Abstract
Cerebellar Purkinje neurons (PNs) receive inhibitory GABAergic input from stellate and basket cells, which are located in the outer and inner portions of the molecular layer, respectively. Ethanol (EtOH) was recently shown to increase GABAergic transmission at PNs via a mechanism that involves enhanced calcium release from presynaptic internal stores (Kelm et al., J Pharmacol Exp Ther. 323:356-64, 2007). Here, we further characterized the effect of EtOH on GABA release and assessed its impact on PN excitability. Using whole-cell patch-clamp electrophysiological techniques in cerebellar vermis parasagittal slices, we found that EtOH acutely increases the frequency but not the amplitude or half-width of miniature and spontaneous inhibitory postsynaptic currents (IPSCs). EtOH significantly increased the amplitude and decreased the paired-pulse ratio of IPSCs evoked by stimulation in the outer but not inner molecular layer. In current-clamp, EtOH decreased both the amplitude of EPSPs evoked in PNs by granule cell axon stimulation and the number of action potentials triggered by these events; these effects depended on GABAA receptor activation as they were not observed in presence of bicuculline. Loose-patch cell-attached PN recordings revealed that neither the spontaneous action potential firing frequency nor the coefficient of variation of the interspike interval were altered by acute EtOH exposure. These findings suggest that EtOH differentially affects GABAergic transmission at stellate cell- and basket cell-to-PN synapses and that it modulates PN firing triggered by granule cell axonal input. These effects could be in part responsible for the cerebellar impairments associated with acute EtOH intoxication.
Introduction
The cerebellum is an important target of the acute and chronic actions of ethanol (EtOH) both during development and at maturity. EtOH-induced alterations of balance, speech, motor coordination and certain cognitive functions are thought to be mediated, in part, by impairment of cerebellar function. In the cerebellar cortex, Purkinje neurons (PNs) have been shown to be EtOH sensitive. PNs are GABAergic neurons that project to deep cerebellar nuclei and constitute the sole output of the cerebellar cortex. PNs receive two types of excitatory inputs: the mossy-parallel fiber and the climbing fiber systems. Inhibitory inputs to PNs are provided, in part, by two types of interneurons that are located in the molecular layer: basket and stellate cells. Basket cells preferentially innervate axonal-initial segments whereas stellate cells preferentially innervate dendrites and spines. GABAergic input from these interneurons, as well as PN collaterals, controls the excitability of PNs and is necessary for their normal functioning (Hausser and Clark, 1997). Synaptic connections between PNs were recently shown to be relatively weak in the parasagittal cerebellar slice preparation (Orduz and Llano, 2007).
The effects of EtOH on GABAergic transmission at PNs have been the focus of a number of studies, which have given disparate results. Harris and Sinclair (1984) showed that a slow intravenous infusion of EtOH antagonized the inhibition of PN single unit activity produced by local micropressure application of GABA in anesthetized rats. Blockade of EtOH-induced inhibition of PN firing by benzodiazepine inverse agonists and the GABAA receptor antagonist, bicuculline, was detected in anesthetized rats (Palmer et al., 1988; but see Lee et al., 1995). In vivo electrophysiological recordings from PNs showed that EtOH does not consistently potentiate responses evoked by exogenous GABA but it was shown to have a more reliable action if β-adrenergic, GABAB, or nicotinic receptors were concomitantly activated (Freund and Palmer, 1997; Yang et al., 1999; Yang et al., 2000). Sapp and Yeh, (1998) demonstrated that EtOH acutely potentiates currents evoked by exogenous GABA in cultured and acutely dissociated PNs, suggesting that GABAA receptors can be directly modulated by acute EtOH exposure under some experimental conditions, in general agreement with the results of experiments performed in preparations from other brain regions (Weiner and Valenzuela, 2006).
In recent years, evidence from a number of laboratories indicates that EtOH indirectly potentiates GABAA receptor function via presynaptic mechanisms in several neuronal populations, including PNs. Criswell and collaborators performed studies with cerebellar vermis slices and found that acute EtOH exposure dose-dependently increases the frequency but not the amplitude or decay of GABAA receptor-mediated miniature inhibitory synaptic currents (mIPSCs) in PNs (Ming et al., 2006). A similar effect was observed in our laboratory (Breese et al., 2006). These findings are consistent with an EtOH-induced increase in quantal GABA release at molecular layer interneuron (MLI)-to-PN synapses. Using cerebellar slices and mechanically dissociated PNs, Kelm et al. (2007) determined that the mechanism responsible for this effect of EtOH involves an increase in Ca2+ release from presynaptic internal stores but not the release of retrograde messengers from PNs.
The purpose of this study was to further characterize the effect of EtOH on GABA release onto PNs. Using patch-clamp electrophysiological techniques and acute cerebellar vermis slices, we investigated the effect of EtOH on action potential-dependent spontaneous IPSCs (sIPSCs) and IPSCs evoked by preferential electrical stimulation of either basket or stellate cells. We next assessed the impact of EtOH on PN excitability both at the level of action potential firing driven by excitatory input from granule cell axons and spontaneous action potential firing.
Methods
For all the experiments, we used EtOH (95%, spectrophotometric grade) from Sigma Chemical Co. (St. Louis, MO). Unless indicated, all other chemicals were from Sigma-RBI-Fluka (St. Louis, MO). Experiments were performed in parasagittal vermis cerebellar slices that were prepared from 19-30 day-old Sprague-Dawley rats (Harlan, Indianapolis, IN). Animals were euthanized by rapid decapitation under deep anesthesia with ketamine (250 mg/kg I.P.) and 200-250 μm thick slices were prepared with a vibratome (Technical Products International, St. Louis, MO). Slices were cut in cold solution containing (in mM) 220 sucrose, 26 NaHCO3, 10 glucose, 6 MgSO4, 3 KCl, 1.25 NaH2PO4, 0.2 CaCl2 and 0.43 ketamine; this solution was pre-equilibrated with 95% O2 plus 5% CO2. Immediately after this procedure, slices were transferred to a chamber containing artificial cerebrospinal fluid (ACSF) and allowed to recover at 35-36 °C for 45 min, followed by storage at room temperature in the same ACSF. ACSF contained (in mM): 126 NaCl, 3 KCl, 1.25 NaH2PO4, 1 MgSO4, 26 NaHCO3, 2 CaCl2, and 10 glucose equilibrated with 95% O2 plus 5% CO2. After a total recovery time ≥ 80 min, slices were transferred to a recording chamber perfused with ACSF at a rate of 2-3 ml/min and maintained at 31-32 °C. Neurons were visualized using infrared-differential interference contrast microscopy and recordings performed with Axopatch 200B or Multiclamp 700B amplifiers (Molecular Devices, Sunnyvale, CA). All agents were bath-applied to the slices. To eliminate a contribution of rapid EtOH tolerance to the results, slices were exposed to EtOH only once.
Recordings of sIPSCs and mIPSCs were obtained in the whole-cell patch-clamp configuration at a holding potential of −65 mV. We used an internal solution containing (in mM): 140 CsCl, 2 MgCl2, 1 CaCl2, 10 ethylene glycol tetraacetic acid (EGTA), 10 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES; pH 7.3), 4 disodium adenosine triphosphate (Na2-ATP) and 4 N-(2,6-Dimethylphenylcarbamoylmethyl)triethylammonium bromide (QX-314; Tocris-Cookson, Ellisville, MO). Patch pipettes had resistances of 3-4 MΩ when filled with this solution; access resistance was between 20 and 40 MΩ and this was not compensated; if access resistance changed more than 25%, the recording was discarded. Recordings of sIPSCs were obtained in ACSF containing 10 μM of 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxaline-2,3-dione (NBQX; Axxora, San Diego, CA). Recordings of mIPSCs were obtained in ACSF containing 10 μM NBQX plus tetrodotoxin (TTX; 0.5 μM; Calbiochem, San Diego, CA).
Evoked IPSCs (eIPSCs; stimulus duration = 100 μs) were elicited every 20 s with a concentric bipolar electrode (outer pole diameter = 125 μm; inner pole diameter = 25 μm; FHC, Bowdoinham, ME) placed in either the outer third (distal stimulation) or the inner third of the molecular layer (proximal stimulation) using an internal solution containing (in mM): 125 potassium gluconate, 10 HEPES (pH 7.25), 10 MgCl2, 1 EGTA, 0.1 CaCl2, 4 Na2-ATP and 4 QX-314. For these studies, the PN membrane potential was held at 0 mV. In some cases, distal or proximal IPSCs were evoked with glass electrodes (3-4 ΩM) that were filled with ACSF (stimulus duration = 100-200 μs). IPSCs evoked in PNs by glass electrode stimulation were recorded at −60 mV with the same CsCl-based internal solution used for the sIPSC and mIPSC experiments. Results obtained with concentric bipolar or glass electrode stimulation were similar and, therefore, the data were pooled. In all cases, an input-output curve was measured at the beginning of each experiment and the stimulation intensity was then set at a value that was 40-50% of maximum for the reminder of the recording. For calculation of the paired pulse ratio, we obtained IPSC average traces corresponding to the control, EtOH, and washout conditions and divided the amplitude of averaged IPSC2 by that of averaged IPSC1 (Kim and Alger, 2001).
Recordings of excitatory postsynaptic potentials (EPSPs) evoked by stimulation of granule cell axons in PNs were obtained in the whole-cell current-clamp configuration; a small amount of current was injected to keep the resting membrane potential near −70 mV using the potassium gluconate-based internal solution described above; this membrane potential is near the calculated ECl– (−68 mV), eliminating IPSPs. Granule cell axons were stimulated with concentric bipolar electrodes (stimulus duration = 100 μs) that were positioned in the molecular layer, 200 μm from the recorded cell. EPSPs were evoked by trains of 5 stimuli at 100 Hz.
Loose-patch cell-attached recordings (seal resistance = 7 to 140 MΩ) from PN somata were obtained with glass pipettes (2-4 MΩ) filled with ACSF at a holding potential of 0 mV; it should be noted that the holding potential in loose-cell attached experiments is unlikely to significantly affect the PN resting membrane potential because most of the current generated by the amplifier will leak across the loose seal rather than passing through the patch.
Data were filtered at 2 kHz and digitized at 5-10 kHz with Digidata 1200 or 1322A and pClamp-8 or 9 (Molecular Devices, Sunnyvale, CA); sIPSCs, mIPSCs, and spontaneous action potential firing were analyzed with Minis Analysis program (Synaptosoft, Decatur, GA). The coefficient of variation of the interspike interval was calculated as the ratio of the standard deviation to the mean. Evoked IPSCs and EPSPs were analyzed with Clampfit 9 (Molecular Devices). The effect of EtOH was calculated with respect to the average of control and washout responses; we only considered neurons that exhibited at least a partial return to baseline activity upon EtOH washout. The Kolmogorov-Smirnov (K-S) test was used in some cases, as indicated. Pooled data were statistically analyzed with Prizm 4 (GraphPad, San Diego, CA). Data are presented as mean ± SEM.
Results
Effect of EtOH on miniature and spontaneous IPSCs
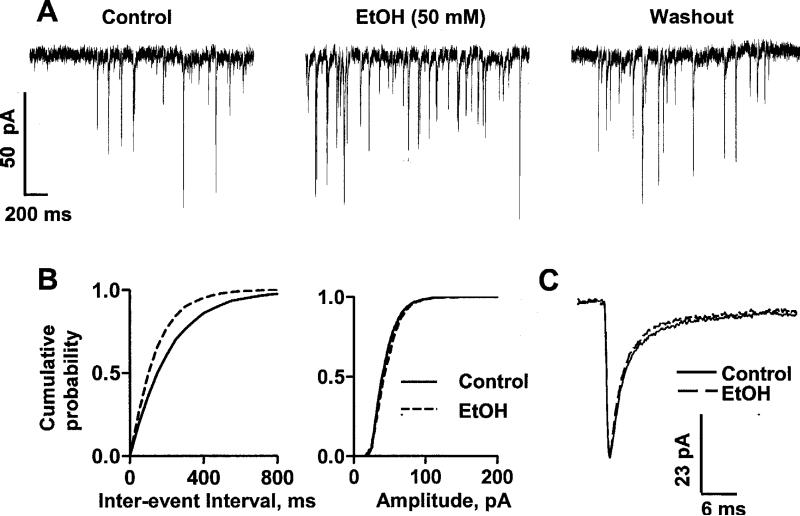
We first recorded miniature IPSCs in the presence of TTX (0.5 μM) and NBQX (10 μM); under these conditions, mIPSCs had a basal frequency of 3.0 ± 0.5 Hz, a basal amplitude of 52.4 ± 3.5 pA and a basal half-width of 3.2 ± 0.3 ms (n = 11; see Fig 1 for example traces). These events were blocked by application of bicuculline (20 μM; not shown) confirming that they were mediated by GABAA receptors. Acute exposure to 50 mM EtOH increased mIPSC frequency (i.e. decreased the inter-event interval) without affecting the amplitude or half-width of these events (Fig 1). Analysis of cumulative probability plots (for an example, see Fig 1B) with the K-S test revealed that 50 mM EtOH significantly (p< 0.01) decreased the inter-event interval in 7 out of 9 neurons. Fig 2A illustrates the time course of the effect of 50 mM EtOH on mIPSC frequency. The effect of EtOH developed gradually over a period of approximately 5 min and it was at least partially reversibly upon washout. Washout of the EtOH effect also took approximately 5 min. Fig 2B shows that the effect of EtOH on mIPSC frequency was concentration dependent.
Fig 1. Acute EtOH exposure increases mIPSC frequency.
A. Sample mIPSC traces obtained under control conditions, in the presence of 50 mM EtOH, and after washout. B. Cumulative probability plots for mIPSC inter-event interval and amplitude corresponding to the recording shown in panel A. EtOH induced a significant (p< 0.01 by K-S test) shift to the left in the inter-event interval cumulative probability plot. The amplitude cumulative probability plot was not significantly affected. C. Average superimposed mIPSC traces, corresponding to the recording shown in panel A.
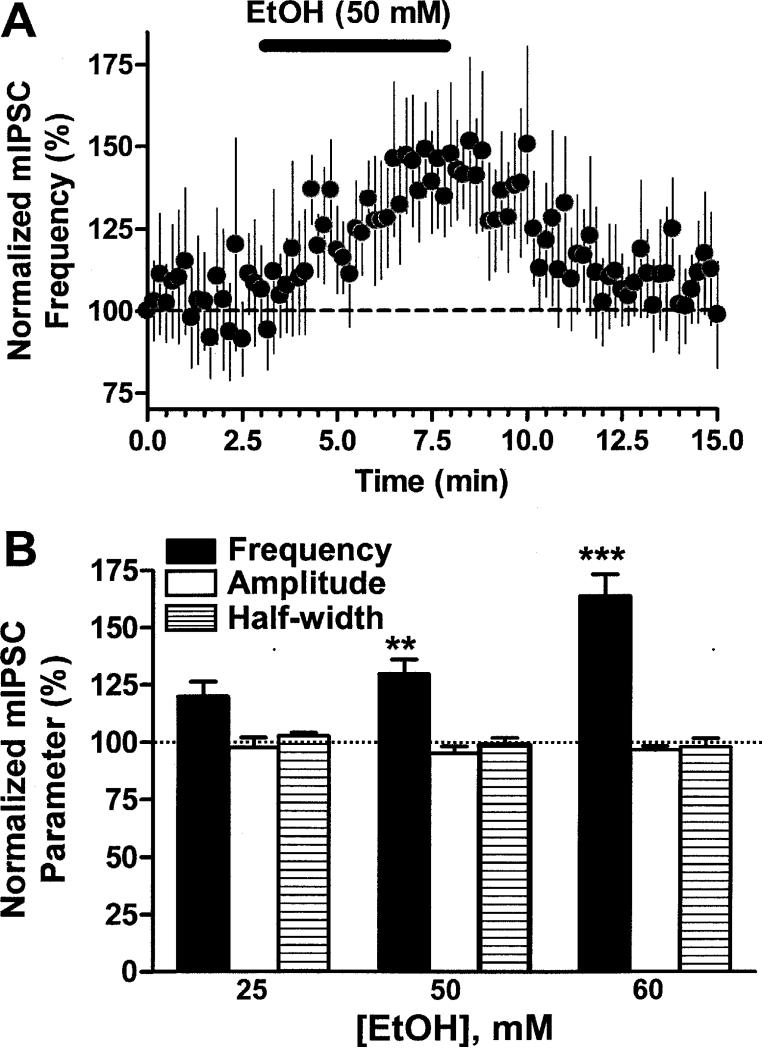
Fig 2. Acute EtOH exposure reversibly and dose-dependently increases mIPSC frequency.
A. Shown is the time-course of the effect of 50 mM EtOH on mIPSC frequency. The average mIPSC frequency in 10 s bins was obtained. These data were subsequently normalized with respect to the average mIPSC frequency obtained during the first 10 s of recording (n = 10). B. EtOH acutely increased mIPSC frequency in a concentration-dependent manner (n = 4, 9 and 7 for 25, 50 and 60 mM, respectively). The effect of EtOH was calculated with respect to the average of baseline and washout responses (represented by the dotted line). **, p<0.002; ***, p<0.001 by one sample t-test vs. 100%
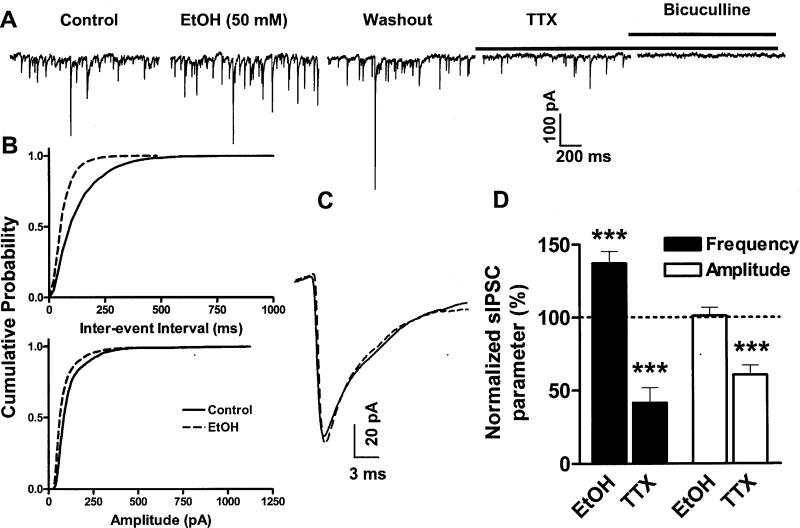
We next recorded sIPSCs in the presence NBQX (10 μM); these events had a basal frequency of 10.5 ± 2.3 Hz, a basal amplitude of 95 ± 13.5 pA and a basal half-width of 4.5 ± 0.6 ms (n = 6; see Fig 3 for example traces). The sIPSCs were blocked by application of bicuculline (20 μM; Fig 3A). EtOH (50 mM) increased sIPSC frequency without affecting amplitude (Fig 3; Table 1). Analysis of cumulative probability plots (for an example, see Fig 3B) with the K-S test revealed that 50 mM EtOH significantly (p< 0.01) decreased the inter-event interval in 5 out of 6 neurons. The normalized sIPSC half-width in presence of 50 mM EtOH was 96.8 ± 3.1% of control (n = 6). For comparison, we also tested the effect of 25 mM EtOH and found that this concentration did not significantly affect sIPSC frequency or amplitude (Table 1). The normalized sIPSC half-width in presence of 25 mM EtOH was 97.6 ± 2 % of control (n = 4)
Fig 3. Acute EtOH exposure increases sIPSC frequency.
A. Sample sIPSC traces obtained under control conditions, in the presence of 50 mM EtOH, and after washout. Subsequently, TTX (0.5 μM) was applied to block action potential dependent IPSCs. At the end of the recording, bicuculline (20 μM) was applied to block GABAA receptors. B. Cumulative probability plots for sIPSC inter-event interval and amplitude corresponding to the recording shown in panel A. EtOH induced a significant (p< 0.0001 by K-S test) shift to the left in the inter-event interval cumulative probability plot. The amplitude cumulative probability plot was not significantly affected. C. Average superimposed sIPSC traces, corresponding to the recording shown in panel A, illustrating that 50 mM EtOH did not affect the amplitude or the decay of these events. D. Summary graph illustrating the effect of 50 mM EtOH and 0.5 μM TTX on sIPSC frequency and amplitude. The effect of EtOH was calculated with respect to the average of control and washout responses whereas the effect of TTX was calculated with respect to the washout responses (represented by the dotted line). ***p<0.001 by one sample t-test vs. a theoretical mean of 100% (n = 6 for EtOH and n = 5 for TTX).
Table 1. Summary of the Effect of Increasing Concentrations of EtOH on sIPSCs, eIPSCs and eEPSPs.
Values represent percent of control (calculated as the average of baseline and washout responses). Number of determinations are given in parentheses.
| |
10 mM |
25 mM |
50 mM |
|---|---|---|---|
| sIPSC | |||
| Frequency | Not determined | 115.6 ± 9.6 (4) | *136.9 ± 8.3 (6) |
| Amplitude |
Not determined |
108.7 ± 8.7 (4) |
101.2 ± 5.5 (6) |
| eIPSC (Proximal) | |||
| eIPSC1-Amplitude | Not determined | Not determined | 95.9 ± 7 (8) |
| Paired-pulse ratio |
Not determined |
Not determined |
98 ± 5.7 (8) |
| eIPSC (Distal) | |||
| eIPSC1-Amplitude | 83 ± 13 (4) | **141.4 ± 5.6 (4) | **126.5 ± 6.7 (8) |
| Paired-pulse ratio |
103.4 ± 5 (4) |
*77.6 ± 7 (4) |
*87.6 ± 4.7 (8) |
| eEPSPs | |||
| eEPSP1-Amplitude | 85.94 ± 5.6 (5) | *62.9 ± 9.4 (5) | ***49.4 ± 11.5 (4) |
| Paired-pulse ratio | 101.3 ± 7.2 (5) | 125.9 ± 11.4 (5) | 117.8 ± 8.8 (4) |
| AP number | 84.7 ± 10.8 (5) | *58.9 ± 14.7 (6) | *53.1 ± 13.3 (4) |
p < 0.05
p < 0.01 by one-sample t-test vs. 100%.
Spontaneous IPSCs are a mixed population of action potential-dependent and independent events. To determine the contribution of each of these populations, we applied TTX (0.5 μM) at the end of most experiments to block the spontaneous action potential-dependent population of events (as well events that are dependent on Na+ channel activation in the axonal terminal). Fig 3D shows that TTX reduced sIPSC frequency by 41.5 ± 10.3% and sIPSC amplitude by 60.6 ± 6.3% (n = 5).
Effect of EtOH on evoked IPSCs
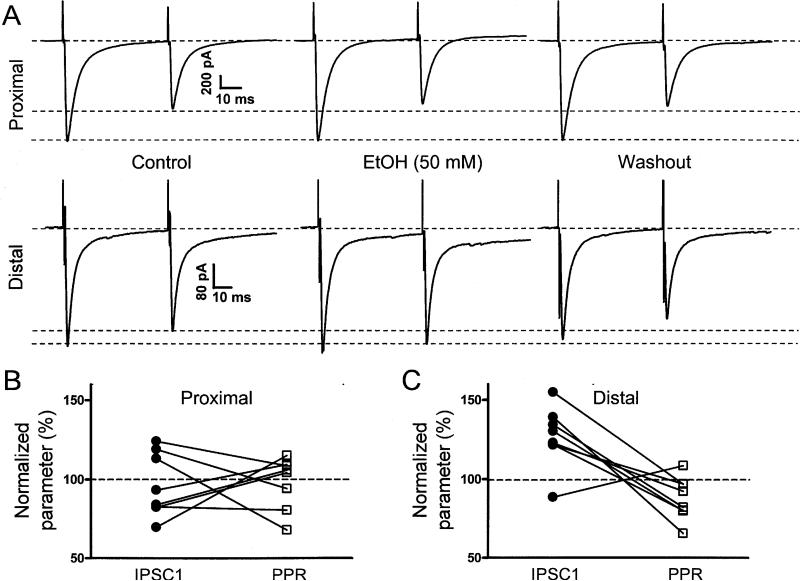
We assessed the acute effect of EtOH on GABAergic transmission at synapses between basket or stellate cells and PNs. Basket cells were preferentially stimulated with pairs of stimuli (interpulse interval = 50 ms) delivered by electrodes placed proximally to the Purkinje layer (i.e. inner third of the molecular layer) (Fig 4A). Proximal eIPSC1 amplitude was 628.3 ± 173.2 pA and the paired-pulse ratio was 0.86 ± 0.05 (n = 8). EtOH (50 mM) did not significantly change the proximal eIPSC1 amplitude nor the paired-pulse ratio (Fig 4B; Table 1).
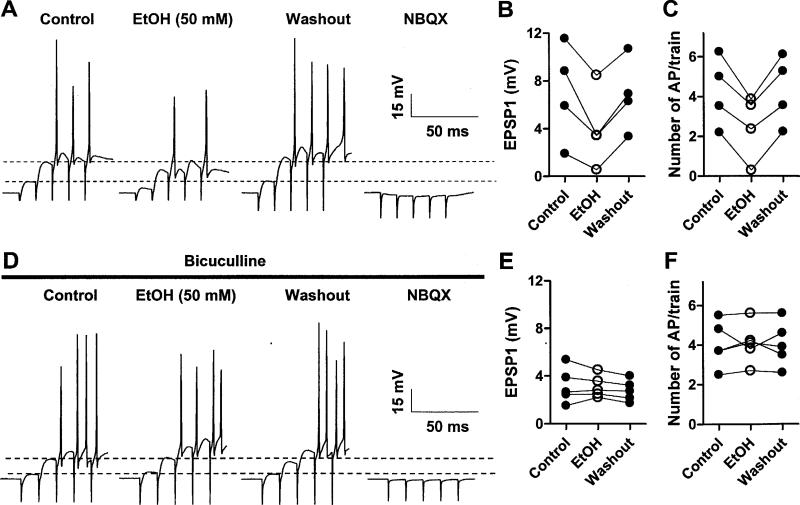
Fig 4. Effects of EtOH on the amplitude and paired-pulse ratio of IPSCs evoked by proximal and distal stimulation in the molecular layer.
A. Sample traces of IPSCs evoked in PNs by pairs of electrical stimuli (50 ms interpulse interval) delivered at the inner (proximal) or outer (distal) thirds of the molecular layer. Note that although 50 mM EtOH minimally affected the proximal IPSCs, it slightly increased the amplitude of the distal IPSC1 and decreased the paired pulse ratio. B. Summary graph illustrating that EtOH did not significantly affect IPSC1 amplitude or the paired-pulse ratio of events evoked by proximal stimulation (n = 8). C. Summary graph illustrating that EtOH significantly affected IPSC1 amplitude (p < 0.006 by one sample t-test vs. a theoretical mean of 100%) and the paired pulse ratio (p < 0.036 by one sample t-test vs. a theoretical mean of 100%) of events evoked by distal stimulation (n = 8).
Stellate cells were preferentially stimulated with pairs of stimuli delivered by electrodes placed distally to the Purkinje layer (Fig 4A) (i.e. outer third of the molecular layer). Distal eIPSC1 amplitude was 305.6 ± 65.1 pA and the paired-pulse ratio was 1.01 ± 0.1 (n = 8). EtOH (50 mM) significantly changed the distal eIPSC1 amplitude and the paired-pulse ratio (n = 8; Table 1). For comparison, we tested the effect of lower concentrations of EtOH and found that 25 mM, but not 10 mM, significantly changes the distal eIPSC1 amplitude and the paired-pulse ratio (Table 1).
Effect of EtOH on action potential firing
PNs fire action potentials either spontaneously or when stimulated by glutamatergic afferents. We initially studied the effect of EtOH on action potential firing evoked by stimulation of granule cell axons. EPSPs were evoked in the whole-cell current-clamp mode by trains of 5 stimuli at 100 Hz to maximize the induction of action potential firing. Under control conditions, repetitive stimulation of the granule cell axons reproducibly induced non-NMDA receptor-mediated EPSPs (peak amplitude of first EPSP = 7.0 ± 2.0 mV; baseline membrane potential = −73.0 ± 0.9 mV; n = 4) that triggered action potential firing (4.2 ± 0.9 action potentials per train) (Fig 5A-C). Acute exposure to 50 mM EtOH significantly reduced both the peak amplitude of the first non-NMDA EPSP and action potential number (n = 4; Fig. 5A-C; Table 1). The paired-pulse ratio of the EPSP2 over the EPSP1 was 2.4 ± 0.5 (n = 4) and it was not significantly affected by EtOH (Table 1; n =4). The resting membrane potential was not affected by EtOH exposure (102.4 ± 8.7 % of control; n =4).
Fig 5. EtOH inhibits PN EPSPs and AP firing evoked by stimulation of granule cell axons.
A. Sample traces of current-clamp recordings from a PN in which a train of five stimuli (100 Hz) delivered in the molecular layer induced non-NMDA EPSPs that triggered a burst of action potentials. Bath application of EtOH (50 mM) reversibly decreased the peak amplitude of the EPSPs and reduced action potential number (these data were digitized at 5 kHz, which explains the variability in AP amplitude). The non-NMDA EPSPs were fully blocked by NBQX (20 μM). B and C. Summary graphs illustrating the inhibitory effect of EtOH (50 mM) on EPSP1 amplitude and action potential number. Analysis by repeated measures ANOVA followed by Tukey's posthoc test revealed that EtOH significantly decreased EPSP1 amplitude (p < 0.01 vs. control and p < 0.05 vs. washout) and the number of action potentials/train (p < 0.001 vs. both control and washout) (n = 4). D. Same as in A but in the continuous presence of bicuculline (20 μM); the stimulation intensity was decreased to obtain a comparable number of action potential/train as in the absence of bicuculline. Note that EtOH neither decreased EPSP1 amplitude nor action potential number in presence of this agent. E and F. Summary graphs illustrating the lack of an effect of EtOH (50 mM) on EPSP1 and action potential number in presence of bicuculline (n = 5).
We next performed similar experiments in the continuous presence of the GABAA receptor antagonist, bicuculline (20 μM). In the presence of this agent, the stimulation intensity was decreased to evoke EPSPs and action potential firing of similar characteristics to those recorded under control conditions (peak amplitude of first EPSP = 3.2 ± 0.7 mV; baseline membrane potential = −68.8 ± 1.4 mV; action potential number = 4.0 ± 0.5; n = 5; not significantly different by unpaired t-test from results obtained in absence of bicuculine). In presence of bicuculline, acute exposure to 50 mM EtOH did not significantly affect the peak amplitude of the first non-NMDA EPSP (109.1 ± 7.0 % of control) or action potential firing (102.6 ± 6.1 % of control) (n = 5; Fig. 5D-F). The paired-pulse ratio of the EPSP2 over the EPSP1 was 3.3 ± 0.3 (n = 5) and it was not significantly affected by EtOH (93.6 ± 2.3 % of control; n = 5). The resting membrane potential was not affected by EtOH exposure (93.8 ± 8.3 % of control; n =4). EPSPs recorded in the absence and presence of bicuculline were abolished by 10 μM NBQX, confirming that these events were mediated by non-NMDA receptors (Fig. 5A, D).
For comparison, we tested the effects of lower EtOH concentrations in absence of bicuculline (Table 1). Acute exposure to 25 mM, but not 10 mM EtOH, significantly reduced the peak amplitude of the first non-NMDA EPSP and reduced action potential number. The paired-pulse ratio was not significantly affected by either 10 or 25 mM EtOH. These data were digitized at 10 kHz (as opposed to 5 kHz for the 50 mM data), which allowed us to more accurately measure AP amplitude and assess the effect of EtOH on this parameter. We found that 25 mM EtOH induced a −3 ± 4.3 % change in AP amplitude with respect the average of baseline and washout responses.
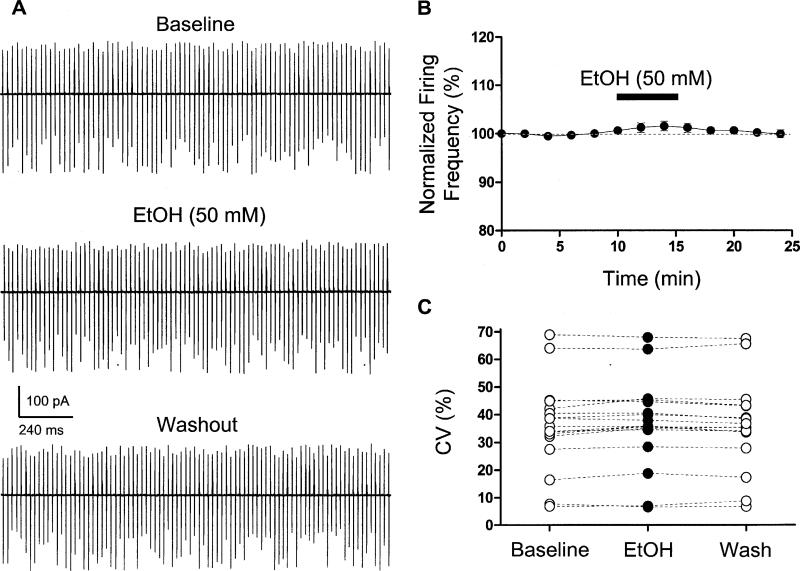
Finally, we examined the effect of EtOH on spontaneous action potential firing recorded using the loose-patch cell-attached configuration. PNs spontaneously fired action potentials at a frequency of 22.9 ± 1.8 Hz, with an interspike interval coefficient of variation of 35.9 ± 3.9 % (n = 17). As shown in Fig 6, EtOH did not significantly affect the frequency or the coefficient of variation of the intespike interval in these neurons.
Fig 6. EtOH does not affect spontaneous action potential firing in PNs.
A. Sample traces of loose-patch cell-attached recordings illustrating that application of 50 mM EtOH does not affect the frequency of spontaneous action potentials. B. Summary of the effect of 50 mM EtOH on firing frequency (note that the error bar is smaller than the symbol in most cases). Data were normalized to the average spontaneous action potential firing frequency obtained during the first 2 min of recording (n = 17). C. Summary graph illustrating the lack of effect of EtOH on the coefficient of variation of the interspike interval (n = 17).
Discussion
We found that EtOH acutely increases spontaneous action potential-dependent and - independent GABAergic transmission in PNs via a presynaptic mechanism. EtOH increased evoked IPSCs and this effect was preferentially observed for events mediated by stellate cells. EtOH also inhibited PN firing in response to granule cell axon stimulation in a GABAA receptor-dependent manner. These acute effects of EtOH were observed at concentrations (25-50 mM = 0.11-0.22 g/dl) that produce significant alterations in motor coordination both in laboratory animals and humans (legal intoxication limit in the U.S.A. is 17.4 mM = 0.08 g/dl).
EtOH increases spontaneous GABA release
EtOH reversibly increased the frequency, but not the amplitude or half-width, of mIPSCs in PNs, suggesting that it does not directly affect postsynaptic GABAA receptor function but increases quantal release from GABAergic terminals. These findings are in agreement with previous reports (Breese et al., 2006; Ming et al., 2006; Kelm et al., 2007). Under our experimental conditions, the maximum effect of 50 mM EtOH on mIPSC frequency was similar to the increase (~35%) observed by Ming et al. (2006), but greater than the effect observed by Kelm et al. (2007) (~10%). The findings of these studies are consistent with other reports indicating that sub-anesthetic concentrations of EtOH increase quantal GABA release in some neuronal populations of the amygdala, hippocampus and ventral tegmental area (Roberto et al., 2003; Galindo et al., 2005; Li et al., 2006; Theile et al., 2008).
EtOH also selectively increased the frequency of sIPSCs and these events were ~3 times more frequent and had approximately double the amplitude than mIPSCs. Consequently, the EtOH-induced increase in sIPSC frequency is predicted to have a larger impact on PN excitability than the increase in mIPSC frequency. The mechanism underlying this effect could be an increase in spontaneous action potential firing frequency of MLIs, as in the case of Golgi interneurons of the cerebellar granule cell layer (Carta et al., 2004; Hanchar et al., 2005). Alternatively, EtOH could increase excitatory or decrease inhibitory neurotransmitter inputs to MLIs. We are currently investigating these possibilities. Increases in sIPSC frequency induced by subanesthetic EtOH concentrations have been observed in cerebellar granule neurons (Carta et al., 2004; Hanchar et al., 2005) and in neuronal populations from other brain regions including the basolateral amygdala (Zhu and Lovinger, 2006) as well as the CA1 and CA3 regions of the hippocampus (Ariwodola and Weiner, 2004; Galindo et al., 2005; Li et al., 2006). A dual effect of 40 mM EtOH on sIPSC frequency was recently reported in dopaminergic neurons of the ventral tegmental area, where it decreased sIPSC frequency under control conditions and increased it in presence of a μ-opioid receptor agonist (Xiao and Ye, 2008; but see Theile et al., 2008).
EtOH preferentially affects the paired-pulse ratio of distal eIPSCs
IPSC amplitude of events evoked by electrical stimulation in the outer third of the molecular layer was increased and this effect was associated with a decrease in the paired-pulse ratio. In contrast, neither the amplitude nor the paired-pulse ratio of IPSCs evoked by proximal stimulation were significantly affected by EtOH application. These findings suggest that EtOH affects evoked GABA release from stellate but not basket cells. These results are somewhat surprising given that the EtOH-sensitive sIPSCs and mIPSCs that we recorded with electrodes placed in the soma of PNs are likely to be primarily mediated by GABA release from basket cell axonal terminals. In PNs in slices from postnatal day 10-13 rats, Llano et al. (2000) determined that somatic mIPSCs have a shorter 10-90% rise time (~ 0.7 ms) than dendritic mIPSCs (~ 2.4 ms) because distal inputs are slowed down by a first order filter with a time constant near 1 ms. In PNs from those juvenile rats, ~40% of the events were mediated by somatic GABA release. We performed our studies in slices from postnatal day 19-30, where PNs have more exhuberant dendritic arborizations and distal mIPSCs are expected to be more significantly filtered even in presence of high intracellular Cs concentrations. Analysis of cumulative probability distributions revealed that 90% of the mIPSCs we recorded had rise times of less than 2 ms and, therefore, these probably correspond to events mediated by quantal GABA release from axonal terminals located in the proximal dendrites, soma or axonal initial segment of PNs; i.e. originating from basket cells. This conclusion is consistent with the finding of Kelm et al. (2007) that EtOH increased mIPSC frequency in mechanically dissociated PNs, where distal dendrites are severed. Therefore, we expected the paired-pulse ratio of proximal eIPSCs to decrease, as an inverse relationship between mIPSC frequency (and also sIPSC frequency in some cases) and the paired-pulse ratio has been observed in PNs (Hirono and Obata, 2006). However, changes in spontaneous and evoked transmitter release are not always correlated (Sara et al., 2005). Thus, it is possible that EtOH selectively affects spontaneous GABA release at basket cell-to-PN synapses, and both spontaneous and evoked GABA release at stellate cell-to-PN synapses.
Differences in EtOH modulation of GABAergic transmission mediated by dendritic- vs. somatic-innervating interneurons have been observed in CA1 hippocampal neurons. Proximal IPSCs (i.e. evoked by stimulation of the stratum pyramidale) were shown to be potentiated by EtOH to a greater extent than distal IPSCs (i.e. evoked in the stratum lacunosum moleculare) (Weiner et al., 1997; Ariwodola and Weiner, 2004; Wu et al., 2005; Proctor et al., 2006). Local vs. paracapsular GABAergic synapses in the rat basolateral amygdala were recently reported to be differentially affected by acute EtOH exposure (Silberman et al., 2008). Taken together with our results, these findings indicate that EtOH can have selective effects on specific populations of GABAergic interneurons within a brain region.
EtOH decreases granule cell axon EPSP-dependent firing but not spontaneous firing
EtOH decreased the amplitude of EPSPs evoked by granule cell axon stimulation as well as the number of action potentials triggered by these events. This effect depended on GABAA receptor activation as it was not observed in presence of bicuculline. Although the somatic membrane potential did not change with EtOH exposure, the effect of GABAA receptors may be a consequence of membrane hyperpolarization in somatodendritic sites that are not under the influence of the patch electrode. In addition, increased GABAA receptor activation in presence of EtOH may cause EPSP shunting (Hausser and Clark, 1997). It should be noted that in parasagittal cerebellar slices, a significant portion of the parallel fibers are cut because these run perpendicular to the sagittal plane. Consequently, it is possible that the EPSPs that we measured correspond primarily to events mediated by glutamate release from the ascending portion of granule cell axons, which have been shown to have different properties to the parallel fiber axons (Gundappa-Sulur et al., 1999).
In agreement with the literature, we found that rat PNs in acute cerebellar vermis slices fire irregular spontaneous action potentials at relatively high frequencies (Hausser and Clark, 1997; Womack and Khodakhah, 2002). In this preparation, PN firing has been shown to be sensitive to GABAergic input. Specifically, blockade of GABAergic input increases average firing frequency and converts irregular firing into regular firing, whereas increases in GABAergic input cause delays in action potential firing and decreases the membrane time constant as well as the membrane resistance (Hausser and Clark, 1997). GABAergic control of PN spontaneous firing likely involves both somatic and dendritic inputs (Hausser and Clark, 1997; Womack and Khodakhah, 2002) so it was somewhat surprising that the EtOH-induced increase of GABAergic tone did not reduce average frequency or increase irregularity of spontaneous action potential firing. However, large changes in GABAergic tone (i.e. complete blockade) produce relatively modest changes in PN spontaneous firing in acute slices and the effect of 50 mM EtOH on sIPSC frequency was comparably small (< 40%). Our findings are in general agreement with results of in vivo and in vitro studies indicating that EtOH does not reliably affect spontaneous firing in PNs (Basile et al., 1983; George and Chu, 1984; Deitrich et al., 1989; Freund et al., 1993; Ming et al., 2006).
Conclusion
The increase in GABAergic input to PNs could be one of the cellular underpinnings responsible for the cerebellar function alterations that are associated with acute intoxication. Other mechanisms that may contribute to these alterations include modulation of the climbing fiber input (Rogers et al., 1986; Svensson et al., 1996; Carta et al., 2006) as well as the mossy-parallel fiber input (Carta et al., 2004; Hanchar et al., 2005; Huang and Huang, 2007) Effects of EtOH on synaptic plasticity may also play a role in its mechanism of action at PNs (Carta et al., 2006; Belmeguenai et al., 2007) and it would be interesting to determine if these effects are modified by increases in GABAergic input.
Acknowledgements
We thank L. Donald Partridge and Mario Carta for critically reading the manuscript.
Abbreviations
- ACSF
artificial cerebrospinal fluid
- AP
action potential
- EGTA
ethylene glycol tetraacetic acid
- EPSP
excitatory postsynaptic potential
- EtOH
ethanol
- GABAA-R
type-A γ-aminobutyric acid receptor
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- IPSC
inhibitory postsynaptic current
- eIPSC
evoked IPSC
- mIPSC
miniature IPSC
- sIPSC
spontaneous IPSC
- K-S
Kolmogorov-Smirnov
- MLI
molecular layer interneuron
- Na2-ATP
disodium adenosine triphosphate
- NBQX
2,3-dihydroxy-6-nitro-7-sulfamoylbenzo[f]quinoxaline-2,3-dione
- NMDA
N-methyl-D-aspartate
- PN
Purkinje neuron
- QX-314
N-(2,6-Dimethylphenylcarbamoylmethyl)triethylammonium bromide
- TTX
tetrodotoxin
Footnotes
This work was supported by NIH Grant AA14973.
References
- Ariwodola OJ, Weiner JL. Ethanol potentiation of GABAergic synaptic transmission may be self-limiting: role of presynaptic GABA(B) receptors. J Neurosci. 2004;24:10679–10686. doi: 10.1523/JNEUROSCI.1768-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basile A, Hoffer B, Dunwiddie T. Differential sensitivity of cerebellar purkinje neurons to ethanol in selectively outbred lines of mice: maintenance in vitro independent of synaptic transmission. Brain Res. 1983;264:69–78. doi: 10.1016/0006-8993(83)91121-6. [DOI] [PubMed] [Google Scholar]
- Belmeguenai A, Botta P, Weber JT, Carta M, De Ruiter M, De Zeeuw CI, Valenzuela CF, Hansel C. Program No 46.17 Abstract Viewer Itinerary Planner. Society for Neuroscience; Washington DC: 2007. Ethanol affects plasticity at cerebellar parallel fiber to Purkinje cell synapses. Online. [Google Scholar]
- Breese GR, Criswell HE, Carta M, Dodson PD, Hanchar HJ, Khisti RT, Mameli M, Ming Z, Morrow AL, Olsen RW, Otis TS, Parsons LH, Penland SN, Roberto M, Siggins GR, Valenzuela CF, Wallner M. Basis of the gabamimetic profile of ethanol. Alcohol Clin Exp Res. 2006;30:731–744. doi: 10.1111/j.0145-6008.2006.00086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carta M, Mameli M, Valenzuela CF. Alcohol enhances GABAergic transmission to cerebellar granule cells via an increase in Golgi cell excitability. J Neurosci. 2004;24:3746–3751. doi: 10.1523/JNEUROSCI.0067-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carta M, Mameli M, Valenzuela CF. Alcohol potently modulates climbing fiber-->Purkinje neuron synapses: role of metabotropic glutamate receptors. J Neurosci. 2006;26:1906–1912. doi: 10.1523/JNEUROSCI.4430-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deitrich RA, Dunwiddie TV, Harris RA, Erwin VG. Mechanism of action of ethanol: initial central nervous system actions. Pharmacol Rev. 1989;41:489–537. [PubMed] [Google Scholar]
- Freund RK, Palmer MR. Beta adrenergic sensitization of gamma-aminobutyric acid receptors to ethanol involves a cyclic AMP/protein kinase A second-messenger mechanism. J Pharmacol Exp Ther. 1997;280:1192–1200. [PubMed] [Google Scholar]
- Freund RK, Wang Y, Palmer MR. Differential effects of ethanol on the firing rates of Golgi-like neurons and Purkinje neurons in cerebellar slices in vitro. Neurosci Lett. 1993;164:9–12. doi: 10.1016/0304-3940(93)90844-b. [DOI] [PubMed] [Google Scholar]
- Galindo R, Zamudio P, Valenzuela C. Alcohol is a potent stimulant of immature neuronal networks: implications for fetal alcohol spectrum disorder. J. Neurochem. 2005;94:1500–1511. doi: 10.1111/j.1471-4159.2005.03294.x. [DOI] [PubMed] [Google Scholar]
- George F, Chu NS. Effects of ethanol on Purkinje cells recorded from cerebellar slices. Alcohol. 1984;1:353–358. doi: 10.1016/0741-8329(84)90002-8. [DOI] [PubMed] [Google Scholar]
- Gundappa-Sulur G, De Schutter E, Bower JM. Ascending granule cell axon: an important component of cerebellar cortical circuitry. J Comp Neurol. 1999;408:580–596. doi: 10.1002/(sici)1096-9861(19990614)408:4<580::aid-cne11>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Hanchar HJ, Dodson PD, Olsen RW, Otis TS, Wallner M. Alcohol-induced motor impairment caused by increased extrasynaptic GABA(A) receptor activity. Nat Neurosci. 2005;8:339–345. doi: 10.1038/nn1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris DP, Sinclair JG. Ethanol-GABA interactions at the rat Purkinje cell. Gen Pharmacol. 1984;15:449–454. doi: 10.1016/0306-3623(84)90198-8. [DOI] [PubMed] [Google Scholar]
- Hausser M, Clark BA. Tonic synaptic inhibition modulates neuronal output pattern and spatiotemporal synaptic integration. Neuron. 1997;19:665–678. doi: 10.1016/s0896-6273(00)80379-7. [DOI] [PubMed] [Google Scholar]
- Hirono M, Obata K. Alpha-adrenoceptive dual modulation of inhibitory GABAergic inputs to Purkinje cells in the mouse cerebellum. J Neurophysiol. 2006;95:700–708. doi: 10.1152/jn.00711.2005. [DOI] [PubMed] [Google Scholar]
- Huang CM, Huang RH. Ethanol inhibits the sensory responses of cerebellar granule cells in anesthetized cats. Alcohol Clin Exp Res. 2007;31:336–344. doi: 10.1111/j.1530-0277.2006.00309.x. [DOI] [PubMed] [Google Scholar]
- Kelm MK, Criswell HE, Breese GR. Calcium release from presynaptic internal stores is required for ethanol to increase spontaneous gamma-aminobutyric acid release onto cerebellum Purkinje neurons. J Pharmacol Exp Ther. 2007;323:356–364. doi: 10.1124/jpet.107.126144. [DOI] [PubMed] [Google Scholar]
- Kim J, Alger BE. Random response fluctuations lead to spurious paired-pulse facilitation. J Neurosci. 2001;21:9608–9618. doi: 10.1523/JNEUROSCI.21-24-09608.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RS, Smith SS, Chapin JK, Waterhouse BD, Shimizu N, Maddux BN, Woodward DJ. Effects of systemic and local ethanol on responses of rat cerebellar Purkinje neurons to iontophoretically applied gamma-aminobutyric acid. Brain Res. 1995;687:1–11. doi: 10.1016/0006-8993(95)00285-x. [DOI] [PubMed] [Google Scholar]
- Li Q, Wilson WA, Swartzwelder HS. Developmental differences in the sensitivity of spontaneous and miniature IPSCs to ethanol. Alcohol Clin Exp Res. 2006;30:119–126. doi: 10.1111/j.1530-0277.2006.00006.x. [DOI] [PubMed] [Google Scholar]
- Llano I, Gonzalez J, Caputo C, Lai FA, Blayney LM, Tan YP, Marty A. Presynaptic calcium stores underlie large-amplitude miniature IPSCs and spontaneous calcium transients. Nat Neurosci. 2000;3:1256–1265. doi: 10.1038/81781. [DOI] [PubMed] [Google Scholar]
- Ming Z, Criswell HE, Yu G, Breese GR. Competing presynaptic and postsynaptic effects of ethanol on cerebellar purkinje neurons. Alcohol Clin Exp Res. 2006;30:1400–1407. doi: 10.1111/j.1530-0277.2006.00167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orduz D, Llano I. Recurrent axon collaterals underlie facilitating synapses between cerebellar Purkinje cells. Proc Natl Acad Sci U S A. 2007;104:17831–17836. doi: 10.1073/pnas.0707489104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer MR, van Horne CG, Harlan JT, Moore EA. Antagonism of ethanol effects on cerebellar Purkinje neurons by the benzodiazepine inverse agonists Ro 15-4513 and FG 7142: electrophysiological studies. J Pharmacol Exp Ther. 1988;247:1018–1024. [PubMed] [Google Scholar]
- Proctor WR, Diao L, Freund RK, Browning MD, Wu PH. Synaptic GABAergic and glutamatergic mechanisms underlying alcohol sensitivity in mouse hippocampal neurons. J Physiol. 2006;575:145–159. doi: 10.1113/jphysiol.2006.112730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Madamba SG, Moore SD, Tallent MK, Siggins GR. Ethanol increases GABAergic transmission at both pre- and postsynaptic sites in rat central amygdala neurons. Proc Natl Acad Sci U S A. 2003;100:2053–2058. doi: 10.1073/pnas.0437926100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J, Madamba SG, Staunton DA, Siggins GR. Ethanol increases single unit activity in the inferior olivary nucleus. Brain Res. 1986;385:253–262. doi: 10.1016/0006-8993(86)91071-1. [DOI] [PubMed] [Google Scholar]
- Sapp DW, Yeh HH. Ethanol-GABAA receptor interactions: a comparison between cell lines and cerebellar Purkinje cells. J Pharmacol Exp Ther. 1998;284:768–776. [PubMed] [Google Scholar]
- Sara Y, Virmani T, Deak F, Liu X, Kavalali ET. An isolated pool of vesicles recycles at rest and drives spontaneous neurotransmission. Neuron. 2005;45:563–573. doi: 10.1016/j.neuron.2004.12.056. [DOI] [PubMed] [Google Scholar]
- Silberman Y, Shi L, Brunso-Bechtold JK, Weiner JL. Distinct mechanisms of ethanol potentiation of local and paracapsular GABAergic synapses in the rat basolateral amygdala. J Pharmacol Exp Ther. 2008;324:251–260. doi: 10.1124/jpet.107.128728. [DOI] [PubMed] [Google Scholar]
- Svensson P, Hesslow G, Winton R. Effect of ethanol on the excitability of the inferior olive in decerebrate ferret. J Pharmacol Exp Ther. 1996;277:761–767. [PubMed] [Google Scholar]
- Theile JW, Morikawa H, Gonzales RA, Morrisett RA. Ethanol enhances GABAergic transmission onto dopamine neurons in the ventral tegmental area of the rat. Alcohol Clin Exp Res. 2008;32:1040–1048. doi: 10.1111/j.1530-0277.2008.00665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner JL, Gu C, Dunwiddie TV. Differential ethanol sensitivity of subpopulations of GABAA synapses onto rat hippocampal CA1 pyramidal neurons. J Neurophysiol. 1997;77:1306–1312. doi: 10.1152/jn.1997.77.3.1306. [DOI] [PubMed] [Google Scholar]
- Weiner JL, Valenzuela CF. Ethanol modulation of GABAergic transmission: the view from the slice. Pharmacol Ther. 2006;111:533–554. doi: 10.1016/j.pharmthera.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Womack M, Khodakhah K. Active contribution of dendrites to the tonic and trimodal patterns of activity in cerebellar Purkinje neurons. J Neurosci. 2002;22:10603–10612. doi: 10.1523/JNEUROSCI.22-24-10603.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu PH, Poelchen W, Proctor WR. Differential GABAB Receptor Modulation of Ethanol Effects on GABA(A) synaptic activity in hippocampal CA1 neurons. J Pharmacol Exp Ther. 2005;312:1082–1089. doi: 10.1124/jpet.104.075663. [DOI] [PubMed] [Google Scholar]
- Xiao C, Ye JH. Ethanol dually modulates GABAergic synaptic transmission onto dopaminergic neurons in ventral tegmental area: Role of mu-opioid receptors. Neuroscience. 2008 doi: 10.1016/j.neuroscience.2008.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Criswell HE, Breese GR. Action of ethanol on responses to nicotine from cerebellar Purkinje neurons: relationship to methyllycaconitine (MLA) inhibition of nicotine responses. Neurochem Int. 1999;35:185–194. doi: 10.1016/s0197-0186(99)00060-1. [DOI] [PubMed] [Google Scholar]
- Yang X, Criswell HE, Breese GR. Ethanol modulation of gamma-aminobutyric acid (GABA)-mediated inhibition of cerebellar Purkinje neurons: relationship to GABAb receptor input. Alcohol Clin Exp Res. 2000;24:682–690. [PubMed] [Google Scholar]
- Zhu PJ, Lovinger DM. Ethanol potentiates GABAergic synaptic transmission in a postsynaptic neuron/synaptic bouton preparation from basolateral amygdala. J Neurophysiol. 2006;96:433–441. doi: 10.1152/jn.01380.2005. [DOI] [PubMed] [Google Scholar]