Abstract
Background
Previous research has demonstrated that heavy prenatal alcohol exposure affects the size and shape of the corpus callosum (CC) and compromises interhemispheric transfer of information. The aim of this study was to confirm the previous reports of poorer performance on a finger localization test (FLT) of interhemispheric transfer in a cohort of heavily exposed children and to extend these findings to a cohort of moderately exposed young adults.
Methods
In Study 1, the FLT was administered to 40 heavily-exposed and 23 non-exposed children from the Cape Coloured community of Cape Town, South Africa, who were evaluated for fetal alcohol syndrome (FAS) dysmorphology and growth. Anatomical images of the CC were obtained using structural MRI on a subset of these children. In Study 2, the FLT was administered to a cohort of 85 moderate-to heavily exposed young adults participating in a 19-year follow-up assessment of the Detroit Prenatal Alcohol Exposure cohort, whose alcohol exposure had been ascertained prospectively during gestation.
Results
In Study 1, children with FAS showed more transfer-related errors than controls after adjustment for confounding, and increased transfer-related errors were associated with volume reductions in the isthmus and splenium of the CC. In Study 2, transfer-related errors were associated with quantity of alcohol consumed per occasion during pregnancy. More errors were made if the mother reported binge drinking (≥ 5 standard drinks) during pregnancy than if she drank regularly (M ≥ 1 drink/per day) without binge drinking.
Conclusions
These findings confirm a previous report of impaired interhemispheric transfer of tactile information in children heavily exposed to alcohol in utero and extend these findings to show that these deficits are also seen in more moderately exposed individuals, particularly those exposed to binge-like pregnancy drinking.
Keywords: Fetal alcohol spectrum disorder, interhemispheric transfer, fetal alcohol syndrome, corpus callosum, pregnancy binge drinking, finger localization test
Introduction
Fetal alcohol spectrum disorder (FASD) is a term used to describe the broad range of adverse outcomes associated with prenatal alcohol exposure (Hoyme et al., 2005). Fetal alcohol syndrome (FAS), which is characterized by a distinctive set of craniofacial dysmorphic features, microcephaly, and pre- and/or postnatal growth retardation is the most severe form of FASD. Less severe forms of FASD include partial FAS (PFAS), in which some but not all of the craniofacial dysmorphic features are present, and alcohol related neurodevelopmental disorder (ARND), in which alcohol-exposed individuals lack the craniofacial dysmorphology but exhibit mild to moderate neurobehavioral deficits (Stratton et al., 1996). Although many children with FAS are mentally retarded, others perform in the low average to average range (Streissguth et al., 1991), and in ARND, effects on IQ have been reported (Streissguth et al., 1990; Mattson et al., 1997), particularly in children of mothers who are older and/or have a history of alcohol abuse (Jacobson et al., 2004).
Findings from several studies suggest that the developing corpus callosum (CC) may be particularly susceptible to the effects of prenatal alcohol exposure. Structural anomalies and reductions in size have been noted (Riley et al., 1995), and cases of partial and complete agenesis have been documented (Jones and Smith, 1973; Wisniewski et al., 1983; Kinney et al., 1980; Clarren et al., 1978). The incidence of agenesis of the corpus callosum among children prenatally exposed to alcohol may be as high as 6.8% (Riley et al., 1995), as compared to a normal population rate of 0.3% and a developmentally delayed population rate of 2.3% (Jeret et al., 1985). Studies have also documented reduced size and displacement of the CC in alcohol exposed children (Sowell et al., 2001; Bookstein et al., 2001; 2002), and microstructural anomalies have been reported in studies using diffusion tensor imaging (DTI) (Ma et al., 2005; Wozniak et al., 2006). Bookstein et al. (2007) have reported that the presence of a large angle of the splenium is strongly associated with heavy prenatal alcohol exposure and suggest that this anomaly may serve as a biomarker of fetal alcohol exposure in newborns.
Two studies have provided evidence that, along with anatomical differences, such as reduced callosal area, children with heavy prenatal alcohol exposure also show differences in function that appear to be related to callosal anatomy. Using the finger localization task (FLT), a neuropsychological test that assesses the efficiency of interhemispheric transfer of tactile information, Roebuck et al. (2002) found that heavily-exposed children performed more poorly than controls. They also found an association between poorer FLT performance and reduced areas in the most anterior and posterior regions of the CC. In a study using functional magnetic resonance imaging, dysmorphic alcohol-exposed adults showed increased activation patterns in the premotor and primary motor cortices during interhemispheric transmission as compared to non-dysmorphic alcohol-exposed and control adults (Santhanam et al., 2007), suggesting that the dysmorphic adults needed additional neuronal resources to perform tasks that require interhemispheric transfer. These studies suggest that the altered anatomical development of the CC associated with prenatal alcohol exposure impairs the function of the CC such that interhemispheric transfer is less efficient.
In this study, we examine the effects of prenatal alcohol exposure on the efficiency of interhemispheric transfer in two cohorts of alcohol-exposed individuals. First, a cohort of heavily-exposed school-aged children from the Cape Coloured (mixed ancestry) population of Cape Town, South Africa, (Jacobson et al., 2008) was examined. This population, which is comprised mainly of descendants of white European, Malaysian, Khoi-San, and black African ancestors, has among the highest rates of FAS in the world (May et al., 2000). The high prevalence of FAS in this community is a consequence of very heavy drinking during pregnancy (Croxford & Viljoen, 1999), which is due to poor psychosocial circumstances and the traditional dop system, in which wine-producing farm laborers were paid, in part, with wine. Although the dop system has been outlawed, regular and heavy alcohol consumption persists in both the urban and rural Cape Coloured communities (Carter et al., 2005; Jacobson et al., 2006). Secondly, we assess finger localization in a sample of moderately-exposed young adults from the Detroit Longitudinal Prenatal Alcohol Exposure cohort (Jacobson et al., 2002; Burden et al., 2005a; 2005b). This study is the first to examine the effects of moderate prenatal exposure to alcohol on the efficiency of interhemispheric transfer of tactile information.
Study 1: Cape Town Cohort
Methods
Participants
Participants were 40 right-handed, heavily alcohol-exposed school-aged children and 23 age- and gender-matched nonexposed controls from the Cape Coloured community. Thirty-six of the children were the older siblings of participants in our Cape Town Longitudinal Cohort Study (Jacobson et al., 2008). The other children in the sample were identified by screening all of the 8- to 12-year old children from an elementary school in a rural section of Cape Town, where there is a very high incidence of alcohol abuse among local farm workers. Mothers were interviewed retrospectively regarding their alcohol consumption, using a timeline follow-back approach to determine incidence and amount of drinking on a day-by-day basis when the mother was pregnant (Sokol et al., 1985; Jacobson et al., 2002). Volume was recorded for each type of beverage consumed and converted to oz of absolute alcohol (AA), using multipliers developed by Bowman et al. (1975). Any child whose mother reported consuming at least 14 standard drinks per week (1.0 oz AA/day) on average or engaged in binge drinking during pregnancy (more than four drinks/occasion) was considered heavily exposed. Most (92%) of the controls abstained during pregnancy, and none drank more than 0.05 oz AA/day on average or more than 1 oz AA on any single occasion.
Procedure
Dysmorphology assessment
In September 2005, we organized a clinic at which each child was independently examined for growth and FAS anomalies using a standard protocol (Hoyme et al., 2005) by two expert FAS dysmorphologists (HEH and LKR), who subsequently reached agreement regarding FAS diagnosis (Jacobson et al., 2008). There was substantial agreement between the two U.S.-based dysmorphologists on their assessments of all dysmorphic features, particularly palpebral fissure length and philtrum and vermilion ratings based on the Astley and Clarren (2001) rating scales (r-values = 0.80, 0.84, and 0.77, respectively). There was also substantial agreement between them and the Cape Town-based dysmorphologist (NK; median r = 0.78), who evaluated the children who could not be scheduled for the clinic.
Among the 40 children whose mothers drank heavily during pregnancy, eight (20%) met the revised Institute of Medicine criteria (Hoyme et al., 2005) for full FAS; that is, at least two of the principal dysmorphic features, small head circumference (bottom 10th percentile), and low weight or short stature (bottom 10th percentile). Four heavily exposed children (10%) met criteria for partial FAS (PFAS); that is, two features and at least one of the following: small head circumference, low weight, short stature, or low IQ (< 70). Data from these four children and the remaining 28 children whose mothers drank heavily were examined together in the Heavily Exposed (HE) group. This group was comparable to the prenatal exposure to alcohol (PEA) group described in Roebuck et al. (2002).
Structural magnetic resonance imaging (MRI)
Fourteen right-handed children were assessed using structural MRI on a 1.5T Magnetom Symphony MRI scanner (Siemens Medical Systems, Erlangen, Germany): the first seven heavily exposed (two with FAS and five with either PFAS or heavy exposure) that were tested and seven non-exposed control children matched for age. High-resolution anatomical images were acquired in the sagittal plane using a three-dimensional inversion recovery gradient echo sequence (72 slices, TR = 1900 ms, TE = 3.93 ms, TI = 1100 ms, slice thickness 2 mm), 250 × 250 mm field of view (resolution 1.4 × 1.0 × 2 mm). Volumetric tracing of the corpus callosum was undertaken following reslicing of the structural images into 3-dimensional space (Magnotta et al., 1995) using Brains2. The structure was defined on 13 contiguous sagittal slices defined medially (six on either side) from the mid-sagittal slice, which provides the clearest visualization of the structure. Structure subdivisions (rostrum, genu, rostral body, anterior body, posterior body, isthmus, and splenium; following Witelson, 1992) were defined on each of the 13 sagittal slices using a geometric definition scheme.
Finger localization task
In this task, the child is lightly touched on the tip of a finger with a sharpened pencil point. S/he is then asked to indicate which finger or fingers were touched by touching that finger with the thumb of the same hand (uncrossed condition) or by touching the corresponding finger on the opposite hand with the thumb of the opposite hand (crossed condition). Three conditions were administered: (1) one finger stimulated, hands in view; (2) one finger stimulated, hands out of view; (3) two fingers consecutively stimulated, hands out of view. Each condition consisted of four 16-trial blocks: (1) right hand, uncrossed; (2) left hand, uncrossed; (3) right hand, crossed; (4) left hand, crossed. In the two-finger condition, the child was asked to touch the fingers in the same order that they were stimulated. His/her vision was occluded for the hands out of view conditions using a 1′× 1′ × 2′ cardboard box with an open end facing the examiner and two small holes at the other end to insert the hands. The child was told which hand and whether one or two fingers were to be stimulated in each condition. The examiner recorded the responses, and no feedback was given concerning performance accuracy. Number of errors was scored in each condition. Following Roebuck et al. (2002), crossed-uncrossed difference (CUD) scores were calculated by subtracting uncrossed errors from crossed errors for each of the three difficulty conditions: hands in view (CUD-visual); hands out of view one-finger stimulation (CUD-1 finger); hands out of view two-finger stimulation (CUD-2 finger). All examiners were blind with respect to prenatal alcohol exposure except in the few instances when evidence of severe FAS was apparent.
Handedness
The children were examined for handedness using the Annett Behavioral Handedness Inventory.
Control variables
Correlational analysis was used to determine which control variables would be controlled statistically as potential confounders. Since a control variable cannot be the true cause of an observed deficit unless it is related to both exposure and outcome (Schlesselman, 1982), association with either exposure or outcome can be used as the criterion for statistical adjustment. In this study, control variables were selected based on their relation to the outcome, which has the additional advantage of increasing precision by also including covariates unrelated to exposure (Kleinbaum et al., 1988). All control variables even weakly related to each outcome (p <.10) were controlled for statistically.
Eight control variables were assessed for consideration as potential confounders of the relation between FASD diagnosis and performance on the FLT: age at testing, gender, maternal cigarette smoking during pregnancy (cigarettes/day), maternal age at delivery and years of education, prenatal cocaine exposure (yes/no), prenatal marijuana exposure (yes/no), and prenatal methaqualone (“mandrax”) exposure (yes/no). In addition to these potential confounding variables, an estimate of IQ was constructed to assess potential mediating effects of IQ on FLT performance. IQ was estimated based on Sattler’s (1992) formula for Short Form IQ, using seven subtests from the Wechsler Intelligence Scales for Children, third edition (WISC-III) — Similarities, Arithmetic, Digit Span, Symbol Search, Coding, Block Design, and Picture Completion — and Matrix Reasoning (WISC-IV). Validity coefficients for the Sattler Short Form IQ based on five or more subtests consistently exceed r = .90.
Informed consent
Informed consent was obtained from each mother at recruitment, and assent was obtained from the child. Approval for human research was obtained from both the Human Investigation Committee at Wayne State University and the Ethics Committee at the University of Cape Town (UCT) Faculty of Health Sciences. Mothers were compensated for their participation and given a photo of their child, mothers and children were given breakfast and lunch during the visit, and the child received a small gift.
Data analysis
To test the hypothesis that children heavily exposed to alcohol in utero have more CC transfer-related errors on the FLT, one-way repeated measures analyses of covariance (ANCOVAs) were performed on each of the three CUD scores calculated as described above, using Transfer (crossed vs. uncrossed) and Hand (right-hand stimulated vs. left-hand stimulated) as repeated measures and FASD diagnosis (FAS/HE/controls) as a between-subjects factor. Potential confounding variables were included as covariates if they met the criteria noted above. Spearman rank order correlation coefficients were used to examine the relation of the three CUD scores to total CC area and the areas of each of the CC regions. Because the anatomical data were available for only a subset of the sample, rank order correlations were used to insure that outliers would not inflate the magnitude of the correlations.
Results
Comparisons of the demographic characteristics of the three diagnostic groups in the Cape Town cohort are presented in Table 1. The three groups did not differ in age at testing or gender. Children in the FAS group were born to mothers who were older at delivery than the HE group (p = .01) and the controls (p = .02), confirming previous studies (May, 1991), and the primary caregivers of the control group had completed more formal education than those in the FAS (p = .02) and HE (p = .01) groups. Mothers in the HE group smoked more during pregnancy than the control mothers (p = .01). Only one mother reported using marijuana during pregnancy, and none reported using cocaine or methaqualone. As expected, the IQs of the children in the three groups differed significantly (p’s <.05) in a dose-dependent fashion.
Table 1.
Comparison of sample characteristics by FASD diagnosis in Cape Town
| FAS (N = 8) | HE (N = 31) | Controls (N = 23) | F or χ2 | |
|---|---|---|---|---|
| Maternal age at delivery | 29.3 (5.7) | 24.0 (5.1) | 24.1 (5.1) | 3.52* |
| Maternal education (years) | 6.4 (3.2) | 6.9 (2.3) | 8.7 (2.0) | 4.85** |
| Prenatal cigarettes (cigarettes/day) | 7.3 (7.2) | 10.2 (8.2) | 4.5 (6.3) | 3.93* |
| Alcohol exposure | ||||
| AA/day (oz) | 2.8 (2.9) | 2.5 (2.3) | 0.0 (0.01) | 13.19*** |
| AA/occasion (oz) | 5.5 (2.8) | 6.3 (5.0) | 0.1 (0.3) | 19.66*** |
| Frequency (days/week) | 2.9 (2.0) | 2.9 (1.6) | 0.01 (0.06) | 31.85*** |
| Child age at testing | 10.3 (1.4) | 10.5 (1.2) | 10.2 (1.2) | 0.27 |
| Gender (% male) | 37.5 | 38.7 | 47.8 | 0.53 |
| IQa | 55.9 (9.5) | 66.5 (10.6) | 77.3 (10.2) | 14.94*** |
Values are M (SD) or %
Estimated from 7 WISC-III subtests — Similarities, Arithmetic, Digit Span, Symbol Search, Coding, Block Design, and Picture Completion — and Matrix Reasoning from the WISC-IV.
p <.10
p <.05
p <.01
p <.001
Finger Localization Task
Table 2 summarizes the performance on the FLT for the Study 1 sample as a whole. For each of the three conditions, a repeated-measures ANOVA was performed using Transfer (crossed vs. uncrossed) and Hand (right-hand stimulated vs. left-hand stimulated) as repeated measures. A significant main effect of Condition was detected in all three conditions; in all cases more errors were made when information had to cross the corpus callosum.
Table 2.
Errors on the FLT in the two cohorts.
| Condition |
Hand |
Condition X Hand | |||||
|---|---|---|---|---|---|---|---|
| Uncrossed | Crossed | F | Left | Right | F | F | |
| Cape Town | |||||||
| Single-finger/hand visiblea | 0.1 (0.0) | 1.8 (0.3) | 42.18** | 1.1 (0.2) | 0.8 (0.1) | 2.66 | 1.69 |
| Single-finger/hand hiddena | 0.4 (0.1) | 2.9 (0.2) | 124.40** | 1.6 (0.2) | 1.6 (0.1) | 0.23 | 0.07 |
| Two-finger/hand hiddena | 6.3 (0.4) | 9.7 (0.4) | 127.77** | 7.7 (0.4) | 8.2 (0.4) | 4.88* | 4.69* |
| Detroit | |||||||
| Single-finger/hand visibleb | 0.0 (0.0) | 0.6 (0.2) | 11.39** | 0.3 (0.1) | 0.4 (0.2) | 1.72 | 1.33 |
| Single-finger/hand hiddenb | 0.1 (0.0) | 1.1 (0.2) | 54.42** | 0.7 (0.1) | 0.5 (0.1) | 11.72** | 6.44* |
| Two-finger/hand hiddenb | 2.3 (0.2) | 4.4 (0.4) | 72.12** | 3.4 (0.3) | 3.4 (0.3) | 0.00 | 21.04** |
Values are M (S.E.)
d.f. = 1, 61
d.f. = 1, 84
p <.05
p <.001
A main effect of hand and an interaction of hand-by-condition were detected in the two-finger/hand hidden condition. The main effect revealed that more errors were made when the right hand was stimulated and the participant had to respond with his/her left hand. Paired-samples t-tests were performed to examine the condition by hand interaction. In the uncrossed condition, no differences in errors were detected for the left or right hand, but in the crossed condition, where the opposite hand responds, more errors were made when the right hand was stimulated and the left hand had to respond (p <.01). This finding may indicate a direction of transfer effect (left-to-right hemisphere), but for the purposes of this study data on both hands were pooled since more errors were made when interhemispheric transfer was required, regardless of which hand was stimulated.
Relation of FLT Performance to FAS Diagnosis and Corpus Callosum Area
Results from the ANCOVA relating FASD diagnosis to FLT performance are presented in Table 3. In the single-finger/hand visible condition, only gender and age met criteria for inclusion as potential confounding variables; girls and older children performed better. No significant effect of diagnostic group was detected. Gender and age were also potential confounders in the single-finger/hand hidden condition. A significant main effect of diagnostic group was seen on single-finger/hand-hidden CUD scores. Post-hoc analysis revealed that the FAS group had significantly higher CUD scores than the HE group (p <.05) and the controls (p <.01), with no significant difference found between the HE group and the controls. When IQ was added to the model, the effect of FASD diagnostic group on CUD scores in the single-finger/hand-hidden condition fell short of statistical significance, F (1,58) = 2.94, p = .09. In the two-finger condition only gender met criteria for inclusion as a confounder, and no significant effect of diagnostic group was detected.
Table 3.
Relation of FASD diagnosis to crossed-uncrossed difference (CUD) scores in the Cape Town cohort.
| FAS | HE | Controls | F | |
|---|---|---|---|---|
| Single-finger/hand visible | 4.5 (1.4) | 3.5 (0.7) | 2.6 (0.8) | 0.83a |
| Single-finger/hand hidden | 7.9 (1.2) | 5.2 (0.6) | 4.0 (0.7) | 4.41b, * |
| Two-finger/hand hidden | 6.4 (1.6) | 6.0 (0.8) | 8.1 (1.0) | 1.49c |
Values are M (S.E.) CUD scores were computed by subtracting the number of errors in the uncrossed condition from those in the crossed condition.
d.f. = 2, 58
d.f. = 2, 59
d.f. = 2, 59
p <.05
CUD scores in the single-finger/hand visible condition were not related to total area or any of the sectional areas of the CC. In the single-finger/hand hidden condition, there was a negative relation between CUD scores and the area of the isthmus of the CC (rs = −.51; p = .03) and the area of the splenium (rs = −.45; p = .05). No significant correlations were detected for the other CC sections. In the two-finger condition, there was a positive relation between CUD scores and area of the rostrum that fell short of statistical significance (rs = .40; p = .08).
Study 2: Detroit Cohort
Methods
Participants
The Detroit cohort was recruited prospectively during pregnancy between September, 1986, and April, 1989, to assess the effects of moderate-to-heavy levels of prenatal alcohol exposure. All African American gravidas were screened for alcohol consumption during their first visit to a prenatal clinic in a large urban maternity hospital in Detroit (Jacobson et al., 2002). All women who averaged at least 7 drinks per week (0.5 oz absolute alcohol (AA) per day) at the time of conception and a random sample of 5% of the lower level drinkers and abstainers were invited to participate in the study. To reduce the risk that alcohol would be confounded with cocaine exposure, a group of heavy cocaine (≥2 days/week), light alcohol (<7 drinks/week) users were also recruited. Infant exclusionary criteria were birth weight <1500g, gestational age <32 weeks, major chromosomal anomalies or neural tube defects, and multiple births.
Maternal alcohol consumption during pregnancy was assessed using the timeline follow-back approach noted above (Sokol et al., 1985; Jacobson et al., 2002). At each prenatal clinic visit (M = 5.2, SD = 3.4), the mother was questioned about how much alcohol she had consumed during the past 2 weeks, with recall linked to certain times of day and activities. Volume was recorded for each type of alcoholic beverage consumed and converted to absolute alcohol (AA), as described above. These values were averaged across the visits to obtain a measure of ounces of absolute alcohol per day across pregnancy. Average alcohol consumed per occasion was measured by dividing total AA by number of drinking days during the pregnancy.
At the 7.5-year follow-up assessment, the children were examined for facial dysmorphology and growth by a trained research psychologist under the supervision of Erawati Bawle, MD, a pediatric geneticist and Director of the Genetics Department, Children’s Hospital of Michigan, who evaluated all suspect cases for FAS diagnosis (see Jacobson et al., 2004, for details). Three children were determined by Dr. Bawle to meet criteria for FAS, diagnoses that were subsequently confirmed independently in blind reviews of a large set of front and profile photographs of children in the cohort by Sterling Clarren, MD, and Kenneth L. Jones, MD, two expert FAS dysmorphologists. Two of the participants with FAS were assessed in Study 2.
This sample for Study 2 was comprised of the first 85 right-handed young adults evaluated in a 19-year follow up of this cohort, who were administered the FLT. Independent-samples t-tests were performed to compare prenatal exposures and demographics between these young adults and the remaining 395 study participants who were recruited prenatally. No differences were found in terms of maternal socioeconomic status (SES), education, or age at delivery, or prenatal alcohol and cigarette exposure (all p’s >.10).
Finger localization task and handedness
Participants were administered the finger localization task and the Annett Behavioral Handedness Index as described in Study 1.
Control variables
Eight control variables were assessed as potential confounders of the association between prenatal alcohol exposure and FLT performance in Study 2: age at testing; gender; SES; mother’s age at delivery; primary caregiver’s years of education; and maternal smoking (cigarettes/day) and cocaine or marijuana use during pregnancy (days/month). The adolescent WISC-III Full-Scale IQ assessed at 14 years was used to determine any mediating effects on FLT performance. For the seven young adults who had not participated in the 14-year follow-up, IQ, SES, and primary caregiver’s years of education were assessed with data from the 7.5-year follow-up visit.
Informed consent
Informed consent was obtained from each mother at recruitment and each follow-up visit and from the young adult at the laboratory visit in Detroit. Approval for human research was obtained from the Wayne State University Human Investigation Committee. Each participant received a gift and lunch, and the mother and young adult received a small remuneration for the visit.
Data Analysis
FLT performance was examined in relation to three continuous measures of prenatal alcohol exposure: oz of absolute alcohol (AA) consumed per day averaged across pregnancy, oz of AA consumed per drinking occasion (AA/occasion), and frequency of drinking (days/week). Multiple regression analyses were performed relating the CUD scores to the continuous alcohol exposure measures and the control variables that met criteria for potential confounders noted above.
Results
Sample Characteristics
Table 4 summarizes the sample characteristics for the Detroit cohort. Median SES was in the fourth level of the Hollingshead scale, the second lowest of five; only 47% of the primary caregivers had completed at least 12 years of education. Fifty-six percent of the mothers reported smoking during pregnancy, and 74% reported using alcohol during gestation. Twenty-eight percent of the mothers reported using marijuana during pregnancy with a mean frequency of 4 occasions per month, and 20% reported using cocaine at least once per week.
Table 4.
Sample characteristics of the Detroit cohort (N = 85).
| M or % | SD | |
|---|---|---|
| Maternal age at delivery | 25.6 | 5.8 |
| Socioeconomic status a | 28.9 | 10.2 |
| Maternal/caregiver education (years) | 12.7 | 1.9 |
| Prenatal cigarettes (cigarettes/day)c | 14.0 | 12.2 |
| Alcohol exposured | ||
| Absolute alcohol/day (oz) | 0.4 | 0.9 |
| Absolute alcohol/occasion (oz) | 2.0 | 3.3 |
| Frequency (days/week) | 1.3 | 1.4 |
| Age at testing | 19.5 | 0.4 |
| Gender (% male) | 61.2 | -- |
| IQ b | 78.4 | 14.4 |
Hollingshead Four Factor Index of Social Status Scale (1975).
14-year WISC-III Full-scale IQ.
Consumers only (N = 48).
Consumers only (N = 63).
Finger Localization Task
As in Study 1, a significant main effect of Condition was detected in all three conditions; in all cases, more errors were made when information had to cross the corpus callosum (Table 2). As also seen in Study 1, there was a hand-by-condition interaction in the two-finger/hand hidden condition (p <.01), and paired-samples t-tests were performed to examine this interaction. In the uncrossed condition, no differences in errors were detected for the left or right hand, whereas in the crossed condition more errors were made when the right hand was stimulated and the left hand had to respond (p <.01). In the single-finger, hand hidden condition, a main effect of hand and an interaction of hand-by-condition were detected, which displayed the opposite pattern from that detected in the two-finger condition. An analysis of this interaction revealed that in the uncrossed condition no differences in errors were detected for the left or right hand, whereas in the crossed condition, more errors were made when the left hand was stimulated and the right hand responded (p <.01). Following the procedure from Study 1, CUD scores were computed from the sums of errors from both hands.
Relation of FLT Performance to Prenatal Alcohol Exposure
Table 5 presents the results of the multiple regression analyses performed to assess the relation of prospectively collected prenatal alcohol exposure data to FLT performance in the Detroit cohort. After controlling for age, oz AA consumed per drinking occasion was related to CUD scores in both the single-finger/hand hidden and two-finger/hand hidden conditions. CUD scores were not related to amount of alcohol averaged across the pregnancy, nor were they related to the frequency with which the mother drank. The relation of oz AA per occasion to CUD scores in the single-finger/hand hidden and the two-finger/hand hidden conditions remained unchanged after IQ was entered into the analysis. Moreover, these effects showing the impact of concentrated drinking also persisted and were significant after omitting the two children who met criteria for full FAS (β = .30, p <.005, and β = .30, p <.005, for one- and two-fingers hidden, respectively), after control for confounders and IQ.
Table 5.
Relation of prenatal alcohol exposure to CUD scores in the Detroit cohort.
| Absolute alcohol/day |
Absolute alcohol/occasion |
Frequency |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| r | β1 | β2 | r | β1 | β2 | r | β1 | β2 | |
| Single-finger/hand visiblea | .05 | .05 | .04 | .15 | .15 | .14 | .04 | .04 | .05 |
| Single-finger/hand hiddenb | .05 | .09 | .08 | .26* | .32** | .32** | .04 | .01 | .01 |
| Two-finger/hand hiddenc | .18† | .16 | .16 | .31** | .26* | .27** | .03 | .08 | .08 |
β1 = Standardized regression coefficient after control for confounders
β2 = Standardized regression coefficient after control for confounders and IQ
Controlling for socioeconomic status
Controlling for age at testing
Controlling for age at testing and primary caregiver’s education
p <.10
p <.05
p <.01
The data linking FLT performance specifically to the amount of alcohol consumed during the days when the mother drank suggest that this impairment is found only when maternal alcohol intake exceeds a given threshold per occasion. To further explore the relation of FLT performance and pregnancy drinking pattern, the sample was divided into four exposure groups: more frequent bingers (4 or more drinks per occasion, ≥ 2 times/week); less frequent bingers (< 2 times/week); chronic non-bingers (at least 1 drink per day on average across pregnancy and no binge drinking); and abstainer/light drinkers. One-way ANOVAs were performed on CUD scores in the single-finger/hand hidden and two-finger/hand hidden conditions with exposure pattern as the between subjects factor. Significant main effects of exposure pattern were found in both conditions (Table 6). Post-hoc analyses revealed that in the single-finger/hand hidden condition, those exposed to more frequent binges performed worse than the non-exposed and the non-binge exposed (p’s <.01). The less frequent bingers made more errors than the non-binge exposed, however, this difference fell short of statistical significance (p = .10), possibly due to small sample size. No significant differences were detected between the other two groups. In the two-finger/hand hidden condition, those exposed to more frequent binges performed worse than the non-exposed (p <.05). No differences were detected between the more frequent and the less frequent binge exposed (p >.50). The less frequent binge exposed performed worse than both the non-binge exposed and non-exposed (p’s <.01). No significant differences were detected between the non-binge exposed and non-exposed in either of the two conditions (p >.50).
Table 6.
Effects of exposure pattern on CUD scores in the Detroit cohort
| Controls (N = 66) |
Non-binge (N = 6) |
Less Frequent Binges (N = 8) |
More Frequent Binges (N = 5) |
||
|---|---|---|---|---|---|
| M S.E. | M S.E. | M S.E. | M S.E. | F | |
| Single-finger/hand hidden | 1.8 (0.3) | 1.2 (1.1) | 3.4 (0.9) | 5.8 (1.2) | 4.49** |
| Two-finger/hand hidden | 3.3 (0.5) | 3.2 (1.7) | 9.6 (1.5) | 8.2 (1.8) | 7.55*** |
p <.01
p <.001
We also examined total AA consumed during pregnancy (in terms of oz AA/day) and found significant differences between the exposure groups (F(3, 81) = 20.44; p <.001). As expected, all three of the exposed groups consumed more alcohol than the non-exposed group. The more frequent binge exposed group consumed significantly more total alcohol across pregnancy (M = 1.8 oz AA/day) than the non-binge exposed (M =0.8 oz) (p <.05) and marginally more than the less frequent binge exposed group (M =1.2 oz) (p = .07). However, the less frequent binge exposed group did not differ from the non-binge exposed (p >.20) in total alcohol consumed. Despite the similarity in total alcohol consumption in these two groups, it is of interest that the young adult participants from the less frequent binge group performed more poorly on the FLT than those from the non-binge exposed group in the two finger/hand hidden condition and marginally more poorly in the single finger/hand hidden condition (Table 6).
Discussion
The first aim of this study was to test the hypothesis that performance on the FLT, a measure that assesses the efficiency of interhemispheric transfer, is impaired in children with very heavy prenatal alcohol exposure. In the elementary school-age Cape Town cohort, we found that performance on the FLT in the single-finger/hand hidden difficulty condition was related to FASD in that children with a diagnosis of FAS committed more transfer-related errors than both the HE and control groups. The lack of differences in the single-finger/hand visible condition is consistent with the interpretation that the observed deficit is related to difficulty in transferring tactile information across the corpus callosum. The failure to detect a deficit in the two-finger/hand hidden condition is likely due to the fact that the Cape Town children found this condition much more difficult than the older Detroit cohort, as indicated by the high rate of errors in the Cape Town non-exposed control group. The children in Roebuck et al. (2002), for whom this effect was also seen in the two-finger condition, were also generally older than the Cape Town cohort. We also found that higher CUD error scores from the single-finger/hand hidden condition were associated with smaller area of the isthmus and splenium of the CC.
Our anatomical data are also consistent with Roebuck et al. (2002), but because in that study the CC was divided into five equiangular regions in contrast to our anatomically defined CC sections, their CC area data are not directly comparable to ours. However, it should be noted that they found relations between FASD and task performance in both the anterior and posterior regions. These findings are consistent with our data indicating that task performance was related to the two most posterior regions — isthmus and splenium — in the single-finger/hand hidden condition although we did not detect a significant relation between task performance and the anterior CC regions, possibly due to our limited sample size.
Previous studies on FASD have documented reduced callosal size (Sowell et al., 2001), callosal shape abnormalities (Bookstein et al., 2001; 2002), and displacement of the CC (Sowell et al., 2001). Notably, the regions that appear to be most affected by prenatal alcohol exposure are the splenium, isthmus, and posterior midbody (Sowell et al., 2001). Our study found correlations of FLT performance in the single-finger/hand hidden condition with the size of two posterior regions — the isthmus and splenium — suggesting that prenatal alcohol-related reductions in the size of these regions may have a functional impact that can be detected by the FLT. The relation of FLT performance to these posterior CC areas is consistent with data from case studies indicating their importance for the transfer of tactile information. Secondary somatosensory cortex (SII) is activated bilaterally to unilateral somatic input, and it has been suggested that the CC allows for unilateral somatic input to reach the ipsilateral SII (Fabri et al., 2005). Thus, in studies of patients who underwent partial callosal resections for drug-resistant epilepsy, when the posterior portions of the CC were ablated, ipsilateral SII activation in response to tactile stimulation was abolished and performance accuracy in the crossed conditions of the FLT was dramatically reduced (Fabri et al., 1999; 2001; 2005).
In addition to macrostructural anomalies of the CC in FASD, studies using DTI have observed microstructural defects within this brain structure (Ma et al., 2005; Wozniak et al., 2006; Sowell et al., 2008; Lebel et al., 2008), although there are inconsistencies regarding which specific areas are impaired. Ma et al. (2005), who examined only the genu and splenium as regions of interest, found lower fractional anisotropy (FA) and an increase in the apparent diffusion coefficient (ADC) in both regions in the alcohol-exposed group, while a second study found an increase in mean diffusivity in the isthmus but not in the genu or splenium (Wozniak et al., 2006). Microstructural defects in the CC in alcohol-exposed children would be expected to contribute to the poorer performance on tasks that assess interhemispheric transfer that we have seen in the two studies reported here. Several studies have provided evidence that microscrutural CC defects measured by traditional DTI measures (FA and ADC) can impair the efficiency and speed of interhemispheric transfer (Muetzel et al., 2008; Johansen-Berg et al., 2007; Sullivan et al., 2001).
The second aim of this study was to test the hypothesis that the effects of prenatal alcohol exposure on efficiency of interhemispheric transfer are also seen in a sample of more moderately-exposed individuals. On average, non-abstaining mothers of the participants in the Detroit cohort drank approximately 7 standard drinks per week during pregnancy, as compared with the exposed children from the Cape Town cohort, who were exposed to an average of 34 standard drinks per week and as many as 12 drinks/occasion, which is more than three times as much as the Detroit mothers were drinking on average per occasion. The markedly lower levels of exposure in the Detroit cohort were not sufficient to produce the characteristic facial dysmorphology that is sometimes observed with heavy exposure except in the two children in this sample who met criteria for FAS, whose mothers drank 9.0 and 12.4 standard drinks/occasion, respectively. It is noteworthy, therefore, that these effects were observed even when these two children with FAS and exposure comparable to those seen in the Cape Town cohort were omitted from the analyses. When IQ was added to the model in Study 1, the effect of FASD diagnostic group on CUD scores fell short of statistical significance, due presumably to the strong correlation between exposure and IQ in children with full FAS. By contrast, at the lower levels of exposure in Detroit, where IQ was less severely affected (Jacobson et al., 2004), the effect of prenatal alcohol on CUD scores was unchanged when IQ was added to the regression, suggesting that interhemispheric transfer of tactile information is a specific target of fetal alcohol exposure rather than a consequence of poorer global intellectual function.
In the Detroit cohort, CUD errors in both the one- and two-finger/hand hidden conditions were associated specifically with a concentrated pattern of alcohol use, suggesting that FLT performance is more sensitive to heavy acute exposure than chronic low-level exposure. Furthermore, when the cohort was divided into groups based on pattern of exposure, FLT performance was affected only in those exposed to binge drinking, whether frequently or infrequently, and not in those exposed to chronic moderate levels (1 drink per day on average with no binges) whose total in utero exposure was similar to that of the infrequent binge exposed group. Moreover, animal studies have shown that the effects of prenatal alcohol exposure are markedly more pronounced in rat pups when exposed to high concentrations of alcohol at discrete times than when administered the equivalent or higher levels at a continuous lower level dose (Bonthius & West, 1990; Goodlett et al., 1987). Early binge-type exposure has also been linked to decreases in myelination (Harris et al., 2000) and white matter lesions (Watari et al., 2006) in animal studies.
It is noteworthy that, although we found a relation of poorer FLT performance to prenatal alcohol exposure in the moderately-exposed Detroit cohort, we did not detect a difference between the HE and control groups in the Cape Town cohort. One possible explanation is the age difference between the two cohorts. It is well documented that the CC maturational process extends well into adolescence and possibly into adulthood (Giedd et al., 1999; Pujols et al. 1993). Whereas the anterior regions of the CC show marked increases in area in children ages 3–6, the posterior regions continue their maturational process through adolescence (Giedd et al., 1999; Thompson et al., 2000). DTI data provide additional support for the later maturation of the posterior CC in that older males show higher fractional anisotropy in the splenium than younger males (Ashtari et al., 2007), and in a study of individuals ages 5–19 years of age, age-related decreases in the apparent diffusion coefficient were observed in the posterior regions of the CC but not the anterior most regions (Bonekamp et al., 2007). Thus, it is possible that less developed posterior regions of the CC in both the exposed and control children in the Cape Town cohort may obscure differences in interhemispheric transfer related to prenatal alcohol exposure that will become apparent once these areas have matured more fully. Our Detroit data suggest that, as individuals grow older and the CC matures, effects on interhemispheric transfer become evident even in relation to markedly lower levels of prenatal alcohol exposure than those found in our Cape Town cohort.
In conclusion, this study confirms previous findings that heavy prenatal alcohol exposure is related to poorer efficiency of interhemispheric transfer and that CC structure and function is particularly sensitive to insult from prenatal alcohol exposure. In addition, this is the first study to demonstrate effects on CC function in individuals with moderate levels of exposure. Given the greater sensitivity to prenatal alcohol related effects on interhemispheric transfer seen in the less exposed but older Detroit cohort, further research is warranted to examine the effects of prenatal alcohol on CC maturational process and the functions that this structure subsumes.
Figure 1.
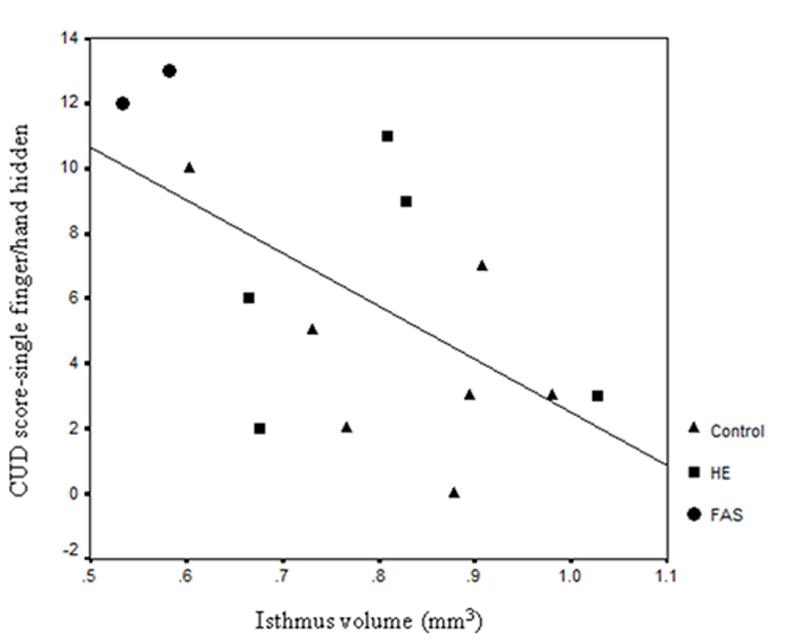
Scatterplot of isthmus area by CUD score in the single-finger/hand hidden condition (rs = −.51).
Acknowledgments
We thank the members of our University of Cape Town staff, Mariska Pienaar, Margaret September, Mandy Cronje, Jan Chamberlain, Lisa Aitken, Moira Raditz, Dickie Naude, and John Minnies for their help in collecting the Cape Town data. We thank Robert J. Sokol and Matthew J. Burden for their collaboration on the Detroit longitudinal study and our Wayne State University research staff, Renee Sun, Audrey Morrison, Julie Croxford, and Douglas Fuller, for their help in the Detroit data collection and analysis. We also thank Erawati Bawle, Sterling Clarren, and Kenneth L. Jones for their assistance in the diagnosis of FAS in the Detroit cohort. The Cape Town neuroimaging study reported here was funded by a NIH Fogarty International Research Collaboration Award from the National Institutes of Health (R03 TW007030), a Focus Area grant (FA2005040800024) from the National Research Foundation of South Africa, the South African Research Chairs Initiative of the Department of Science and Technology and National Research Foundation of South Africa, a Children’s Bridge grant from the Office of the President of Wayne State University, and a seed money grant from the University of Cape Town. The Cape Town dysmorphology assessments were conducted in conjunction with the National Institute on Alcohol Abuse and Alcoholism (NIAAA) Collaborative Initiative on Fetal Alcohol Spectrum Disorder (U01-AA014790 to S.W. Jacobson and U24AA014815 to K.L. Jones). The Detroit longitudinal study was funded by grants from NIAAA (R01-AA06966, R01-AA09524, and P50-AA0706) and the National Institute on Drug Abuse (R21-DA021034). Both the Detroit and Cape Town studies were supplemented by a grant from the Joseph Young, Sr., Fund from the State of Michigan.
References
- Ashtari M, Cervellione KL, Hasan KM, Wu J, McIlree C, Kester H, Ardekani BA, Roofeh D, Szezko PR, Kumra S. White matter development during late adolescence in healthy males: A cross-sectional diffusion tensor imaging study. Neuroimage. 2007;35:501–510. doi: 10.1016/j.neuroimage.2006.10.047. [DOI] [PubMed] [Google Scholar]
- Astley SJ, Clarren SK. Measuring the facial phenotype of individuals with prenatal alcohol exposure: correlations with brain dysfunction. Alcohol Alcohol. 2001;36:147–59. doi: 10.1093/alcalc/36.2.147. [DOI] [PubMed] [Google Scholar]
- Bonekamp D, Nagae LM, Degaonkar M, Matson M, Abdalla WMA, Barker PB, Mori S, Horska A. Diffusion tensor imaging in children and adolescents: Reproducibility, hemispheric, and age-related differences. Neuroimage. 2007;34:733–742. doi: 10.1016/j.neuroimage.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonthius DJ, West JR. Alcohol-induced neuronal loss in developing rats: Increased brain damage with binge exposure. Alcohol Clin Exp Res. 1990;14:107–118. doi: 10.1111/j.1530-0277.1990.tb00455.x. [DOI] [PubMed] [Google Scholar]
- Bookstein FL, Sampson PD, Streissguth AP, Connor PD. Geometric morphometrics of corpus callosum and subcortical structures in the fetal-alcohol-affected brain. Teratol. 2001;64:4–32. doi: 10.1002/tera.1044. [DOI] [PubMed] [Google Scholar]
- Bookstein FL, Streissguth AP, Sampson PD, Connor PD, Barr HM. Corpus callosum shape and neuropsychological deficits in adult males with heavy fetal alcohol exposure. Neuroimage. 2002;15:233–251. doi: 10.1006/nimg.2001.0977. [DOI] [PubMed] [Google Scholar]
- Bookstein FL, Connor PD, Huggins JE, Barr HM, Pimental KD, Streissguth AP. Many infants prenatally exposed to high levels of alcohol show one particular anomaly of the corpus callosum. Alcohol Clin Exp Res. 2007;31:868–879. doi: 10.1111/j.1530-0277.2007.00367.x. [DOI] [PubMed] [Google Scholar]
- Bowman RS, Stein LI, Newton JR. Measurement and interpretation of drinking behavior. Q J Stud Alcohol. 1975;36:1154–1172. doi: 10.15288/jsa.1975.36.1154. [DOI] [PubMed] [Google Scholar]
- Burden MJ, Jacobson SW, Jacobson JL. The relation of prenatal alcohol exposure to cognitive processing speed and efficiency in childhood. Alcohol Clin Exp Res. 2005a;29:1473–1483. doi: 10.1097/01.alc.0000175036.34076.a0. [DOI] [PubMed] [Google Scholar]
- Burden MJ, Jacobson SW, Sokol RJ, Jacobson JL. Effects of prenatal alcohol exposure on attention and working memory at 7.5 years of age. Alcohol Clin Exp Res. 2005b;29:443–452. doi: 10.1097/01.alc.0000156125.50577.ec. [DOI] [PubMed] [Google Scholar]
- Carter RC, Jacobson SW, Molteno CD, Chiodo LM, Viljoen D, Jacobson JL. Effects of prenatal alcohol exposure on infant visual acuity. J Pediatr. 2005;147:473–479. doi: 10.1016/j.jpeds.2005.04.063. [DOI] [PubMed] [Google Scholar]
- Clarren SK, Smith DW. The fetal alcohol syndrome. N Eng J Med. 1978;298:1063–1067. doi: 10.1056/NEJM197805112981906. [DOI] [PubMed] [Google Scholar]
- Croxford J, Viljoen D. Alcohol consumption by pregnant women in the Western Cape. South African Med J. 1999;89:962–965. [PubMed] [Google Scholar]
- Fabri M, Polonara G, Quattrini A, Salvolini U, Del Pesce M, Manzoni T. Role of the corpus callosum in the somatosensory activation of the ipsilateral cerebral cortex: An fMRI study of collosotomized patients. Eur J Neurosci. 1999;11:3983–3994. doi: 10.1046/j.1460-9568.1999.00829.x. [DOI] [PubMed] [Google Scholar]
- Fabri M, Polonara G, Del Pesce M, Quattrini A, Salvolini U, Manzoni T. Posterior corpus callosum and interhemispheric transfer of somatosensory information: An fMRI and neuropsychological study of a partially callosotomized patient. J Cog Neurosci. 2001;13:1071–1079. doi: 10.1162/089892901753294365. [DOI] [PubMed] [Google Scholar]
- Fabri M, Del Pesce M, Paggi A, Polonara G, Bartolini M, Salvotini U, Manzoni T. Contribution of posterior corpus callosum to the interhemispheric transfer of tactile information. Cog Brain Res. 2005;24:73–80. doi: 10.1016/j.cogbrainres.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Rajapakse JC, Vaituzis AC, Liu H, Berry YC, Tobin M, Nelson J, Castellanos FX. Development of the human corpus callosum during childhood and adolescence: A longitudinal MRI study. Prog Neuro-Psychopharmocol Biol Psychiatr. 1999;23:571–588. doi: 10.1016/s0278-5846(99)00017-2. [DOI] [PubMed] [Google Scholar]
- Good CD, Ashburner J, Frackowiak RS. Computational neuroanatomy: new perspectives for neuroradiology. Rev Neuro (Paris) 2001;157:797–806. [PubMed] [Google Scholar]
- Goodlett CR, Kelly SJ, West JR. Early postnatal alcohol exposure that produces high blood alcohol levels impairs development of spatial navigation learning. Psychobiol. 1987;15:64–74. [Google Scholar]
- Harris SJ, Wilce P, Bedi KS. Exposure of rats to a high but not low dose of ethanol during early postnatal life increases the rate of loss of optic nerve axons and decreases the rate of myelination. J Anat. 2000;197:477–485. doi: 10.1046/j.1469-7580.2000.19730477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead AB. Four factor index of social status. Yale University; 1975. Unpublished manuscript. [Google Scholar]
- Hoyme HE, May PA, Kalberg WO, Kodituwakku P, Gossage JP, Trujillo PM, Buckley DG, Miller JH, Aragon AS, Khaole N, Viljoen DL, Jones KL, Robinson LK. A practical clinical approach to diagnosis of fetal alcohol spectrum disorders: Clarification of the 1996 Institute of Medicine criteria. Pediatr. 2005;115:39–47. doi: 10.1542/peds.2004-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson JL, Jacobson SW, Molteno CD, Odendaal H. A prospective examination of the incidence of heavy drinking during pregnancy among Cape Coloured South African women. Alcohol Clin Exp Res. 2006;30:233A. [Google Scholar]
- Jacobson SW, Chiodo LM, Jacobson JL, Sokol RJ. Validity of maternal report of alcohol, cocaine, and smoking during pregnancy in relation to infant neurobehavioral outcome. Pediatr. 2002;109:815–825. doi: 10.1542/peds.109.5.815. [DOI] [PubMed] [Google Scholar]
- Jacobson SW, Jacobson JL, Sokol RJ, Chiodo LM, Corobana R. Maternal age, alcohol abuse history, and quality of parenting as moderators of the effects of prenatal alcohol exposure on 7.5-year intellectual function. Alcohol Clin Exp Res. 2004;28:1732–1745. doi: 10.1097/01.alc.0000145691.81233.fa. [DOI] [PubMed] [Google Scholar]
- Jacobson SW, Stanton ME, Molteno CD, Burden MJ, Fuller DS, Hoyme HE, Robinson LK, Khaole N, Jacobson JL. Impaired eyeblink conditioning in children with fetal alcohol syndrome. Alcohol Clin Exp Res. 2008;32:365–372. doi: 10.1111/j.1530-0277.2007.00585.x. [DOI] [PubMed] [Google Scholar]
- Jeret JS, Serur D, Wisniewski K, Fisch C. Frequency of agenesis of the corpus callosum in the developmentally disabled population as determined by computerized tomography. Pediatr Neurosci. 1985;12:101–103. doi: 10.1159/000120229. [DOI] [PubMed] [Google Scholar]
- Johansen-Berg H, Della-Maggiore V, Behrens TEJ, Smith SM, Paus T. Integrity of white matter in the corpus callosum correlates with bimanual coordination skills. Neuroimage. 2007;36:T16–T21. doi: 10.1016/j.neuroimage.2007.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KL, Smith DW. Recognition of the fetal alcohol syndrome in early infancy. Lancet. 1973;2:999–1001. doi: 10.1016/s0140-6736(73)91092-1. [DOI] [PubMed] [Google Scholar]
- Kinney H, Faix R, Brazy J. The fetal alcohol syndrome and neuroblastoma. Pediatr. 1980;66:130–132. [PubMed] [Google Scholar]
- Kleinbaum DG, Kupper LL, Muller KE. Applied regression analysis and other mutivariable methods. 2. PWS-Kent; Boston: 1988. [Google Scholar]
- Lebel C, Rasmussen C, Wyper K, Walker L, Andrew G, Yager J, Beaulieu C. Brain diffusion abnormalities in children with fetal alcohol spectrum disorder. Alcohol Clin Exp Res. 2008;32:1732–1740. doi: 10.1111/j.1530-0277.2008.00750.x. [DOI] [PubMed] [Google Scholar]
- Ma X, Coles CD, Lynch ME, LaConte SM, Zurkiya O, Wang D, Hu X. Evaluation of corpus callosum anisotropy in young adults with fetal alcohol syndrome according to diffusion tensor imaging. Alcohol Clin Exp Res. 2005;29:1214–1222. doi: 10.1097/01.alc.0000171934.22755.6d. [DOI] [PubMed] [Google Scholar]
- Magnotta VA, Harris G, Andreason NC, O’Leary DS, Yuh WT, Heckel D. Structural MR image processing using the BRAINS2 toolbox. Comput Med Imaging Graph. 2002;26:251–264. doi: 10.1016/s0895-6111(02)00011-3. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Riley EP, Gramling L, Delis DC, Jones KL. Heavy prenatal alcohol exposure with or without physical features of fetal alcohol syndrome leads to IQ deficits. J Pediatr. 1997;131:718–721. doi: 10.1016/s0022-3476(97)70099-4. [DOI] [PubMed] [Google Scholar]
- May PA. Fetal alcohol effects among North American Indians: evidence and implications for society. Alcohol Health Res World. 1991;15:239–247. [Google Scholar]
- May PA, Brooke L, Gossage JP, Croxford J, Adnams C, Jones KL, Robinson L, Viljoen D. Epidemiology of fetal alcohol syndrome in a South African community in the Western Cape Province. Am J Pub Health. 2000;90:1905–1912. doi: 10.2105/ajph.90.12.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muetzel RL, Collins PF, Mueller BA, Schissel AM, Lim KO, Luciana M. The development of corpus callosum microstructure and associations with bimanual task performance in healthy adolescents. Neuroimage. 2008;39:1918–1925. doi: 10.1016/j.neuroimage.2007.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol J, Vendrell P, Junque C, Marti-Vilalta JL, Capdevila A. When does human brain development end? Evidence of corpus callosum growth up to adulthood. Ann Neurol. 1993;34:71–75. doi: 10.1002/ana.410340113. [DOI] [PubMed] [Google Scholar]
- Riley EP, Mattson SN, Sowell ER, Jernigan TL, Sobel DF, Jones KL. Abnormalities of the corpus callosum in children prenatally exposed to alcohol. Alcohol Clin Exp Res. 1995;19:1198–1202. doi: 10.1111/j.1530-0277.1995.tb01600.x. [DOI] [PubMed] [Google Scholar]
- Roebuck TM, Mattson SN, Riley EP. Interhemispheric transfer in children with heavy prenatal alcohol exposure. Alcohol Clin Exp Res. 2002;26:1863–1871. doi: 10.1097/01.ALC.0000042219.73648.46. [DOI] [PubMed] [Google Scholar]
- Santhanam P, Hu X, Peltier SJ, Li Z, Lynch ME, Coles CD. Interhemispheric transmission in adults prenatally exposed to alcohol: An fMRI study. Alcohol Clin Exp Res. 2007;31:186A. [Google Scholar]
- Sattler JM. Assessement of Children. 3. Jerome M. Sattler, Inc; San Diego: 1992. [Google Scholar]
- Schlesselman J. Case-control studies: Design, conduct, analysis. Oxford University Press; New York: 1982. [Google Scholar]
- Sokol RJ, Martier S, Ernhart C. Identification of alcohol abuse in the prenatal clinic, in Early Identification of Alcohol Abuse. In: Chang NC, Chao HM, editors. Alcohol, Drug Abuse, and Mental Health Administration Research Monograph No. 17. Rockville, MD: 1985. [Google Scholar]
- Sowell ER, Mattson SN, Thompson PM, Jernigan TL, Riley EP, Toga AW. Mapping callosal morphology and cognitive correlates: Effects of heavy prenatal alcohol exposure. Neurol. 2001;57:235–244. doi: 10.1212/wnl.57.2.235. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Mattson SN, Kan E, Thompson PM, Riley EP, Toga AW. Abnormal cortical thickness and brain-behavior correlation patterns in individuals with heavy prenatal alcohol exposure. Cereb Cortex. 2008;18:136–144. doi: 10.1093/cercor/bhm039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratton K, Howe C, Battaglia F. Fetal alcohol syndrome: Diagnosis, epidemiology, prevention, and treatment. National Academy Press; Washington, DC: 1996. [Google Scholar]
- Streissguth AP, Barr HM, Sampson PD. Moderate prenatal alcohol exposure: Effects on child IQ and learning problems at age 7 1/2 years. Alcohol Clin Exp Res. 1990;14:662–669. doi: 10.1111/j.1530-0277.1990.tb01224.x. [DOI] [PubMed] [Google Scholar]
- Streissguth AP, Aase JM, Clarren SK, Randels SP, LaDue RA, Smith DF. Fetal Alcohol Syndrome in adolescents and adults. JAMA. 1991;265:1961–1967. [PubMed] [Google Scholar]
- Sullivan EV, Adalsteinsson E, Hedehus M, Ju C, Moseley M, Lim KO, Pfefferbaum A. Equivalent disruption of regional white matter microstructure in ageing healthy men and women. Neuroreport. 2001;12:99–104. doi: 10.1097/00001756-200101220-00027. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Giedd JN, Woods RP, MacDonald D, Evans AC, Toga AW. Growth patterns in the developing brain detected by using continuum mechanical tensor maps. Nature. 2000;404:190–193. doi: 10.1038/35004593. [DOI] [PubMed] [Google Scholar]
- Watari H, Born DE, Gleason CA. Effects of first trimester binge alcohol exposure on developing white matter in fetal sheep. Pediatr Res. 2006;59:560–564. doi: 10.1203/01.pdr.0000203102.01364.de. [DOI] [PubMed] [Google Scholar]
- Wisniewski K, Dambska M, Sher JH, Qazi Q. A clinical neuropathological study of the fetal alcohol syndrome. Neuropediatr. 1983;14:197–201. doi: 10.1055/s-2008-1059578. [DOI] [PubMed] [Google Scholar]
- Witelson SF. Hand and sex differences in the isthmus and genu of the human corpus callosum. Brain. 1989;112:799–835. doi: 10.1093/brain/112.3.799. [DOI] [PubMed] [Google Scholar]
- Wozniak JR, Mueller BA, Chang P, Muetzel RL, Caros L, Lim KO. Diffusion tensor imaging in children with fetal alcohol spectrum disorders. Alcohol Clin Exp Res. 2006;30:1799–1806. doi: 10.1111/j.1530-0277.2006.00213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


