrane antigens from EBV-superinfected Raji cells. Int J Cancer. 1979;23:808–817. doi: 10.1002/ijc.2910230612. [DOI] [PubMed] [Google Scholar]
- Rickinson AB, Kieff E. Epstein-Barr Virus, Epstein-Barr Virus and Its Replication. Philadelphia PA: Lippincott WIlliams & Wilkins; 2007. [Google Scholar]
- Ross LJ, Sanderson M, Scott SD, Binns MM, Doel T, Milne B. Nucleotide sequence and characterization of the Marek’s disease virus homologue of glycoprotein B of herpes simplex virus. Journal of General Virology. 1989;70:1789–1804. doi: 10.1099/0022-1317-70-7-1789. [DOI] [PubMed] [Google Scholar]
- Silva AL, Omerovic J, Jardetzky TS, Longnecker R. Mutational analyses of Epstein-Barr virus glycoprotein 42 reveal functional domains not involved in receptor binding but required for membrane fusion. Journal of Virology. 2004;78:5946–5956. doi: 10.1128/JVI.78.11.5946-5956.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear PG, Longnecker R. Herpesvirus entry: an update. J Virol. 2003;77:10179–10185. doi: 10.1128/JVI.77.19.10179-10185.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speck P, Longnecker R. Infection of breast epithelial cells with Epstein-Barr virus via cell-to-cell contact. Journal of the National Cancer Institute. 2000;92:1849–1851. doi: 10.1093/jnci/92.22.1849. [DOI] [PubMed] [Google Scholar]
- Strive T, Borst E, Messerle M, Radsak K. Proteolytic processing of human cytomegalovirus glycoprotein B is dispensable for viral growth in culture. Journal of Virology. 2002;76:1252–1264. doi: 10.1128/JVI.76.3.1252-1264.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan DC, Allen GP, O’Callaghan DJ. Synthesis and processing of equine herpesvirus type 1 glycoprotein 14. Virology. 1989;173:638–646. doi: 10.1016/0042-6822(89)90576-x. [DOI] [PubMed] [Google Scholar]
- van Drunen Littel-van den Hurk S, Babiuk LA. Synthesis and processing of bovine herpesvirus 1 glycoproteins. Journal of Virology. 1986;59:401–410. doi: 10.1128/jvi.59.2.401-410.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vey M, Schafer W, Reis B, Ohuchi R, Britt W, Garten W, Klenk HD, Radsak K. Proteolytic processing of human cytomegalovirus glycoprotein B (gpUL55) is mediated by the human endoprotease furin. Virology. 1995;206:746–749. doi: 10.1016/s0042-6822(95)80002-6. [DOI] [PubMed] [Google Scholar]
- Whealy ME, Robbins AK, Enquist LW. The export pathway of the pseudorabies virus gB homolog gII involves oligomer formation in the endoplasmic reticulum and protease processing in the Golgi apparatus. Journal of Virology. 1990;64:1946–1955. doi: 10.1128/jvi.64.5.1946-1955.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfer U, Kruft V, Sawitzky D, Hampl H, Wittmann-Liebold B, Habermehl KO. Processing of pseudorabies virus glycoprotein gII. Journal of Virology. 1990;64:3122–3125. doi: 10.1128/jvi.64.6.3122-3125.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]