Abstract
Background
The caudate nucleus might contribute to the psychopathological and cognitive deficits observed in schizotypal personality disorder (SPD), a schizophrenia spectrum disorder. Here we focused on female patients, because this group is underrepresented in studies of SPD and schizophrenia, and we might learn more about the caudate and clinical and cognitive impairments that are unique to female patients diagnosed with SPD.
Methods
Magnetic resonance imaging scans, obtained on a 1.5-T magnet with 1.5-mm contiguous slices, were used to measure the caudate in 32 neuroleptic-naïve women with SPD and in 29 female normal comparison subjects. Subjects were group-matched for age, parental socioeconomic status, and intelligence quotient.
Results
We found significantly reduced left and right caudate relative volume (8.3%, 7.7%) in female SPD subjects compared with normal comparison subjects. In female SPD subjects, we found significant correlations between smaller total caudate relative volume and worse performance on the Wisconsin Card Sorting test (nonperseverative errors) and on the California Verbal Learning Test (verbal memory and learning), and significant correlations between smaller total caudate relative volume and both positive and negative symptoms on the Structured Interview for Schizotypy.
Conclusions
These findings demonstrate that, for female SPD subjects, smaller caudate volume is associated with poorer cognitive performance and more schizotypal symptomatology.
Keywords: Schizotypal personality disorder, female, caudate nucleus, MRI, neuroleptic naïve, psychopathology
The basal ganglia modulate cerebral cortical functioning through basal ganglia–thalamo–cortical feedback loops (Joel and Weiner 2000). One such loop, for example, originates in the dorsolateral prefrontal cortex (DLPFC) and projects to the caudate nucleus (Alexander et al 1986; Cummings 1993). The caudate nucleus, in turn, sends projections to the globus pallidus and substantia nigra, pars reticulata, which then project, through the thalamus, back to the DLPFC (Alexander et al 1986, 1990). It has been proposed that the caudate nucleus, acting through such frontal–subcortical circuits, helps to modulate executive functioning, including working memory (Cummings 1993). Furthermore, the limbic circuit of the basal ganglia, originating in the anterior cingulate gyrus and orbitofrontal cortex, innervates the nucleus accumbens, part of the basal ganglia (Alexander et al 1990). It is through these latter projections that the caudate nucleus as we define it in this article, which includes part of the nucleus accumbens, might play an important role in the modulation of emotion. In addition, the caudate nucleus, through its connections with other cortical regions, including the temporal lobe, might help to mediate other memory and learning functions (Calabresi et al 1997).
Evidence supporting a role for the caudate nucleus in cognitive functioning is further provided by clinical case reports (Bokura and Robinson 1997; Kessels et al 1999; Pickett et al 1998). In addition, Richfield et al (1987) published a case report demonstrating that bilateral destruction of the caudate nucleus produced deficits in memory, learning, motivation, and behavior without producing any motor deficits. Furthermore, both functional and structural neuroimaging studies have implicated abnormalities in basal ganglia structures in disorders affecting cognition and emotion, such as schizophrenia or schizotypal personality disorder (SPD) (Buchsbaum et al 1992; Cohen et al 1997; Hokama et al 1995; Levitt et al 2002; Shihabuddin et al 2001).
We have focused on SPD, instead of schizophrenia, for our study of the caudate because individuals with SPD have less severe behavioral and neuropsychological symptoms than do those with schizophrenia (Voglmaier et al 2000) and hence do not require neuroleptic treatment, nor do they typically become hospitalized. Thus, by studying SPD, we avoid the twin confounds on the caudate of the potential effects of chronic hospitalization and of the use of neuroleptic medications (Chakos et al 1994; Gur et al 1998; Keshavan et al 1998). Furthermore, genetic studies support the idea that SPD is genetically related to schizophrenia (i.e., is in the schizophrenia spectrum) (Kendler et al 1993a; Siever et al 1990, 1993). Specifically, prior studies suggest a greater prevalence of SPD in the relatives of schizophrenic patients than in comparison groups (Kendler 1985; Kendler et al 1993b; Kety et al 1994). Furthermore, the deficit-like symptoms (negative or cognitive symptoms) (Torgersen et al 2002; Tsuang et al 1999) and positive symptoms (Fanous et al 2001) of SPD show a genetic relationship with schizophrenia. Subjects diagnosed with SPD also share a number of psychophysiological abnormalities observed in schizophrenia, including deficits in prepulse inhibition (Cadenhead et al 2000) and numerous cognitive deficits (Niznikiewicz et al 1999). Additionally structural and functional studies have suggested that temporal lobe (Dickey et al 2002, 2003), striatum and thalamus (Byne et al 2001; Shihabuddin et al 2001), and ventricle volume (Buchsbaum et al 1997) are abnormal in SPD, as well as in schizophrenia. Taken together, the genetic, cognitive, and brain morphological similarities reported between schizophrenia and SPD (Dickey et al 2002; Siever and Davis 2004) suggest that the pathology found in SPD has implications for understanding pathology in schizophrenia.
For the above reasons, we performed our initial study of the caudate nucleus in male SPD subjects, in which we found a reduction in its volume (Levitt et al 2002). Recent studies, however, have reported findings that the volume of the caudate nucleus in schizophrenia (Gunduz et al 2002) and in SPD subjects (Suzuki et al 2004) was not significantly different compared with normal control (NC) subjects, which contradicted our findings (Levitt et al 2002) and other prior studies (Corson et al 1999; Keshavan et al 1998). There are several possible reasons for this lack of agreement. First, caudate nucleus volume is affected by drugs, particularly neuroleptic medications, and thus medication status is an important variable that must be controlled (Chakos et al 1994; Keshavan et al 1994, 1998). Of note, however, even in studies with drug-naïve subjects, there are inconsistencies in the literature with regard to caudate nucleus volume in schizophrenia. For instance, there were no differences in caudate nucleus volume reported in neuroleptic-naïve, first-episode patients (Chakos et al 1994; Gunduz et al 2002; Gur et al 1998) compared with control subjects. Furthermore, the inconsistency in these studies might be due to the differences in their sampling, scanning, and measurement methods. For this reason, it is necessary to replicate the caudate nucleus volume study with a bigger sample size, under strict control of confounding factors. Second, gender is an additional variable that might affect both the size (Goldstein et al 2002) and function of the caudate nucleus. Thus, the mixture of male and female subjects in studies might influence results. More specifically, several groups have examined the influence of gender on caudate nucleus volume and function. For example, neuroimaging studies of the caudate nucleus have reported relatively larger caudate volume (Filipek et al 1994; Goldstein et al 2001; Murphy et al 1996) and higher caudate presynaptic dopamine synthesis capacity (Laakso et al 2002) in healthy women compared with healthy men. Moreover, Voglmaier et al (2005) reported that, compared with male SPD subjects, female SPD subjects showed less verbal learning deficits, which is in accord with findings in schizophrenia (Goldstein et al 1998). Studies of gender difference in schizophrenia also have suggested that female schizophrenic patients are prone to show more positive and fewer negative symptoms and a better response to neuroleptics (Goldstein and Levine 2000; Tamminga 1997), suggesting that gender might have an effect on cognitive and clinical symptoms (Davatzikos and Resnick 1998) in SPD or schizophrenia.
Generally, there has been less research on the caudate nucleus in female subjects with either schizophrenia or SPD. Prior studies have included female subjects but generally only in small samples. For example, in a study of SPD subjects, Suzuki et al (2004) found that the volume of the caudate nucleus in female SPD subjects was larger (although not significantly so) compared with female NC subjects. This study, however, had only small numbers of female subjects mixed together with the male subjects. Hence, on the basis of these prior studies, it remains unclear whether the size of the caudate nucleus of female subjects with either schizophrenia or SPD is different from that in normal female subjects.
In this study, we selected neuroleptic-naïve, female subjects alone, to control for the potentially confounding effects of medication and gender. Furthermore, we obtained neuropsychological and clinical data for our subjects, to test possible associations between caudate nucleus volume and psychopathology. To date, there has been little work on the relationship between the cognitive functions and volume of the caudate nucleus or subcortical structures in female SPD. For this reason, we tested the hypothesis that female SPD subjects, similar to their male SPD counterparts, would have smaller caudate nucleus volume. Previous research by our group had shown that the neuropsychological deficits in female SPD subjects exist, though they differ from those in male subjects. For example, female SPD subjects showed abnormal verbal learning on the California Verbal Learning Test, but these deficits were less severe than those found in male SPD subjects (Voglmaier et al 2005). Nonetheless, given that executive functioning can be adversely affected by caudate nucleus lesions, given the anatomical connections with the DLPFC (see above) and that such disturbances might impact on verbal learning, we thought it important to test whether neuroleptic-naïve female SPD subjects would similarly show abnormalities in the caudate nucleus, as we previously showed in male SPD subjects (Levitt et al 2002). Conversely, a prior study by Shihabuddin et al (2001) in both male and female SPD subjects found a positive association between caudate nucleus size and clinical symptoms. These results suggested to us that further research on the psychopathological implications of caudate nucleus volume, especially in female SPD subjects, was warranted. In the present study of neuroleptic-naïve female SPD, and on the basis of our prior findings of reduced caudate volume in male neuroleptic-naïve SPD subjects, which was associated with more perseverative errors (Levitt et al 2002), we formulated several hypotheses. First, we hypothesized that the caudate nucleus volume in neuroleptic-naïve female SPD subjects would be smaller than in female NC subjects. Second, we hypothesized that diminished caudate nucleus volume in female SPD would correlate with poorer neuropsychological functioning. Third, we hypothesized that worse clinical symptomatology in female SPD would parallel worse neuropsychological functioning and, in turn, would correlate with smaller caudate nucleus volume.
Methods and Materials
Subjects
Thirty-two neuroleptic-naïve female subjects diagnosed with SPD and 29 female NC subjects, right-handed, underwent magnetic resonance imaging (MRI) scanning. Both SPD and NC subjects were recruited from the community through advertisements. Subjects underwent a telephone screening, which reduced the total subjects from 911 to 294 women according to the following inclusion criteria: 1) age between 18 and 55 years; 2) English as the primary language; 3) no history of neurological disorder with loss of consciousness for >2 min; 4) no history of electroconvulsive therapy, no drug or alcohol dependence, and no abuse in the last year; and 5) no history of current or past use of neuroleptics ever and psychotropic medications in the past year (including serotonin reuptake inhibitors). The Structured Clinical Interview for DSM-IV–Patient Edition (First et al 1997) and its personality disorder version were used to make DSM-IV diagnoses and to exclude any Axis I psychotic or bipolar disorder from both groups and Axis I or II diagnoses from the NC subjects. Interviews were conducted by either a licensed psychiatrist (CCD) or a psychologist (MMV). Interrater reliability for the diagnosis of SPD has been high (κ = .89, n = 25). Of the 294 subjects, 32 met DSM-IV criteria for SPD and completed the neuropsychological and clinical evaluation. Subjects with SPD met criteria for other comorbid personality and Axis I disorders, including paranoid (n = 10), borderline (n = 9), and narcissistic personality disorder (n = 3), depression (n = 7), dysthymia (n = 2), panic disorder (n = 2), generalized anxiety disorder (n = 1), phobic disorder (n = 1), and alcohol or substance abuse (n = 2); all substance abuse occurred more than 2 years before testing.
Both groups were group-matched for demographic variables; however, the groups differed in self-assessed socioeconomic status (SES) (Table 1). After a full description of the study to the subjects, written informed consent was obtained. Handedness was evaluated with the Edinburgh inventory (Oldfield 1971). Socioeconomic status of SPD and NC subjects and their parents were evaluated by the Hollingshead two-factor index (Hollings-head 1965), whereby a higher score indicates lower SES.
Table 1.
Demographic Characteristics of Subjects with Schizotypal Personality Disorder and Normal Comparison Subjects
| Subjects with Schizotypal Personality Disorder (n = 32) |
Comparison Subjects (n = 29) |
Analysis by Student’s t Test (Two-Tailed) |
|||||
|---|---|---|---|---|---|---|---|
| Characteristic | Mean | SD | Mean | SD | t | df | p |
| Age (y) | 30.0 | 9.2 | 32.0 | 10.5 | −.79 | 59 | .43 |
| Education (y) | 15.6 | 2.1 | 16.5 | 1.7 | −1.82 | 59 | .07 |
| IQ | 115.8 | 10.5 | 118.5 | 9.8 | −1.03 | 59 | .31 |
| Socioeconomic Statusa | |||||||
| Parental | 4.1 | 1.0 | 4.4 | .7 | −1.61 | 59 | .11 |
| Self | 2.8 | 1.2 | 4.0 | 1.0 | −4.11 | 59 | <.01 |
| Edinburgh Handed Inventoryb | 63.7 | 23.5 | 72.8 | 21.9 | −1.48 | 53 | .15 |
| Race | |||||||
| African American | 3 | 2 | |||||
| White | 27 | 27 | |||||
| Asian | 2 | 0 | |||||
IQ, intelligence quotient.
Higher numbers represent higher socioeconomic status, based on the Hollingshead two-factor index of socioeconomic status.
Data were missing for three schizotypal personality disorder and three normal comparison subjects.
Neuropsychological and Clinical Measures
Subjects with SPD underwent a neuropsychological and psychopathological evaluation, performed as follows. We used two cognitive domains. One was executive function, tested by perseverative or nonperseverative errors. This function was measured with the Wisconsin Card Sorting Test (WCST) and the Delayed Alternation Test (Oscar-Berman 1991; Seidman et al 1995). Another domain we assessed was verbal learning capacity, measured by the California Verbal Learning Test (CVLT). The CVLT included trial 5 raw scores and total words learned (trials 1–5) raw scores for verbal learning and memory. Additionally, a modified Structured Interview for Schizotypy (SIS; Kendler et al 1989) was used for the assessment of subtle positive and negative symptoms in subjects with SPD. For clinical measurements of schizotypal symptoms, we used the SIS instead of the Scale for the Assessment of Negative Symptoms (Andreasen 1981) and Positive Symptoms (Andreasen 1984) because we assumed that the SIS was more appropriate for measuring subtle symptoms of SPD. The use of the SIS interview began in the middle of our project, and for this reason only 16 of the total 32 subjects with SPD completed the SIS. For the SIS, symptoms such as the magical thinking, ideas of references, illusions, suspiciousness, and psychotic-like symptoms constitute the positive symptoms because of their phenomenological similarities with schizophrenia. On the other hand, symptoms of restricted emotion, social isolation, introversion, and sensitivity to rejection were characterized as negative symptoms or deficit-related symptoms.
MRI Image Acquisition and Postprocessing
Images were acquired from a 1.5-T General Electric scanner (GE Medical Systems, Milwaukee, Wisconsin). The MRI methodology used in this study has been previously described (Dickey et al 2000). Briefly, a 1.5-mm-thick coronal series of contiguous spoiled-gradient recalled acquisition (SPGR) images (repetition time, 35 msec; echo time, 5 msec; voxel dimensions, .9375 × .9375 × 1.5 mm) was used for delineating and measuring caudate nucleus. Then an axial series of contiguous double-echo (proton density and T2-weighted) images (repetition time, 3000 msec; echo time, 30 and 80 msec; voxel dimensions, .9375 × .9375 × 3.0 mm) was acquired. An anisotropic diffusion filter was applied to the images to reduce noise before processing each set of scans. The intensity information from both the SPGR and T2 images was then used in a fully automated segmentation program to evaluate total intracranial content (ICC).
Caudate Nucleus Regions of Interest: Anatomical Landmarks
The caudate nucleus regions of interest (ROIs) were manually outlined on the SPGR without knowledge of diagnosis and other demographic features of the subjects, with a software package for three-dimensional medical image analysis (3D Slicer; Pieper et al 2004) on a work station. The head, body, and tail portions were included up to the point where the tail curved ventrally to border the lateral aspect of the atrium of the lateral ventricles. The anterior coursing of the tail was excluded because it was indistinct as a result of partial voluming (Figure 1). Because of difficulty separating the nucleus accumbens (ventral striatum) from the merged caudate and putamen nuclei anteriorly, a vertical line was drawn from the most ventral point of the internal capsule inferiorly to the external capsule and was considered to be the lateral bound of the caudate nucleus (Hokama et al 1995). We also parcellated the caudate nucleus into its head (anterior) and body (posterior) portions for separate analysis, because the head portion of the caudate nucleus is located most anteriorly, hence it receives its major innervation from the more anterior part of the cortex, namely, dorsolateral, orbitofrontal, and limbic area of cortex (Alexander et al 1986). We used the interventricular foramen of Monroe, bilaterally, as the anatomical landmark for this division (Levitt et al 2002). This foramen was defined, bilaterally, by the most posterior coronal slice where any part of the anterior column of the fornix could still be visualized. Our posterior boundary, for this parcellation, was the coronal slice before the atrium.
Figure 1.
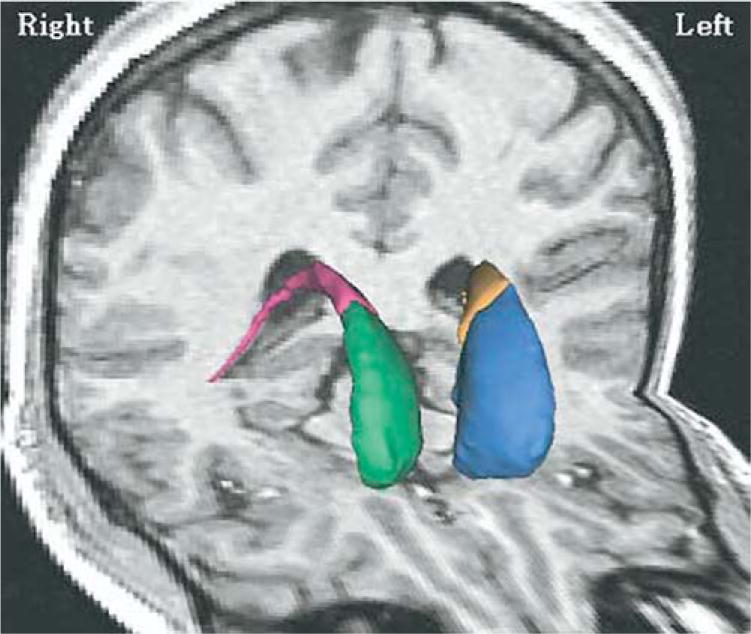
Three-dimensional renderings of left and right anterior (head) and posterior portions of the caudate nucleus superimposed on a magnetic resonance imaging coronal and axial slice of a normal comparison subject. The left and right anterior portion (head) of the caudate nuclei are color-coded in blue and green, and the left and right posterior caudate nuclei are color-coded in yellow and red.
Interrater reliability (based on intraclass correlation coefficients) among three raters (MK, KRL, PZ) for whole left (r = .95), right (r = .94), and total caudate volume (r = .97) were high; interrater reliabilities were also high for left (r =.91) and right (r =.87) anterior and left (r =.97) and right (r =.91) posterior caudate nucleus ROIs. Interrater reliabilities were computed by three raters on the brain scans of five randomly selected subjects from the pool of subjects. All ROI measurements were performed by one researcher (MK).
Statistical Analyses
Relative brain volumes were used for statistical analyses of MRI structural measures to correct for variation in head size. Relative volumes were obtained by dividing absolute volumes by total ICC and multiplying by 100. The effects of laterality (left vs. right caudate) alone on the caudate nucleus, and then laterality (left vs. right) plus portion (anterior vs. posterior caudate) on the caudate nucleus were examined by repeated-measures analysis of covariance (ANCOVA), with group (subjects with SPD vs. NC subjects) as the between-subject factor, laterality (left vs. right) or laterality plus portion (anterior vs. posterior) as the within-subject factors, and age or age and SES as covariates. If a main effect for group was found, then follow-up planned Student’s t tests, with significance set at p < .05 (two-tailed), were used to test the mean difference between groups in relative volumes of each caudate ROI. We also performed similar analyses of absolute brain volumes, covarying for ICC and age or ICC, age, and SES.
To control for the effect of age, a partial correlation analysis was used, with two-tailed p values, for all within-group correlations between total caudate relative volume and measures of neuropsychological and clinical scores.
Results
Caudate Nucleus Volume Measures
As shown in Table 2, repeated-measures ANCOVA of caudate nucleus relative volumes revealed a significant difference, showing a main effect of group (SPD vs. NC subjects) [F(1,56) = 13.5, p = .001], with no effect of laterality (left vs. right side) [F(1,56) = .7, p = .4]. There was no interaction between group and laterality [F(1,56) = 1.9, p = .17], laterality and age [F(1,56) = .7, p = .4], or laterality and SES [F(1,56) = 1.8, p = .17]. We also performed repeated-measures ANCOVA of absolute volumes with the two covariates age and ICC or with the three covariates age, SES, and ICC, which parallels the results with relative volumes. We also performed a repeated-measures analysis with a mixed-models approach for confirmatory purposes, which did not alter the above results. An additional analysis without age as a covariate was also carried out, which parallels the results from repeated measures ANCOVA with covariates, showing a main effect of group [F (1,59) = 12.1, p = .001], with no interaction between group and laterality [F (1,59) = .5, p = .48].
Table 2.
Absolute and Relative Volume of Caudate Nuclei Regions of Interest in Subjects with Schizotypal Personality Disorder and Normal Comparison Subjects
| Subjects with Schizotypal Personality Disorder (n = 32) |
Comparison Subjects (n = 29) |
Analysis with Student’s t Test (Two-Tailed) |
|||||
|---|---|---|---|---|---|---|---|
| Measure | Mean | SD | Mean | SD | t | df | p |
| Total Intracranial Volume (mL) | 1360.90 | 91.99 | 1365.49 | 100.41 | −.19 | 59 | .85 |
| Right Caudate Nucleus | |||||||
| Absolute volume (mL) | 4.41a | .36 | 4.79 | .48 | −3.47 | 59 | <.01 |
| Relative volume (%) | .325b | .024 | .352 | .039 | −3.23 | 45.9 | .01 |
| Left Caudate Nucleus | |||||||
| Absolute volume (mL) | 4.35a | .38 | 4.75 | .49 | −3.65 | 59 | <.01 |
| Relative volume (%) | .320b | .025 | .349 | .039 | −3.43 | 46.6 | <.01 |
| Right Anterior Caudate Nucleus | |||||||
| Absolute volume (mL) | 3.63c | .27 | 3.91 | .45 | −2.83 | 44.6 | <.01 |
| Relative volume (%) | .267d | .018 | .287 | .036 | −2.66 | 40.2 | .01 |
| Left Anterior Caudate Nucleus | |||||||
| Absolute volume (mL) | 3.56c | .30 | 3.86 | .44 | −3.04 | 48.4 | <.01 |
| Relative volume (%) | .262d | .018 | .284 | .036 | −2.91 | 39.9 | <.01 |
| Right Posterior Caudate Nucleus | |||||||
| Absolute volume (mL) | .78c | .18 | .89 | .15 | −2.42 | 59 | .02 |
| Relative volume (%) | .058d | .013 | .065 | .011 | −2.36 | 59 | .02 |
| Left Posterior Caudate Nucleus | |||||||
| Absolute volume (mL) | .79c | .17 | .89 | .15 | −2.59 | 59 | .01 |
| Relative volume (%) | .058d | .013 | .062 | .012 | −2.39 | 59 | .02 |
Repeated-measures analysis of covariance (ANCOVA) of absolute volume with age, total intracranial volume, and socioeconomic status score as covariates and group (subjects with schizotypal personality disorder vs. comparison subjects) as the between-subjects factor, and laterality (left vs. right) as the within-subjects factor revealed a main effect for group [F(1,56) = 12.6, p < .01].
Repeated-measures ANCOVA of relative volume with group as the between-subjects factor, laterality as the within-subjects factor, and age and socioeconomic status score as covariates revealed a main effect for group [F(1, 56) = 13.5, p < .01].
Repeated-measures ANCOVA of absolute volume with age, total intracranial volume, and socioeconomic status score as covariates, group as the between-subjects factor, and laterality and portion (anterior versus posterior) as the within-subjects factor revealed a main effect for group [F(1, 56) = 12.6, p < .01].
Repeated-measures ANCOVA of relative volume with group as the between-subjects factor, laterality and portion as the within-subjects factor, and age and socioeconomic status score as covariates revealed a main effect for group [F(1, 56) = 13.5, p < .01].
Follow-up planned contrasts applying Student’s t tests showed that left [t (46.6) = −3.43, p = .001] and right [t (45.9) = −3.23, p = .011] caudate nucleus relative volumes were significantly smaller in SPD than in NC subjects; however, there was no significant difference [t(59) = .79, p = .43] in asymmetry [(right − left relative volumes) × 100/(right + left relative volumes)] between SPD and NC subjects. Repeating the same analysis for absolute volume yielded similar results for absolute volumes (Table 2 and Figure 2).
Figure 2.
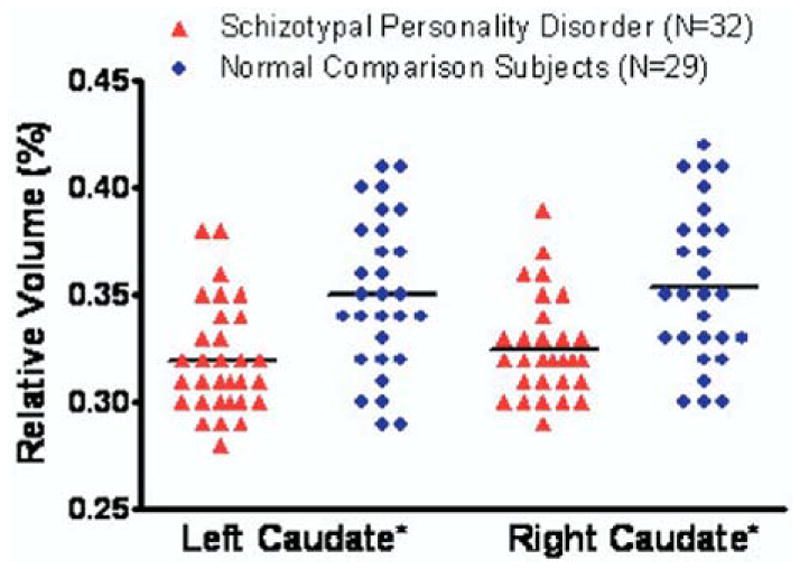
Scatter plots of left and right relative caudate nucleus volumes in female schizotypal personality disorder and normal comparison subjects. *Follow-up planned contrasts applying Student’s t tests showed that left [t(46.6) = −3.43, p < .01] and right [t(45.9) = −3.23, p = .01] caudate relative volumes were significantly smaller in subjects with schizotypal personality disorder than in normal comparison subjects. Horizontal lines represent means.
The Shapiro and Wilk tests showed normal distributions for the volumes of both SPD and NC subjects: left [W(df = 32) = .95, p = .35] and right [W(32) = .97, p = .45] caudate of SPDs; left [W(29) = .97, p = .53] and right [W(29) = .98, p = .77] caudate of NC subjects. In percentage terms, we found that the left and right caudate nucleus relative volumes in the subjects with SPD were, respectively, 8.3% and 7.7% smaller than in NC subjects.
We performed an analysis of relative volumes of the caudate nucleus, parcellated into anterior and posterior portions. Repeated-measures ANCOVA revealed significant differences for group [F(1,56) = 13.5, p = .001], with no effect of laterality [F(1,56) = .5, p = .48]. There were no statistically significant interactions between group and laterality [F (1,56) = 1.5, p = .23], group and portion (anterior or posterior portions) [F(1,56) = 3.2, p = .07], or portion and laterality [F(1,56) = .2, p = .68]. Follow-up Student’s t tests yielded significant differences for left [t (39.9) = −2.91, p = .005] and right [t (40.2) = −2.66, p = .01] anterior caudate relative volumes and also for left [t (59) = −2.39, p = .02] and right [t (59) = −2.36, p = .02] posterior caudate relative volumes (Table 2). The results of the same statistical analyses performed on absolute volumes in parcellated caudate portions of interest and covarying for intracranial contents fully paralleled those for relative volumes. There were no significant group differences between subjects with SPD and NC subjects in total intracranial volumes [t (59) = −.19, p = .85].
Of note, we did not find any difference in caudate volume between SPD subjects without depression and those who were comorbid for depression [t (30) = .02, p = .41].
Correlations Between Caudate Nucleus Volumes and Neuropsychological Measures in Subjects with Schizotypal Personality Disorder
We found significant correlations between caudate nucleus volumes and cognitive measures of abstract concept formation and verbal memory (learning) capacity, which showed that smaller caudate volumes were associated with worse cognitive performance in SPD subjects (Table 3). Specifically, we found negative correlations between nonperseverative errors [r(28) = − .50, p = .005] on WCST and total caudate relative volumes. Moreover, we found positive correlations between list A trial 5 raw scores in the CVLT and total caudate relative volumes [r(28) = .38, p = .04] and trend-level correlations between list A total words learned (trials 1–5) raw scores and total caudate relative volumes [r(28) = .37, p = .051]. In contrast, there were no significant correlations in the control group. Furthermore, we compared the difference of correlation coefficients between groups using Fisher’s z transformation. The results showed that the WCST nonperseveration error (p = .01) and CVLT 1–5 raw scores (p = .05) showed significant differences between groups (Table 3).
Table 3.
Partial Correlations Between Caudate Nucleus Relative Volumes and Scores for Neuropsychological Test
| Subjects with Schizotypal Personality Disorder (df = 28) |
Comparison Subjects (df = 26) |
Comparison Between Groupsa |
||||
|---|---|---|---|---|---|---|
| Measure | Correlation Coefficientsb | p | Correlation Coefficientsb | p | z | p |
| WCST | ||||||
| Perseverative errors | −.26 | .173 | .04 | .840 | −1.1 | .27 |
| Nonperseverative errors | −.50 | .005 | .11 | .588 | −2.38 | .01 |
| CVLT | ||||||
| Trial 5 raw | .38 | .040 | .21 | .289 | .67 | .50 |
| Trial 1–5 raw | .37 | .051 | −.05 | .819 | 1.62 | .05 |
| DAT | ||||||
| Perseveration errors | −.26 | .174 | −.32 | .133 | .24 | .81 |
WCST, Wisconsin Card Sorting Test; CVLT, California Verbal Learning Test; DAT, Delayed Alternation Test.
Comparison between correlation coefficients of two groups using Fisher’s z-transformation.
Age is partialled out in the partial correlation analysis (two-tailed).
Correlations Between Caudate Nucleus Volumes and Measures of Psychopathology in Subjects with Schizotypal Personality Disorder
For clinical measures, we found significant inverse correlations between the caudate nucleus relative volumes and clinical symptoms, which revealed that smaller caudate volumes were associated with worse symptoms (Table 4). Specifically, we found negative correlations between total caudate relative volumes with SIS positive symptom scores of illusion (r = −.64, n = 15, p = .005) and psychotic-like symptoms (r = −.70, n = 15, p = .002). In addition, we found a negative correlation between total caudate relative volumes and the negative symptom of sensitivity (to criticism) on the SIS (r = −.62, n = 15, p = .007).
Table 4.
Partial Correlations Between Caudate Nucleus Relative Volumes and Scores for Structured Interview for Schizotypy of Subjects with Schizotypal Personality Disorder
| SIS Measure | Correlation Coefficients (p Value)a |
|---|---|
| Positive Symptoms | |
| Idea of reference | −.26 (.175) |
| Suspiciousness | −.43 (.055) |
| Magical thinking | −.36 (.092) |
| Illusion | −.64 (.005) |
| Psychotic-like symptoms | −.70 (.002) |
| Negative Symptoms | |
| Sensitivity | −.62 (.007) |
| Restricted emotion | −.01 (.485) |
| Social isolation | −.35 (.103) |
| Introversion | −.23 (.204) |
SIS, Structured Interview for Schizotypy.
Age is partialled out in the partial correlation analysis (df = 13, two-tailed).
Discussion
There were three major findings. First, we found that left and right caudate nucleus relative volumes in female subjects with SPD were significantly smaller than those of NC subjects by 8.3% and 7.7%, respectively. These data are consistent with findings of reduced caudate nucleus volume reported in our prior study of male SPD (Levitt et al 2002) and in studies of neuroleptic-naïve patients with schizophrenia (Corson et al 1999; Keshavan et al 1998). Second, in our female SPD subjects, we found that smaller total relative caudate volume correlated with more nonperseverative errors on the WCST and poorer scores on the CVLT. Third, we found that smaller relative caudate volume correlated with more severe clinical symptoms. Of particular note, and as other data suggest, the absence of prefrontal cortex volume reduction in SPD (Buchsbaum et al 2002; Siever et al 2002), as well as neuropsychological and clinical data indicating prefrontal cortex-like abnormalities in SPD, suggest that the abnormalities might arise elsewhere in the fronto–subcortico–thalamo circuitry. Our findings of decreased MRI volumes in both female and male (Levitt et al 2002) subjects with SPD point to the caudate nucleus as a likely potential source.
Our findings are especially noteworthy because, to our knowledge, this is the largest study sample of the caudate nucleus in female SPD and the first such findings in a neuroleptic-naïve group of female subjects with a disorder that is considered to be in the schizophrenia spectrum. Furthermore, studies of SPD in female subjects, alone, help to elucidate specific associations between caudate nucleus volume and psychopathology in women, which might or might not resemble the specific associations found in male subjects. Of further note, as previously reviewed in the Introduction, some studies of neuroleptic-naïve patients diagnosed with schizophrenia have not shown significant caudate volume reduction (Chakos et al 1994; Gunduz et al 2002; Gur et al 1998). Of these studies, Gunduz et al (2002) reported larger caudate nucleus volume in patients diagnosed with schizophrenia compared with control subjects, although this was not statistically significant.
With regard to our specific neuropsychological findings, in our female SPD sample, caudate nucleus volume was associated with concept formation errors, that is, nonperseverative errors, in the WCST (Spreen and Strauss 1998), whereas in our prior male sample (Levitt et al 2002), we found correlations between caudate nucleus volume and perseverative errors on a delayed alternation task and on a verbal working memory task. Hence, in both male and female samples we found that smaller caudate nucleus volume was associated with worse executive functioning, yet the specific cognitive impairments were not identical. Moreover, Gabrieli (1998) has reviewed findings indicating that patients with basal ganglia lesions, as well as those with prefrontal cortical lesions, have reduced working memory capacity and that this reduced working memory capacity limits reasoning ability. These findings are consistent with our finding, which shows poorer abstract concept formation performance on the WCST in female SPD subjects as their caudate nucleus volume decreases, reflecting that WCST performance is closely related to working memory ability (Fristoe et al 1997; Spreen and Strauss 1998). This association might be explained by pathology in the caudate nucleus that impairs working memory and, in turn, is expressed, especially in female subjects, as poorer performance in abstract reasoning. As we mentioned earlier, through its anatomic connections to the prefrontal cortex, the caudate nucleus might also help to modulate executive functioning, including working memory, through basal ganglia–thalamo–cortical feedback loops, such as the loop originating in the DLPFC (Alexander et al 1986; Cummings 1993).
With respect to performance on the CVLT in female SPD subjects, we found that reduced verbal learning performance was associated with reduced caudate nucleus volume. Of note, however, the CVLT total trial 1–5 raw scores correlation with total caudate nucleus volume was only a trend-level finding, whereas the trial 5 raw scores, which significantly correlated with total caudate volume, are a post hoc finding and thus should be interpreted cautiously. As a possible explanation for these findings, the caudate nucleus might mediate memory and learning processes through its anatomic connections with the temporal lobe (Calabresi et al 1997; Sakai and Passingham 2004). Taken together, these correlations suggest that in female SPD subjects the caudate nucleus might be involved with both concept formation and with verbal learning, two of the key deficits in this population (Voglmaier et al 1997, 2000).
Of further interest are the number of correlations we found between clinical symptoms from the SIS, a useful measure for the type of subtle symptoms characteristic of SPD, and caudate nucleus relative volumes. Other investigators have also reported interesting clinical correlates of caudate nucleus MR volumes. For example, Scheepers et al (2001) found correlations between an increase in caudate volume, over time, and clinical symptom (negative and general symptoms) improvement in schizophrenic patients taking clozapine. In contrast to these findings in which larger volumes predict higher functioning, Shihabuddin et al (2001) reported that DSM-III psychotic-like symptoms in SPD and caudate nucleus volume were positively correlated, suggesting that higher volume predicted worse symptoms. The findings in these two studies, however, were based primarily on male subjects, and the methods they used for measuring caudate nucleus volume, as well as the demographic characteristics of their samples, differed from ours. Furthermore, both of these studies used different clinical measures than we did to assess positive and negative symptoms. We used the SIS, which, we believe, is particularly well suited for measuring the type of subtle psychotic symptoms found in SPD.
The association between negative and positive symptoms and caudate nucleus volumes that we found might be explained in the following manner. Because the limbic loop of the fronto–striato–thalamic circuitry originates, in part, from the anterior cingulate gyrus and sends projections to the caudate nucleus (Alexander et al 1986, 1990), we believe that caudate abnormalities might indirectly impact on the functioning of the anterior cingulate gyrus, thereby resulting in negative symptoms. Neurological lesions in the anterior cingulate, for example, can lead to the syndrome of akinetic mutism (Cummings 1993). Similarly, as regards the inverse correlations we found between positive symptoms and caudate nucleus volume, because of the extensive connections between the DLPFC and the caudate nucleus, abnormal DLPFC functioning, secondary to pathology in the caudate nucleus, might result. We hypothesized that such abnormal DLPFC function, with consequent abnormal executive functioning, could result in both cognitive (Delazer et al 2004) and positive symptoms (Park and McTigue 1997; Sabri et al 1997).
A key strength of our study is that we eliminated the possible confound of neuroleptic treatment, which affects the size of the basal ganglia. Prior MRI studies of the striatum have shown that first-episode schizophrenic and schizoaffective subjects showed enlargement of caudate nucleus volume while taking conventional neuroleptics (Chakos et al 1994; Keshavan et al 1998) or decreases in caudate nucleus volume when typical neuroleptics were replaced by an atypical antipsychotic, clozapine (Gur et al 1998). A potential shortcoming of our study is that we obtained subjects from the community, which might yield less severely affected subjects than if subjects were chosen from clinic groups. This approach, however, has the important advantage of finding subjects not yet exposed to neuroleptics or other psychotropic medications. Another possible limitation of our study is that we did not eliminate the potential effect of having comorbid depression in seven of our SPD subjects. Of note here, prior work (Parashos et al 1998) has demonstrated decreased caudate nucleus volume in patients with depression. We, however, did evaluate SPD subjects with and without depression, and we did not find a statistically significant difference in caudate volume between these two groups. In addition, we did not apply corrections for multiple testing of correlation analyses, which is a potential limitation in our findings, although we performed a Fisher’s z-transformation analysis for comparisons between correlations found in SPD and normal control groups, showing group differences in the WCST nonperseveration error and CVLT 1–5 raw scores, as we mentioned earlier in Table 3.
In summary, we found reduced caudate nucleus volume in female SPD that significantly correlated with worse cognitive performance (e.g., more nonperseverative errors and worse verbal learning scores) and more severe clinical symptoms (e.g., illusion, psychotic-like symptoms, and sensitivity). These findings, taken together with our prior findings in male SPD (Levitt et al 2002), suggest that the caudate nucleus is potentially intrinsically abnormal in schizophrenia spectrum disorders. Moreover, our findings suggest that the caudate nucleus, through its effect on cortical–subcortical circuitry, might play a key role in affecting the clinical and cognitive symptoms that are observed in both male and female SPD subjects.
Acknowledgments
This study was supported by the Department of Veterans Affairs’ Merit Awards (MES, RWM), a Research Enhancement Award Program (RWM, MES), and an Advanced Career Development Award (CCD), and by grants from the National Institute of Mental Health (K05 MH 070047 and R01 MH 50740 to MES, R01 MH 063360 to MN, and R01 MH 40799 and R01 MH 052807 to RWM).
Some of these data were presented at the 34th Annual Meeting of the Society for Neuroscience, San Diego, California, October 26th, 2004.
We thank Marie Fairbanks for administrative support; and Noriomi Kuroki, M.D., for helpful suggestions.
References
- Alexander GE, Crutcher MD, DeLong MR. Basal ganglia-thalamocortical circuits: Parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. Prog Brain Res. 1990;85:119 –146. [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Andreasen NC. Scale for the Assessment of Negative Symptoms (SANS) Iowa City: University of Iowa; 1981. [Google Scholar]
- Andreasen NC. Scale for the Assessment of Positive Symptoms (SAPS) Iowa City: University of Iowa; 1984. [Google Scholar]
- Bokura H, Robinson RG. Long-term cognitive impairment associated with caudate stroke. Stroke. 1997;28:970 –975. doi: 10.1161/01.str.28.5.970. [DOI] [PubMed] [Google Scholar]
- Buchsbaum MS, Haier RJ, Potkin SG, Nuechterlein K, Bracha HS, Katz M, et al. Frontostriatal disorder of cerebral metabolism in never-medicated schizophrenics. Arch Gen Psychiatry. 1992;49:935–942. doi: 10.1001/archpsyc.1992.01820120023005. [DOI] [PubMed] [Google Scholar]
- Buchsbaum MS, Shihabuddin L, Hazlett EA, Schroder J, Haznedar MM, Powchik P, et al. Kraepelinian and non-Kraepelinian schizophrenia subgroup differences in cerebral metabolic rate. Schizophr Res. 2002;55:25–40. doi: 10.1016/s0920-9964(01)00206-7. [DOI] [PubMed] [Google Scholar]
- Buchsbaum MS, Yang S, Hazlett E, Siegel BV, Jr, Germans M, Haznedar M, et al. Ventricular volume and asymmetry in schizotypal personality disorder and schizophrenia assessed with magnetic resonance imaging. Schizophr Res. 1997;27:45–53. doi: 10.1016/S0920-9964(97)00087-X. [DOI] [PubMed] [Google Scholar]
- Byne W, Buchsbaum MS, Kemether E, Hazlett EA, Shinwari A, Mitropoulou V, et al. Magnetic resonance imaging of the thalamic mediodorsal nucleus and pulvinar in schizophrenia and schizotypal personality disorder. Arch Gen Psychiatry. 2001;58:133–140. doi: 10.1001/archpsyc.58.2.133. [DOI] [PubMed] [Google Scholar]
- Cadenhead KS, Swerdlow NR, Shafer KM, Diaz M, Braff DL. Modulation of the startle response and startle laterality in relatives of schizophrenic patients and in subjects with schizotypal personality disorder: evidence of inhibitory deficits. Am J Psychiatry. 2000;157:1660 –1668. doi: 10.1176/appi.ajp.157.10.1660. [DOI] [PubMed] [Google Scholar]
- Calabresi P, De Murtas M, Bernardi G. The neostriatum beyond the motor function: Experimental and clinical evidence. Neuroscience. 1997;78:39–60. doi: 10.1016/s0306-4522(96)00556-8. [DOI] [PubMed] [Google Scholar]
- Chakos MH, Lieberman JA, Bilder RM, Borenstein M, Lerner G, Bogerts B, et al. Increase in caudate nuclei volumes of first-episode schizophrenic patients taking antipsychotic drugs. Am J Psychiatry. 1994;151:1430 –1436. doi: 10.1176/ajp.151.10.1430. [DOI] [PubMed] [Google Scholar]
- Cohen RM, Nordahl TE, Semple WE, Andreason P, Litman RE, Pickar D. The brain metabolic patterns of clozapine- and fluphenazine-treated patients with schizophrenia during a continuous performance task. Arch Gen Psychiatry. 1997;54:481– 486. doi: 10.1001/archpsyc.1997.01830170107014. [DOI] [PubMed] [Google Scholar]
- Corson PW, Nopoulos P, Andreasen NC, Heckel D, Arndt S. Caudate size in first-episode neuroleptic-naive schizophrenic patients measured using an artificial neural network. Biol Psychiatry. 1999;46:712–720. doi: 10.1016/s0006-3223(99)00079-7. [DOI] [PubMed] [Google Scholar]
- Cummings JL. Frontal-subcortical circuits and human behavior. Arch Neurol. 1993;50:873– 880. doi: 10.1001/archneur.1993.00540080076020. [DOI] [PubMed] [Google Scholar]
- Davatzikos C, Resnick SM. Sex differences in anatomic measures of interhemispheric connectivity: Correlations with cognition in women but not men. Cereb Cortex. 1998;8:635– 640. doi: 10.1093/cercor/8.7.635. [DOI] [PubMed] [Google Scholar]
- Delazer M, Domahs F, Lochy A, Karner E, Benke T, Poewe W. Number processing and basal ganglia dysfunction: A single case study. Neuropsychologia. 2004;42:1050 –1062. doi: 10.1016/j.neuropsychologia.2003.12.009. [DOI] [PubMed] [Google Scholar]
- Dickey CC, McCarley RW, Shenton ME. The brain in schizotypal personality disorder: A review of structural MRI and CT findings. Harv Rev Psychiatry. 2002;10:1–15. doi: 10.1080/10673220216201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickey CC, McCarley RW, Voglmaier MM, Niznikiewicz MA, Seidman LJ, Demeo S, et al. An MRI study of superior temporal gyrus volume in women with schizotypal personality disorder. Am J Psychiatry. 2003;160:2198 –2201. doi: 10.1176/appi.ajp.160.12.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickey CC, Shenton ME, Hirayasu Y, Fischer I, Voglmaier MM, Niznikiewicz MA, et al. Large CSF volume not attributable to ventricular volume in schizotypal personality disorder. Am J Psychiatry. 2000;157:48 –54. doi: 10.1176/ajp.157.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanous A, Gardner C, Walsh D, Kendler KS. Relationship between positive and negative symptoms of schizophrenia and schizotypal symptoms in nonpsychotic relatives. Arch Gen Psychiatry. 2001;58:669 – 673. doi: 10.1001/archpsyc.58.7.669. [DOI] [PubMed] [Google Scholar]
- Filipek PA, Richelme C, Kennedy DN, Caviness VS., Jr The young adult human brain: An MRI-based morphometric analysis. Cereb Cortex. 1994;4:344 –360. doi: 10.1093/cercor/4.4.344. [DOI] [PubMed] [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Williams JB, Benjamin L. Structured Clinical Interview for DSM-IV Personality Disorders (SCID-II): Interview and Questionnaire. Washington, DC: American Psychiatric Press; 1997. [Google Scholar]
- Fristoe NM, Salthouse TA, Woodard JL. Examination of age-related deficits on the Wisconsin Card Sorting Test. Neuropsychology. 1997;11:428 –436. doi: 10.1037//0894-4105.11.3.428. [DOI] [PubMed] [Google Scholar]
- Gabrieli J. Cognitive neuroscience of human memory. Annu Rev Psychol. 1998;49:87–115. doi: 10.1146/annurev.psych.49.1.87. [DOI] [PubMed] [Google Scholar]
- Goldstein JM, Levine RRJ. Overview of sex differences in schizophrenia: Where have we been and where are we do we go from here? In: Castle DJ, McGrath JJ, Kulkarni J, editors. Women and Schizophrenia. Cambridge, United Kingdom: Cambridge University Press; 2000. pp. 111–153. [Google Scholar]
- Goldstein JM, Seidman LJ, Goodman JM, Koren D, Lee H, Weintraub S, et al. Are there sex differences in neuropsychological functions among patients with schizophrenia? Am J Psychiatry. 1998;155:1358 –1364. doi: 10.1176/ajp.155.10.1358. [DOI] [PubMed] [Google Scholar]
- Goldstein JM, Seidman LJ, Horton NJ, Makris N, Kennedy DN, Caviness VS, Jr, et al. Normal sexual dimorphism of the adult human brain assessed by in vivo magnetic resonance imaging. Cereb Cortex. 2001;11:490 –497. doi: 10.1093/cercor/11.6.490. [DOI] [PubMed] [Google Scholar]
- Goldstein JM, Seidman LJ, O’Brien LM, Horton NJ, Kennedy DN, Makris N, et al. Impact of normal sexual dimorphisms on sex differences in structural brain abnormalities in schizophrenia assessed by magnetic resonance imaging. Arch Gen Psychiatry. 2002;59:154 –164. doi: 10.1001/archpsyc.59.2.154. [DOI] [PubMed] [Google Scholar]
- Gunduz H, Wu H, Ashtari M, Bogerts B, Crandall D, Robinson DG, et al. Basal ganglia volumes in first-episode schizophrenia and healthy comparison subjects. Biol Psychiatry. 2002;51:801– 808. doi: 10.1016/s0006-3223(01)01345-2. [DOI] [PubMed] [Google Scholar]
- Gur RE, Maany V, Mozley PD, Swanson C, Bilker W, Gur RC. Subcortical MRI volumes in neuroleptic-naive and treated patients with schizophrenia. Am J Psychiatry. 1998;155:1711–1717. doi: 10.1176/ajp.155.12.1711. [DOI] [PubMed] [Google Scholar]
- Hokama H, Shenton ME, Nestor PG, Kikinis R, Levitt JJ, Metcalf D, et al. Caudate, putamen, and globus pallidus volume in schizophrenia: A quantitative MRI study. Psychiatry Res. 1995;61:209 –229. doi: 10.1016/0925-4927(95)02729-h. [DOI] [PubMed] [Google Scholar]
- Hollingshead A. Two Factor Index of Social Position. New Haven, Connecticut: Yale University Press; 1965. [Google Scholar]
- Joel D, Weiner I. The connections of the dopaminergic system with the striatum in rats and primates: An analysis with respect to the functional and compartmental organization of the striatum. Neuroscience. 2000;96:451– 474. doi: 10.1016/s0306-4522(99)00575-8. [DOI] [PubMed] [Google Scholar]
- Kendler KS. Diagnostic approaches to schizotypal personality disorder: A historical perspective. Schizophr Bull. 1985;11:538 –553. doi: 10.1093/schbul/11.4.538. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Lieberman JA, Walsh D. The Structured Interview for Schizotypy (SIS): A preliminary report. Schizophr Bull. 1989;15:559 –571. doi: 10.1093/schbul/15.4.559. [DOI] [PubMed] [Google Scholar]
- Kendler KS, McGuire M, Gruenberg AM, O’Hare A, Spellman M, Walsh D. The Roscommon Family Study. I. Methods, diagnosis of pro-bands, and risk of schizophrenia in relatives. Arch Gen Psychiatry. 1993a;50:527–540. doi: 10.1001/archpsyc.1993.01820190029004. [DOI] [PubMed] [Google Scholar]
- Kendler KS, McGuire M, Gruenberg AM, O’Hare A, Spellman M, Walsh D. The Roscommon Family Study. III. Schizophrenia-related personality disorders in relatives. Arch Gen Psychiatry. 1993b;50:781–788. doi: 10.1001/archpsyc.1993.01820220033004. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Bagwell WW, Haas GL, Sweeney JA, Schooler NR, Pettegrew JW. Changes in caudate volume with neuroleptic treatment. Lancet. 1994;344:1434. doi: 10.1016/s0140-6736(94)90599-1. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Rosenberg D, Sweeney JA, Pettegrew JW. Decreased caudate volume in neuroleptic-naive psychotic patients. Am J Psychiatry. 1998;155:774 –778. doi: 10.1176/ajp.155.6.774. [DOI] [PubMed] [Google Scholar]
- Kessels RP, van Zandvoort MJ, de Haan EH, Kappelle LJ. Memory dysfunction and caudate stroke. Stroke. 1999;30:1734 –1735. doi: 10.1161/01.str.30.8.1734. [DOI] [PubMed] [Google Scholar]
- Kety SS, Wender PH, Jacobsen B, Ingraham LJ, Jansson L, Faber B, et al. Mental illness in the biological and adoptive relatives of schizophrenic adoptees. Replication of the Copenhagen Study in the rest of Denmark. Arch Gen Psychiatry. 1994;51:442–455. doi: 10.1001/archpsyc.1994.03950060006001. [DOI] [PubMed] [Google Scholar]
- Laakso A, Vilkman H, Bergman J, Haaparanta M, Solin O, Syvalahti E, et al. Sex differences in striatal presynaptic dopamine synthesis capacity in healthy subjects. Biol Psychiatry. 2002;52:759 –763. doi: 10.1016/s0006-3223(02)01369-0. [DOI] [PubMed] [Google Scholar]
- Levitt JJ, McCarley RW, Dickey CC, Voglmaier MM, Niznikiewicz MA, Seidman LJ, et al. MRI study of caudate nucleus volume and its cognitive correlates in neuroleptic-naive patients with schizotypal personality disorder. Am J Psychiatry. 2002;159:1190 –1197. doi: 10.1176/appi.ajp.159.7.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy DG, DeCarli C, McIntosh AR, Daly E, Mentis MJ, Pietrini P, et al. Sex differences in human brain morphometry and metabolism: An in vivo quantitative magnetic resonance imaging and positron emission tomography study on the effect of aging. Arch Gen Psychiatry. 1996;53:585–594. doi: 10.1001/archpsyc.1996.01830070031007. [DOI] [PubMed] [Google Scholar]
- Niznikiewicz MA, Voglmaier M, Shenton ME, Seidman LJ, Dickey CC, Rhoads R, et al. Electrophysiological correlates of language processing in schizotypal personality disorder. Am J Psychiatry. 1999;156:1052–1058. doi: 10.1176/ajp.156.7.1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Oscar-Berman M, McNamara P, Freedman M. Delayed response tasks: Parallels between experimental ablation studies and findings in patients with frontal lesions. In: Levin H, Eisenberg HM, Benton AL, editors. Frontal Lobe Function and Injury. New York: Oxford University Press; 1991. pp. 230–255. [Google Scholar]
- Parashos IA, Tupler LA, Blitchington T, Krishnan KR. Magnetic-resonance morphometry in patients with major depression. Psychiatry Res. 1998;84:7–15. doi: 10.1016/s0925-4927(98)00042-0. [DOI] [PubMed] [Google Scholar]
- Park S, McTigue K. Working memory and the syndromes of schizotypal personality. Schizophr Res. 1997;26:213–220. doi: 10.1016/s0920-9964(97)00051-0. [DOI] [PubMed] [Google Scholar]
- Pickett ER, Kuniholm E, Protopapas A, Friedman J, Lieberman P. Selective speech motor, syntax and cognitive deficits associated with bilateral damage to the putamen and the head of the caudate nucleus: A case study. Neuropsychologia. 1998;36:173–188. doi: 10.1016/s0028-3932(97)00065-1. [DOI] [PubMed] [Google Scholar]
- Pieper S, Halle M, Kikinis R. 3D Slicer. IEEE International Symposium on Biomedical Imaging: From Nano to Macro; 2004. pp. 632–635. [Google Scholar]
- Richfield EK, Twyman R, Berent S. Neurological syndrome following bilateral damage to the head of the caudate nuclei. Ann Neurol. 1987;22:768–771. doi: 10.1002/ana.410220615. [DOI] [PubMed] [Google Scholar]
- Sabri O, Erkwoh R, Schreckenberger M, Owega A, Sass H, Buell U. Correlation of positive symptoms exclusively to hyperperfusion or hypoperfusion of cerebral cortex in never-treated schizophrenics. Lancet. 1997;349:1735–1739. doi: 10.1016/S0140-6736(96)08380-8. [DOI] [PubMed] [Google Scholar]
- Sakai K, Passingham RE. Prefrontal selection and medial temporal lobe reactivation in retrieval of short-term verbal information. Cereb Cortex. 2004;14:914 –921. doi: 10.1093/cercor/bhh050. [DOI] [PubMed] [Google Scholar]
- Scheepers FE, Gispen de Wied CC, Hulshoff Pol HE, Kahn RS. Effect of clozapine on caudate nucleus volume in relation to symptoms of schizophrenia. Am J Psychiatry. 2001;158:644 – 646. doi: 10.1176/appi.ajp.158.4.644. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Oscar-Berman M, Kalinowski AG, Ajilore O, Kremen WS, Faraone SV, et al. Experimental and clinical neuropsychological measures of prefrontal dysfunction in schizophrenia. Neuropsychology. 1995;9:481– 490. [Google Scholar]
- Shihabuddin L, Buchsbaum MS, Hazlett EA, Silverman J, New A, Brickman AM, et al. Striatal size and relative glucose metabolic rate in schizotypal personality disorder and schizophrenia. Arch Gen Psychiatry. 2001;58:877– 884. doi: 10.1001/archpsyc.58.9.877. [DOI] [PubMed] [Google Scholar]
- Siever LJ, Davis KL. The pathophysiology of schizophrenia disorders: Perspectives from the spectrum. Am J Psychiatry. 2004;161:398 – 413. doi: 10.1176/appi.ajp.161.3.398. [DOI] [PubMed] [Google Scholar]
- Siever LJ, Kalus OF, Keefe RS. The boundaries of schizophrenia. Psychiatr Clin North Am. 1993;16:217–244. [PubMed] [Google Scholar]
- Siever LJ, Koenigsberg HW, Harvey P, Mitropoulou V, Laruelle M, Abi-Dargham A, et al. Cognitive and brain function in schizotypal personality disorder. Schizophr Res. 2002;54:157–167. doi: 10.1016/s0920-9964(01)00363-2. [DOI] [PubMed] [Google Scholar]
- Siever LJ, Silverman JM, Horvath TB, Klar H, Coccaro E, Keefe RS, et al. Increased morbid risk for schizophrenia-related disorders in relatives of schizotypal personality disordered patients. Arch Gen Psychiatry. 1990;47:634 –640. doi: 10.1001/archpsyc.1990.01810190034005. [DOI] [PubMed] [Google Scholar]
- Spreen O, Strauss E. A Compendium of Neuropsychological Tests. New York: Oxford University Press; 1998. [Google Scholar]
- Suzuki M, Zhou SY, Hagino H, Takahashi T, Kawasaki Y, Nohara S, et al. Volume reduction of the right anterior limb of the internal capsule in patients with schizotypal disorder. Psychiatry Res. 2004;130:213–225. doi: 10.1016/j.pscychresns.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Tamminga CA. Gender and schizophrenia. J Clin Psychiatry. 1997;58(suppl 15):33–37. [PubMed] [Google Scholar]
- Torgersen S, Edvardsen J, Oien PA, Onstad S, Skre I, Lygren S, et al. Schizotypal personality disorder inside and outside the schizophrenic spectrum. Schizophr Res. 2002;54:33–38. doi: 10.1016/s0920-9964(01)00349-8. [DOI] [PubMed] [Google Scholar]
- Tsuang MT, Stone WS, Faraone SV. Schizophrenia: A review of genetic studies. Harv Rev Psychiatry. 1999;7:185–207. [PubMed] [Google Scholar]
- Voglmaier MM, Seidman LJ, Niznikiewicz MA, Dickey CC, Shenton ME, McCarley RW. Verbal and nonverbal neuropsychological test performance in subjects with schizotypal personality disorder. Am J Psychiatry. 2000;157:787–793. doi: 10.1176/appi.ajp.157.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voglmaier MM, Seidman LJ, Niznikiewicz MA, Dickey CC, Shenton ME, McCarley RW. A comparative profile analysis of neuropsychological function in men and women with schizotypal personality disorder. Schizophr Res. 2005;74:43– 49. doi: 10.1016/j.schres.2004.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voglmaier MM, Seidman LJ, Salisbury D, McCarley RW. Neuropsychological dysfunction in schizotypal personality disorder: A profile analysis. Biol Psychiatry. 1997;41:530 –540. doi: 10.1016/s0006-3223(96)00056-x. [DOI] [PubMed] [Google Scholar]


