Abstract
Background:
Myelosuppression has been observed with several multikinase angiogenesis inhibitors in clinical studies, although the frequency and severity varies among the different agents. Inhibitors targeting vascular endothelial growth factor receptor (VEGFR) often inhibit other kinases, which may contribute to their adverse-event profiles.
Methods:
Kinase selectivity of pazopanib, sorafenib, and sunitinib was evaluated in a panel of 242 kinases. Cellular potency was measured using autophosphorylation assays. Effect on human bone marrow progenitor growth in the presence of multiple growth factors was evaluated and correlated with the kinase selectivity.
Results:
Sunitinib inhibited more kinases than pazopanib and sorafenib, at potencies within 10-fold of VEGFR-2. All three compounds potently inhibited VEGFR-2, platelet-derived growth factor receptor-β and c-Kit, However, pazopanib was less active against Flt-3 in both kinase and cellular assays. The inhibitory properties of pazopanib, sorafenib, and sunitinib were dependent on the growth factor used to initiate bone marrow colony formation. Addition of stem cell factor and/or Flt-3 ligand with granulocyte-macrophage colony stimulating factor resulted in significant shifts in potency for sorafenib and sunitinib but less so for pazopanib.
Conclusion:
Activity against c-kit and Flt-3 by multikinase angiogenesis inhibitors provide a potential explanation for the differences in myelosuppression observed with these agents in patients.
Keywords: kinase inhibitors, selectivity, myelosuppression
Kinase inhibitors often show off-target activity against additional kinases, along with the primary intended target (Baselga, 2006; Sebolt-Leopold and English, 2006; Karaman et al, 2008). The activity against other kinases provides additional therapeutic opportunities, for example, imatinib was originally developed as a Bcr-abl kinase inhibitor; however, its inhibitory activity against c-Kit and platelet-derived growth factor (PDGF) receptors led to its successful clinical evaluation against gastrointestinal stromal tumour (Buchdunger et al, 2000). However, off-target activity of kinase inhibitors have more often been considered to be a potential liability, which can add to the toxicities of the drug (Liebler and Guengerich, 2005; Force et al, 2007; Campillos et al, 2008; Hasinoff et al, 2008). Knowledge of the kinase selectivity of small-molecule kinase inhibitors can provide a better understanding of the biological activity and potential insights into the mechanisms of adverse effects, thereby allowing the design of optimally targeted multikinase inhibitors.
Myelosuppression has been observed with several vascular endothelial growth factor receptor (VEGFR) tyrosine kinase inhibitors (TKIs) in human clinical trials, although the frequency and severity vary among the different agents, with sunitinib having the highest frequency of neutropenia in 72% of patients (12% grade 3/4), whereas pazopanib and sorafenib treatment was associated with neutropenia in 34% (1% grade 3/4) and 18% (5% grade 3/4) of patients, respectively. Similarly, thrombocytopenia was observed in 65% of patients (8% grade 3/4) with sunitinib, whereas only 32% and 12% of patients showed thrombocytopenia with pazopanib and sorafenib (1% grade 3/4 with both agents) (Motzer et al, 2006, 2007; Escudier et al, 2007; Bhojani et al, 2008; Sternberg et al, 2009). Vascular endothelial growth factor and its receptors are essential for developmental haematopoiesis, and emerging evidence suggests a role of VEGF signalling in adult haematopoietic stem cells (Shalaby et al, 1995; Ferrara et al, 1996; Gerber et al, 2002; Larrivee et al, 2003). Vascular endothelial growth factor is secreted by haematopoietic stem cells and it inhibits total colony formation from less mature progenitor cells and at the same time promotes the formation of myeloid, mixed, and erythroid colonies from lineage-committed progenitors (Gabrilovich et al, 1998; Dikov et al, 2001).
Most TKIs targeting VEGFR have been reported to inhibit other kinases, some of which are expressed on haematopoietic progenitor cells and have been shown to modulate their growth and differentiation (Karaman et al, 2008). Receptors of Flt-3 and c-Kit are two such tyrosine kinases that are expressed on early haematopoietic progenitor cells and are involved in their proliferation and differentiation (reviewed in Lyman and Jacobsen, 1998). The two receptors are activated by their respective ligands, stem cell factor (SCF) for c-Kit and Flt-3 ligand for Flt-3. Further, the haematopoietic activities of these ligands require a synergistic interaction with other early acting or lineage-selective cytokines such as granulocyte-macrophage colony stimulating factor (GM-CSF). The signalling through c-Kit and Flt-3 appears to be essential for optimal production of mature haematopoietic cells from stem cells. Flt-3 ligand is more critical for the generation of lymphoid progenitors, whereas SCF regulates erythroid and myeloid progenitor cells.
In this report, we investigated the effects of three VEGFR TKIs, sunitinib (Motzer et al, 2006, 2007), sorafenib (Escudier et al, 2007), and pazopanib (Kumar et al, 2007) against a large panel of kinases and measured their ability to inhibit VEGFR, PDGFR, c-Kit, and Flt-3 in vitro and in cellular assays. Further, their ability to inhibit human bone marrow progenitor growth in colony forming assay formats induced by multiple growth factors was tested to evaluate their potential for myelosuppression.
Materials and methods
Compounds
Pazopanib, sunitinib, and sorafenib were synthesized at GlaxoSmithKline and dissolved in DMSO for treatment of cells.
Kinase selectivity screen
All three kinase inhibitors were tested against 242 kinases at 0.3 μM and 10 μM concentration using KinaseProfiler (Millipore, Billerica, MA, USA). Enzyme assays were carried out in duplicate under optimized conditions for each enzyme. Data were represented as percent inhibition. For IC50 values, the compounds were tested at various concentrations ranging from 0.001 to 10 μM using IC50 Profiler Express (Millipore).
Determination of in vitro potency against VEGFR-1/2/3, PDGFR-α/β, Flt-3, and c-Kit
Human VEGFR-1, VEGFR-2, VEGFR-3, and PDGFR-β enzymes were produced at GlaxoSmithKline. Human PDGFR-α (aa 550–1089) was obtained from Invitrogen (Carlsbad, CA, USA). Human Flt-3 (aa 564–end) was obtained from Millipore, and human c-Kit (aa 544–947) was obtained from Cell Signaling Technology (Beverly, MA, USA). For VEGFR-1/2/3, PDGFR-α/β, and Flt-3, 200–500 nM enzyme was preactivated at room temperature for 45 min in the presence of 0.1 mM ATP. After activation, each enzyme was diluted in assay buffer (0.2–2 nM final); 30 μl enzyme mix (1.3 × ) was added to 1 μl (40 × ) of test compound for 60 min followed by addition of 10 μl (4 × ) substrate mix (ATP at Kmapp for each enzyme and 20 μM peptide C, Ahx-RAHEEIYHFFFAKKK-CONH2) in assay plates. Final assay conditions were: 100 mM HEPES (pH 7.2), 10 mM MgCl2, 0.3 mM DTT, 0.1 mg ml−1 BSA, and 2.5% DMSO, plus 33P-ATP 0.02–0.08 μCi μl−1. Assay plates were incubated for 30 min before addition of 0.5% phosphoric acid (40 μl) to stop the enzymatic reaction. Samples in plates were mixed and 60 μl transferred to a phosphocellulose filter plate for 45 min before vacuum filtration and washing (4 × 100 μl) with 0.5% phosphoric acid. Plates were dried and scintillation fluid added before radioactive detection using a TopCount scintillation plate reader (PerkinElmer, Waltham, MA, USA).
Activity against the c-Kit enzyme was evaluated using an HTRF assay kit (CisBio, Bedford, MA, USA). In brief, the assay was initiated by addition of 10 μl (2 × ) c-Kit (0.5–1 nM) to assay plates containing 0.1 μl (200 × ) compound. Reactions were started by addition of 10 μl (2 × ) substrate mix (280 μM ATP, 1 μM TK biotinylated peptide) to assay plates. Final assay conditions were: 50 mM HEPES (pH 7.0), 0.1% NaN3, 5 mM MgCl2, 1 mM TCEP, 0.8 mM CHAPS, 0.1 mM orthovanadate, 0.1 mg ml−1 BSA, and 0.5% DMSO. Assay plates were then incubated for 50 min at room temperature before addition of 20 μl quench buffer (containing EDTA, strepavidin-XL665, TK antibody Eu3+-cryptate) to stop the reaction and detection of phosphorylated substrate. Assay plates were read on an EnVision multilabel plate reader (PerkinElmer) and fluorescence was measured at 620 nM (Cryptate) and 665 nM (XL665) with the ratio calculated as (665/620) for each well.
The percent maximal inhibition at VEGFR-1/2/3, PDGFR-α/β, Flt-3, and c-Kit was plotted as a function of inhibitor concentration to yield a concentration–response curve. Results were fit to the IC50 equation below:
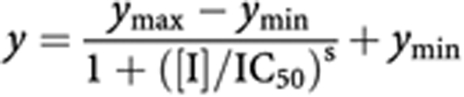 |
where the ymin was fixed at 0 and the ymax at 100, with IC50, and hill slope (‘s’) parameters floating (two-parameter fit). The Cheng–Prusoff equation (Cheng and Prusoff, 1973) was used to convert IC50 values to Ki values. This conversion was done assuming competitive inhibition vs ATP, as described by the equation below:
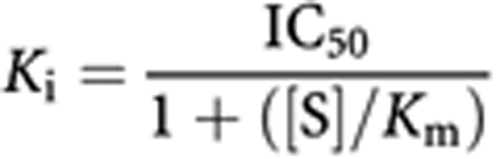 |
All reactions were run at an ATP concentration (‘S’) for each enzyme listed in Supplementary Table 1.
Cellular autophosphorylation assay
Ligand-induced receptor autophosphorylation assays were done to evaluate the cellular effect of kinase inhibitors against different receptor tyrosine kinases. For VEGFR-2 phosphorylation, human umbilical vein endothelial cells (HUVECs) were treated with DMSO or TKIs (ranging from 0.01 to 10 μM) for 2 h and then stimulated with VEGF165 (50 ng ml−1) for 10 min at 37°C before cell harvesting. For c-Kit, PDGFR-β, and Flt-3 autophosphorylation, NCI-H526, human foreskin fibroblasts, and RS4;11 cells were stimulated with SCF (100 ng ml−1), PDGF-BB (50 ng ml−1), and Flt-3 ligand (1 μg ml−1), respectively. Whole-cell lysate from cells treated with various compounds and stimulated with ligands was prepared in radioimmunoprecipitation assay buffer containing protease and phosphatase inhibitors. Cell lysates from NCI-H526 (200 μg), HFF (200 μg), HUVEC (800 μg), and RS4;11 cells (1600 μg) were immunoprecipitated with 2 μg of anti-c-Kit, anti-PDGFR-β, anti-VEGFR-2, or anti-Flt-3 antibodies, respectively, by overnight incubation at 4°C. Subsequently, the antigen–antibody complex was captured with 50 μl protein A/G sepharose (Pierce, Rockford, IL, USA) and was denatured in 0.05% β-mercaptoethanol sample loading buffer, then boiled for 10 min before loading onto 8% Tris-Glycine SDS–PAGE (Invitrogen). The resolved proteins were transferred onto PVDF membrane (Invitrogen) and probed with 1 : 1000 diluted antiphosphotyrosine, anti-VEGFR-2, anti-c-Kit, anti-PDGFR-β, or anti-Flt-3 antibodies. Secondary, fluorescently labelled antibodies were used at 1 : 10 000 dilutions. All blots were imaged on a LI-COR Odyssey instrument as directed by the manufacturer (LI-COR Biosciences, Lincoln, NE, USA).
Bone marrow progenitor growth assay
Low-density cells from cooled human bone marrow collected <24 h before usage (purchased from Lonza Walkersville, Inc., Gaithersburg, MD, USA) were isolated by Ficoll density gradient centrifugation (1.077 g ml−1; Ficoll-Paque). Three different human donor cells were used for these studies. Low-density marrow cells were washed in RPMI 1640 medium with 10% FBS and plated in T-75 flasks at 37°C to remove adherent cells. After 1 h incubation, nonadherent cells were collected and 5 × 104 cells ml−1 were mixed thoroughly with methocult (Stemcell Technologies, Vancouver, Canada), recombinant growth factors, TKIs (ranging from 0.1 nM to 10 μM), and media and dispensed in 12-well plates (0.5 ml well−1). Plates were incubated in a humidified low-oxygen incubator (7.5% CO2, 5% O2) for 10 days, and colonies of >50 cells were counted microscopically. The growth factors used were human GM-CSF (90 ng ml−1), SCF (50 ng ml−1), and Flt-3 ligand (200 ng ml−1) alone, and combinations of growth factors GM-CSF+SCF, GM-CSF+SCF+Flt-3 ligand, and SCF+Flt-3 ligand. The numbers of progenitors that would grow in the presence of SCF or Flt-3 alone were anticipated to be very low; therefore, marrow-cell density plated was doubled to 1 × 105 cells ml−1 for these growth factors. Inhibitory concentration (IC)50 and IC90 values were calculated from the dose–response curves using ExcelFit 4.0 (Microsoft, San Francisco, CA, USA) software tools (four-parameter fits). Statistical analysis was performed with GraphPad InStat (GraphPad, San Diego, CA, USA) software.
Results
Kinase selectivity of various TKIs
Pazopanib, sorafenib, and sunitinib were tested in a panel of 242 kinases at 0.3 and 10 μM (Supplementary Table 2). At 0.3 μM, 29 kinases were inhibited >50% by pazopanib, whereas 49 and 26 kinases were inhibited by sunitinib and sorafenib, respectively. At 10 μM, a total of 94 kinases were inhibited >50% by pazopanib, whereas 147 and 82 kinases were inhibited by sunitinib and sorafenib, respectively. A set of 61 kinases that were inhibited by >50% at 0.3 μM by at least one drug was selected for IC50 determination (Table 1). Pazopanib inhibited 32 kinases with an IC50 <1 μM, whereas sunitinib and sorafenib inhibited 54 and 25 kinases, respectively. As reported in the literature, the three TKIs were potent inhibitors of VEGFR-1 (Flt-1), VEGFR-2 (KDR), and VEGFR-3 (Flt-4), with very similar potency among compounds. Pazopanib and sunitinib inhibited c-Kit activity with IC50 of 48 and 40 nM, respectively, whereas sorafenib was much less active in this assay (Table 1). Sunitinib was a more potent inhibitor of Flt-3 compared with pazopanib. Looking at the kinases selectively targeted by these TKIs, sunitinib inhibited the activity of Mer, CaMKIIδ, CaMKIIγ, DRAK1, CHK2, PhKγ2, and ARK5 kinases quite potently (IC50<100 nM), whereas pazopanib and sorafenib had little to no activity against these kinases (IC50>10 000 nM). Pazopanib was more potent against MLK1 and TAO3 kinases compared with the potency of sunitinib and sorafenib. Sorafenib uniquely inhibited SAPK2a mutant and Mnk2 kinases with submicromolar potency. Further, DDR2, TAO2, and c-RAF were inhibited by pazopanib and sorafenib, but not by sunitinib. The results indicate that all three inhibitors had activity against several kinases in this panel, beyond those involved in tumour angiogenesis, which could modulate the cellular and antitumour activity of these compounds.
Table 1. IC50 values of pazopanib, sunitinib, and sorafenib against 61 kinases.
| IC50 (nM) | |||
|---|---|---|---|
| Kinasea | Pazopanib | Sunitinib | Sorafenib |
| Abl | 624 | 556 | 1133 |
| Abl(T315I) | 406 | 222 | 957 |
| ALK | 5377 | 293 | >10 000 |
| ARK5 | 1108 | 85 | >10 000 |
| Aurora-A | 64 | 508 | 1411 |
| Axl | >10 000 | 259 | 6273 |
| CaMKIIβ | >10 000 | 519 | >10 000 |
| CaMKIIγ | >10 000 | 20 | >10 000 |
| CaMKIIδ | >10 000 | 20 | >10 000 |
| CDK7/cyclinH/MAT | >10 000 | 133 | >10 000 |
| CHK2 | >10 000 | 59 | >10 000 |
| CHK2(I157T) | >10 000 | 79 | >10 000 |
| CHK2(R145W) | >10 000 | 83 | >10 000 |
| CK1δ | >10 000 | 206 | >10 000 |
| c-Kit(D816H) | 45 | 24 | 95 |
| c-Kit | 48 | 40 | 1862 |
| c-Kit(V560G) | 1 | <1 | 13 |
| c-Kit(V654A) | 1 | 1 | 170 |
| c-RAF | 92 | >10 000 | 110 |
| DDR2 | 474 | >10 000 | 51 |
| DRAK1 | >10 000 | 54 | >10 000 |
| FGFR1 | 80 | 437 | 64 |
| FGFR1(V561M) | 4266 | 180 | 3908 |
| FGFR2 | 350 | 852 | 825 |
| FGFR2(N549H) | 3 | 211 | 12 |
| FGFR3 | 138 | 314 | 1019 |
| Fgr | 498 | 142 | 5260 |
| Flt-3(D835Y) | 186 | 3 | 1981 |
| Flt-3 | 619 | 4 | 45 |
| Fms | 6 | 5 | 29 |
| Lck | 379 | 95 | 1495 |
| MARK1 | >10 000 | 475 | >10 000 |
| MELK | >10 000 | 145 | >10 000 |
| Mer | 2096 | 12 | 2096 |
| MLK1 | 21 | 987 | >10 000 |
| Mnk2 | >10 000 | >10 000 | 302 |
| MST1 | >10 000 | 221 | >10 000 |
| MuSK | 5937 | 206 | 53 |
| PDGFR-α(D842V) | 296 | 161 | 61 |
| PDGFR-α | 73 | 143 | 933 |
| PDGFR-α(V561D) | <1 | 1 | 5 |
| PDGFR-β | 215 | 75 | 1129 |
| PhKγ2 | >10 000 | 75 | >10 000 |
| PrKX | >10 000 | >10 000 | >10 000 |
| PTK5 | 97 | 748 | 124 |
| Pyk2 | 1664 | 480 | >10 000 |
| Ret | 232 | 37 | 2 |
| Rse | >10 000 | 251 | >10 000 |
| Rsk2 | >10 000 | 380 | >10 000 |
| Rsk3 | >10 000 | 215 | 6 166 |
| Rsk4 | >10 000 | 238 | >10 000 |
| SAPK2a(T106M) | >10 000 | >10 000 | 201 |
| SAPK2b | 5656 | >10 000 | > 10 000 |
| TAK1 | >10 000 | 150 | 1226 |
| TAO2 | 382 | >10 000 | 67 |
| TAO3 | 181 | >10 000 | >10 000 |
| TrkA | 937 | 23 | 218 |
| TrkB | 4282 | 158 | 832 |
| VEGFR-1 | 7 | 21 | 9 |
| VEGFR-2 | 15 | 34 | 28 |
| VEGFR-3 | 2 | 3 | 7 |
| Yes | 667 | 45 | >10 000 |
Abbreviations: IC=inhibitory concentration; PDGFR=platelet-derived growth factor receptor; VEGFR=vascular endothelial growth factor receptor.
All human kinases.
For a more accurate determination of the potency of pazopanib, sunitinib, and sorafenib against VEGFR-1, VEGFR-2, VEGFR-3, PDGFR-α/β, Flt-3, and c-Kit kinases, affinity (Kiapp) values were determined using either filter-binding or HTRF assays. All three compounds showed comparable activity against VEGFR-2, VEGFR-3, PDGFR-α, and PDGFR-β (Table 2). Pazopanib possessed the weakest affinity for Flt-3 with a mean Ki value of 230 nM, which is 25-fold weaker compared with VEGFR-2. In contrast, sunitinib possessed 85-fold and 100-fold greater affinity for Flt-3 and c-Kit compared with VEGFR-2 with Kiapp values of 0.6, 0.45, and 51 nM, respectively. Sorafenib showed the least selectivity among these tyrosine kinase receptors with affinity values ranging from 2 to 22 nM.
Table 2. Activity of pazopanib, sunitinib, and sorafenib against purified kinases.
|
Kiapp (nM)
|
|||
|---|---|---|---|
| Enzyme | Pazopanib | Sunitinib | Sorafenib |
| VEGFR-1 | 15 | 229 | 10 |
| VEGFR-2 | 8 | 51 | 4 |
| VEGFR-3 | 10 | 30 | 6 |
| PDGFR-α | 30 | 28 | 2 |
| PDGFR-β | 14 | 7 | 5 |
| c-Kit | 2.4 | 0.45 | 15 |
| Flt-3 | 230 | 0.6 | 22 |
Abbreviations: Kiapp=apparent inhibition constant; PDGFR=platelet-derived growth factor receptor; VEGFR=vascular endothelial growth factor receptor.
Cellular activity against various receptor tyrosine kinases
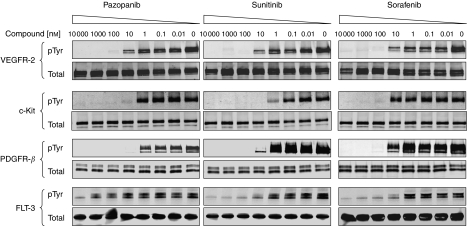
To compare the cellular activities of pazopanib, sunitinib, and sorafenib, we evaluated their inhibitory effect against wild-type VEGFR-2, c-Kit, PDGFR-β, and Flt-3 receptors in HUVEC, NCI-H526, HFF, and RS4;11 cells, respectively. Serum-starved cells were treated with inhibitors at various concentrations for 2 h. Cells were then stimulated with respective ligands as described in Materials and Methods. Whole-cell lysates were collected after 10 min of ligand stimulation, and the total receptor was immunoprecipitated using antireceptor antibodies. The ligand-induced receptor activation was detected by anti-pTyr antibody following western blot analysis. All three compounds inhibited the ligand-induced activation of VEGFR-2, c-Kit, PDGFR-β, and Flt-3 receptors (p-Tyr) in a dose-dependent manner, whereas total receptor levels remained constant (Figure 1). The three compounds exhibited similar potency in suppressing activation of VEGFR-2 and PDGFR-β (Table 3). However, sunitinib showed 10-fold greater potency than pazopanib and 100-fold greater potency than sorafenib against c-Kit activation (Figure 1; Table 3). Sunitinib and sorafenib both potently inhibited wild-type Flt-3 receptor activation with IC50 of 1 nM, whereas pazopanib was 1000-fold less active against Flt-3 with IC50⩾1 μM.
Figure 1.
Inhibition of receptor autophosphorylation by various tyrosine kinase inhibitors. To compare the activities of pazopanib, sunitinib, and sorafenib, we evaluated their inhibitory effects against wild-type VEGFR-2, c-Kit, PDGFR-β, and Flt-3 receptors in HUVEC (for VEGFR-2), NCI-H526 (c-Kit), HFF (PDGFR-β), and RS4;11 (Flt-3). Cells were serum-starved overnight and then treated with DMSO or different concentrations of pazopanib, sunitinib, or sorafenib for 2 h. Cells were then stimulated with respective ligands as described in the cellular autophosphorylation section under Materials and Methods. Total receptor was immunoprecipitated using antireceptor antibodies and phosphorylation was detected using anti-pTyr antibody following western blot analysis.
Table 3. Cellular IC50 for inhibition of ligand-induced receptor autophosphorylation.
|
IC50 (nM)
|
||||
|---|---|---|---|---|
| Receptors | Cells | Pazopanib | Sunitinib | Sorafenib |
| VEGFR-2 | HUVEC | 8 | 5 | 10 |
| PDGFR-β | HFF | 3 | 2 | 7 |
| c-Kit | NCI-H526 | 2.6 | 0.3 | 29 |
| Flt-3 | RS4;11 | ⩾1000 | 1 | 1 |
Abbreviations: HUVEC=human umbilical vein endothelial cells; IC=inhibitory concentration; PDGFR=platelet-derived growth factor receptor; VEGFR=vascular endothelial growth factor receptor.
Effect on human bone marrow progenitor cell growth
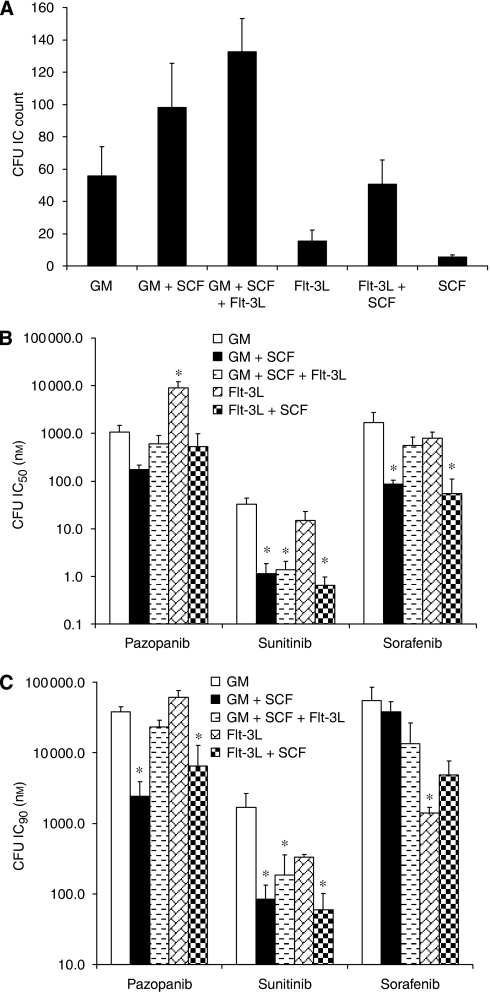
As haematopoietic progenitor cell proliferation and differentiation are regulated by various growth factors, including SCF and Flt-3 ligand, the human bone marrow progenitor cell growth was evaluated in the presence of various ligands using colony formation assay. The number of colony forming units (CFUs) formed was growth factor dependent. Use of GM-CSF resulted in an average CFU count of 56 in three studies (Figure 2A). The addition of SCF to GM-CSF increased the number of CFUs to an average of 98, consistent with the known synergistic activity of SCF on CFU growth. Addition of Flt-3 ligand to GM-CSF and SCF resulted in further augmented CFU formation. The combination of SCF with Flt-3 ligand resulted in the formation of 51 CFUs. The number of CFUs formed in the presence of SCF or Flt-3 ligand alone was determined in cultures plated with double the number of enriched marrow cells. The presence of SCF alone resulted in very few CFUs, with only six CFUs formed in one study; Flt-3 alone resulted in an average of 15 CFU in three studies. Of note, less than one CFU formed in cultures without any growth factor addition; therefore, no background correction was needed in these studies.
Figure 2.
Effect of tyrosine kinase inhibitors (TKIs) on ligand-stimulated progenitor cell growth. (A) Human bone marrow progenitor cells were stimulated with GM-CSF (90 ng ml−1), SCF (50 ng ml−1), Flt-3 ligand (200 ng ml−1), and their various combinations for colony stimulating unit (CFU) formation. Less than one CFU formed in cultures without any growth factor addition, thus no background correction was needed. (B and C) Bone marrow progenitor cells were treated with pazopanib, sunitinib, and sorafenib at concentrations ranging from 0.1 nM to 10 μM in the presence of various ligands. Inhibitory potentials, IC50 (B) and IC90 (C), of TKIs were calculated for ligand-induced CFU formation. Data represent mean+s.d. from three human bone marrow preparations. *Significantly different than GM-CSF alone; P<0.01 by Dunnett's Multiple Comparison test.
The inhibitory properties of pazopanib, sorafenib, and sunitinib were dependent on the growth factor(s)/ligand(s) used to initiate colony formation (Figure 2B and C). Pazopanib and sorafenib had modest effect on GM-CSF–driven CFU-GM formation with an IC50 of 1 and 1.7 μM, respectively. Growth factor additions, which contained SCF, resulted in a shift in potency of pazopanib by 2- to 6-fold to the nM range, whereas CFU inhibition by Flt-3 ligand alone was similar to CFU inhibition driven by GM-CSF. Addition of SCF or Flt-3 ligand resulted in modest shifts in potency of sorafenib to the nM range, whereas CFU inhibition by Flt-3 ligand alone was similar to CFU inhibition driven by GM-CSF. Sunitinib was more potent than the other two compounds in inhibiting CFU-GM formation induced by GM-CSF (IC50=32.7 nM). Addition of SCF or Flt-3 ligand with GM-CSF resulted in significant shifts in potency of sunitinib to the low nM range.
The drug concentration that inhibited the CFU-GM by 90% (IC90) was more predictive of clinical measurement of the myelosuppressive dose than the often published IC50 values (Pessina et al, 2001). Therefore, we calculated both IC50 and IC90 values for pazopanib, sunitinib, and sorafenib in bone marrow progenitor growth assays in the presence of various ligands. The IC90 values for pazopanib and sorafenib inhibition of CFU were often extrapolated because CFU formation occurred at the highest concentration tested (10 μM). Compared with GM-CSF, the shift in IC90 values for pazopanib after the addition of SCF was 15-fold for GM-CSF+SCF and six-fold for SCF+Flt-3 ligand. For sorafenib, none of the IC90 values were statistically significantly shifted compared with GM-CSF; however, the average IC90 values were 30- and 11-fold more potent for the Flt-3 ligand alone and Flt-3 ligand + SCF groups. Similar to IC50 results, sunitinib showed 9- to 28-fold shifts in CFU IC90 values with SCF and/or Flt-3 ligands compared with GM-CSF alone. The results suggest that sunitinib is a potent inhibitor of CFUs driven by GM-CSF and Flt-3 ligand, and that sunitinib's inhibitory activity is greatly enhanced with the activation of c-Kit receptor signalling.
Discussion
Off-target activity of drugs often results in unwanted and generally harmful effects, and a better understanding of the target profile of drugs can help design agents that may avoid/minimize activities against undesired targets (Liebler and Guengerich, 2005; Campillos et al, 2008). Large-scale molecular profiling of agents against a panel of related proteins is often the first step in evaluating selectivity. A number of kinase inhibitors have shown promising clinical activity and several are now approved (Baselga, 2006; Sebolt-Leopold and English, 2006; Zhang et al, 2009). All of these agents have activity against other kinases to various degrees (Karaman et al, 2008), which may be associated with their adverse-event profiles. Comparing three TKIs targeting VEGF signalling, against a large panel of kinases, we observed differences in their inhibitory potential against various kinases, although they have relatively similar potency against VEGFR-2. Sunitinib had the broadest kinase activity among the three TKIs, with 20% of the kinases (49 of 242) inhibited by >50% at 0.3 μM, whereas pazopanib and sorafenib inhibited 12% and 11% of the tested kinases, respectively. Although there is overlap in the specific kinases inhibited by pazopanib and sorafenib, there are also clear distinction for some of the kinases, for example, sorafenib is a more potent inhibitor of DDR2, MuSK, MnK2, Ret, and so on, whereas pazopanib is more potent against Aurora-A and MLK1. Our results are generally consistent with published selectivity results for the three compounds using binding assays against a panel of 290 kinases, wherein sunitinib bound to five times more kinases than pazopanib, with a Kd<100 nM (Karaman et al, 2008). The selectivity was more evident for serine/threonine kinases, where sunitinib interacted with 36 times more kinases than pazopanib. For tyrosine kinases, the difference was only 1.8-fold. On the other hand, the selectivity ratio of sorafenib was only slightly higher compared with pazopanib (1.3- to 2-fold) in the same data set.
Among the tyrosine kinases, related to VEGFR-2, there were subtle differences in the potencies of the three TKIs against c-Kit and Flt-3, with sunitinib having the highest affinity for both of these targets. Sorafenib had modest activity against both c-Kit and Flt-3 kinases; however, pazopanib was much less active against Flt-3, although it inhibited c-Kit kinase (Table 2). The selectivity against in vitro kinases translated into the ability of TKIs to inhibit ligand-induced receptor autophosphorylation, where pazopanib was a very weak inhibitor of Flt-3 activation (Figure 1). The differences in the activity of these TKIs against such closely related receptor tyrosine kinases clearly demonstrate the need to broadly profile drugs to understand their true selectivity and potential off-targets.
As GM-CSF, Flt-3, and c-Kit are involved in the development of various haematopoietic lineage cells, we evaluated the reported adverse-effect profiles of these TKIs in clinical trials. All three TKIs have been shown to cause myelosuppression, although the frequency and severity vary (Motzer et al, 2006, 2007; Escudier et al, 2007; Bhojani et al, 2008; Sternberg et al, 2009). Sunitinib appeared to have higher rates of lymphopenia and neutropenia compared with sorafenib and pazopanib, although most were grade 1/2 events and not dose limiting. It is important to highlight that although all three agents were tested in patients with renal cell carcinoma, no comparative study was reported and thus caution should be used in comparing the results from different studies.
Human bone marrow progenitor cell growth using GM-CSF (CFU-GM) has been used as a predictive assay to identify compounds with myelosuppression capacity (Pessina et al, 2001, 2005). The interaction of GM-CSF with its receptor on human bone marrow progenitors, including various kinases, signals a series of biochemical events with resultant growth and differentiation. The addition of synergistic growth factors such as Flt-3 ligand and SCF are known to increase the number of responding progenitors that grow in the presence of GM-CSF. The increased number and continued discovery of more targeted agents that specifically inhibit key biochemical components of the receptor signalling pathways may require modification of the standard CFU-GM assay to better predict the inhibitory nature of these TKIs.
Different TKIs inhibited the bone marrow colony formation with different potency, with sunitinib having the highest activity (Figure 2). Addition of SCF and/or Flt-3 ligand with GM-CSF resulted in increased colony formation as expected. Consistent with the strong inhibition of c-Kit and Flt-3 kinases by sunitinib, it showed enhanced inhibitory activity in the CFU assay done in the presence of SCF and Flt-3 ligand. The changes in the IC50 and IC90 values with the addition of SCF and Flt-3 ligand were less pronounced for pazopanib and sorafenib, and were in general agreement with their activity against these kinases. Sunitinib induced inhibition of bone marrow progenitors was broader than both pazopanib and sorafenib. The mechanism for this broad-spectrum inhibition of progenitors in vitro in not completely understood, but is likely due to the potent inhibition of both c-KIT and flt-3 kinases. Both c-kit and flt-3 are important kinases in early stem and progenitor cell development; therefore, inhibition of both of these kinases may result in the observed sensitivity of haematopoietic progenitors in vitro especially with the addition of SCF and FLT-3 ligand to further augment progenitor growth. As sunitinib inhibits a larger number of kinases than pazopanib and sorafenib, the contribution from other kinases cannot be ruled out. The data presented in this report clearly indicate that the testing of TKIs (such as pazopanib, sorafenib, and sunitinib) in the standard GM-CSF–induced CFU-GM assay, although useful, does not represent the inhibitory potential of these targeted kinase inhibitors in human bone marrow assays. For a better evaluation of the myelosuppressive potential of TKIs, the CFU assay should be done in the presence of various ligands.
In summary, activity against other targets can explain the differences in clinical effects for various kinase inhibitors, and a better understanding of the contributions of various kinases to the different adverse effects may help in designing optimally targeted inhibitors. The differences in the activity against c-Kit and Flt-3 kinases among sunitinib, sorafenib, and pazopanib provide a likely explanation for the observed difference in clinical myelosuppression with these antiangiogenic TKIs.
Conflict of interest
All authors are current or former employees of GlaxoSmithKline.
Footnotes
Supplementary Information accompanies the paper on British Journal of Cancer website (http://www.nature.com/bjc)
Supplementary Material
References
- Baselga J (2006) Targeting tyrosine kinases in cancer: the second wave. Science 312(5777): 1175–1178 [DOI] [PubMed] [Google Scholar]
- Bhojani N, Jeldres C, Patard JJ, Perrotte P, Suardi N, Hutterer G, Patenaude F, Oudard S, Karakiewicz PI (2008) Toxicities associated with the administration of sorafenib, sunitinib, and temsirolimus and their management in patients with metastatic renal cell carcinoma. Eur Urol 53(5): 917–930 [DOI] [PubMed] [Google Scholar]
- Buchdunger E, Cioffi CL, Law N, Stover D, Ohno-Jones S, Druker BJ, Lydon NB (2000) Abl protein-tyrosine kinase inhibitor STI571 inhibits in vitro signal transduction mediated by c-kit and platelet-derived growth factor receptors. J Pharmacol Exp Ther 295(1): 139–145 [PubMed] [Google Scholar]
- Campillos M, Kuhn M, Gavin AC, Jensen LJ, Bork P (2008) Drug target identification using side-effect similarity. Science 321(5886): 263–266, doi: 10.1126/science.1158140 [DOI] [PubMed] [Google Scholar]
- Cheng Y, Prusoff WH (1973) Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol 22(23): 3099–3108 [DOI] [PubMed] [Google Scholar]
- Dikov MM, Oyama T, Cheng P, Takahashi T, Takahashi K, Sepetavec T, Edwards B, Adachi Y, Nadaf S, Daniel T, Gabrilovich DI, Carbone DP (2001) Vascular endothelial growth factor effects on nuclear factor-kappaB activation in hematopoietic progenitor cells. Cancer Res 61(5): 2015–2021 [PubMed] [Google Scholar]
- Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, Negrier S, Chevreau C, Solska E, Desai AA, Rolland F, Demkow T, Hutson TE, Gore M, Freeman S, Schwartz B, Shan M, Simantov R, Bukowski RM (2007) Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med 356(2): 125–134 [DOI] [PubMed] [Google Scholar]
- Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O’Shea KS, Powell-Braxton L, Hillan KJ, Moore MW (1996) Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature 380(6573): 439–442, doi:10.1038/380439a0 [DOI] [PubMed] [Google Scholar]
- Force T, Krause DS, Van Etten RA (2007) Molecular mechanisms of cardiotoxicity of tyrosine kinase inhibition. Nat Rev Cancer 7(5): 332–344 [DOI] [PubMed] [Google Scholar]
- Gabrilovich D, Ishida T, Oyama T, Ran S, Kravtsov V, Nadaf S, Carbone DP (1998) Vascular endothelial growth factor inhibits the development of dendritic cells and dramatically affects the differentiation of multiple hematopoietic lineages in vivo. Blood 92(11): 4150–4166 [PubMed] [Google Scholar]
- Gerber HP, Malik AK, Solar GP, Sherman D, Liang XH, Meng G, Hong K, Marsters JC, Ferrara N (2002) VEGF regulates haematopoietic stem cell survival by an internal autocrine loop mechanism. Nature 417(6892): 954–958 [DOI] [PubMed] [Google Scholar]
- Hasinoff BB, Patel D, O’Hara KA (2008) Mechanisms of myocyte cytotoxicity induced by the multiple receptor tyrosine kinase inhibitor sunitinib. Mol Pharmacol 74(6): 1722–1728, doi: 10.1124/mol.108.050104 [DOI] [PubMed] [Google Scholar]
- Karaman MW, Herrgard S, Treiber DK, Gallant P, Atteridge CE, Campbell BT, Chan KW, Ciceri P, Davis MI, Edeen PT, Faraoni R, Floyd M, Hunt JP, Lockhart DJ, Milanov ZV, Morrison MJ, Pallares G, Patel HK, Pritchard S, Wodicka LM, Zarrinkar PP (2008) A quantitative analysis of kinase inhibitor selectivity. Nat Biotechnol 26(1): 127–132 [DOI] [PubMed] [Google Scholar]
- Kumar R, Knick VB, Rudolph SK, Johnson JH, Crosby RM, Crouthamel MC, Hopper TM, Miller CG, Harrington LE, Onori JA, Mullin RJ, Gilmer TM, Truesdale AT, Epperly AH, Boloor A, Stafford JA, Luttrell DK, Cheung M (2007) Pharmacokinetic-pharmacodynamic correlation from mouse to human with pazopanib, a multikinase angiogenesis inhibitor with potent antitumor and antiangiogenic activity. Mol Cancer Ther 6(7): 2012–2021 [DOI] [PubMed] [Google Scholar]
- Larrivee B, Lane DR, Pollet I, Olive PL, Humphries RK, Karsan A (2003) Vascular endothelial growth factor receptor-2 induces survival of hematopoietic progenitor cells. J Biol Chem 278(24): 22006–22013 [DOI] [PubMed] [Google Scholar]
- Liebler DC, Guengerich FP (2005) Elucidating mechanisms of drug-induced toxicity. Nat Rev Drug Discov 4(5): 410–420, doi:10.1038/nrd1720 [DOI] [PubMed] [Google Scholar]
- Lyman SD, Jacobsen SE (1998) c-kit ligand and Flt3 ligand: stem/progenitor cell factors with overlapping yet distinct activities. Blood 91(4): 1101–1134 [PubMed] [Google Scholar]
- Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, Oudard S, Negrier S, Szczylik C, Kim ST, Chen I, Bycott PW, Baum CM, Figlin RA (2007) Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med 356(2): 115–124 [DOI] [PubMed] [Google Scholar]
- Motzer RJ, Michaelson MD, Redman BG, Hudes GR, Wilding G, Figlin RA, Ginsberg MS, Kim ST, Baum CM, DePrimo SE, Li JZ, Bello CL, Theuer CP, George DJ, Rini BI (2006) Activity of SU11248, a multitargeted inhibitor of vascular endothelial growth factor receptor and platelet-derived growth factor receptor, in patients with metastatic renal cell carcinoma. J Clin Oncol 24(1): 16–24 [DOI] [PubMed] [Google Scholar]
- Pessina A, Albella B, Bueren J, Brantom P, Casati S, Gribaldo L, Croera C, Gagliardi G, Foti P, Parchment R, Parent-Massin D, Sibiril Y, Van Den Heuvel R (2001) Prevalidation of a model for predicting acute neutropenia by colony forming unit granulocyte/macrophage (CFU-GM) assay. Toxicol In Vitro 15(6): 729–740 [DOI] [PubMed] [Google Scholar]
- Pessina A, Malerba I, Gribaldo L (2005) Hematotoxicity testing by cell clonogenic assay in drug development and preclinical trials. Curr Pharm Des 11(8): 1055–1065 [DOI] [PubMed] [Google Scholar]
- Sebolt-Leopold JS, English JM (2006) Mechanisms of drug inhibition of signalling molecules. Nature 441(7092): 457–462 [DOI] [PubMed] [Google Scholar]
- Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu XF, Breitman ML, Schuh AC (1995) Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature 376(6535): 62–66 [DOI] [PubMed] [Google Scholar]
- Sternberg CN, Szczylik C, Lee E, Salman PV, Mardiak J, Davis ID, Pandite L, Chen M, McCann L, Hawkins R (2009) A randomized, double-blind phase III study of pazopanib in treatment-naive and cytokine-pretreated patients with advanced renal cell carcinoma (RCC). J Clin Oncol 27(Suppl): 15s (abstr 5021) [Google Scholar]
- Zhang J, Yang PL, Gray NS (2009) Targeting cancer with small molecule kinase inhibitors. Nat Rev Cancer 9(1): 28–39 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.