Abstract
Background:
This study quantified the risk of urinary bladder neoplasms in cancer patients taking into account the age at first diagnosis, the gender of the patients and the lead time between diagnoses.
Methods:
We used standardised incidence ratios (SIRs) to compare the incidence of bladder tumours in 967 767 cancer patients with the incidence rate in the general Swedish population. A total of 3324 male and 1560 female patients developed bladder tumours at least 1 year after first cancer diagnosis.
Results:
After bladder and renal pelvis cancers, the SIRs of bladder neoplasms were higher in female than in male patients. Men affected by lung, stomach and larynx tumours belonged to the population at high risk for bladder cancer. Treatment of breast, ovarian and cervical cancers seems to contribute to the subsequent development of bladder neoplasms. Long latencies (16–25 years) were observed after testicular, cervical and endometrial cancers. Detection bias had an important role after prostate cancer. Chemotherapy with cyclophosphamide and cisplatin, and also radiotherapy, seem to increase the risk of subsequent neoplasms in the bladder.
Conclusions:
These population-based results may help urologists to assess the risk of bladder neoplasms in cancer survivors. Our data should guide ongoing studies that investigate the effectiveness of bladder cancer screening in cancer patients.
Keywords: urinary bladder cancer, second primary tumours, recurrence risk, population based studies
The investigation of the development of urinary bladder neoplasms in cancer patients may provide unique clues to advance the understanding of the aetiology of bladder cancer. Tobacco smoking, occupational exposure to aromatic and heterocyclic amines, and probably chronic bladder infections are established risk factors for bladder cancer (Nordlund et al, 1997; Brennan et al, 2000, 2001; IARC, 2004; Gandini et al, 2008; Kiemeney et al, 2008). Variants in genes coding for xenobiotic-transforming enzymes and polymorphisms in DNA repair genes may also modify cancer susceptibility (Easton et al, 2007; Kellen et al, 2007; Murta-Nascimento et al, 2007b; Sanderson et al, 2007; Andrew et al, 2008). In addition to detection bias and risk factors shared by cancer of distinct types, for example, tobacco smoking is a risk factor for both lung and bladder cancers, second primary neoplasms may be related to the treatment of the first tumour. Radiotherapy to the pelvic area and chemotherapy with cyclophosphamide increase the risk of bladder cancer (Murta-Nascimento et al, 2007a).
After laryngeal cancer, bladder cancer shows the highest excess of risk for men compared with that for women in Sweden and the excess risk increases with age (National Board of Health and Welfare, 2007; Bermejo et al, 2008). In Sweden in 2005, the incidence ratio of bladder cancer in men compared with that in women was 3.1 in the age band 50 to 54 years and this ratio increased to 4.1 for the age band 80–84 years (Engholm et al, 2007). Recent meta-analyses imply that, at least in the western world, smoking can only partially explain this gender difference in the incidence of bladder cancer (Hemelt et al, 2008).
The objective of this article was to examine the risk of bladder neoplasms after cancer diagnosis, taking into account the gender of the patients, the age of diagnosis of the first cancer and the time since first diagnosis. These data may be relevant for clinical counselling, future development of screening programmes, and to advance the understanding of the aetiology of the disease. The present results were based on around 3000 male and 1500 female cancer patients who developed bladder tumours at least 1 year after first cancer diagnosis. In addition to the large sample size from a single country, the nationwide complete coverage and the reliability of cancer data represented important advantages of this study.
Material and methods
The Swedish Family-Cancer Database includes persons born in Sweden after 1931, totalling more than 11.8 million persons and more than 1.2 million tumour notifications – for a detailed description of the Database and its last update see reference Bermejo et al (2008). Cancer cases were retrieved from the Swedish Cancer Registry, which relies on separate compulsory notifications of cases from clinicians who diagnosed a neoplasm and from pathologists/cytologists. Second cancers were classified as such by the Cancer Registry, including synchronous tumours. The percentage of histologically or cytologically verified cases of cancer has been close to 100% (National Board of Health and Welfare, 2007). Unfortunately, the Swedish Cancer Registry lacks historic clinical and treatment data. In this study, 967 767 cancer patients were followed up from first cancer diagnosis until death, recurrence, detection of a second primary cancer, emigration or 31 December 2006, whichever came first.
The incidences of second primary urinary bladder malignancies among cancer patients were compared with the rates of first primary bladder cancers in the general Swedish population by standardised incidence ratios (SIRs) and 95% confidence intervals (CIs), adjusting for covariates age (5-year bands), sex, socioeconomic index (six groups), region (four groups) and calendar year (1961 to 1964, 1965 to 1969, and so on to 2000 to 2006). Separate analyses were carried out according to age at first cancer diagnosis (before age 20 years, 20 to 39, 40 to 59 and after 60 years). The SIR applies indirect standardisation, which is particularly suitable for cells with small numbers of subjects. In this method the observed number of cases is divided by the expected number of cases, calculated from the whole background population of 11.8 million individuals. The investigation of 36 types of cancer may result in false positive associations due to multiple comparisons. To alleviate this problem, associations were reported according to 0.05 and 0.01 significance levels. Kidney cancers were separated into cancers of the renal pelvis (International Classification of Diseases, 7th revision (ICD7)=1801) and the renal parenchyma (ICD7=1800). Cancer types were classified as ‘recurrent sites’ (urinary bladder and renal pelvis), ‘smoking-related sites’ and ‘non-smoking-related sites’.
Results
Table 1 shows gender-specific SIRs of bladder tumours in cancer patients. Results are presented for ‘any time’ and ‘at least 1 year’ between the two diagnoses. For example, 14 women developed bladder cancer after upper aerodigestive tract cancer. Their risk of bladder cancer was 2.22 times higher than the averaged risk in the general female population. When follow-up was started 1 year after first diagnosis, the number of patients decreased to 13, the SIR was 2.54. To limit the possible effect of surveillance bias due to first diagnosis, following description focuses on tumours diagnosed at least one year apart. Significant findings at the 0.01 confidence level are underlined in Table 1.
Table 1. Number and SIRs of second bladder tumours in cancer patients.
|
Women
|
Men
|
|||||||
|---|---|---|---|---|---|---|---|---|
|
Time between two diagnoses
|
Time between two diagnoses
|
|||||||
|
Any time
|
At least 1 year
|
Any time
|
At least 1 year
|
|||||
| First cancer type | N | SIR (95% CI) | N | SIR (95% CI) | N | SIR (95% CI) | N | SIR (95% CI) |
| Recurrent neoplasms | ||||||||
| Urinary bladder | 189 | 19.6 (17.0–22.7) | 132 | 10.5 (8.87–12.5) | 655 | 6.47 (5.98–6.99) | 501 | 5.66 (5.19–6.19) |
| Renal pelvis | 93 | 205 (167–251) | 48 | 61.4 (46.3–81.6) | 187 | 61.8 (53.5–71.3) | 108 | 23.4 (19.4–28.3) |
| Smoking-related cancer sites (excluding recurrent neoplasms) | ||||||||
| Upper aerodigestive tract | 14 | 2.22 (1.32–3.76) | 13 | 2.54 (1.47–4.37) | 105 | 1.18 (0.98–1.43) | 93 | 1.18 (0.96–1.45) |
| Oesophagus | 10 | 2.17 (1.17–4.03) | 6 | 2.64 (1.19–5.87) | ||||
| Stomach | 19 | 2.87 (1.83–4.51) | 16 | 3.29 (2.01–5.37) | 103 | 2.56 (2.11–3.11) | 80 | 2.57 (2.06–3.20) |
| Anus | 7 | 3.16 (1.51–6.63) | 5 | 2.12 (0.88–5.09) | 7 | 2.75 (1.31–5.77) | 5 | 2.63 (1.10–6.33) |
| Pancreas | 1 | 0.69 (0.10–4.93) | 9 | 1.47 (0.77–2.83) | 4 | 0.67 (0.25–1.80) | ||
| Nose | 2 | 1.23 (0.31–4.91) | 1 | 0.51 (0.07–3.60) | 13 | 1.60 (0.93–2.75) | 11 | 1.48 (0.82–2.66) |
| Larynx | 96 | 2.32 (1.90–2.84) | 88 | 2.34 (1.90–2.89) | ||||
| Lung | 24 | 2.69 (1.80–4.02) | 18 | 2.21 (1.39–3.51) | 151 | 2.95 (2.51–3.46) | 101 | 2.91 (2.40–3.54) |
| Cervix | 278 | 5.60 (4.97–6.31) | 258 | 5.45 (4.82–6.17) | ||||
| Renal parenchyma | 38 | 2.82 (2.05–3.88) | 25 | 1.57 (1.06–2.33) | 119 | 2.33 (1.94–2.78) | 84 | 1.38 (1.12–1.71) |
| Any smoking-related | 383 | 4.01 (3.62–4.43) | 336 | 3.84 (3.45–4.27) | 613 | 2.08 (1.92–2.25) | 472 | 1.75 (1.60–1.91) |
| Non-smoking-related cancer sites | ||||||||
| Salivary glands | 5 | 1.85 (0.77–4.45) | 5 | 1.97 (0.82–4.75) | 18 | 1.59 (1.00–2.53) | 15 | 1.48 (0.89–2.46) |
| Small intestine | 4 | 1.06 (0.40–2.83) | 4 | 1.22 (0.46–3.26) | 16 | 1.43 (0.88–2.34) | 11 | 0.96 (0.53–1.72) |
| Colon | 102 | 1.87 (1.54–2.27) | 89 | 1.71 (1.39–2.10) | 294 | 1.71 (1.52–1.92) | 253 | 1.86 (1.65–2.11) |
| Rectum | 43 | 1.77 (1.31–2.38) | 38 | 1.77 (1.28–2.43) | 174 | 2.50 (2.15–2.90) | 135 | 2.63 (2.22–3.11) |
| Liver | 6 | 2.66 (1.19–5.91) | 1 | 0.29 (0.04–2.04) | 20 | 14.1 (9.11–21.9) | 11 | 3.56 (1.97–6.43) |
| Breast | 378 | 1.77 (1.60–1.96) | 352 | 1.42 (1.28–1.58) | 4 | 0.44 (0.17–1.18) | 3 | 0.37 (0.12–1.14) |
| Endometrium | 212 | 2.80 (2.45–3.21) | 206 | 3.13 (2.73–3.60) | ||||
| Ovary | 85 | 2.63 (2.13–3.26) | 82 | 2.85 (2.29–3.54) | ||||
| Other female genital | 25 | 4.45 (3.01–6.59) | 19 | 3.99 (2.54–6.25) | ||||
| Prostate | 1721 | 3.99 (3.81–4.19) | 1000 | 2.05 (1.93–2.19) | ||||
| Testis | 70 | 2.91 (2.30–3.68) | 65 | 2.65 (2.08–3.38) | ||||
| Other male genital | 24 | 1.90 (1.28–2.84) | 17 | 1.39 (0.86–2.24) | ||||
| Melanoma | 39 | 1.24 (0.90–1.70) | 37 | 1.26 (0.92–1.75) | 139 | 1.29 (1.10–1.53) | 127 | 1.36 (1.14–1.62) |
| Skin, squamous cell | 52 | 1.66 (1.26–2.18) | 46 | 1.35 (1.01–1.80) | 258 | 1.17 (1.03–1.32) | 226 | 1.27 (1.12–1.45) |
| Eye | 2 | 0.63 (0.16–2.53) | 2 | 0.66 (0.16–2.64) | 16 | 6.00 (3.68–9.80) | 13 | 3.09 (1.80–5.33) |
| Nervous system | 33 | 1.44 (1.02–2.02) | 31 | 1.42 (1.00–2.01) | 59 | 1.13 (0.87–1.45) | 52 | 1.08 (0.82–1.41) |
| Thyroid gland | 15 | 0.93 (0.56–1.55) | 15 | 0.97 (0.59–1.61) | 15 | 2.00 (1.20–3.31) | 14 | 1.96 (1.16–3.31) |
| Endocrine glands | 44 | 1.12 (0.84–1.51) | 40 | 1.08 (0.79–1.47) | 53 | 1.21 (0.93–1.59) | 49 | 1.28 (0.96–1.69) |
| Bone | 1 | 0.57 (0.08–4.07) | 1 | 0.60 (0.09–4.29) | 7 | 1.56 (0.74–3.27) | 6 | 1.45 (0.65–3.23) |
| Connective tissue | 8 | 1.15 (0.58–2.30) | 8 | 1.24 (0.62–2.49) | 25 | 1.57 (1.06–2.32) | 21 | 1.53 (1.00–2.34) |
| Hodgkin disease | 3 | 1.25 (0.40–3.87) | 2 | 0.93 (0.23–3.70) | 17 | 1.39 (0.86–2.23) | 17 | 1.55 (0.96–2.49) |
| Non-Hodgkin lymphoma | 29 | 1.51 (1.05–2.18) | 27 | 1.61 (1.10–2.35) | 122 | 1.98 (1.66–2.37) | 102 | 1.95 (1.60–2.36) |
| Myeloma | 8 | 1.08 (0.54–2.17) | 4 | 0.69 (0.26–1.84) | 18 | 0.60 (0.38–0.95) | 14 | 0.47 (0.28–0.79) |
| Leukaemia | 22 | 2.18 (1.44–3.32) | 17 | 1.39 (0.87–2.24) | 92 | 1.83 (1.50–2.25) | 67 | 1.66 (1.31–2.11) |
| Any non-smoking related | 1116 | 1.80 (1.70–1.91) | 1026 | 1.77 (1.66–1.88) | 3162 | 2.04 (1.97–2.12) | 2218 | 1.52 (1.46–1.58) |
| Any site | 1807 | 2.47 (2.35–2.60) | 1560 | 2.22 (2.11–2.34) | 4657 | 2.40 (2.33–2.48) | 3324 | 1.86 (1.79–1.92) |
Abbreviations: CI=confidence interval; SIR=standardised incidence ratio.
Bold type represents a significant increase at the 5% confidence level, underlined SIRs were higher than 1.00 at the 1% confidence level.
Among female patients, the highest increases in risk were observed after renal pelvis (SIR=61.4) and bladder (SIR=10.5) neoplasms. The SIRs higher than 3.0 were found in women affected by cancer in the cervix, other female genital organs, stomach and endometrium. Statistically significant (P<0.01) increases were also observed after ovarian, lung, rectal, colonic and breast cancers. The SIR after cancer at any smoking-related site was 3.84 and it equalled SIR=1.77 after any non-smoking-related site. The averaged SIR of bladder neoplasms in women 1 year after diagnosis of any type of cancer was 2.22. Among men, the highest increases in the risk of bladder cancer were also observed after renal pelvis (SIR=23.4) and bladder (SIR=5.27) neoplasms. Interestingly, SIRs of recurrent neoplasms were significantly lower for male than for female patients. The highest SIRs in men were found after liver, eye and lung cancers. The SIRs in Table 1 permit to compare the risks of bladder neoplasms after diagnosis of different types of cancer. For example, men affected by lung cancer were at a higher risk of bladder tumours than prostate cancer patients (disjoint CIs).
In addition to shared risk factors, the diagnosis of two cancers in the same individual may be related to the treatment of the first tumour. Results in Table 2, based on tumours diagnosed at least 1 year apart, may help to discriminate between these two components. For example, Table 1 shows the SIR of 2.57 after stomach cancer in men. Bladder cancer patients did not show an increased risk of stomach cancer, thus favouring the effect of stomach cancer treatment over shared risk factors. The association between colorectal and bladder cancers was noticed in both directions, with similar risk increase in women. In men, the SIRs after bladder cancer were statistically lower than the SIRs in the opposite sequence. The association between lung and bladder tumours was observed in both directions. Female patients diagnosed with bladder cancer did not show an increased risk of breast and ovarian tumours, and a preventive effect was found for cervical cancer (SIR=0.41). These data probably signalise a contribution of treatment of breast, ovarian and cervical cancers to the development of subsequent bladder neoplasms. The SIR of endometrial cancer after bladder tumours was 4.83, higher than the SIR of bladder cancer after endometrial cancer. The relative risk of prostate neoplasms in bladder cancer patients (SIR=1.41) was significantly lower than the risk increase in the opposite direction.
Table 2. Number and SIRs of second tumours in urinary bladder cancer patients.
|
Women
|
Men
|
|||
|---|---|---|---|---|
| Second cancer type | N | SIR (95% CI) | N | SIR (95% CI) |
| Recurrent neoplasms | ||||
| Urinary bladder | 132 | 10.5 (8.87–12.5) | 501 | 5.66 (5.19–6.19) |
| Renal pelvis | 48 | 40.1 (30.0–53.5) | 154 | 34.5 (29.2–40.7) |
| Smoking-related cancer sites (excluding recurrent neoplasms) | ||||
| Upper aerodigestive tract | 6 | 0.73 (0.33–1.63) | 70 | 1.28 (1.01–1.61) |
| Oesophagus | 7 | 4.53 (2.16–9.51) | 47 | 0.87 (0.66–1.16) |
| Stomach | 19 | 0.95 (0.60–1.49) | 188 | 1.04 (0.90–1.20) |
| Anus | 2 | 1.36 (0.34–5.44) | 6 | 1.23 (0.55–2.76) |
| Pancreas | 42 | 1.54 (1.14–2.09) | 120 | 1.23 (1.02–1.47) |
| Nose | 1 | 0.38 (0.05–2.73) | 7 | 1.13 (0.54–2.38) |
| Larynx | 1 | 0.83 (0.12–5.89) | 48 | 1.35 (1.02–1.79) |
| Lung | 115 | 3.32 (2.76–3.99) | 645 | 2.00 (1.85–2.17) |
| Cervix | 13 | 0.41 (0.24–0.71) | ||
| Renal parenchyma | 16 | 0.60 (0.37–0.98) | 91 | 1.38 (1.12–1.69) |
| Any smoking-related | 222 | 1.58 (1.38–1.80) | 1222 | 1.50 (1.42–1.59) |
| Non-smoking-related cancer sites | ||||
| Salivary glands | 3 | 2.16 (0.70–6.71) | 7 | 1.35 (0.64–2.85) |
| Small intestine | 5 | 0.82 (0.34–1.97) | 32 | 1.81 (1.27–2.56) |
| Colon | 95 | 1.76 (1.44–2.15) | 352 | 1.21 (1.09–1.34) |
| Rectum | 46 | 1.67 (1.25–2.24) | 187 | 1.22 (1.06–1.41) |
| Liver | 34 | 2.06 (1.47–2.88) | 112 | 1.96 (1.63–2.36) |
| Breast | 195 | 0.98 (0.85–1.13) | 5 | 0.95 (0.39–2.29) |
| Endometrium | 52 | 4.83 (3.68–6.34) | ||
| Ovary | 31 | 0.71 (0.50–1.01) | ||
| Other female genital | 6 | 2.04 (0.91–4.54) | ||
| Prostate | 1454 | 1.41 (1.34–1.49) | ||
| Testis | 2 | 0.98 (0.24–3.91) | ||
| Other male genital | 11 | 0.81 (0.45–1.47) | ||
| Melanoma | 23 | 2.61 (1.74–3.93) | 107 | 1.18 (0.98–1.43) |
| Skin, squamous cell | 53 | 1.64 (1.26–2.15) | 272 | 1.30 (1.15–1.47) |
| Eye | 5 | 1.98 (0.82–4.76) | 13 | 3.62 (2.10–6.25) |
| Nervous system | 24 | 1.24 (0.83–1.85) | 70 | 26.3 (20.8–33.3) |
| Thyroid gland | 6 | 0.72 (0.32–1.60) | 19 | 1.69 (1.07–2.65) |
| Endocrine glands | 27 | 1.87 (1.28–2.72) | 34 | 1.72 (1.23–2.41) |
| Bone | 3 | 0.40 (0.13–1.25) | ||
| Connective tissue | 8 | 2.18 (1.09–4.37) | 27 | 1.30 (0.89–1.90) |
| Hodgkin disease | 2 | 2.54 (0.63–10.2) | 9 | 6.92 (3.60–13.3) |
| Non-Hodgkin lymphoma | 35 | 1.36 (0.98–1.90) | 110 | 0.97 (0.80–1.17) |
| Myeloma | 6 | 0.41 (0.18–0.92) | 58 | 9.97 (7.70–12.9) |
| Leukaemia | 36 | 4.53 (3.27–6.28) | 121 | 1.16 (0.97–1.38) |
| Any non-smoking related | 653 | 1.38 (1.28–1.49) | 3005 | 2.85 (2.75–2.95) |
| Any site | 1138 | 1.91 (1.80–2.03) | 5060 | 2.77 (2.69–2.84) |
Abbreviations: CI=confidence interval; SIR=standardised incidence ratio.
Only tumours diagnosed at least one year apart were included in the calculations.
Bold type represents a significant increase at the 5% confidence level.
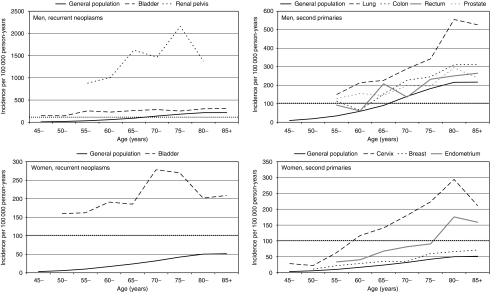
Figure 1 shows absolute incidence rates for cancer types with at least 100 patients affected by second bladder neoplasms, and for age intervals with at least five observed cases. The incidence rates of bladder cancer in the general population are also shown. Among men, the highest recurrence rate was observed among renal pelvis cancer patients (around 2 cases per 1000 person-years in the age band 75 to 85 years). The incidence was higher than 200 per 105 person-years in men with bladder cancer older than 55 years. Men affected by colon cancer reached an incidence of 300 per 105 person-years at age 80–85 years. More modest incidences were found among female patients. Recurrence rates after urinary bladder cancer were the highest (around 270 cases per 105 person-years) at age 70–79 years. Cervical cancer survivors showed the highest rate of bladder neoplasms at age 80–85 years. The estimated incidence of second bladder neoplasms in endometrial cancer patients was around 180 per 105 person-years in the age period of 80–85 years.
Figure 1.
Age- and gender-specific incidence rates of urinary bladder cancer in the general population and in patients affected by selected types of cancer. Only tumours diagnosed at least 1 year apart were included in the calculations. Results are presented for cancer sites with at least 100 patients affected by bladder cancer as second malignancy and for age intervals with at least five observed cases.
Consideration of the age of diagnosis of the first malignancy, together with patient's age, may result in more accurate estimates of the risk to develop secondary bladder neoplasms. Table 3 shows SIRs according to these categories. Results are only presented for cancer types with at least 100 patients affected by second bladder tumours, for testicular cancer and for cells with observed cases. Only tumours diagnosed at least 1 year apart were included in the calculations.
Table 3. Number and SIRs of urinary bladder tumours in cancer patients according to age and age-of-diagnosis of first malignancy.
|
Age of diagnosis of the first cancer
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|
|
Any
|
20–39 years
|
40–59 years
|
60+ years
|
||||||
| Cancer type (Gender) | Age (years) | N | SIR (95% CI) | N | SIR (95% CI) | N | SIR (95% CI) | N | SIR (95% CI) |
| Recurrent neoplasms | |||||||||
| Urinary bladder (females) | 46–55 | 6 | 31.7 (14.2–70.8) | 3 | 74.8 (24.1–232) | 3 | 20.4 (6.56–63.3) | ||
| 56–65 | 21 | 16.4 (10.7–25.2) | 4 | 44.0 (16.5–118) | 15 | 18.0 (10.8–29.9) | 2 | 7.03 (1.76–28.1) | |
| 66–75 | 46 | 7.64 (5.71–10.2) | 16 | 8.53 (5.22–13.9) | 30 | 7.16 (5.00–10.3) | |||
| 76–85 | 45 | 5.34 (3.97–7.16) | 8 | 5.76 (2.88–11.5) | 37 | 4.80 (3.47–6.64) | |||
| 85+ | 14 | 3.73 (2.20–6.32) | 14 | 3.95 (2.33–6.70) | |||||
| Urinary bladder (males) | <45 | 7 | 68.6 (32.6–144) | 4 | 40.9 (15.3–109) | 2 | 99.4 (24.8– 399) | ||
| 46–55 | 22 | 11.2 (7.37–17.0) | 8 | 13.3 (6.66–26.7) | 14 | 11.7 (6.92–19.8) | |||
| 56–65 | 94 | 5.26 (4.29–6.44) | 3 | 6.14 (1.98–19.0) | 80 | 5.14 (4.12–6.40) | 11 | 4.40 (2.43–7.95) | |
| 66–75 | 178 | 2.41 (2.08–2.80) | 2 | 6.87 (1.72–27.5) | 60 | 3.07 (2.38–3.96) | 116 | 2.09 (1.74–2.51) | |
| 76–85 | 155 | 1.28 (1.09–1.50) | 25 | 3.62 (2.44–5.36) | 130 | 1.16 (0.98–1.38) | |||
| 85+ | 45 | 1.38 (1.03–1.86) | 3 | 2.43 (0.78–7.55) | 42 | 1.33 (0.98–1.81) | |||
| Renal pelvis (females and males) | <45 | 1 | 189 (26.6–1344) | 1 | 618 (86.6–4413) | ||||
| 46–55 | 11 | 114 (63.3–207) | 1 | 11.6 (1.63–82.4) | 10 | 343 (184–638) | |||
| 56–65 | 27 | 35.1 (24.1–51.2) | 1 | 15.6 (2.19–110) | 13 | 23.7 (13.8–40.8) | 13 | 164 (95.4–283) | |
| 66–75 | 56 | 23.3 (17.9–30.2) | 5 | 5.65 (2.35–13.6) | 51 | 32.7 (24.8–43.0) | |||
| 76–85 | 54 | 15.6 (11.9–20.4) | 3 | 10.1 (3.25–31.2) | 51 | 17.6 (13.3–23.1) | |||
| 85+ | 7 | 8.97 (4.27–18.8) | 7 | 9.34 (4.45–19.6) | |||||
| Smoking-related cancer sites (excluding recurrent neoplasms) | |||||||||
| Lung (females and males) | 46–55 | 2 | 6.58 (1.65–26.3) | 2 | 9.90 (2.47–39.6) | ||||
| 56–65 | 21 | 2.93 (1.91–4.50) | 1 | 14.8 (2.08–105) | 17 | 2.83 (1.76–4.55) | 3 | 1.80 (0.58–5.57) | |
| 66–75 | 46 | 2.30 (1.72–3.07) | 9 | 1.46 (0.76–2.81) | 37 | 2.49 (1.81–3.44) | |||
| 76–85 | 44 | 2.21 (1.65–2.98) | 5 | 1.92 (0.80–4.62) | 39 | 2.18 (1.59–2.99) | |||
| 85+ | 6 | 2.58 (1.16–5.74) | 6 | 2.78 (1.25–6.20) | |||||
| Cervix (females) | <45 | 1 | 5.62 (0.79–40.0) | 1 | 5.63 (0.79–40.1) | ||||
| 46–55 | 17 | 5.42 (3.36–8.76) | 10 | 5.70 (3.05–10.6) | 7 | 5.46 (2.59–11.5) | |||
| 56–65 | 63 | 5.83 (4.54–7.49) | 23 | 6.97 (4.62–10.5) | 39 | 4.88 (3.56– 6.7) | 1 | 2.73 (0.38–19.4) | |
| 66–75 | 89 | 5.40 (4.37–6.67) | 19 | 6.07 (3.87–9.54) | 61 | 6.09 (4.73–7.85) | 9 | 2.59 (1.35–4.98) | |
| 76–85 | 75 | 5.64 (4.48–7.09) | 10 | 6.53 (3.51–12.2) | 47 | 6.72 (5.04–8.97) | 18 | 6.54 (4.11–10.4) | |
| 85+ | 13 | 4.00 (2.32–6.92) | 1 | 138 (19.4–981) | 6 | 5.33 (2.39–11.9) | 6 | 3.52 (1.58–7.87) | |
| Renal parenchyma (females and males) | 46–55 | 1 | 0.63 (0.09–4.44) | 1 | 0.87 (0.12–6.19) | ||||
| 56–65 | 16 | 1.65 (1.01–2.69) | 13 | 1.75 (1.01–3.01) | 3 | 2.40 (0.77–7.45) | |||
| 66–75 | 45 | 1.72 (1.28–2.30) | 19 | 1.99 (1.27–3.12) | 26 | 1.65 (1.12–2.42) | |||
| 76–85 | 42 | 1.42 (1.05–1.93) | 1 | 12.8 (1.80–90.9) | 6 | 1.13 (0.51–2.51) | 35 | 1.46 (1.05–2.04) | |
| 85+ | 5 | 0.94 (0.39–2.27) | 1 | 1.56 (0.22–11.1) | 4 | 0.88 (0.33–2.35) | |||
| Non-smoking-related cancer sites | |||||||||
| Colorectum (females and males) | <45 | 3 | 4.57 (1.47–14.2) | 1 | 4.45 (0.63–31.6) | 2 | 27.1 (6.75–108) | ||
| 46–55 | 10 | 2.92 (1.57–5.44) | 1 | 1.38 (0.19–9.80) | 9 | 4.12 (2.14–7.93) | |||
| 56–65 | 35 | 1.57 (1.12–2.18) | 3 | 2.15 (0.69–6.68) | 24 | 1.38 (0.92–2.06) | 8 | 1.54 (0.77–3.07) | |
| 66–75 | 161 | 1.50 (1.28–1.75) | 41 | 1.63 (1.20–2.21) | 120 | 1.60 (1.34–1.92) | |||
| 76–85 | 236 | 1.33 (1.17–1.52) | 26 | 1.60 (1.09–2.35) | 210 | 1.32 (1.15–1.51) | |||
| 85+ | 78 | 1.23 (0.98–1.54) | 2 | 0.67 (0.17–2.67) | 76 | 1.25 (1.00–1.57) | |||
| Breast (females) | 46–55 | 16 | 2.10 (1.28–3.45) | 2 | 1.29 (0.32–5.16) | 14 | 2.07 (1.22–3.50) | ||
| 56–65 | 72 | 1.71 (1.36–2.17) | 3 | 1.01 (0.33–3.15) | 59 | 1.63 (1.26–2.11) | 10 | 2.44 (1.31–4.54) | |
| 66–75 | 101 | 1.25 (1.03–1.53) | 2 | 1.10 (0.28–4.41) | 38 | 0.89 (0.64–1.22) | 61 | 1.42 (1.10–1.83) | |
| 76–85 | 122 | 1.38 (1.15–1.66) | 30 | 1.75 (1.22–2.51) | 92 | 1.34 (1.09–1.65) | |||
| 85+ | 41 | 1.33 (0.97–1.82) | 3 | 0.76 (0.25–2.37) | 38 | 1.37 (0.99–1.90) | |||
| Endometrium (females) | <45 | 1 | 17.7 (2.49–126) | 1 | 32.4 (4.54–231) | ||||
| 46–55 | 2 | 1.72 (0.43–6.87) | 2 | 1.85 (0.46–7.42) | |||||
| 56–65 | 27 | 2.70 (1.84–3.94) | 2 | 8.03 (2.01–32.1) | 20 | 2.35 (1.51–3.65) | 5 | 3.59 (1.49–8.65) | |
| 66–75 | 70 | 2.97 (2.34–3.76) | 49 | 3.58 (2.70–4.75) | 21 | 2.13 (1.39–3.27) | |||
| 76–85 | 82 | 2.44 (1.96–3.04) | 43 | 4.19 (3.10–5.66) | 39 | 1.74 (1.27–2.39) | |||
| 85+ | 24 | 2.75 (1.83–4.13) | 13 | 6.88 (3.98–11.9) | 11 | 1.56 (0.86–2.84) | |||
| Prostate (males) | 46–55 | 4 | 6.07 (2.28–16.2) | 4 | 6.07 (2.28–16.2) | ||||
| 56–65 | 69 | 3.10 (2.44–3.93) | 45 | 2.80 (2.09–3.75) | 24 | 3.21 (2.15–4.79) | |||
| 66–75 | 290 | 1.30 (1.16–1.46) | 17 | 1.16 (0.72–1.86) | 273 | 1.31 (1.16–1.48) | |||
| 76–85 | 495 | 1.12 (1.02–1.22) | 4 | 0.93 (0.35–2.49) | 491 | 1.12 (1.02–1.23) | |||
| 85+ | 142 | 1.03 (0.87–1.22) | 142 | 1.03 (0.87–1.22) | |||||
| Testis (males) | <45 | 3 | 2.81 (0.91–8.74) | 3 | 3.15 (1.01–9.78) | ||||
| 46–55 | 10 | 2.43 (1.31–4.52) | 8 | 3.12 (1.56–6.24) | 2 | 1.66 (0.42–6.65) | |||
| 56–65 | 21 | 2.93 (1.91–4.50) | 13 | 6.20 (3.60–10.7) | 7 | 1.84 (0.88–3.86) | 1 | 13.1 (1.84–92.9) | |
| 66–75 | 22 | 2.41 (1.59–3.67) | 13 | 6.33 (3.67–10.9) | 9 | 1.96 (1.02–3.77) | |||
| 76–85 | 8 | 2.68 (1.34–5.37) | 6 | 3.72 (1.67–8.28) | 2 | 3.23 (0.81–12.9) | |||
| 85+ | 1 | 2.99 (0.42–21.3) | 1 | 4.07 (0.57–28.9) | |||||
| Melanoma (females and males) | <45 | 1 | 0.75 (0.10–5.30) | 1 | 0.95 (0.13–6.73) | ||||
| 46–55 | 12 | 2.91 (1.65–5.13) | 3 | 2.24 (0.72–6.96) | 9 | 3.00 (1.56–5.77) | |||
| 56–65 | 31 | 1.45 (1.02–2.06) | 4 | 1.13 (0.43–3.02) | 25 | 1.66 (1.12–2.45) | 2 | 0.70 (0.18–2.80) | |
| 66–75 | 52 | 1.18 (0.90–1.54) | 1 | 0.89 (0.12–6.30) | 16 | 2.01 (1.23–3.28) | 35 | 2.03 (1.46–2.83) | |
| 76–85 | 53 | 1.07 (0.82–1.40) | 7 | 0.91 (0.43–1.91) | 46 | 1.12 (0.84–1.50) | |||
| 85+ | 15 | 1.01 (0.61–1.68) | 2 | 1.70 (0.43–6.81) | 13 | 0.91 (0.53–1.57) | |||
| Skin, squamous cell (females and males) | 56–65 | 13 | 1.33 (0.77–2.29) | 1 | 0.40 (0.06–2.82) | 10 | 1.32 (0.71–2.45) | 2 | 1.17 (0.29–4.68) |
| 66–75 | 58 | 1.44 (1.11–1.86) | 13 | 1.15 (0.67–1.98) | 45 | 1.62 (1.21–2.16) | |||
| 76–85 | 131 | 1.60 (1.34–1.90) | 9 | 1.84 (0.96–3.54) | 122 | 1.59 (1.33–1.90) | |||
| 85+ | 70 | 1.56 (1.23–1.98) | 1 | 1.01 (0.14–7.20) | 69 | 1.56 (1.23–1.98) | |||
| Non-Hodgkin lymphoma (females and males) | <45 | 4 | 6.43 (2.41–17.1) | 3 | 7.07 (2.28–22.0) | ||||
| 46–55 | 5 | 1.97 (0.82–4.75) | 3 | 4.83 (1.56–15.0) | 2 | 0.96 (0.24–3.86) | |||
| 56–65 | 24 | 1.95 (1.31–2.91) | 4 | 4.36 (1.64–11.6) | 19 | 1.89 (1.20–2.96) | 1 | 0.96 (0.14–6.82) | |
| 66–75 | 49 | 1.87 (1.41–2.48) | 1 | 10.8 (1.52–76.5) | 19 | 3.02 (1.93–4.74) | 29 | 1.42 (0.99–2.04) | |
| 76–85 | 33 | 1.07 (0.76–1.50) | 3 | 7.07 (2.28–22.0) | 6 | 2.61 (1.17–5.81) | 27 | 0.92 (0.63–1.34) | |
| 85+ | 14 | 2.20 (1.30–3.72) | 2 | 4.24 (1.06–17.0) | 12 | 2.00 (1.13–3.52) | |||
Abbreviations: CI=confidence interval; SIR=standardised incidence ratio.
Results are presented for categories with observed cases. Only tumours diagnosed at least one year apart were included in the calculations.
Bold type represents a significant increase at the 5% confidence level.
The SIRs of recurrent bladder neoplasms in bladder cancer patients decreased with age in both men and women, and they were markedly higher in women compared with those in men. The SIRs after renal pelvis cancer also decreased with increasing ages and with increasing lead times. The SIRs in lung cancer patients showed a U-shaped pattern. Cervical cancer patients showed the highest SIRs of bladder neoplasms in the age period of 56–65 years (SIR=5.83) and women diagnosed with cervical cancer at age 40–59 years showed the highest increase in risk of bladder neoplasms 16–25 years after the first diagnosis. The SIR after renal parenchyma cancer was almost constant.
Colorectal cancer patients showed decreasing SIRs with increasing ages. The SIRs according to the age of diagnosis of the first malignancy are presented in the three right columns of Table 3. For example, the SIR of bladder cancer was 1.63 in patients aged 66–75 years who were diagnosed with colorectal cancer at the age of 40–59 years. Women affected by breast cancer showed decreasing SIRs with increasing ages. The risk pattern was bimodal in women diagnosed at age 40–59 years, with the first maximum shortly after first diagnosis and a second maximum after 16–25 years. Long latency times were also noticed in women affected by endometrial cancer. Prostate cancer patients showed decreasing SIRs with increasing ages, and with increasing time since first diagnosis. By contrast, the pattern of risk in men affected by testicular cancer favoured treatment effects. Men affected by non-Hodgkin lymphoma showed SIR maxima in the age intervals ‘before age 45 years’, ‘56–65 years’ and ‘after 85 years of age’.
Discussion
In the near future, urologists will face an increasing number of cancer survivors visiting their practices. This study investigates the risk of second bladder neoplasms in cancer patients. We used two different strategies to discriminate between risk factors shared by subsequent malignancies and the effect of first cancer treatment. First, we compared the SIRs of bladder tumours in cancer patients with the SIRs of second cancers in patients affected by bladder tumours. The second approach investigated the pattern of risk after first tumours: asynchronous constant or decreasing relative risks may favour shared risk factors, whereas increasing risks with increasing lead time should point to treatment effects (Heard et al, 2005). There is an excellent literature on the development of bladder tumours after specific types of cancer, for example after prostate, oesophageal, testicular, cervical and lung tumours, and after lymphomas (Kaldor et al, 1987; Pettersson et al, 1990; Pathak et al, 1992; Bjorge et al, 1995; Bokemeyer and Schmoll, 1995; Travis et al, 1995, 1997; Levi et al, 1996, 1999; Fisher et al, 1997; Teppo et al, 2001; Dores et al, 2002; Brennan et al, 2005; Heard et al, 2005; Liauw et al, 2006; Bostrom and Soloway, 2007; Chaturvedi et al, 2007; Kellen et al, 2007; Landgren et al, 2007; Muller et al, 2007; Chuang et al, 2008; Singh et al, 2008). The main advantage of this study was the simultaneous investigation of the most common types of cancer before bladder tumours using a uniform reference population. Unfortunately, information on disease grade, treatment and smoking history was not available. The number of affected patients was small for some combinations of first cancer types and age categories, and chance due to multiple comparisons probably explains some of the detected associations.
In addition to lung and bladder cancers, tobacco smoking has been related to an increased risk of kidney, oral cavity, larynx, oesophagus, pancreas and stomach tumours (IARC, 2004). Table 4 shows a summary of risk factors that may result in the development of urinary bladder neoplasms in cancer patients. Although this list is not comprehensive and some readers would attribute a rather minor effect to some of the enumerated factors, we consider that the table may facilitate the interpretation of present results in the following discussion.
Table 4. Summary of major risk factors that may result in the development of urinary bladder neoplasms in cancer patients.
| Risk factor | Type of malignancy |
|---|---|
| Smoking | Lung, larynx, oral cavity, oesophagus, anus, stomach, pancreas, cervix and kidney cancers |
| Workplace | Leukaemia, stomach and lung cancers (rubber workers) |
| exposures | Cancer in the nasal cavity (textile workers) |
| Lung, renal pelvis and liver cancers (printing companies) | |
| Chemotherapy | Lymphomas, leukaemia, ovary and breast cancers |
| Radiation therapy | Cervix, rectum, anus, testis and prostate cancers |
| Arsenic in water | Lung and liver cancers |
With the exception of pancreatic cancer, characterised by a very poor prognosis, our data confirmed that patients affected by tobacco-related malignancies were at an increased risk of bladder tumours. Tobacco smoking confers the highest risk to lung cancer, which was reflected in SIRs of 2.21 for women and 2.91 for men. The estimated SIRs were in agreement with a previous Finnish study that investigated the risk of new primary tumours in lung cancer patients and suggested that the increased use of cytostatic drugs may increase the risk of second tumours (Teppo et al, 2001). Recent studies have shown that patients with non-muscle invasive bladder cancer suffer a high incidence of mortality from lung cancer and they might constitute a suitable population for a lung screening trial (Rusthoven et al, 2008). These data indicate that men affected by other tobacco-related cancers, in particular by stomach, larynx and lung tumours, belong to the population at high-risk for bladder cancer.
We found a borderline increased risk of bladder cancer (SIR=1.61) in women affected by non-Hodgkin lymphoma, the SIR was 1.95 for male patients. These estimates were slightly higher than the overall SIR of 1.50 found in an international study, which included part of the Swedish cohort (Brennan et al, 2005; Hemminki et al, 2008). International studies take benefit from a large number of patients, but standard registration and homogeneity in treatment and exposure were important plus factors of this study. The increase in risk with time since diagnosis in the two studies underlines the effect of treatment for non-Hodgkin lymphoma on the risk of second bladder neoplasms. Treatment regimes for non-Hodgkin lymphoma typically involve chemotherapy with cyclophosphamide, which has been associated with a 4.5- to 10-fold increased risk of bladder cancer (Travis et al, 1995). Localised radiotherapy may be also applied for low-grade subtypes of lymphoma, which may additively interact with cyclophosphamide treatment. The present simultaneous consideration of age at first diagnosis and time since diagnosis suggested that treatment effects are particularly important when lymphomas are diagnosed after 40 years of age. Cyclophosphamide is also used, often in combination with other drugs, to treat leukaemia and this may contribute to the increased SIRs we found among male leukaemia patients (Simister, 1971).
Women affected by cervical cancer showed a large increase in their risk of bladder tumours, and reversed analyses suggested an important contribution of cervical cancer treatment. A recent study that included Swedish data found an increased risk in patients treated with and without radiotherapy (Chaturvedi et al, 2007). The SIRs around 4 have been reported in studies based on women from the United States (Fisher et al, 1997). These data were in agreement with these studies and indicated that survivors of cervical cancer older than 65 years clearly belong to the population at high-risk for bladder cancer. In general, cervical cancer patients smoke more than women in the general population, and some of the malignancies that were diagnosed close in time were probably related to tobacco smoking. We observed that the risk of bladder cancer increased among women affected by tumours at other genital organs than the cervix, for example, the endometrium.
Previous studies have shown that men with testicular cancer continue to be at significantly an elevated risk of second bladder cancer for more than two decades after initial diagnosis (Travis et al, 1997). These data corroborated this result and showed that, even 26–35 years after testicular cancer diagnosed before the age of 40 years, the SIR was around 6. Although the estimated dose of radiation to the bladder is generally higher after non-seminoma than after seminoma treatment, studies that have taken the histological type into account show similar SIRs after the two types of germ-cell tumours. The use of radiotherapy fields to treat testicular cancer has decreased in recent decades and lower doses are used now. However, it has been hypothesised that cisplatin may act as radiation enhancer and contribute to a shortened latency periods for radiogenic bladder cancer. Histological information is available in the Swedish Family-Cancer Database, and future studies may add population-based evidence to this respect.
The within-patient clustering of bladder and prostate tumours has been extensively explored (Liauw et al, 2006; Bostrom and Soloway, 2007; Kellen et al, 2007; Bostrom et al, 2008; Singh et al, 2008). In contrast with testicular cancer, which showed increasing SIRs with increasing latency times, the SIRs of bladder neoplasms decreased with the time after diagnosis of prostate cancer. The reversed analyses also suggested a major contribution of risk factors shared by bladder and prostate tumours. Relevant to the urological clinical practice, our results indicated that men older than 60 years affected by prostate cancer show an excess of second primary bladder tumours. Previous studies have shown that detection bias may have an important role in the first year of follow-up, and our results confirmed this statement (Kellen et al, 2007). Out of 1721 patients affected by bladder after prostate cancer, (1721−1000)/1721=42% were diagnosed with the two tumours within 1 year. A similar proportion was observed when the opposite sequence was investigated. Prostate cancer radiotherapy has been suggested to induce secondary malignancies in the bladder, but if radiation were a central issue, SIRs would increase with follow-up time, and this trend was not observed (Liauw et al, 2006).
Mutations in DNA repair genes (XRCC3) and variants in genes coding for xenobiotic-transforming enzymes (NAT2, GSTM1, GSTP1 and NQO1) have been shown to modify the susceptibility to bladder cancer (Chao et al, 2006; Chaturvedi et al, 2007; Easton et al, 2007; Figueroa et al, 2007; Kellen et al, 2007; Sanderson et al, 2007; Murta-Nascimento et al, 2007b). These variants may be associated with an increased risk of cancer at additional sites. For example, two meta-analyses that explored the relevance of GSTM1 null status on stomach cancer found a modest risk increase (La Torre et al, 2005; Saadat, 2006). A recent genome-wide association study identified a novel variant, which confers an increased risk for both urinary bladder and lung cancers (Kiemeney et al, 2008). These polymorphisms are relatively common in Swedes, but the low penetrances conferred by the risk alleles (genotype relative risks between 1.2 and 1.5) result in a limited contribution of the variants to the increased risk of bladder neoplasms among cancer patients.
These data may be used to design future studies that investigate the effectiveness of bladder cancer screening in cancer patients. Although the definition of the population at high risk depends on multiple interacting factors, men older than 60 years of age and a smoking history have been traditionally considered to be at high risk (Grossman et al, 2005). The incidence of bladder cancer among men of 60 years of age in the Swedish Family-Cancer Database was around 60 cases per 105 person-years. The combination of this figure with a smoking prevalence of 40% (Stegmayr et al, 2005), and with an odds ratio of bladder cancer for smokers versus non-smokers of five, results in an incidence of bladder cancer of 115 cases per 105 person-years for men older than 60 years with a smoking history. We drew a horizontal line on Figure 1 to characterise the groups of high-risk patients according to this criterion. As expected, bladder and renal pelvis cancer patients show very high recurrence risks. After lung cancer, for every 1000 survivors, approximately 5 developed secondary bladder cancer in the age interval 80–85 years. The threshold of 100 cases per 105 person-years was reached at the age of 60–65 years by male patients with colorectal and prostate cancers. Among women, high-risk groups consisted of cervical cancer patients older than 55 years and endometrial cancer patients older than 75 years. Note that absolute incidences among cancer patients can be approximated by combining the SIRs in Table 3 with population-specific incidence rates.
The present data may help urologists to assess the risk of bladder neoplasms in cancer survivors, who are going to ask for clinical advice with increasing frequency in the near future. Our results should also guide future studies that investigate the effectiveness of bladder cancer screening in cancer patients. The contribution of known genetic variants to the observed associations is probably minor. Treatment of the first malignancy, in particular chemotherapy with cyclophosphamide and cisplatin, and also radiotherapy, probably have a larger impact. Smoking can be instrumental in the development of bladder cancer after tumours in tobacco-related sites.
Acknowledgments
Supported by Deutsche Krebshilfe, the Swedish Cancer Society, The Swedish Council for Working Life and Social Research and the EU, LSHC-CT-2004-503465. The Family-Cancer Database was created by linking registers maintained at Statistics Sweden and the Swedish Cancer Registry.
References
- Andrew AS, Karagas MR, Nelson HH, Guarrera S, Polidoro S, Gamberini S, Sacerdote C, Moore JH, Kelsey KT, Demidenko E, Vineis P, Matullo G (2008) DNA repair polymorphisms modify bladder cancer risk: a multi-factor analytic strategy. Hum Hered 65: 105–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermejo JL, Sundquist J, Hemminki K (2008) Sex-specific familial risks of urinary bladder cancer and associated neoplasms in Sweden. Int J Cancer 124(9): 2166–2171 [DOI] [PubMed] [Google Scholar]
- Bjorge T, Hennig EM, Skare GB, Soreide O, Thoresen SO (1995) Second primary cancers in patients with carcinoma in situ of the uterine cervix. The Norwegian experience 1970–1992. Int J Cancer 62: 29–33 [DOI] [PubMed] [Google Scholar]
- Bokemeyer C, Schmoll HJ (1995) Treatment of testicular cancer and the development of secondary malignancies. J Clin Oncol 13: 283–292 [DOI] [PubMed] [Google Scholar]
- Bostrom PJ, Soloway MS (2007) Secondary cancer after radiotherapy for prostate cancer: should we be more aware of the risk? Eur Urol 52: 973–982 [DOI] [PubMed] [Google Scholar]
- Bostrom PJ, Soloway MS, Manoharan M, Ayyathurai R, Samavedi S (2008) Bladder cancer after radiotherapy for prostate cancer: detailed analysis of pathological features and outcome after radical cystectomy. J Urol 179: 91–95; discussion 95 [DOI] [PubMed] [Google Scholar]
- Brennan P, Bogillot O, Cordier S, Greiser E, Schill W, Vineis P, Lopez-Abente G, Tzonou A, Chang-Claude J, Bolm-Audorff U, Jockel KH, Donato F, Serra C, Wahrendorf J, Hours M, T’Mannetje A, Kogevinas M, Boffetta P (2000) Cigarette smoking and bladder cancer in men: a pooled analysis of 11 case-control studies. Int J Cancer 86: 289–294 [DOI] [PubMed] [Google Scholar]
- Brennan P, Bogillot O, Greiser E, Chang-Claude J, Wahrendorf J, Cordier S, Jockel KH, Lopez-Abente G, Tzonou A, Vineis P, Donato F, Hours M, Serra C, Bolm-Audorff U, Schill W, Kogevinas M, Boffetta P (2001) The contribution of cigarette smoking to bladder cancer in women (pooled European data). Cancer Causes Control 12: 411–417 [DOI] [PubMed] [Google Scholar]
- Brennan P, Scelo G, Hemminki K, Mellemkjaer L, Tracey E, Andersen A, Brewster DH, Pukkala E, McBride ML, Kliewer EV, Tonita JM, Seow A, Pompe-Kirn V, Martos C, Jonasson JG, Colin D, Boffetta P (2005) Second primary cancers among 109 000 cases of non-Hodgkin's lymphoma. Br J Cancer 93: 159–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao C, Zhang ZF, Berthiller J, Boffetta P, Hashibe M (2006) NAD(P)H:quinone oxidoreductase 1 (NQO1) Pro187Ser polymorphism and the risk of lung, bladder, and colorectal cancers: a meta-analysis. Cancer Epidemiol Biomarkers Prev 15: 979–987 [DOI] [PubMed] [Google Scholar]
- Chaturvedi AK, Engels EA, Gilbert ES, Chen BE, Storm H, Lynch CF, Hall P, Langmark F, Pukkala E, Kaijser M, Andersson M, Fossa SD, Joensuu H, Boice JD, Kleinerman RA, Travis LB (2007) Second cancers among 104,760 survivors of cervical cancer: evaluation of long-term risk. J Natl Cancer Inst 99: 1634–1643 [DOI] [PubMed] [Google Scholar]
- Chuang SC, Hashibe M, Scelo G, Brewster DH, Pukkala E, Friis S, Tracey E, Weiderpass E, Hemminki K, Tamaro S, Chia KS, Pompe-Kirn V, Kliewer EV, Tonita JM, Martos C, Jonasson JG, Dresler CM, Boffetta P, Brennan P (2008) Risk of second primary cancer among esophageal cancer patients: a pooled analysis of 13 cancer registries. Cancer Epidemiol Biomarkers Prev 17: 1543–1549 [DOI] [PubMed] [Google Scholar]
- Dores GM, Metayer C, Curtis RE, Lynch CF, Clarke EA, Glimelius B, Storm H, Pukkala E, van Leeuwen FE, Holowaty EJ, Andersson M, Wiklund T, Joensuu T, van’t Veer MB, Stovall M, Gospodarowicz M, Travis LB (2002) Second malignant neoplasms among long-term survivors of Hodgkin's disease: a population-based evaluation over 25 years. J Clin Oncol 20: 3484–3494 [DOI] [PubMed] [Google Scholar]
- Easton DF, Pooley KA, Dunning AM, Pharoah PD, Thompson D, Ballinger DG, Struewing JP, Morrison J, Field H, Luben R, Wareham N, Ahmed S, Healey CS, Bowman R, Meyer KB, Haiman CA, Kolonel LK, Henderson BE, Le Marchand L, Brennan P, Sangrajrang S, Gaborieau V, Odefrey F, Shen CY, Wu PE, Wang HC, Eccles D, Evans DG, Peto J, Fletcher O, Johnson N, Seal S, Stratton MR, Rahman N, Chenevix-Trench G, Bojesen SE, Nordestgaard BG, Axelsson CK, Garcia-Closas M, Brinton L, Chanock S, Lissowska J, Peplonska B, Nevanlinna H, Fagerholm R, Eerola H, Kang D, Yoo KY, Noh DY, Ahn SH, Hunter DJ, Hankinson SE, Cox DG, Hall P, Wedren S, Liu J, Low YL, Bogdanova N, Schurmann P, Dork T, Tollenaar RA, Jacobi CE, Devilee P, Klijn JG, Sigurdson AJ, Doody MM, Alexander BH, Zhang J, Cox A, Brock IW, MacPherson G, Reed MW, Couch FJ, Goode EL, Olson JE, Meijers-Heijboer H, van den Ouweland A, Uitterlinden A, Rivadeneira F, Milne RL, Ribas G, Gonzalez-Neira A, Benitez J, Hopper JL, McCredie M, Southey M, Giles GG, Schroen C, Justenhoven C, Brauch H, Hamann U, Ko YD, Spurdle AB, Beesley J, Chen X, Mannermaa A, Kosma VM, Kataja V, Hartikainen J, Day NE, Cox DR, Ponder BA (2007) Genome-wide association study identifies novel breast cancer susceptibility loci. Nature 447: 1087–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engholm G, Storm HH, Ferlay J, Christensen N, Bray F, Olafsdottir E, Pukkala E, Talbäck M (2007) Software reference NORDCAN: Cancer Incidence And Mortality In The Nordic Countries, Version 3.0. Association of Nordic Cancer Registries. Danish Cancer Society
- Figueroa JD, Malats N, Rothman N, Real FX, Silverman D, Kogevinas M, Chanock S, Yeager M, Welch R, Dosemeci M, Tardon A, Serra C, Carrato A, Garcia-Closas R, Castano-Vinyals G, Garcia-Closas M (2007) Evaluation of genetic variation in the double-strand break repair pathway and bladder cancer risk. Carcinogenesis 28: 1788–1793 [DOI] [PubMed] [Google Scholar]
- Fisher G, Harlow SD, Schottenfeld D (1997) Cumulative risk of second primary cancers in women with index primary cancers of uterine cervix and incidence of lower anogenital tract cancers, Michigan, 1985–1992. Gynecol Oncol 64: 213–223 [DOI] [PubMed] [Google Scholar]
- Gandini S, Botteri E, Iodice S, Boniol M, Lowenfels AB, Maisonneuve P, Boyle P (2008) Tobacco smoking and cancer: a meta-analysis. Int J Cancer 122: 155–164 [DOI] [PubMed] [Google Scholar]
- Grossman HB, Messing E, Soloway M, Tomera K, Katz G, Berger Y, Shen Y (2005) Detection of bladder cancer using a point-of-care proteomic assay. JAMA 293: 810–816 [DOI] [PubMed] [Google Scholar]
- Heard A, Roder D, Luke C (2005) Multiple primary cancers of separate organ sites: implications for research and cancer control (Australia). Cancer Causes Control 16: 475–481 [DOI] [PubMed] [Google Scholar]
- Hemelt M, Yamamoto H, Cheng KK, Zeegers MP (2008) The effect of smoking on the male excess of bladder cancer: a meta-analysis and geographical analyses. Int J Cancer 124(2): 412–419 [DOI] [PubMed] [Google Scholar]
- Hemminki K, Lenner P, Sundquist J, Bermejo JL (2008) Risk of subsequent solid tumors after non-Hodgkin's lymphoma: effect of diagnostic age and time since diagnosis. J Clin Oncol 26: 1850–1857 [DOI] [PubMed] [Google Scholar]
- IARC (2004) Tobacco Smoke And Involuntary Smoking Vol. 83. World Health Organisation. International Agency for Research on Cancer: Lyon, France [Google Scholar]
- Kaldor JM, Day NE, Band P, Choi NW, Clarke EA, Coleman MP, Hakama M, Koch M, Langmark F, Neal FE, Pettersson F, Pompe-Kim V, Prior P, Storm HH (1987) Second malignancies following testicular cancer, ovarian cancer and Hodgkin's disease: an international collaborative study among cancer registries. Int J Cancer 39: 571–585 [DOI] [PubMed] [Google Scholar]
- Kellen E, Zeegers MP, Dirx M, Houterman S, Droste J, Lawrence G, Truyers C, Bruckers L, Molenberghs G, Joniau S, Buntinx F (2007) Occurrence of both bladder and prostate cancer in five cancer registries in Belgium, The Netherlands and the United Kingdom. Eur J Cancer 43: 1694–1700 [DOI] [PubMed] [Google Scholar]
- Kiemeney LA, Thorlacius S, Sulem P, Geller F, Aben KK, Stacey SN, Gudmundsson J, Jakobsdottir M, Bergthorsson JT, Sigurdsson A, Blondal T, Witjes JA, Vermeulen SH, Hulsbergen-van de Kaa CA, Swinkels DW, Ploeg M, Cornel EB, Vergunst H, Thorgeirsson TE, Gudbjartsson D, Gudjonsson SA, Thorleifsson G, Kristinsson KT, Mouy M, Snorradottir S, Placidi D, Campagna M, Arici C, Koppova K, Gurzau E, Rudnai P, Kellen E, Polidoro S, Guarrera S, Sacerdote C, Sanchez M, Saez B, Valdivia G, Ryk C, de Verdier P, Lindblom A, Golka K, Bishop DT, Knowles MA, Nikulasson S, Petursdottir V, Jonsson E, Geirsson G, Kristjansson B, Mayordomo JI, Steineck G, Porru S, Buntinx F, Zeegers MP, Fletcher T, Kumar R, Matullo G, Vineis P, Kiltie AE, Gulcher JR, Thorsteinsdottir U, Kong A, Rafnar T, Stefansson K (2008) Sequence variant on 8q24 confers susceptibility to urinary bladder cancer. Nat Genet 40: 1307–1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Torre G, Boccia S, Ricciardi G (2005) Glutathione S-transferase M1 status and gastric cancer risk: a meta-analysis. Cancer Lett 217: 53–60 [DOI] [PubMed] [Google Scholar]
- Landgren O, Pfeiffer RM, Stewart L, Gridley G, Mellemkjaer L, Hemminki K, Goldin LR, Travis LB (2007) Risk of second malignant neoplasms among lymphoma patients with a family history of cancer. Int J Cancer 120: 1099–1102 [DOI] [PubMed] [Google Scholar]
- Levi F, Randimbison L, La Vecchia C, Franceschi S (1996) Incidence of invasive cancers following carcinoma in situ of the cervix. Br J Cancer 74: 1321–1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi F, Randimbison L, Te VC, La Vecchia C (1999) Second primary cancers in patients with lung carcinoma. Cancer 86: 186–190 [DOI] [PubMed] [Google Scholar]
- Liauw SL, Sylvester JE, Morris CG, Blasko JC, Grimm PD (2006) Second malignancies after prostate brachytherapy: incidence of bladder and colorectal cancers in patients with 15 years of potential follow-up. Int J Radiat Oncol Biol Phys 66: 669–673 [DOI] [PubMed] [Google Scholar]
- Muller AC, Ganswindt U, Bamberg M, Belka C (2007) Risk of second malignancies after prostate irradiation? Strahlenther Onkol 183: 605–609 [DOI] [PubMed] [Google Scholar]
- Murta-Nascimento C, Schmitz-Drager BJ, Zeegers MP, Steineck G, Kogevinas M, Real FX, Malats N (2007a) Epidemiology of urinary bladder cancer: from tumor development to patient's death. World J Urol 25: 285–295 [DOI] [PubMed] [Google Scholar]
- Murta-Nascimento C, Silverman DT, Kogevinas M, Garcia-Closas M, Rothman N, Tardon A, Garcia-Closas R, Serra C, Carrato A, Villanueva C, Dosemeci M, Real FX, Malats N (2007b) Risk of bladder cancer associated with family history of cancer: do low-penetrance polymorphisms account for the increase in risk? Cancer Epidemiol Biomarkers Prev 16: 1595–1600 [DOI] [PubMed] [Google Scholar]
- National Board of Health and Welfare, Sweden (2007) Cancer Incidence in Sweden, 2006
- Nordlund LA, Carstensen JM, Pershagen G (1997) Cancer incidence in female smokers: a 26-year follow-up. Int J Cancer 73: 625–628 [DOI] [PubMed] [Google Scholar]
- Pathak AB, Advani SH, Gopal R, Nadkarni KS, Saikia TK (1992) Urinary bladder cancer following cyclophosphamide therapy for Hodgkin's disease. Leuk Lymphoma 8: 503–504 [DOI] [PubMed] [Google Scholar]
- Pettersson F, Ryberg M, Malker B (1990) Second primary cancer after treatment of invasive carcinoma of the uterine cervix, compared with those arising after treatment for in situ carcinomas. An effect of irradiation? A cancer registry study. Acta Obstet Gynecol Scand 69: 161–174 [DOI] [PubMed] [Google Scholar]
- Rusthoven KE, Flaig TW, Raben D, Kavanagh BD (2008) High incidence of lung cancer after non-muscle-invasive transitional cell carcinoma of the bladder: implications for screening trials. Clin Lung Cancer 9: 106–111 [DOI] [PubMed] [Google Scholar]
- Saadat M (2006) Genetic polymorphisms of glutathione S-transferase T1 (GSTT1) and susceptibility to gastric cancer: a meta-analysis. Cancer Sci 97: 505–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson S, Salanti G, Higgins J (2007) Joint effects of the N-acetyltransferase 1 and 2 (NAT1 and NAT2) genes and smoking on bladder carcinogenesis: a literature-based systematic HuGE review and evidence synthesis. Am J Epidemiol 166: 741–751 [DOI] [PubMed] [Google Scholar]
- Simister JM (1971) Cyclophosphamide and the bladder. Br Med J 3: 248–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A, Kinoshita Y, Rovito Jr PM, Landas S, Silberstein J, Nsouli I, Wang CY, Haas GP (2008) Higher than expected association of clinical prostate and bladder cancers. J Urol 179: S2–S5 [DOI] [PubMed] [Google Scholar]
- Stegmayr B, Eliasson M, Rodu B (2005) The decline of smoking in northern Sweden. Scand J Public Health 33: 321–324; discussion 243 [DOI] [PubMed] [Google Scholar]
- Teppo L, Salminen E, Pukkala E (2001) Risk of a new primary cancer among patients with lung cancer of different histological types. Eur J Cancer 37: 613–619 [DOI] [PubMed] [Google Scholar]
- Travis LB, Curtis RE, Glimelius B, Holowaty EJ, Van Leeuwen FE, Lynch CF, Hagenbeek A, Stovall M, Banks PM, Adami J, Gospodarowicz MK, Wacholder S, Inskip PD, Tucker MA, Boice JD (1995) Bladder and kidney cancer following cyclophosphamide therapy for non-Hodgkin's lymphoma. J Natl Cancer Inst 87: 524–530 [DOI] [PubMed] [Google Scholar]
- Travis LB, Curtis RE, Storm H, Hall P, Holowaty E, Van Leeuwen FE, Kohler BA, Pukkala E, Lynch CF, Andersson M, Bergfeldt K, Clarke EA, Wiklund T, Stoter G, Gospodarowicz M, Sturgeon J, Fraumeni Jr JF, Boice Jr JD (1997) Risk of second malignant neoplasms among long-term survivors of testicular cancer. J Natl Cancer Inst 89: 1429–1439 [DOI] [PubMed] [Google Scholar]