Abstract
Schizophrenia is a serious and disabling mental disorder that affects approximately 1% of the general population, with often devastating effects on the psychological and financial resources of the patient, family, and larger community. The etiology of schizophrenia is not known, although it likely involves several interacting biological and environmental factors that predispose an individual to schizophrenia. However, although the underlying pathology remains unknown, it has been believed that brain abnormalities would ultimately be linked to the etiology of schizophrenia. This theory was rekindled in the 1970s, when the first computer-assisted tomography (CT) study showed enlarged lateral ventricles in schizophrenia. Since that time, there have been many improvements in MR acquisition and image processing, including the introduction of positron emission tomography (PET), followed by functional MR (fMRI), and diffusion tensor imaging (DTI). These advances have led to an appreciation of the critical role that brain abnormalities play in schizophrenia. While structural MRI has proven to be useful in investigating and detecting gray matter abnormalities in schizophrenia, the investigation of white matter has proven to be more challenging as white matter appears homogeneous on conventional MRI and the fibers connecting different brain regions cannot be appreciated. With the development of DTI, we are now able to investigate white matter abnormalities in schizophrenia.
Keywords: schizophrenia, diffusion tensor imaging (DTI), frontal-temporal connections, white matter
INTRODUCTION
Schizophrenia is a serious and disabling mental disorder that affects approximately 1% of the general population, with often devastating effects on the psychological and financial resources of the patient, family, and larger community. It generally afflicts individuals in early adulthood, at a time when they are on the threshold of entering the most productive and formative years of life. The overt, or positive, symptoms of the disorder include auditory hallucinations, disordered thinking, and delusions, while the negative symptoms include avolition, anhedonia, blunted affect, and apathy. Additionally, broad areas of functioning are frequently disturbed including attention, memory, emotion, motivation, thought and language processes, social functioning, and mood regulation.
The etiology of schizophrenia is not known, although it likely involves several interacting biological (e.g., genetic and neurodevelopmental) and environmental factors (e.g., viral infection, fetal insult, drug abuse) that predispose an individual to schizophrenia. Of note, however, although the underlying pathology remains unknown, both Kraepelin1 and Bleuler2 (pioneers of schizophrenia) believed that brain abnormalities would ultimately be linked to the etiology of schizophrenia. This theory was rekindled in the 1970s, when the first computer-assisted tomography (CT) study showed enlarged lateral ventricles in schizophrenia.3 With the introduction of magnetic resonance imaging (MRI) studies, the first MRI study of schizophrenia was conducted in 1984.4 Since that time, there have been many improvements in MR acquisition and image processing, including the introduction of positron emission tomography (PET), followed by functional MR (fMRI) and diffusion tensor imaging (DTI), all of which have enabled us to exploit more fully information contained in MR and other medical images. These advances have led to an appreciation of the critical role that brain abnormalities play in schizophrenia.
MRI findings in schizophrenia include lateral ventricle enlargement, medial temporal lobe volume reduction (including amygdala-hippocampal complex and parahippocampal gyrus), neocortical superior temporal gyrus volume reduction, frontal and parietal lobe abnormalities, and subcortical abnormalities affecting the cavum septi pellucidi, basal ganglia, corpus callosum, thalamus, and cerebellum (see review in ref. 5). These findings further suggest the involvement of a large number of functionally related brain regions. Of note here, Wernicke6 and Kraepelin1 both suggested that schizophrenia might be a disease of insufficient or ineffective communication between these brain regions. This hypothesis has been refueled by recent functional imaging studies, which have demonstrated functional connectivity abnormalities between temporolimbic and prefrontal regions (e.g., refs. 7–11). These findings, as well as recent postmortem and genetic studies that demonstrate myelin-related abnormalities in schizophrenia,12–14 suggest that not only functional, but also anatomical disconnection between brain regions may be involved in schizophrenia. This latter speculation has led to an interest in investigating white matter fiber tract abnormalities in schizophrenia. Here, the focus is on long, association fiber tracts, consisting of heavily myelinated axons, which interconnect distant brain regions. Of particular interest, and based on earlier speculations, are white matter fiber tracts connecting the frontal and temporal lobes.
Finally, while structural MRI has proven to be useful in investigating and detecting gray matter abnormalities in schizophrenia, the investigation of white matter has proven to be more challenging as white matter appears homogeneous on conventional MRI and the fibers connecting different brain regions cannot be appreciated. With the development of diffusion tensor imaging (DTI), we are now able to investigate white matter abnormalities in schizophrenia.
DTI AND SCHIZOPHRENIA
DTI is the first imaging tool that makes it possible to evaluate white matter fiber tracts in the brain. Moreover, the ability to visualize and to quantify white matter fiber tracts makes DTI a particularly attractive research tool. The first DTI study of schizophrenia was published in 1998,15 only 4 years after DTI was introduced.16 Since then, over 20 DTI studies of schizophrenia have been conducted.
Early applications of DTI to schizophrenia investigations focused on the quantification and group comparison of large regions of white matter and thus did not focus on specific anatomically defined fiber tracts (e.g., refs. 17–19). With more recent advances in DT image processing, the field is clearly moving towards a focus on the quantification and group comparison of specific fiber tracts in the brain.
This work has developed along two main directions: (1) a voxel by voxel evaluation of whole brain white matter and (2) measurements of specific fiber bundles based on their anatomical definition. The first type of analysis involves the coregistration of each subject’s scan to a common template and can be performed without a priori hypotheses. This method is very popular in gray matter volumetric studies (termed “voxel-based morphometry analyses”) and it has also been applied to FA maps in schizophrenia. Findings from these studies show decreased FA within the cingulate bundle,20,21 uncinate fasciculus,20 arcuate fasciculus,21 and corpus callosum22 in schizophrenia compared to control subjects. Results, however, are not consistent, with some negative findings.23 Low resolution of the images, heavy partial volume effects, and imperfect registration of the fiber tracts likely contribute to such inconsistent findings. Of note, this method is still in the development phase and, currently, intensity-based registration of FA maps to a common template is being replaced by more precise, higher-order multicomponent registration (e.g., ref. 24).
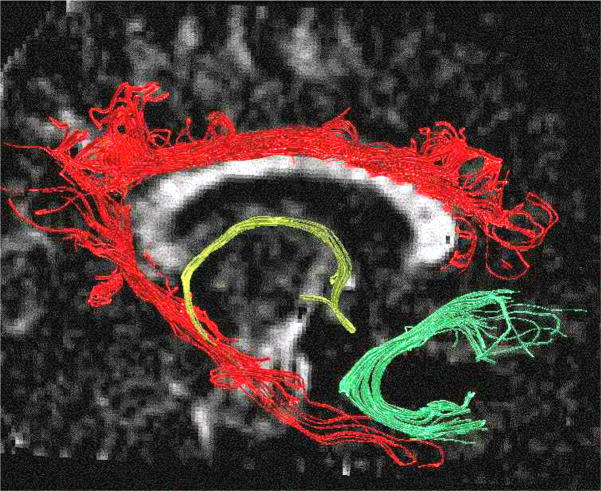
The second type of analysis involves focusing on testing specific hypotheses about particular connections that may be disrupted in schizophrenia. These studies use small regions of interest (ROIs), defined either manually or automatically, and based on anatomical definitions of the fiber tract. These ROIs have been used to investigate FA within the cingulum bundle, uncinate fasciculus, corpus callosum, and cerebellar peduncles. Because the anatomical definition of ROIs is difficult, particularly given that early DTI images had low resolution, and the measurements were generally taken from one or a small number of slices, results tended to also be equivocal (for review, see ref. 25). This type of analysis, however, is rapidly developing and now takes advantage of new image processing tools such as fiber tracking, where specific fiber bundles can be parcellated based on their anatomical connections (see Fig. 1 and Future Directions, below, for further details). Note that, in Figure 1, fiber tracts have been delineated using in-house software (www.slicer.org). Here, seed points have been placed manually on a single coronal slice, within three fiber tracts (cingulate bundle, fornix, and uncinate fasciculus), and fiber tracking has been performed using the Runge-Kutta algorithm.
FIGURE 1.
Three large frontal-temporal fiber tracts are visualized: the cingulum bundle in red, the fornix in yellow, and the uncinate fasciculus in green.
FRONTOTEMPORAL CONNECTIONS IN SCHIZOPHRENIA
Evidence from the Literature
Our laboratory has used DTI techniques to investigate frontotemporal connections in schizophrenia. The focus of this work has evolved from evidence in the literature, reviewed above, which suggests the importance of temporal and frontal lobe abnormalities in MRI studies of schizophrenia (see also review in ref. 5). While most of these studies highlight gray matter abnormalities in schizophrenia, several studies have reported prefrontal white matter volume reduction,26,27 temporal white matter volume reduction,28–30 or a relationship between prefrontal white matter volume reduction and temporal gray matter volume reduction (e.g., STG, amygdala-hippocampal complex, and parahippocampal gyrus).26,31
Of further note, Weinberger and coworkers8 were among the first to report an association between functional deficits observed in the prefrontal cortex in schizophrenic patients and structural abnormalities observed in the medial temporal lobe (i.e., reduced volume of the hippocampus). Since then, several functional studies have reported a disruption of functional connectivity between frontal and temporal lobes in schizophrenia (e.g., refs. 32–35), again emphasizing the likely importance of frontotemporal circuitry abnormalities in the pathophysiology of schizophrenia.
Postmortem investigations of schizophrenia have also reported abnormalities in white matter neuronal distribution in both prefrontal and temporal regions, as well as abnormalities in the density, distribution, and genetics of oligodendrocytes in schizophrenia, further suggesting a disruption in frontal-temporal connections. Moreover, oligodendrocyte abnormalities, involved in the formation of a protective sheath around the axons, as well as an abnormal distribution of interstitial neurons, responsible for neuronal migration guidance, suggest that perhaps not only functional, but also structural disconnectivity may be involved in the pathophysiology of schizophrenia.
The frontal and temporal lobes are connected by multiple long, association fiber tracts. Among the most important of these connections are (1) the uncinate fasciculus, a fiber tract connecting amygdala, uncus, and temporal pole with the subcallosal region and the orbitofrontal gyrus,36–38 and involved in decision-making, social behavior, and autobiographical and episodic memory—all functions that are disturbed in schizophrenia; (2) the cingulum bundle, a fiber tract interconnecting limbic structures (e.g., dorsolateral prefrontal cortex, cingulate gyrus, parahippocampal gyrus) and involved in attention, emotions, spatial orientation, and memory—functions that are also disrupted in schizophrenia; (3) the fornix, a fiber tract connecting the hippocampus with other brain regions (e.g., thalamus, prefrontal cortex) and involved in spatial learning and memory—functions disrupted in schizophrenia; and finally (4) the arcuate fasciculus, a fiber tract that connects Broca’s and Wernicke’s areas and is involved in language processing—this fiber tract may also be involved in language and thought disturbances observed in schizophrenia.
Findings from Our Laboratory
Thus far, in our laboratory, we have used DTI to examine the uncinate fasciculus and the cingulate bundle in schizophrenia, and in an additional study we have demonstrated arcuate fasciculus abnormalities in schizophrenia using a voxel-based analysis of DTI. Below, we review these findings.
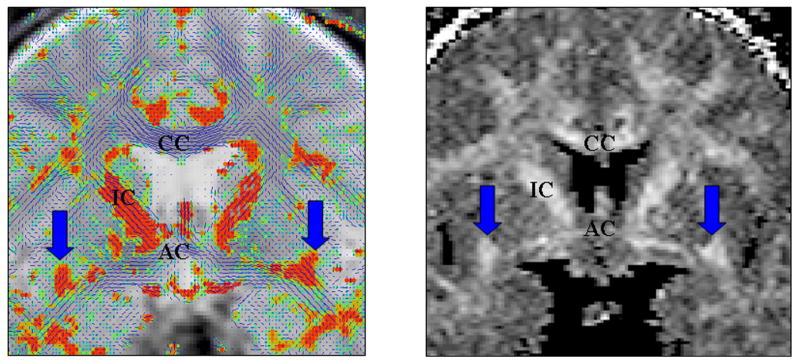
The uncinate fasciculus (UF), one of the largest connections between the frontal and temporal lobes, may play an important role in emotions, decision-making, and episodic memory, and in pathophysiology of schizophrenia. The UF pathology in schizophrenia has been described in two DTI investigations.20,39 The ROI study from our laboratory showed a lack of left greater than right fractional anisotropy (FA) asymmetry in schizophrenia that was present in control subjects.39 In addition, a voxel-based morphometry analysis of FA maps, in controls and schizophrenics, showed FA abnormalities in the UF in schizophrenia.20 Figure 2 shows an example of both an FA and a tensor map showing the UF in a normal control subject.
FIGURE 2.
The left panel shows a tensor map with the largest in-plane component of the diffusion in blue and the out-of-plane component in orange. The arrows point to the uncinate fasciculus. The right panel shows an FA map: CC, corpus callosum; IC, internal capsule; AC, anterior commissure.
The cingulum bundle (CB) is a fiber tract interconnecting limbic structures involved in attention, emotions, spatial orientation, and memory, and has been implicated in numerous studies of schizophrenia. Noteworthy are structural MR studies in schizophrenia that show cingulate gyri volume decrease,28,40,41 histopathological studies that show changes in neuronal organization within the cingulate gyrus,42–44 and functional studies that show abnormal activation of the cingulate gyri in numerous tasks.45–47 In addition, functional connectivity, as measured by fMRI, tends to be abnormally modulated by the cingulate gyrus,48,49 thus furnishing a possible basis for hallucinations. CB integrity abnormalities have thus far also been described in three DTI ROI studies.50–52 Our laboratory used automatic ROI definition and measured CB on 8 coronal slices covering the anterior part of the fiber bundle. We found decreased mean FA as well as decreased area of this bundle in schizophrenia, bilaterally. In addition, left > right asymmetry was present in both groups. Moreover, decreased diffusion in CB was associated with poorer performance on the Wisconsin Card Sorting Test, which is heavily dependent upon intact communication between prefrontal and anterior cingulate gyri. Figure 3 provides an example of a CB ROI drawn in a control subject superimposed over the FA map.
FIGURE 3.
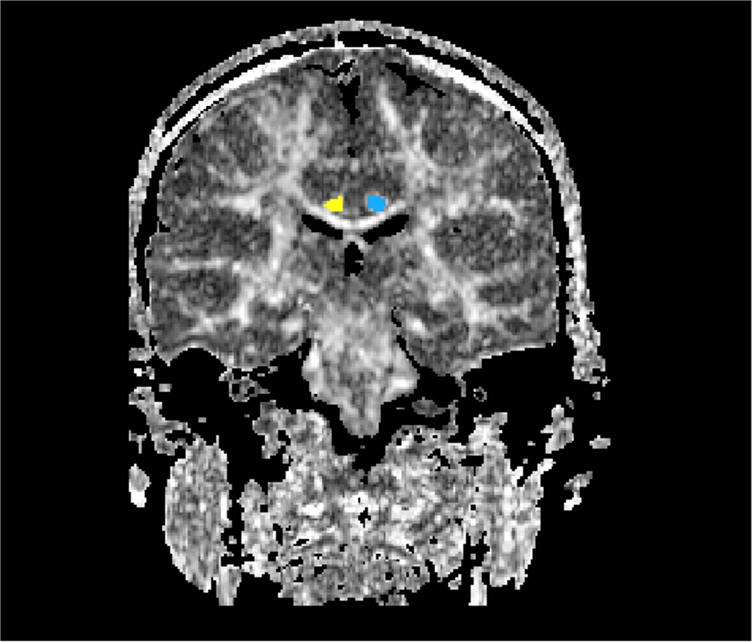
An FA map is displayed of a normal control subject, with the ROI for the cingulum bundle displayed as blue on the left and yellow on the right.
The arcuate fasciculus connects Wernicke’s and Broca’s areas and is the major language processing tract in the brain. Patients diagnosed with schizophrenia often suffer from deficits in verbal memory, auditory hallucinations, delusions, and thought disorder, with the latter likely related to language deficits observed in behavioral studies, although verbal memory and auditory hallucinations may also be linked to areas of the brain involving auditory perception and language. Functional studies show abnormal activation within Broca’s and Wernicke’s areas in schizophrenia during various verbal tasks.35,53–56 Of note here, abnormalities within the arcuate fasciculus have been reported in schizophrenia in two separate voxel-based FA analyses.20,21 The Hubl et al. study21 also showed an association between arcuate fasciculus abnormalities and auditory hallucinations. Studies from our laboratory also indicate arcuate fasciculus abnormalities in schizophrenia. More specifically, a VBM study, using a higher-order tensorial coregistration method, showed a left arcuate fasciculus FA decrease in schizophrenia compared with control subjects. Figure 4 shows results of a voxel-based comparison between controls and schizophrenics,57 where FA decrease is present within the arcuate fasciculus in schizophrenics.
FIGURE 4.
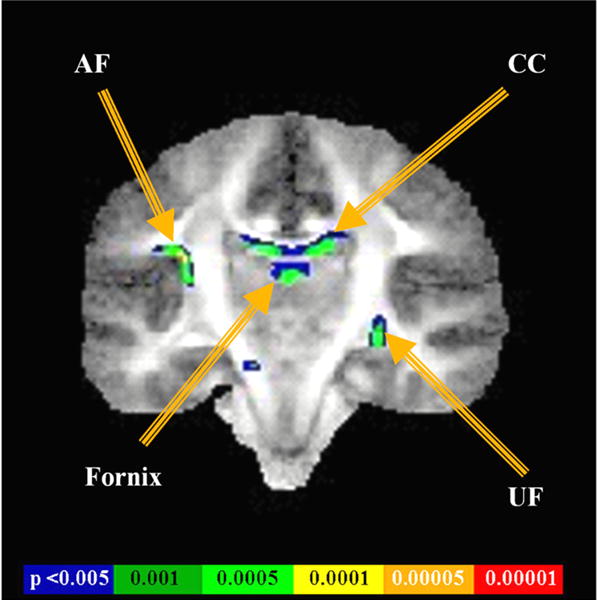
Results of a voxel-based analysis show the FA differences between controls and schizophrenics, located primarily in the arcuate fasciculus (AF), corpus callosum (CC), fornix, and uncinate fasciculus (UF).
We have also begun to evaluate the fornix in patients diagnosed with schizophrenia compared with normal controls, where we have shown a decrease in FA in the patient group.58
Thus, in summary, empirical data have amassed that clearly support the notion that frontal-temporal connectivity in the brain is likely disrupted in schizophrenia. As DTI provides us with the opportunity to directly visualize and measure these connections, it has become important for studying white matter fiber tracts that connect frontal and temporal lobes. To date, all our investigations have used a small ROI approach, measuring only a small segment of the fiber connection of interest. As this is just the beginning of such investigations, it is important now to develop tools that will enable us to improve the methods used in investigating white matter abnormalities in schizophrenia as well as in other neuropsychiatric disorders (see below under Future Directions for DTI Studies of Schizophrenia).
LIMITATIONS OF DTI IN SCHIZOPHRENIA STUDIES
To date, over 20 studies have investigated white matter abnormalities in schizophrenia using DTI. Results of these studies are frequently inconsistent as there are no accepted gold standards for data acquisition, processing, and analysis. In addition, small sample size, different ROI definitions, low S/N ratio, different number of acquisition angles, and different scanner gradient performance and field strength, as well as partial volume effect due to the low image resolution, all affect the final results. DTI findings in schizophrenia must therefore be understood within the context of the acquisition parameters used as well as in the context of the different methodological strategies adopted by different investigators.
Positive DTI findings in schizophrenia obtained to date are interesting, but methodological limitations, discussed here as well as elsewhere, limit our ability to conclude, with certainty, the nature of white matter pathology in schizophrenia. There is no question that DTI provides a new window of opportunity to evaluate white matter in a manner not possible with conventional MRI. However, we do not as yet know whether the abnormalities observed reflect a decrease in number of axons, a decrease in axonal diameter, thinner myelination sheaths, less coherent fibers, more fiber crossings, or simply more noise in the DTI data. All of these possibilities are supported to some extent by empirical data that show abnormal neuronal number and density,59,60 as well as myelin abnormalities in both postmortem anatomical studies12,61 and genetic studies.13,62,63 Moreover, DTI is not sufficiently specific to differentiate among these aforementioned pathologic processes, and thus we need to consider additional imaging techniques such as magnetization transfer imaging (MTI), MR spectroscopy, and relaxation time measurements in order to increase the specificity of white matter findings.
Another issue that needs to be addressed in DTI studies of schizophrenia is the effect of medication. For example, several DTI studies report a correlation between medication and FA in some regions of the brain,64,65 while other studies report no such association.15,19,52,66 Further clarification regarding medication effects is thus needed in order to understand further white matter pathology of schizophrenia. This suggests that unmedicated first-episode patients, a group that has not been studied with DTI, should be investigated.
Finally, there is evidence to suggest that some brain abnormalities progress over time in schizophrenia.67 In order to better understand such changes over time, and in particular in order to better understand changes observed in DTI, we need to look more closely at the effects of aging on DTI in normal control subjects and adjust for these effects when searching for neurodegenerative signs in schizophrenia.
FUTURE DIRECTIONS FOR DTI STUDIES OF SCHIZOPHRENIA
Over the last 5 years, DTI has become the most important imaging technique to investigate white matter changes in schizophrenia. This technique, however, is not free of limitations, the major two being the relatively low resolution and image distortions. Use of high magnet fields, and the development of fast, low distortion techniques that produce high resolution and high SNR diffusion images, will thus be important in furthering our understanding of white matter pathology in schizophrenia.
Fiber tracking is one of the most promising image processing techniques in terms of both the visualization of fiber pathways in the brain as well as in the quantitative analysis of specific fiber bundles. Future studies will likely be heavily dependent upon the further development and validation of these methods. Their application to schizophrenia will also likely reveal disruptions in connections between brain regions that heretofore could not be evaluated quantitatively. Such studies will also likely benefit from the acquisition of small, isotropic voxels, with diffusion encoded in multiple directions. With reliable fiber tracking, it will become easier to define and to measure whole fibers of interest.
Figure 5 provides an example of work that will be critical for evaluating fiber bundles in schizophrenia. Here, an automated fiber tracking procedure (described in detail in ref. 24) was used to create all of the major white matter fiber tracts in the brain. Two ROIs were then manually drawn in order to define and to extract the entire uncinate fasciculus fiber tract, which previously was evaluated using only one coronal slice.39
FIGURE 5.
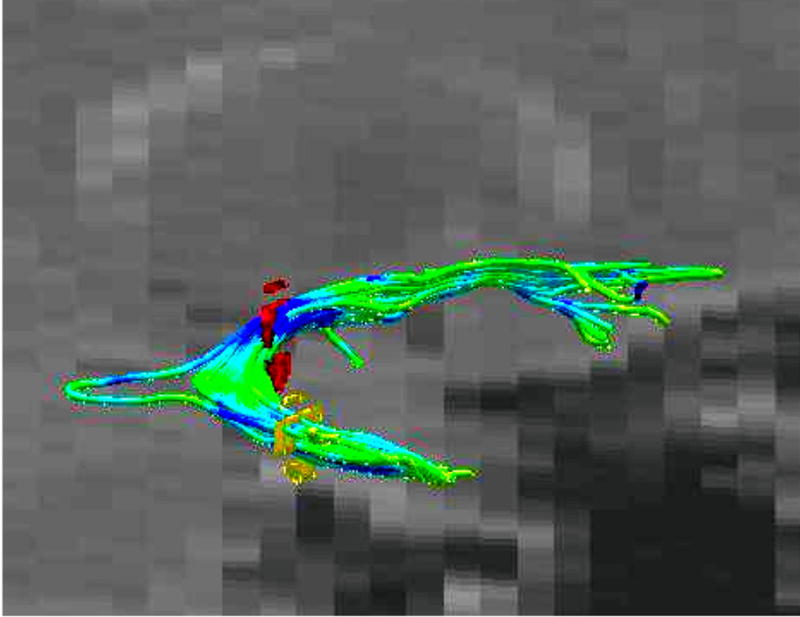
Three-dimensional model of the uncinate fasciculus (UF) is created using fiber tracking. This fiber tracking was created using two ROIs (red and yellow circles on the picture). The UF is colored according to the FA values (green: higher FA values; blue: lower FA values).
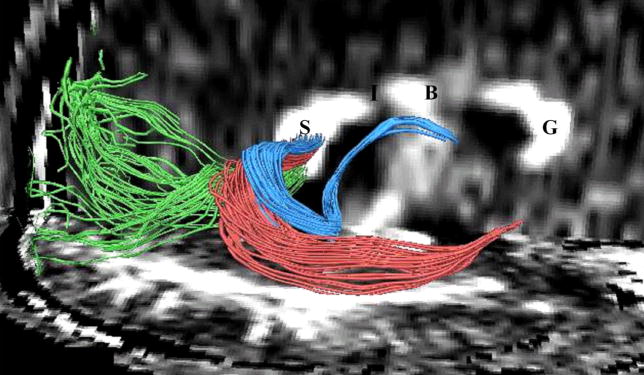
Another example of the way fiber tracking will be useful in future studies is displayed in Figure 6. Here, manual seeding within the splenium of the corpus callosum reveals fiber traces that are located in close proximity to each other within the corpus callosum, but which nonetheless belong to fiber bundles that connect anatomically distinct brain areas. This work, however, will need to be validated, perhaps in anatomical postmortem studies.
FIGURE 6.
Fibers traveling through the splenium of the corpus callosum are shown, where fibers connecting the left and right occipital lobe are displayed in green, fibers interconnecting lateral temporal regions are displayed in red, and fibers connecting medial temporal regions are displayed in blue. Additionally, the genu of the corpus callosum is labeled as “G”, the body as “B”, the isthmus as “I”, and the splenium as “S”.
The examples in Figures 5 and 6 show the potential strength of using tractography to define fiber bundles of interest. The regions defined by these “fibers of interests”, or FOIs, can then further be analyzed using fiber analysis methods such as performing statistics on diffusion-related values along the fibers. If the spatial extent of the FOI region is projected back to the voxel space, that will define the collection of voxels that is traversed with at least one fiber from the FOI, and this set of voxels can then be analyzed using traditional voxel-based methods.
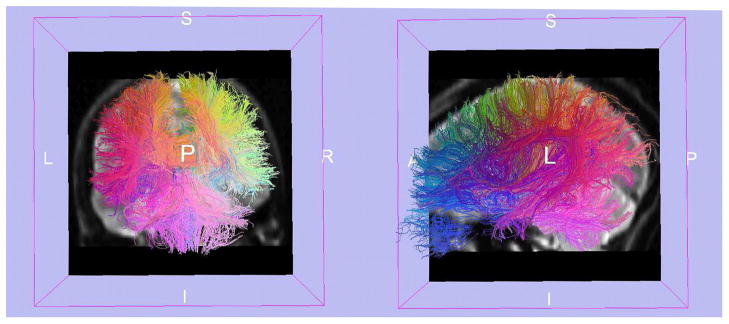
Future DTI analyses will also likely be performed in some fiber-related space, utilizing both fiber tract atlases and precise registration strategies, which will further increase the anatomical accuracy of such studies and allow a direct comparison of the whole fiber bundles. There is, however, still an open question as to how best to construct fiber spaces and atlases. Concepts from traditional voxel-based approaches do not directly translate to fibers. For example, averaging of information in voxel space normally produces an output that is also voxel-based (i.e., average values of a set of aligned MRI scans will produce a new smoother MRI looking scan). In contrast, the average of a set of fibers does not straightforwardly produce a set of smooth fibers. New methods that will be able to infer local coordinate systems defining anatomically relevant positions along the fibers are thus a direction of research that will likely produce interesting results. Automated methods for fiber clustering are an important direction for this type of work68,69 (Fig. 7).
FIGURE 7.
Fiber traces from a human brain are colored such that fibers with similar endpoints are assigned similar colors. Slices from a T2-weighted volume add additional understanding of the anatomy. The view of the fiber traces on the left is coronal, while the view on the right is sagittal. [Courtesy of Anders Brun.]
Future studies investigating white matter abnormalities in schizophrenia should also be combined not only with other structural imaging techniques, but also with functional MRI and PET imaging in order to characterize and to understand more fully the relationship between functional and structural abnormalities in schizophrenia. Using multiple images to evaluate brain abnormalities in schizophrenia will provide a wealth of information that will likely lead to an increased understanding of the neuropathology of schizophrenia, and perhaps even targeted treatments, as we begin to understand brain circuitry better with the use of multiple imaging techniques.
Finally, DTI was introduced to clinical imaging in 1995 and has thus only begun to be used to explore white matter abnormalities in schizophrenia. Nevertheless, it has fast become one of the most popular imaging techniques used in schizophrenia research. With still newer technological advances, we will likely learn even more about white matter abnormalities in this devastating disorder.
Acknowledgments
We would like to thank Marie Fairbanks for her administrative assistance. Additionally, we gratefully acknowledge the support of the National Alliance for Research on Schizophrenia and Depression (to M. Kubicki), The Wodecroft Foundation (to M. Kubicki), the National Institutes of Health (R03 MH 068464-02 to M. Kubicki, R01 NS 39335 and R01 MH 40799 to R. W. McCarley, and K02 MH 01110 and R01 MH 50747 to M. E. Shenton), and the Department of Veterans Affairs Merit and REAP Awards (to M. E. Shenton and R. W. McCarley). In addition, we thank Anders Brun for his figure showing fiber traces (Fig. 7).
References
- 1.Kraepelin E. Dementia Praecox. Churchill Livingstone; New York: 19191971. [Google Scholar]
- 2.Bleuler M. The concept of schizophrenia. Am J Psychiatry. 19111954;111:382–383. doi: 10.1176/ajp.111.5.382. [DOI] [PubMed] [Google Scholar]
- 3.Johnstone EC, et al. Cerebral ventricular size and cognitive impairment in chronic schizophrenia. Lancet. 1976;2:924–926. doi: 10.1016/s0140-6736(76)90890-4. [DOI] [PubMed] [Google Scholar]
- 4.SMITH RC, et al. Nuclear magnetic resonance in schizophrenia: a preliminary study. Psychiatry Res. 1984;12:137–147. doi: 10.1016/0165-1781(84)90013-1. [DOI] [PubMed] [Google Scholar]
- 5.Shenton ME, et al. A review of MRI findings in schizophrenia. Schizophr Res. 2001;49:1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wernicke C. Grundrisse der Psychiatrie. Thieme; Leipzig: 1906. [Google Scholar]
- 7.Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry. 1987;44:660–669. doi: 10.1001/archpsyc.1987.01800190080012. [DOI] [PubMed] [Google Scholar]
- 8.Weinberger DR, et al. Evidence of dysfunction of a prefrontal-limbic network in schizophrenia: a magnetic resonance imaging and regional cerebral blood flow study of discordant monozygotic twins. Am J Psychiatry. 1992;149:890–897. doi: 10.1176/ajp.149.7.890. [DOI] [PubMed] [Google Scholar]
- 9.Weinberger DR. From neuropathology to neurodevelopment. Lancet. 1995;346:552–557. doi: 10.1016/s0140-6736(95)91386-6. [DOI] [PubMed] [Google Scholar]
- 10.Yurgelun-Todd DA, et al. Functional magnetic resonance imaging of schizophrenic patients and comparison subjects during word production. Am J Psychiatry. 1996;153:200–205. doi: 10.1176/ajp.153.2.200. [DOI] [PubMed] [Google Scholar]
- 11.Shergill SS, et al. Mapping auditory hallucinations in schizophrenia using functional magnetic resonance imaging. Arch Gen Psychiatry. 2000;57:1033–1038. doi: 10.1001/archpsyc.57.11.1033. [DOI] [PubMed] [Google Scholar]
- 12.Uranova N, et al. Electron microscopy of oligodendroglia in severe mental illness. Brain Res Bull. 2001;55:597–610. doi: 10.1016/s0361-9230(01)00528-7. [DOI] [PubMed] [Google Scholar]
- 13.Hakak Y, et al. Genome-wide expression analysis reveals dysregulation of myelination-related genes in chronic schizophrenia. Proc Natl Acad Sci USA. 2001;98:4746–4751. doi: 10.1073/pnas.081071198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davis KL, et al. White matter changes in schizophrenia: evidence for myelin-related dysfunction. Arch Gen Psychiatry. 2003;60:443–456. doi: 10.1001/archpsyc.60.5.443. [DOI] [PubMed] [Google Scholar]
- 15.Buchsbaum MS, et al. MRI white matter diffusion anisotropy and PET metabolic rate in schizophrenia. Neuroreport. 1998;9:425–430. doi: 10.1097/00001756-199802160-00013. [DOI] [PubMed] [Google Scholar]
- 16.Basser PJ, Mattiello J, Lebihan D. MR diffusion tensor spectroscopy and imaging. Biophys J. 1994;66:259–267. doi: 10.1016/S0006-3495(94)80775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lim KO, et al. Compromised white matter tract integrity in schizophrenia inferred from diffusion tensor imaging. Arch Gen Psychiatry. 1999;56:367–374. doi: 10.1001/archpsyc.56.4.367. [DOI] [PubMed] [Google Scholar]
- 18.Hoptman MJ, et al. Frontal white matter microstructure, aggression, and impulsivity in men with schizophrenia: a preliminary study. Biol Psychiatry. 2002;52:9–14. doi: 10.1016/s0006-3223(02)01311-2. [DOI] [PubMed] [Google Scholar]
- 19.Steel RM, et al. Diffusion tensor imaging (DTI) and proton magnetic resonance spectroscopy (H MRS) in schizophrenic subjects and normal controls. Psychiatry Res Neuroimaging Sect. 2001;106:161–170. doi: 10.1016/s0925-4927(01)00080-4. [DOI] [PubMed] [Google Scholar]
- 20.Burns J, et al. Structural disconnectivity in schizophrenia: a diffusion tensor magnetic resonance imaging study. Br J Psychiatry. 2003;182:439–443. [PubMed] [Google Scholar]
- 21.Hubl D, et al. Pathways that make voices: white matter changes in auditory hallucinations. Arch Gen Psychiatry. 2004;61:658–668. doi: 10.1001/archpsyc.61.7.658. [DOI] [PubMed] [Google Scholar]
- 22.Ardekani BA, et al. MRI study of white matter diffusion anisotropy in schizophrenia. Neuroreport. 2003;14:2025–2029. doi: 10.1097/00001756-200311140-00004. [DOI] [PubMed] [Google Scholar]
- 23.Foong J, et al. Investigating regional white matter in schizophrenia using diffusion tensor imaging. Neuroreport. 2002;13:333–336. doi: 10.1097/00001756-200203040-00017. [DOI] [PubMed] [Google Scholar]
- 24.Park HJ, et al. Spatial normalization of diffusion tensor MRI using multiple channels. Neuroimage. 2003;20:1995–2009. doi: 10.1016/j.neuroimage.2003.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kubicki M, et al. Diffusion tensor imaging and its application to neuropsychiatric disorders. Harv Rev Psychiatry. 2002;10:324–336. doi: 10.1080/10673220216231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Breier A, et al. Brain morphology and schizophrenia: a magnetic resonance imaging study of limbic, prefrontal cortex, and caudate structures [see comments] Arch Gen Psychiatry. 1992;49:921–926. doi: 10.1001/archpsyc.1992.01820120009003. [DOI] [PubMed] [Google Scholar]
- 27.Paillere-Martinot M, et al. Cerebral gray and white matter reductions and clinical correlates in patients with early onset schizophrenia. Schizophr Res. 2001;50:19–26. doi: 10.1016/s0920-9964(00)00137-7. [DOI] [PubMed] [Google Scholar]
- 28.Sigmudsson T, et al. Structural abnormalities in frontal, temporal, and limbic regions and interconnecting white matter tracts in schizophrenic patients with prominent negative symptoms. Am J Psychiatry. 2001;158:234–243. doi: 10.1176/appi.ajp.158.2.234. [DOI] [PubMed] [Google Scholar]
- 29.Spalletta G, et al. Chronic schizophrenia as a brain misconnection syndrome: a white matter voxel-based morphometry study. Schizophr Res. 2003;64:15–23. doi: 10.1016/s0920-9964(03)00010-0. [DOI] [PubMed] [Google Scholar]
- 30.Mitelman SA, et al. MRI assessment of gray and white matter distribution in Brodmann’s areas of the cortex in patients with schizophrenia with good and poor outcomes. Am J Psychiatry. 2003;160:2154–2168. doi: 10.1176/appi.ajp.160.12.2154. [DOI] [PubMed] [Google Scholar]
- 31.Wible CG, et al. Prefrontal cortex and schizophrenia: a quantitative magnetic resonance imaging study. Arch Gen Psychiatry. 1995;52:279–288. doi: 10.1001/archpsyc.1995.03950160029007. [DOI] [PubMed] [Google Scholar]
- 32.Friston KJ, Frith CD. Schizophrenia: a disconnection syndrome? Clin Neurosci. 1995;3:89–97. [PubMed] [Google Scholar]
- 33.McGuire PK, Frith CD. Disordered functional connectivity in schizophrenia [editorial] Psychol Med. 1996;26:663–667. doi: 10.1017/s0033291700037673. [DOI] [PubMed] [Google Scholar]
- 34.Fletcher P, et al. Abnormal cingulate modulation of fronto-temporal connectivity in schizophrenia. Neuroimage. 1999;9:337–342. doi: 10.1006/nimg.1998.0411. [DOI] [PubMed] [Google Scholar]
- 35.Kubicki M, et al. An fMRI study of semantic processing in men with schizophrenia. Neuroimage. 2003;20:1923–1933. doi: 10.1016/s1053-8119(03)00383-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klingler J, Gloor P. The connections of the amygdala and of the anterior temporal cortex in the human brain. J Comp Neurol. 1960;115:333–369. doi: 10.1002/cne.901150305. [DOI] [PubMed] [Google Scholar]
- 37.Ebeling U, von Cramon D. Topography of the uncinate fascicle and adjacent temporal fiber tracts. Acta Neurochir (Wien) 1992;115:143–148. doi: 10.1007/BF01406373. [DOI] [PubMed] [Google Scholar]
- 38.Kier EL, et al. MR imaging of the temporal stem: anatomic dissection tractography of the uncinate fasciculus, inferior occipitofrontal fasciculus, and Meyer’s loop of the optic radiation. AJNR Am J Neuroradiol. 2004;25:677–691. [PMC free article] [PubMed] [Google Scholar]
- 39.Kubicki M, et al. Uncinate fasciculus findings in schizophrenia: a magnetic resonance diffusion tensor imaging study. Am J Psychiatry. 2002;159:813–820. doi: 10.1176/appi.ajp.159.5.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crespo-Facorro B, et al. Insular cortex abnormalities in schizophrenia: a structural magnetic resonance imaging study of first-episode patients. Schizophr Res. 2000;46:35–43. doi: 10.1016/s0920-9964(00)00028-1. [DOI] [PubMed] [Google Scholar]
- 41.Goldstein JM, et al. Cortical abnormalities in schizophrenia identified by structural magnetic resonance imaging. Arch Gen Psychiatry. 1999;56:537–547. doi: 10.1001/archpsyc.56.6.537. [DOI] [PubMed] [Google Scholar]
- 42.Benes FM, et al. Increased GABAA receptor binding in superficial layers of cingulate cortex in schizophrenics. J Neurosci. 1992;12:924–929. doi: 10.1523/JNEUROSCI.12-03-00924.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Benes FM, Vincent SL, Todtenkopf M. The density of pyramidal and nonpyramidal neurons in anterior cingulate cortex of schizophrenic and bipolar subjects. Biol Psychiatry. 2001;50:395–406. doi: 10.1016/s0006-3223(01)01084-8. [DOI] [PubMed] [Google Scholar]
- 44.Bouras C, et al. Anterior cingulate cortex pathology in schizophrenia and bipolar disorder. Acta Neuropathol (Berl) 2001;102:373–379. doi: 10.1007/s004010100392. [DOI] [PubMed] [Google Scholar]
- 45.Kiehl KA, Liddle PF, Hopfinger JB. Error processing and the rostral anterior cingulate: an event-related fMRI study. Psychophysiology. 2000;37:216–223. [PubMed] [Google Scholar]
- 46.Carter CS, et al. Anterior cingulate gyrus dysfunction and selective attention deficits in schizophrenia: [15O]H2O PET study during single-trial Stroop task performance. Am J Psychiatry. 1997;154:1670–1675. doi: 10.1176/ajp.154.12.1670. [DOI] [PubMed] [Google Scholar]
- 47.Nordahl TE, et al. Anterior cingulate metabolism correlates with Stroop errors in paranoid schizophrenia patients. Neuropsychopharmacology. 2001;25:139–148. doi: 10.1016/S0893-133X(00)00239-6. [DOI] [PubMed] [Google Scholar]
- 48.Frith CD, et al. Regional brain activity in chronic schizophrenic patients during the performance of a verbal fluency task. Br J Psychiatry. 1995;167:343–349. doi: 10.1192/bjp.167.3.343. [DOI] [PubMed] [Google Scholar]
- 49.Ford JM, et al. Neurophysiological evidence of corollary discharge dysfunction in schizophrenia. Am J Psychiatry. 2001;158:2069–2071. doi: 10.1176/appi.ajp.158.12.2069. [DOI] [PubMed] [Google Scholar]
- 50.Wang F, et al. Anterior cingulum abnormalities in male patients with schizophrenia determined through diffusion tensor imaging. Am J Psychiatry. 2004;161:573–575. doi: 10.1176/appi.ajp.161.3.573. [DOI] [PubMed] [Google Scholar]
- 51.Sun Z, et al. Abnormal anterior cingulum in patients with schizophrenia: a diffusion tensor imaging study. Neuroreport. 2003;14:1833–1836. doi: 10.1097/00001756-200310060-00015. [DOI] [PubMed] [Google Scholar]
- 52.Kubicki M, et al. Cingulate fasciculus integrity disruption in schizophrenia: a magnetic resonance diffusion tensor imaging study. Biol Psychiatry. 2003;54:1171–1180. doi: 10.1016/s0006-3223(03)00419-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Niznikiewicz MA, et al. ERP assessment of visual and auditory language processing in schizophrenia. J Abnorm Psychol. 1997;106:85–94. doi: 10.1037//0021-843x.106.1.85. [DOI] [PubMed] [Google Scholar]
- 54.Guillem F, et al. Memory impairment in schizophrenia: a study using event-related potentials in implicit and explicit tasks. Psychiatry Res. 2001;104:157–173. doi: 10.1016/s0165-1781(01)00305-5. [DOI] [PubMed] [Google Scholar]
- 55.Hazlett EA, et al. Hypofrontality in unmedicated schizophrenia patients studied with PET during performance of a serial verbal learning task. Schizophr Res. 2000;43:33–46. doi: 10.1016/s0920-9964(99)00178-4. [DOI] [PubMed] [Google Scholar]
- 56.Fletcher PC, et al. Brain activations in schizophrenia during a graded memory task studied with functional neuroimaging. Arch Gen Psychiatry. 1998;55:1001–1008. doi: 10.1001/archpsyc.55.11.1001. [DOI] [PubMed] [Google Scholar]
- 57.Kubicki M, et al. DTI and MTR abnormalities in schizophrenia: analysis of white matter integrity. Neuropsychopharmacology. 2004;29:167. doi: 10.1016/j.neuroimage.2005.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kuroki N, et al. Fornix integrity and hippocampal volume in schizophrenia. Biol Psychiatry. 2004;55:72S. doi: 10.1016/j.biopsych.2005.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Eastwood SL, Harrison PJ. Interstitial white matter neurons express less reelin and are abnormally distributed in schizophrenia: towards an integration of molecular and morphologic aspects of the neurodevelopmental hypothesis. Mol Psychiatry. 2003;8:821–831. doi: 10.1038/sj.mp.4001399. [DOI] [PubMed] [Google Scholar]
- 60.Kirkpatrick B, et al. Interstitial cells of the white matter in the dorsolateral prefrontal cortex in deficit and nondeficit schizophrenia. J Nerv Ment Dis. 2003;191:563–567. doi: 10.1097/01.nmd.0000087181.61164.e1. [DOI] [PubMed] [Google Scholar]
- 61.Hof PR, et al. Loss and altered spatial distribution of oligodendrocytes in the superior frontal gyrus in schizophrenia. Biol Psychiatry. 2003;53:1075–1085. doi: 10.1016/s0006-3223(03)00237-3. [DOI] [PubMed] [Google Scholar]
- 62.Tkachev D, et al. Oligodendrocyte dysfunction in schizophrenia and bipolar disorder. Lancet. 2003;362:798–805. doi: 10.1016/S0140-6736(03)14289-4. [DOI] [PubMed] [Google Scholar]
- 63.Aston C, Jiang L, Sokolov BP. Microarray analysis of postmortem temporal cortex from patients with schizophrenia. J Neurosci Res. 2004;77:858–866. doi: 10.1002/jnr.20208. [DOI] [PubMed] [Google Scholar]
- 64.Minami T, et al. Diffusion tensor magnetic resonance imaging of disruption of regional white matter in schizophrenia. Neuropsychobiology. 2003;47:141–145. doi: 10.1159/000070583. [DOI] [PubMed] [Google Scholar]
- 65.Okugawa G, et al. Subtle disruption of the middle cerebellar peduncles in patients with schizophrenia. Neuropsychobiology. 2004;50:119–123. doi: 10.1159/000079101. [DOI] [PubMed] [Google Scholar]
- 66.Foong J, et al. Neuropathological abnormalities of the corpus callosum in schizophrenia: a diffusion tensor imaging study. J Neurol Neurosurg Psychiatry. 2000;68:242–244. doi: 10.1136/jnnp.68.2.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kasai K, et al. Progressive decrease of left superior temporal gyrus gray matter volume in patients with first-episode schizophrenia. Am J Psychiatry. 2003;160:156–164. doi: 10.1176/appi.ajp.160.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brun A, et al. Coloring of DT-MRI fiber traces using Laplacian eigenmaps: computer aided systems theory (EUROCAST’03) Lect Notes Comput Sci. 2003;2809:564–572. [Google Scholar]
- 69.Brun A, et al. Seventh International Conference on Medical Image Computing and Computer-Assisted Intervention. Springer-Verlag; Berlin/New York: 2004. Clustering fiber tracts using normalized cuts. [DOI] [PMC free article] [PubMed] [Google Scholar]