Abstract
Chronic, excessive ethanol intake can increase retinoic acid (RA) catabolism by inducing cytochrome P450 2E1 (CYP2E1). Vitamin E (VE) is an antioxidant implicated in CYP2E1 inhibition. In the current study, we hypothesized that VE supplementation inhibits CYP2E1 and decreases RA catabolism, thereby preventing ethanol-induced hepatocyte hyperproliferation. For 1 month, four groups of Sprague-Dawley rats were fed a Lieber-DeCarli liquid ethanol (36% of the total calories) diet as follows: either ethanol alone (Alc group) or ethanol in combination with 0.1 mg/kg body wt of all-trans RA (Alc+RA group), 2 mg/kg body wt of VE (Alc+VE group), or both together (Alc+RA+VE group). Control rats were pair-fed a liquid diet with an isocaloric amount of maltodextrin instead of ethanol. The ethanol-fed groups had three-fold higher hepatic CYP2E1 levels, 50% lower hepatic RA levels, and significantly increased hepatocyte proliferation when compared with the controls. The ethanol-fed rats given VE had more than four-fold higher hepatic VE concentrations than did ethanol-fed rats without VE, but this did not prevent ethanol induction of CYP2E1, lower hepatic retinoid levels, or hepatocellular hyperproliferation. Further, VE supplementation could not prevent RA catabolism in liver microsomal fractions of the ethanol-fed rats in vitro. These results show that VE supplementation can neither inhibit ethanol-induced changes in RA catabolism nor prevent ethanol-induced hepatocyte hyperproliferation in the rat liver.
Keywords: Retinoic acid, vitamin E, CYP2E1, alcohol intake, cell proliferation, rat
1. Introduction
Chronic, excessive ethanol intake increases the risk of developing various liver diseases such as cirrhosis and cancer, and an early and key characteristic in the pathogenesis of ethanol-induced liver injury is hepatocyte hyperproliferation [1-2]. One suggested mechanism by which ethanol induces hepatic cell proliferation involves the interference of ethanol with retinoid (retinol, retinoic acid, and retinyl ester) metabolism and signaling [3]. Lower hepatic vitamin A (retinol and retinyl ester) levels have been well documented in alcoholics [4]. Moreover, both plasma and hepatic concentrations of retinoic acid (RA), the most physiologically active retinoid, are significantly decreased in rats after 1 month of ethanol feeding [5]. RA, which functions as a ligand for the nuclear retinoid receptors (RARs and RXRs), plays an important role in controlling cell cycle progression, thereby regulating cell proliferation and differentiation [6]. Indeed, RA is of clinical interest for use in cancer chemoprevention and treatment, and retinoids have been shown to be effective against acute promyelocytic leukemia, skin cancer, and breast cancer [7-9]. Previous studies in our laboratory have demonstrated that ethanol-induced hepatocyte hyperproliferation can be prevented by RA supplementation [1, 10], indicating that low levels of retinoids could be an important factor in ethanol-induced chronic liver diseases.
Increased oxidative stress, which occurs in the liver as a result of chronic ethanol intake [11], could also contribute to pathogenic alterations in hepatic cells, including hyperproliferation. Vitamin E (VE) is a potent antioxidant and has been shown to effectively decrease in vitro and in vivo susceptibility to lipid peroxidation [12-14]. Research has documented synergistic interactions between vitamins A and E against lipid peroxidation in vitro, with the combination offering reciprocal protection against oxidation [15].
The generation of reactive oxygen species and oxidative stress by ethanol in the liver appears to be mediated mainly by the induction of cytochrome P450 2E1 (CYP2E1). CYP2E1 is highly reactive in oxidizing various substrates and producing free radicals, and the hepatic content of CYP2E1 is significantly increased in alcoholics and rats fed an ethanol diet [16,17]. The CYP2E1 induction in the liver of ethanol-fed rats closely coincides with the production of 1-hydroxyethyl radicals [18]. Moreover, transgenic mice that overexpress CYP2E1 are more susceptible to oxidative liver injury after ethanol treatment than nontransgenic controls [19]. CYP2E1 has been shown to play a major role in depleting liver RA stores by facilitating RA degradation into polar metabolites [20]. Further, treatment with chlormethiazole, a CYP2E1 inhibitor, effectively inhibits liver pathology in ethanol-fed rats [21] and restores hepatic RA and retinol levels to normal [20, 22], thus providing evidence that CYP2E1-mediated reactions are likely to contribute to ethanol-related liver injury. Administration of a VE analogue, the tris salt of α-tocopheryl hemisuccinate, to rats has been shown to decrease CYP2E1 activity and protect against CCl4-induced hepatotoxicity [23]. Therefore, we hypothesized that VE supplementation could prevent ethanol-related alterations in the liver, such as CYP2E1 induction, decreased RA concentrations, or increased cellular proliferation. To test this hypothesis, we investigated the effects of dietary VE supplementation, with or without RA, on hepatic retinoid concentrations, CYP2E1 levels, RA oxidation to polar metabolites, and hepatocyte proliferation in rat liver tissues after 1-month of ethanol feeding.
2. Methods and materials
2.1. Animals and diets
Twenty-five male Sprague-Dawley rats (Charles River Breeding Laboratory, Kingston, NY) were provided free access to Teklad 7012 rodent meal (Harlan Teklad, Madison, WI) and water for a 1-week acclimation period. Subsequently, all animals were fed a Lieber-DeCarli liquid diet (Dyets, Inc., Bethlehem, PA) without ethanol for five days. Animals were then distributed, by wt-matching, into five groups: 1) control [C]; 2) ethanol-fed [Alc]; 3) ethanol-fed with all-transRA (0.1 mg/kg body wt) supplementation [Alc+RA]; 4) ethanol-fed with vitamin E (2 mg/kg body wt) supplementation [Alc+VE]; or (5) ethanol-fed with both all-trans-RA and vitamin E supplementation [Alc+RA+VE]. Ethanol was fed with the Lieber-DeCarli liquid diet containing 36% of total calories as ethanol, yielding a concentration of 6.2% (vol/vol) [24]. Ethanol was gradually introduced into the experimental diets over a 10-day period before providing animals with the final concentration. Control animals were maintained on the Lieber-DeCarli liquid diet without ethanol. In the control diet, ethanol was replaced by an isocaloric amount of maltodextrin (Purina Test diets, Richmond, IN). Both diets contained 16.4% of total calories as protein and 35% as fat; 48.6% of total calories were provided from carbohydrates in the control diet, whereas 12.6% of total calories were from carbohydrates in the ethanol diet. The dosage of RA supplementation was determined based on the data from our previous study in ethanol-fed rats [1], which showed that RA supplementation at the dose of 0.1 mg/kg body wt/day restored hepatic RA concentrations similar to those of control rats. In addition, there was no evidence of toxicity as determined by lack of changes in wt gain or liver histology. The dosage of vitamin E supplementation was determined based on the data from a previous study by Chow et al. [25], which showed that 2 mg vitamin E supplementation (/kg body wt/day) significantly increased the levels of vitamin E in tissues. For RA supplementation, all-trans-RA (Sigma, St. Louis, MO) was dissolved in 95% ethanol. Then, the precise concentration of the RA stock solution was determined by spectral analyses and the appropriate volume of the RA stock solution was directly added into the liquid diet. For VE supplementation, α-tocopherol acetate (Hoffmann-La Roche Ltd, Basel, Switzerland) was dissolved in corn oil and mixed with the liquid diet. To protect from degradation, the stock solutions of RA and VE were filled with nitrogen gas and stored in colored tubes at 4°C. The liquid diet was made fresh every two to three day and stored in colored bottles at 4°C. Since the liquid diet provided physiological amounts of fluid, extra water was not given. Rats were pair-fed using Alc+RA+VE as the leading group for one month. Body wts were recorded weekly. At the end of experimental period, all rats were terminally exsanguinated under AErrane® (Fort Dodge Animal Health, Fort Dodge, IO) anesthesia. Liver tissues were collected, frozen under liquid nitrogen, and stored at − 80°C for further analysis.
All animals were maintained in an American Association for the Accreditation of Laboratory Animal Care accredited facility, in an environmentally controlled atmosphere (temperature, 23°C, 45% relative humidity) with 15 air changes of 100% fresh filtered air per hour and a 12/12h light/dark cycle. All animals were observed daily for clinical signs of illness. This study protocol was approved by the Jean Mayer USDA Human Nutrition Research Center on Aging Animal Care and Use Committee.
2.2. HPLC analysis
Liver sample extractions were done as described previously [1]. Briefly, liver tissue was homogenized with ice-cold HEPES buffer:methanol (2:1, vol/vol) mixture. After adding the internal standard (100 μL of retinyl acetate), liver samples were extracted twice without saponification using 6.0 ml of chloroform:methanol (2:1, vol/vol) mixture followed by hexane. The extracts were evaporated under N2 gas, and resuspended in 50 μL ethanol for injection into the HPLC system. A gradient reverse phase HPLC system was used as follows: flow rate was 1 mL/min; 10% solvent A (acetonitrile:tetrahydrofuran:water = 50:20:30, vol/vol/vol, with 0.35% acetic acid and 1% ammonium acetate in water) for 3 minutes, followed by a 6-minute linear gradient to 40% solvent A and 60% solvent B (acetonitrile: tetrahydrofuran: water = 50:44:6, vol/vol/vol, with 0.35% acetic acid and 1% ammonium acetate in water), a 12-minute hold at 40% solvent A/60% solvent B, and then a 7-minute gradient back to 100% solvent A. Individual retinoids and α-tocopherol were identified by co-elution with standards and absorption spectrum analysis, and quantified by determining peak areas calibrated against known amounts of standards. Levels were corrected for extraction and handling losses by monitoring the recovery of the internal standard.
2.3. Western blot analysis
Western blot analysis was carried out as described earlier [26]. Protein was transferred onto polyvinylidene diflouride membranes, which were incubated with polyclonal antibody against CYP2E1 (Chemicon International Inc, Temecula, CA) at a 1:200 dilution in Tris-buffered saline-Tween 20 (TBS-T) buffer at room temperature for 2-3 hours. The membranes were then washed and incubated for 1 hour in TBS-T buffer containing a 1:5000 dilution of the horseradish peroxidase-labeled secondary antibody (Bio-Rad, Hercules, CA). The blots were developed using the ECL Western blotting system (Amersham, Piscataway, NJ) and analyzed by densitometry (Bio-Rad model 710). The equal loadings of proteins were verified by checking that the intensities of non-specific bands were the same among wells. All samples in each pair-feeding group were run on the same blot. The band intensities of samples in four treatment groups were compared to the band intensity of a control sample on the same blot, and expressed as fold increase compared with the control.
2.4. Immunohistochemistry
Hepatocytes in the S phase were quantified by immunohistochemical analysis of proliferating cellular nuclear antigen (PCNA) as described earlier [1]. Briefly, liver samples were fixed with 10% buffered formalin and embedded in paraffin wax. After deparaffinization and rehydration, the liver sections were incubated with 3% H2O2 to quench endogenous peroxide activity, and the slides were heated in 10 mM citrate buffer to retrieve the antigen. The slides were then blocked with horse serum and incubated with the primary antibody, monoclonal anti-PCNA (clone PC10, Dako, Carpinteria, CA), and then with the biotinylated horse anti-mouse immunoglobulin G and streptavidin conjugated to a horseradish peroxidase label. Slides were developed using DAB (diaminobenzidine) substrate and counterstained with hematoxylin. Hepatocytes with dark brown stained nuclei and cytoplasm were counted as S phase cells. The investigators were blinded, and the cells were viewed under X400 magnification. A total of 20 fields were counted and S phase cells were expressed as positive cells per 100 hepatocytes.
2.5. In vitro assays for measuring RA catabolism
Hepatic microsomal protein (2 mg) was incubated under red light in glass vials at 37°C in a shaking water bath for a 3-min preincubation with the following additions: buffer [Hepes, 20 mmol/L; KCl, 150 mmol/L; MgCl2, 5 mmol/L; EDTA, 1 mmol/L (pH, 7.35)] and substrate (RA dissolved in dimethyl sulfoxide, 5 nmol) in a final volume of 1 mL. The incubation mixtures were preincubated with either α-tocopherol or CYP inhibitors (chlormethiazole for CYP2E1, and liarozole for nonspecific CYP) for 10 min before incubation. After preincubation, the reaction was started by adding NADPH (1 mmol/L) and the mixtures were incubated for 10 min at 37°C. Two control vials were run lacking either substrate or microsomes. The vials were uncovered and the incubation mixtures were exposed to room air as the gas phase. After incubation, RA and its polar metabolites were extracted and analyzed by the method described previously [20]. Individual retinoids (4-oxo-RA, 18-hydroxy-RA, RA) were identified by coelution with standards and absorption spectrum analysis. Retinoids were quantified relative to the internal standard (retinyl acetate), by determining peak areas calibrated against known amounts of standards.
2.6. Statistical Analyses
All group values are expressed means ± standard deviation. Group means were compared using analysis of variance (ANOVA) followed by Tukey's Honest Significant Difference test. All analyses were performed using the SAS software version 9.1 (SAS Institute Inc, Cary NC). Significance of difference was determined at P <0.05.
3. Results
3.1. Body wt
No significant differences were observed in the body wt after 1 month of alcohol feeding and supplementation with RA, VE, or both. The final mean body wt of the rats in each group was as follows: Alc, 256 ± 6 g; Alc+RA, 228 ± 13 g; Alc+VE, 225 ± 8 g; and Alc+RA+VE, 223 ± 9 g. The wt of the control rats at the end of the study was 237 ± 4 g.
3.2. Effects of ethanol, all-trans RA, and VE treatments on hepatic concentrations of VE and retinoids
The hepatic VE concentration was slightly lower in the ethanol-fed rats than in the controls, but this change did not reach statistical significance (Table 1). The ethanol-fed rats given a VE supplement, with or without RA, had hepatic VE concentrations more than four-fold higher than the animals given alcohol alone and three-fold higher than the control rats. Ethanol feeding for 1 month resulted in approximately 50% lower hepatic RA concentrations in these rats than in the controls, and VE supplementation alone could not prevent this decline (Table 1). However, RA treatment, alone or with VE, resulted in hepatic RA concentrations in the ethanol-fed rats that were not significantly different from those in the control animals. There were no differences in the hepatic retinol concentrations among the groups except in the Alc+RA+VE group, where the hepatic retinol levels were significantly higher than in the rest of the ethanol-fed groups (Table 1). Concentrations of retinyl palmitate were 71% lower in the ethanol-fed animals that in the controls (Table 1). Hepatic retinyl palmitate concentrations were slightly higher in the Alc+RA and Alc+VE groups than in the Alc group, but these differences were not significant. However, the hepatic retinyl palmitate concentrations in the ethanol-fed rats cotreated with RA and VE were closer to the levels in the control animals.
Table 1.
Hepatic concentrations (nmol/g) of vitamin E, retinoic acid, retinol and retinyl palmitate after feeding ethanol with or without vitamin E and retinoic acid for one month
| Control | Ethanol-fed | Ethanol-fed + Retinoic Acid (0.1mg/kg body wt) |
Ethanol-fed + Vitamin E (2mg/kg body wt) |
Ethanol-fed + Retinoic Acid + Vitamin E |
|
|---|---|---|---|---|---|
| Vitamin E | 51.7 ± 0.4a | 33.3 ± 3.2a | 31.0 ± 5.6a | 160.4 ± 16.9b | 132.2 ± 18.6b |
| Retinoic Acid | 0.157 ± 0.006a | 0.081 ± 0.003b | 0.110 ± 0.009ab | 0.083 ± 0.013b | 0.123 ± 0.012a |
| Retinol | 28.4 ± 6.4ab | 19.5 ± 0.5a | 19.2 ± 2.9a | 26.7 ± 3.2a | 36.4 ± 2.6b |
| Retinyl Palmitate | 608.8 ± 58.5a | 174.3 ± 4.0b | 293.3 ± 122.8b | 281.1 ± 68.3b | 358.6 ± 56.1ab |
Hepatic vitamin E and retinoids were extracted with hexane and analyzed by HPLC as described in Methods. Values are expressed as means ± SD (n=3-5 in each group). Within rows, treatments not sharing the same superscript are significantly different from each other (P<0.05).
3.3. Effects of all-trans RA, VE, and ethanol on CYP2E1 protein levels
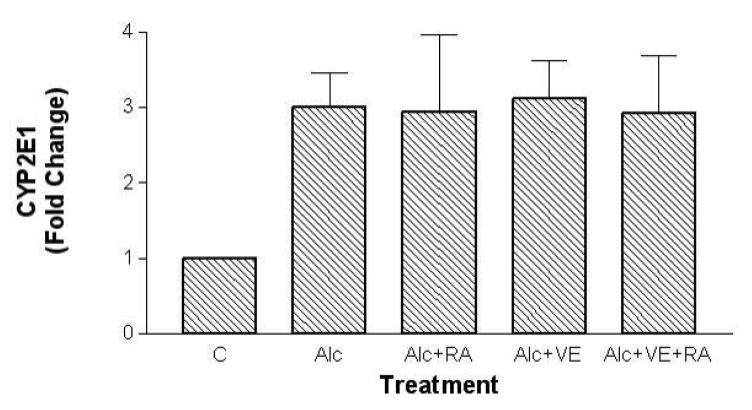
Hepatic CYP2E1 levels were approximately three-fold higher in the ethanol-fed rats than in the controls. VE supplementation, with or without RA, did not affect the expression of hepatic CYP2E1 when compared with ethanol feeding alone (Figure 1).
Fig. 1.
Effects of retinoic acid (0.1 mg/kg body weight) and vitamin E (2.0 mg/kg body weight) supplementation on hepatic CYP2E1 protein levels in rats fed with ethanol for a one-month period. Western Blotting analyses were followed by densitometric analysis. All samples in each pair-feeding group were run on the same blot. The band intensities of samples in four treatment groups were compared to the band intensity of a control sample on the same blot, and expressed as fold increase compared with the control. Mean ± SD (n= 3 - 5 per group). C, control; Alc, ethanol; RA, retinoic acid; VE, vitamin E.
3.4. Effects of ethanol, all-trans RA, and VE on hepatocyte proliferation
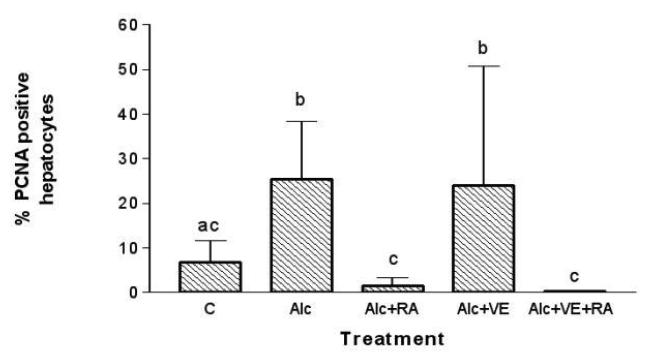
The effect of ethanol, RA, and VE supplementation for a 1-month period on hepatocyte proliferation was assessed by immunohistochemical staining of liver tissue for PCNA (Figure 2). Liver sections from the ethanol-fed rats had a significantly higher number of PCNA-labeled hepatocytes than the rats fed the control diet. The groups receiving RA treatment, with or without VE, had markedly lower numbers of PCNA-positive hepatocytes than the rats fed alcohol alone (p < 0.05). In the ethanol-fed rats, VE supplementation alone did not result in a lower number of positively stained hepatocytes than by ethanol feeding alone.
Fig. 2.
Effects of retinoic acid (0.1 mg/kg body weight) and vitamin E (2.0 mg/kg body weight) supplementation on hepatocyte proliferation in rats fed with ethanol for a one-month period. Hepatocytes in S-phase were determined by immunohistochemical staining for proliferating cell nuclear antigen (PCNA) in liver sections. A total of 20 fields were screened per animal and examined under light microscopy. C, control; Alc, ethanol; RA, retinoic acid; VE, vitamin E. Treatments not sharing the same superscript are significantly different from each other (P<0.05).
3.5. Effects of ethanol, VE, and CYP2E1 inhibitors on RA catabolism
The effects of ethanol, VE, and CYP2E1 inhibitors on RA catabolism were examined by quantification of polar oxidative metabolites of RA, namely, 4-oxo-retinoic acid (4-oxo-RA) and 18-hydroxy-retinoic acid (18-hydroxy-RA), after incubation of liver microsomal fractions from various groups with 5 mmol/L RA (Table 2). The ethanol-fed rats had significantly higher levels of 4-oxo-RA and 18-hydroxy-RA (eight-fold and six-fold higher, respectively) than the controls. Neither in vivo VE supplementation nor exogenous VE addition to the incubation mixture had any protective effect against ethanol-induced vitamin A catabolism. On the other hand, addition of liazarole, a non-specific CYP enzyme inhibitor, to the incubation mixture resulted in significantly lower levels of 4-oxo-RA and 18-hydroxy-RA compared with the levels in both ethanol-fed (>90% reduction) and control (~50% reduction) animals. Addition of chlormethiazole, a specific inhibitor of CYP2E1, to the incubation mixture resulted in lower levels of vitamin A polar metabolites than those achieved with ethanol feeding alone (~50% reduction); however, the 4-oxo-RA and 18-hydroxy-RA levels were still significantly higher than the levels in the controls.
Table 2.
The production of 4-oxo-retinoic acid (RA) and 18-hydroxy-RA from the incubation of RA (5 μmol/L) with liver microsomal fraction of ethanol-fed rats with or without vitamin E supplementation
| Ethanol-fed + Vitamin E supplemented |
|||||||
|---|---|---|---|---|---|---|---|
| Non-ethanol- fed |
Ethanol-fed | Ethanol-fed + Vitamin E supplemented |
Add additional α-tocopherol (5 μmol/L) |
Add additional α-tocopherol (10 μmol/L) |
Add CYP inhibitor liarozole (10 μmol/L) |
Add CYP inhibitor chloromethiazole (5 μmol/L) |
|
| 4-oxo-RA (pmol/mg protein) |
8.3 ± 1.6 c | 68.1 ± 7.2 a | 65.4 ± 6.5 a | 62.3 ± 7.7 a | 64.4 ± 8.3 a | 3.8 ± 0.6 d | 31.4 ± 3.3 b |
| 18-hydroxy-RA (pmol/mg protein) |
9.2 ±2.1 c | 54.6 ± 8.1 a | 58.5 ± 4.9 a | 59.4 ± 8.9 a | 65.9 ± 9.5 a | 5.1 ± 0.8 d | 28.7 ± 4.9 b |
Data represent three determinations from three rats. Values are expressed as means ± SD. Within rows, treatments not sharing the same superscript are significantly different from each other (P<0.05).
4. Discussion
Chronic, excessive ethanol consumption can lead to increased RA degradation to polar metabolites, largely due to oxidative metabolism by CYP2E1 [20]. In the present study, we hypothesized that VE prevents alcohol-reduced RA concentrations by blocking CYP2E1 activity, thereby preventing alcohol-induced hepatocyte hyperproliferation. As observed in our previous studies [1, 10], chronic ethanol intake in rats significantly decreased the hepatic RA concentrations, induced CYP2E1, and increased hepatocyte hyperproliferation. The hepatic VE level in the ethanol-fed rats was 36% lower than in the pair-fed controls. Although the difference was not statistically significant, these data are in agreement with the results of previous studies, which showed that the hepatic VE concentrations were 25-38% lower in ethanol-fed rats than in control rats [27, 28]. Low levels of VE in the liver have also been reported in patients with alcoholic cirrhosis [29]. Mechanisms that may lead to reduced hepatic levels of VE by ethanol include increased degradation of VE via ethanol-induced free radical formation and lipid peroxidation [30], and malabsorption resulting from β-lipoprotein deficiency [31]. Further, Meydani and colleagues [32] observed that chronic ethanol feeding of rats significantly decreased the hepatic VE level when expressed as per milligram of total lipid but not when expressed as per milligram of tissue, indicating ethanol-induced redistribution of VE in hepatic tissue.
In the ethanol-fed rats, although VE supplementation at a dose of 2 mg/kg body wt resulted in significantly higher hepatic VE concentrations, than without VE supplementation, the hepatic RA levels were not affected by the supplementation, and no significant difference was observed in the hepatocyte proliferation rate between the ethanol-fed rats with and without VE supplementation. Consistent with these findings is the fact that the CYP2E1 levels were higher in all the alcohol-fed animals than in the controls, despite VE supplementation in two groups. In addition, the data from our in vitro incubation studies support the lack of a VE effect on RA metabolism. Neither in vivo VE supplementation nor exogenous VE addition to the incubation mixture had any protective effect against ethanol-induced RA catabolism in the liver microsomal fractions. We confirmed that liazarole, a nonspecific CYP enzyme inhibitor, completely prevented ethanol-induced catabolism of vitamin A; in fact, it also seemed to partially inhibit basal catabolism of vitamin A. Chlormethiazole, a specific inhibitor of CYP2E1, partially inhibited ethanol-induced catabolism of vitamin A. This result suggests that although CYP2E1 is the major CYP enzyme induced by chronic ethanol feeding [33], there are probably additional enzymes induced by ethanol that contribute to the oxidative catabolism of vitamin A. For example, the family of CYP26 enzymes was shown to metabolize RA [34-36]. Taken together, above data confirm that CYP inhibition, and inhibition of CYP2E1 particularly, can prevent ethanol-induced catabolism of vitamin A to polar metabolites but suggest that the effects are not achieved by VE supplementation.
Our results are in contrast to several reports regarding the protective effect of VE in other tissues of ethanol-treated animals, including prevention of ethanol-induced hyperregeneration of colon crypt cells in rats [37] and inhibition of a promotional effect of ethanol on chemically induced esophageal cancers with dietary VE supplementation [38]. This suggests that the effect of VE on cell proliferation may vary depending on the organ or cell type. For example, VE enhances ethanol-suppressed splenocyte proliferation but not thymocyte proliferation [39].
In contrast, RA supplementation of the ethanol-fed rats restored the ethanol-reduced hepatic RA levels and was correlated with decreased hepatocyte proliferation. Interestingly, slightly higher hepatic concentrations of RA and slightly lower levels of hepatocyte proliferation were achieved with VE and RA co-supplementation than with RA supplementation alone. Although these differences did not reach statistical significance, they suggest a potentially useful interaction between these two nutrients, which deserves further study. Our study is limited by relatively small number of animals in each treatment group. Further, studies with several doses or different ratios of VE and RA combinations are needed. Nevertheless, our data demonstrated that supplementing RA and VE together partially restored both retinol and retinyl palmitate levels in ethanol-fed rats, whereas RA or VE alone had little effect. RA has been shown to regulate retinoid metabolism in the liver by stimulating retinol esterification [40,41]. It may be that the regulatory effect of RA in ethanol-fed rats can be enhanced in the presence of VE, although the mechanism for this effect needs further investigation.
Taken together, our data support the concept of RA as a key regulator of hepatocyte proliferation. VE supplementation, however, does not protect against ethanol-induced CYP2E1 induction, RA catabolism, or hepatocyte hyperproliferation in vivo. VE supplementation, in conjunction with RA, appears to improve ethanol-induced alterations in retinoid metabolism, but the difference was not statistically significant. Further studies are warranted to fully understand the potential beneficial role of the VE and RA combinations in preventing ethanol-induced changes in retinoid metabolism.
Acknowledgment
This work was supported by the Korea Research Foundation Grant funded by the Korean Government (KRF-2008-331-C00307) to J.C., and by the NIH/NIAAA R01AA12628 and U.S. Department of Agriculture, under agreement No. 1950-51000-056 to X-D.W.. Any opinions, findings, conclusion, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the views of the U.S. Department of Agriculture.
List of abbreviations
- Alc
alcohol (ethanol)
- RA
retinoic acid
- CYP2E1
cytochrome P450 2E1
- VE
vitamin E
- HPLC
high performance liquid chromatography
- PCNA
proliferating cellular nuclear antigen
- RP
retinyl palmitate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chung J, Liu C, Smith DE, Seitz HK, Russell RM, Wang XD. Restoration of retinoic acid concentration suppresses ethanol-enhanced c-Jun expression and hepatocyte proliferation in rat liver. Carcinogenesis. 2001;22:1213–9. doi: 10.1093/carcin/22.8.1213. [DOI] [PubMed] [Google Scholar]
- 2.Baumgardner JN, Shankar K, Korourian S, Badger TM, Ronis MJ. Undernutrition enhances alcohol-induced hepatocyte proliferation in the liver of rats fed via total enteral nutrition. Am J Physiol Gastrointest Liver Physiol. 2007;293:G355–64. doi: 10.1152/ajpgi.00038.2007. [DOI] [PubMed] [Google Scholar]
- 3.Wang XD. Alcohol, vitamin A, and cancer. Alcohol. 2005;35:251–258. doi: 10.1016/j.alcohol.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 4.Leo MA, Lieber CS. Hepatic vitamin A depletion in alcoholic liver injury. N Engl J Med. 1982;307:597–601. doi: 10.1056/NEJM198209023071006. [DOI] [PubMed] [Google Scholar]
- 5.Wang XD, Liu C, Chung J, Stickel F, Seitz HK, Russell RM. Chronic alcohol intake reduces retinoic acid concentration and enhances AP-1 (c-Jun and c-Fos) expression in rat liver. Hepatology. 1998;28:744–50. doi: 10.1002/hep.510280321. [DOI] [PubMed] [Google Scholar]
- 6.Lippman SM, Lotan R. Advances in the development of retinoids as chemopreventive agents. J Nutr. 2000;130:479S–82S. doi: 10.1093/jn/130.2.479S. [DOI] [PubMed] [Google Scholar]
- 7.Kurie JM. The biologic basis for the use of retinoids in cancer prevention and treatment. Curr Opin Oncol. 1999;11:497–502. doi: 10.1097/00001622-199911000-00011. [DOI] [PubMed] [Google Scholar]
- 8.Campbell RM, DiGiovanna JJ. Skin cancer chemoprevention with systemic retinoids: an adjunct in the management of selected high-risk patients. Dermatol Ther. 2006;19:306–14. doi: 10.1111/j.1529-8019.2006.00088.x. [DOI] [PubMed] [Google Scholar]
- 9.Zanardi S, Serrano D, Argusti A, Barile M, Puntoni M, Decensi A. Clinical trials with retinoids for breast cancer chemoprevention. Endocr Relat Cancer. 2006;13:51–68. doi: 10.1677/erc.1.00938. [DOI] [PubMed] [Google Scholar]
- 10.Chung J, Chavez PR, Russell RM, Wang XD. Retinoic acid inhibits hepatic Jun N-terminal kinase-dependent signaling pathway in ethanol-fed rats. Oncogene. 2002;21:1539–47. doi: 10.1038/sj.onc.1205023. [DOI] [PubMed] [Google Scholar]
- 11.Seitz HK, Stickel F. Risk factors and mechanisms of hepatocarcinogenesis with special emphasis on alcohol and oxidative stress. Biol Chem. 2006;387:349–360. doi: 10.1515/BC.2006.047. [DOI] [PubMed] [Google Scholar]
- 12.Pirozhkov SV, Eskelson CD, Watson RR, Hunter GC, Piotrowski JJ, Bernhard V. Effect of chronic consumption of ethanol and vitamin E on fatty acid composition and lipid peroxidation in rat heart tissue. Alcohol. 1992;9:329–34. doi: 10.1016/0741-8329(92)90076-m. [DOI] [PubMed] [Google Scholar]
- 13.Mileva M, Bakalova R, Tancheva L, Galabov A, Ribarov S. Effect of vitamin E supplementation on lipid peroxidation in blood and lung of influenza virus infected mice. Comp Immunol Microbiol Infect Dis. 2002;25:1–11. doi: 10.1016/s0147-9571(01)00010-8. [DOI] [PubMed] [Google Scholar]
- 14.Brockes C, Buchli C, Locher R, Koch J, Vetter W. Vitamin E prevents extensive lipid peroxidation in patients with hypertension. Br J Biomed Sci. 2003;60:5–8. doi: 10.1080/09674845.2003.11783669. [DOI] [PubMed] [Google Scholar]
- 15.Tesoriere L, Bongiorno A, Pintaudi AM, D'Anna R, D'Arpa D, Livrea MA. Synergistic interactions between vitamin A and vitamin E against lipid peroxidation in phosphatidylcholine liposomes. Arch Biochem Biophys. 1996;326:57–63. doi: 10.1006/abbi.1996.0046. [DOI] [PubMed] [Google Scholar]
- 16.Liu C, Russell RM, Seitz HK, Wang XD. Ethanol enhances retinoic acid metabolism into polar metabolites in rat liver via induction of cytochrome P4502E1. Gastroenterology. 2001;120:179–89. doi: 10.1053/gast.2001.20877. [DOI] [PubMed] [Google Scholar]
- 17.Lu Y, Cederbaum AI. CYP2E1 and oxidative liver injury by alcohol. Free Radic Biol Med. 2008;44:723–738. doi: 10.1016/j.freeradbiomed.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Navasumrit P, Ward TH, Dodd NJ, O'Connor PJ. Ethanol-induced free radicals and hepatic DNA strand breaks are prevented in vivo by antioxidants: effects of acute and chronic ethanol exposure. Carcinogenesis. 2000;21:93–9. doi: 10.1093/carcin/21.1.93. [DOI] [PubMed] [Google Scholar]
- 19.Morgan K, French SW, Morgan TR. Production of a cytochrome P450 2E1 transgenic mouse and initial evaluation of alcoholic liver damage. Hepatology. 2002;36:122–34. doi: 10.1053/jhep.2002.33720. [DOI] [PubMed] [Google Scholar]
- 20.Liu C, Russell RM, Seitz HK, Wang XD. Ethanol enhances retinoic acid metabolism into polar metabolites in rat liver via induction of cytochrome P4502E1. Gastroenterology. 2001;120:179–89. doi: 10.1053/gast.2001.20877. [DOI] [PubMed] [Google Scholar]
- 21.Gouillon Z, Lucas D, Li J, Hagbjork AL, French BA, Fu P, Fang C, Ingelman-Sundberg M, Donohue TM, Jr., French SW. Inhibition of ethanol-induced liver disease in the intragastric feeding rat model by chlormethiazole. Proc Soc Exp Biol Med. 2000;224:302–8. doi: 10.1046/j.1525-1373.2000.22435.x. [DOI] [PubMed] [Google Scholar]
- 22.Liu C, Chung J, Seitz HK, Russell RM, Wang XD. Chlormethiazole treatment prevents reduced hepatic vitamin A levels in ethanol-fed rats. Alcohol Clin Exp Res. 2002;26:1703–9. doi: 10.1097/01.ALC.0000037135.09289.69. [DOI] [PubMed] [Google Scholar]
- 23.Tirmenstein MA, Ge X, Elkins CR, Fariss MW. Administration of the tris salt of alpha-tocopheryl hemisuccinate inactivates CYP2E1, enhances microsomal alpha-tocopherol levels and protects against carbon tetrachloride-induced hepatotoxicity. Free Radic Biol Med. 1999;26:825–35. doi: 10.1016/s0891-5849(98)00265-2. [DOI] [PubMed] [Google Scholar]
- 24.Lieber CS, DeCarli LM. Liquid diet technique of ethanol administration: 1989 update. Alcohol Alcohol. 1989;24:197–211. [PubMed] [Google Scholar]
- 25.Chow CK, Ibrahim W, Wei Z, Chan AC. Vitamin E regulates mitochondrial hydrogen peroxide generation. Free Radic Biol Med. 1999;27:580–7. doi: 10.1016/s0891-5849(99)00121-5. [DOI] [PubMed] [Google Scholar]
- 26.Liu C, Russell RM, Wang XD. Exposing ferrets to cigarette smoke and a pharmacological dose of beta-carotene supplementation enhance in vitro retinoic acid catabolism in lungs via induction of cytochrome P450 enzymes. J Nutr. 2003;133:173–179. doi: 10.1093/jn/133.1.173. [DOI] [PubMed] [Google Scholar]
- 27.Bjorneboe GE, Bjorneboe A, Hagen BF, Morland J, Drevon CA. Reduced hepatic alpha-tocopherol content after long-term administration of ethanol to rats. Biochim Biophys Acta. 1987;918:236–41. doi: 10.1016/0005-2760(87)90226-8. [DOI] [PubMed] [Google Scholar]
- 28.Odeleye OE, Eskelson CD, Alak JI, Watson RR, Chvapil M, Mufti SI, Earnest D. The effect of vitamin E (alpha-tocopherol) supplementation on hepatic levels of vitamin A and E in ethanol and cod liver oil fed rats. Int J Vitam Nutr Res. 1991;61:143–8. [PubMed] [Google Scholar]
- 29.Bell H, Bjorneboe A, Eidsvoll B, Norum KR, Raknerud N, Try K, Thomassen Y, Drevon CA. Reduced concentration of hepatic alpha-tocopherol in patients with alcoholic liver cirrhosis. Alcohol Alcohol. 1992;27:39–46. [PubMed] [Google Scholar]
- 30.Kasdallah-Grissa A, Mornagui B, Aouani E, Hammami M, Gharbi N, Kamoun A, et al. Protective effect of resveratrol on ethanol-induced lipid peroxidation in rats. Alcohol Alcohol. 2006;41:236–9. doi: 10.1093/alcalc/agh256. [DOI] [PubMed] [Google Scholar]
- 31.Bonjour JP. Vitamins and alcoholism. X. Vitamin D, XI, Vitamin E, XII. Vitamin K. Int J Vitam Nutr Res. 1981;51:307–18. [PubMed] [Google Scholar]
- 32.Meydani M, Seitz HK, Blumberg JB, Russell RM. Effect of chronic ethanol feeding on hepatic and extrahepatic distribution of vitamin E in rats. Alcohol Clin Exp Res. 1991;15:771–4. doi: 10.1111/j.1530-0277.1991.tb00598.x. [DOI] [PubMed] [Google Scholar]
- 33.Lieber CS. Cytochrome P-4502E1: its physiological and pathological role. Physiol Rev. 1997;77:517–44. doi: 10.1152/physrev.1997.77.2.517. [DOI] [PubMed] [Google Scholar]
- 34.White JA, Ramshaw H, Taimi M, Stangle W, Zhang A, Everingham S, et al. Identification of the human cytochrome P450, P450RAI-2, which is predominantly expressed in the adult cerebellum and is responsible for all-trans-retinoic acid metabolism. Proc Natl Acad Sci U S A. 2000;97:6403–8. doi: 10.1073/pnas.120161397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taimi M, Helvig C, Wisniewski J, Ramshaw H, White J, Amad M, et al. A novel human cytochrome P450, CYP26C1, involved in metabolism of 9-cis and all-trans isomers of retinoic acid. J Biol Chem. 2004;279:77–85. doi: 10.1074/jbc.M308337200. [DOI] [PubMed] [Google Scholar]
- 36.Lutz JD, Dixit V, Yeung CK, Dickmann LJ, Zelter A, Thatcher JE, et al. Expression and functional characterization of cytochrome P450 26A1, a retinoic acid hydroxylase. Biochem Pharmacol. 2009;77:258–68. doi: 10.1016/j.bcp.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vincon P, Wunderer J, Simanowski UA, Koll M, Preedy VR, Peters TJ, Werner J, Waldherr R, Seitz HK. Inhibition of alcohol-associated colonic hyperregeneration by alpha-tocopherol in the rat. Alcohol Clin Exp Res. 2003;27:100–6. doi: 10.1097/01.ALC.0000046341.31828.A4. [DOI] [PubMed] [Google Scholar]
- 38.Eskelson CD, Odeleye OE, Watson RR, Earnest DL, Mufti SI. Modulation of cancer growth by vitamin E and alcohol. Alcohol Alcohol. 1993;28:117–25. [PubMed] [Google Scholar]
- 39.Wang Y, Huang DS, Watson RR. Dietary vitamin E modulation of cytokine production by splenocytes and thymocytes from alcohol-fed mice. Alcohol Clin Exp Res. 1994;18:355–62. doi: 10.1111/j.1530-0277.1994.tb00025.x. [DOI] [PubMed] [Google Scholar]
- 40.Matsuura T, Ross AC. Regulation of hepatic lecithin: retinol acyltransferase activity by retinoic acid. Arch Biochem Biophys. 1993;301:221–7. doi: 10.1006/abbi.1993.1137. [DOI] [PubMed] [Google Scholar]
- 41.Ross AC, Li NQ. Retinol combined with retinoic acid increases retinol uptake and esterification in the lungs of young adult rats when delivered by the intramuscular as well as oral routes. J Nutr. 2007;137:2371–6. doi: 10.1093/jn/137.11.2371. [DOI] [PubMed] [Google Scholar]