Abstract
The importance of chronic immune activation in progression to AIDS has been inferred by correlative studies in HIV-infected individuals and in nonhuman primate models of SIV infection. Using the SIVmac251 macaque model, we directly address the impact of immune activation by inhibiting CTLA-4, an immunoregulatory molecule expressed on activated T cells and a subset of regulatory T cells. We found that CTLA-4 blockade significantly increased T cell activation and viral replication in primary SIVmac251 infection, particularly at mucosal sites, and increased IDO expression and activity. Accordingly, protracted treatment with anti-CTLA-4 Ab of macaques chronically infected with SIVmac251 decreased responsiveness to antiretroviral therapy and abrogated the ability of therapeutic T cell vaccines to decrease viral set point. These data provide the first direct evidence that immune activation drives viral replication, and suggest caution in the use of therapeutic approaches for HIV infection in vivo that increase CD4+ T cell proliferation.
The pathogenesis of AIDS is not fully understood. One suggestion is that the ability of HIV to directly kill CD4+ T cells is central in the progressive demise of the immune system (1–7). An alternative proposal is that indirect effects linked to the host immune response, including production of proinflammatory cytokines and chemokines and increased CD4+ T cell activation and turnover, are pivotal to progression to AIDS (8, 9). Early loss of CCR5+CD4+ T cells from mucosal compartments, particularly the GALT, is another hallmark of HIV and SIV infection (1–5, 9), but “per se” may not be sufficient to determine disease development as it also occurs in nonpathogenic models of SIV infection (10–12). Thus, in the pathogenic SIV model, the relative contribution of direct CD4+ T cell killing and/or immune activation in disease progression, particularly at mucosal sites, remains unclear. In contrast, natural SIV infection of African nonhuman primates is asymptomatic and the lack of disease progression in this model has been linked to a low level of immune activation (13).
The initiation, maintenance, and contraction of adaptive immune responses are highly regulated by positive and negative molecules expressed on T cells, APCs, and CD4+CD25highFoxP3+ regulatory T cells (Treg).5 Upon TCR ligation, the relative expression of costimulatory CD28 molecules and negative regulatory CTLA-4 molecules dictate IL-2 production and T cell proliferation (14).
CD4+ Treg that constitutively express CTLA-4, FoxP3, and glucocorticoid-induced TNFR have evolved to regulate immune responses and limit the tissue damage caused by CTL and proinflammatory cytokines (15). Through the induction of TGF-β and IDO, Treg block cell cycle progression of T cells and reduce responsiveness to Ags (15). Thus, in principle, Treg may delay or impair the elimination of pathogens and diminish responses to self-Ags, including those expressed by tumor cells.
In the case of HIV/SIV infection, the role of Treg is unclear (16). Some reports conclude that Treg or Treg-associated markers are decreased in blood of HIV-1-infected individuals while others indicate an increase of Treg in tissues (17–28). Even during the stages of SIV infection, the dynamics of Treg distribution are unclear. Some reports have shown that the frequency of FoxP3+ T cells increases rapidly in tissues, suggesting that Treg may impair the establishment of an effective adaptive immune response (22), whereas a recent study showed depletion of Treg from the intestinal lamina propria during acute SIV infection, similar to that observed for CCR5+CD4+ T cells (29). Studies on gene expression in tissue RNA demonstrate a significant increase in the expansion of CTLA-4, IDO, and TGF-β in chronically infected macaques (19, 23). However, transient expression of these markers by newly activated T cells, not only by Treg, precludes an accurate assessment of the importance of Treg in AIDS pathogenesis using gene expression or phenotypic markers.
Because of the central role played by CTLA-4 in mediating both Treg function and directly modulating T cell costimulation, we reasoned that the use of a blocking Ab to CTLA-4 could provide clues on the importance of T cell activation in the pathogenesis of AIDS. For our study, we have chosen the human MDX-010 Ab because of its known ability to block B7 binding to CTLA-4 (30). MDX-010 administration augments vaccine-induced immune responses in mice and macaques and, despite repeated inoculations, monkeys do not produce Abs to the human Ig (30).
In this study, we found that protracted CTLA-4 blockade treatment during both primary and chronic SIVmac251 infection of macaques caused increased T cell activation and virus levels in tissues and plasma. Surprisingly, this treatment did not improve virus-specific T cell responses or decrease IDO activity. Similarly, the titers of Ab responses to SIV were not affected. Accordingly, CD4+ T cell loss at mucosal sites was exacerbated by the treatment.
Altogether, these data suggest that in SIV-infected macaques, the effect on T cell costimulation of anti-CTLA-4 Abs likely outweighed its effect on Treg and highlight the importance of T cell activation in AIDS pathogenesis.
Materials and Methods
Animals
All animals were colony-bred Indian rhesus macaques (Macaca mulatta) obtained from Covance Research Products. The animals were housed and handled in accordance with the standards of the Association for the Assessment and Accreditation of Laboratory Animal Care International, and the study was reviewed and approved by the animal care and use committees at Advanced BioScience Laboratories. The care and use of the animals were in compliance with all relevant institutional (National Institutes of Health) guidelines.
At the time of purchase, all animals were in good health, 2–4 years of age, and weighed 4–9 kg. All animals were seronegative for SIV, simian T cell lymphotropic virus type 1, and herpes virus B. All animals were infected intrarectally with the same stock of SIVmac251 as previously described (31). Antiretroviral therapy (ART), given in condition of mild sedation, was initiated at week 14 and halted at week 37 in the second study. ART consisted of i.v. administration of didanosine (10 mg/kg/day), oral administration of stavudine (d4T) twice a day (1.2 mg/kg/dose), and s.c. administration of (R)-9-(2-phosphonylmethoxypropyl) adenine (20 mg/kg/day). Treatment with MDX-010 (Medarex) was performed i.v. at a dose of 10 mg/kg. Repeated treatments of macaques with MDX-010 do not induce anti-human Abs. This was demonstrated by bridge ELISA (our unpublished data with Medarex). In this assay, the plates were coated with macaque sera, reacted with biotinylated MDX-010 and the complexes were revealed with streptavidin-alkaline phosphatase, eliminating concerns that the detection reagent reacted with the MDX-010. This assay has been accepted by the Food and Drug Administration as a means to validate the presence of anti-MDX-010 Abs in all of phase III studies performed.
RNA extraction, reverse transcription, and real-time PCR
Total RNA was extracted from macaques’ lymph nodes using the TRIzol LS Reagent (Invitrogen Life Technologies), according to the manufacturer’s instructions. RNA (1 µg) was reverse transcribed into first-strand cDNA in a 20-µl reaction containing 1 µM random hexanucleotide primers, 1 µM oligo dT, and 200 U Moloney murine leukemia virus reverse transcriptase (Promega).
cDNA quantification for IDO, SIV Gag RNA, and GAPDH was performed by real-time PCR conducted with the ABI Prism 7900HT (Applied Biosystems). All reactions were performed using a SYBR green PCR mix (Qiagen), according to the following thermal profile: denaturation at 95°C for 15 s, annealing at 60°C for 15 s, extension at 72°C for 15 s (data collection was performed during the extension step). Data analysis was performed with SDS2.1 software, provided with ABI Prism 7900HT (Applied Biosystems). Primer sequences were designed using the Primer3 software, available online (http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi), and were previously described (23).
Viral load measurement
A real-time nucleic acid sequence-based amplification (NASBA) assay was used to quantitate SIV viral RNA in plasma. Plasma was clarified by centrifugation at 2,300 × g for 3 min. Clarified plasma (0.1 ml) was lysed in 0.9 ml of lysis buffer. For samples expected to have low viral load, the clarified plasma (0.5–1 ml) was centrifuged at 49,100 × g for 60 min. The virus pellet was then lysed in 1 ml of lysis buffer. Nucleic acid was isolated as described previously (32) and then analyzed by real-time NASBA as described previously (33). The real-time NASBA assay had a lower limit of sensitivity of 50 copies of RNA.
Preparation of lymphocytes from blood and tissues
Mononuclear cells from blood and lymph nodes were isolated by densitygradient centrifugation on Ficoll and resuspended in RPMI 1640 medium (Invitrogen Life Technologies) containing 10% FBS (R-10). Tissues from the rectum were treated with 1 mM Ultra Pure DTT (Invitrogen Life Technologies) for 30 min followed by incubation in calcium/magnesium-free HBSS (Invitrogen Life Technologies) three to four times for 60 min with stirring at room temperature to remove the epithelial layer. Lamina propria lymphocytes were separated following the removal of the epithelium and intraepithelial lymphocytes. The remaining tissue was cut into small pieces and incubated with collagenase D (400 U/ml; Boehringer Mannheim) and DNase (1 µg/ml; Invitrogen Life Technologies) for 2.5 h at 37°C in IMDM (Invitrogen Life Technologies) supplemented with 10% FBS and penicillin-streptomycin. The dissociated mononuclear cells were than placed over 42% Percoll (General Electric Healthcare) and centrifuged at 800 × g for 25 min at 4°C. Lamina propria lymphocytes were collected from the cell pellet.
Measurement of T cell number, activation, and memory phenotype
CD4+ and CD8+ counts were periodically determined on 100 µl of whole blood and by FACS analysis, according to the FACS/Lyse kit (BD Immunocytometry Systems) with minor modifications. Briefly, after incubating 10 µl of a mixture containing PerCP-CD4, allophycocyanin-CD8, PECD45, and FITC-CD3 mAbs (BD Biosciences) for 30 min at room temperature, red cells were lysed by adding 2 ml of FACS lysing solution (BD Biosciences) for 15 min. Samples were then centrifuged for 5 min at 1200 rpm at room temperature, washed (1% FCS, 0.05% NaN3 in PBS), resuspended in 500 µl of wash buffer, and stored at 4°C until analysis using a FACSCalibur flow cytometer (BD Biosciences).
The frequency of activated T cells was measured by FACS analysis. Briefly, cells (1 × 106) were surface stained with anti-human CD8β-PE (Immunotech), anti-human CD4-PerCP (BD Pharmingen), and anti-human CD69-allophycocyanin (BD Pharmingen). After 30 min of incubation in the dark at room temperature, cells were washed with cold 1% FCS in PBS and permeabilized with FACSPerm (BD Pharmingen) for 10 min at room temperature in the dark. Following two further washes, cells were intracellularly stained with FITC-conjugated anti-Ki67 (BD Pharmingen), incubated for 20 min at 37°C, fixed with 1% paraformaldehyde in PBS, and analyzed on a FACSCalibur.
The frequency of memory T cells in lymph nodes was determined by FACS analysis. Briefly, cells (1 × 106) were surface stained with antihuman CD28-FITC (BD Pharmingen), anti-human CD8β-PE (Immunotech), anti-human CD95-PeCy5 (BD Pharmingen), and anti-human CD4- allophycocyanin (BD Pharmingen) and incubated for 30 min at room temperature in the dark. Cells were washed with cold 1% FCS in PBS, fixed with 1% paraformaldehyde in PBS, and analyzed on a FACSCalibur. For the analysis, the central memory and effector memory T cell populations were identified as CD28+CD95+ and CD28−CD95+, respectively. The CCR7 marker was not used to define memory cells, as we have previously demonstrated that CD28 and CD95 identify similar T cell populations (34).
Tetramer staining
We screened rhesus macaques for the presence of the Mamu-A*01 allele using a PCR-based technique (35). Freshly prepared PBMC (5 × 105/animal) were stained with anti-human CD3-FITC (BD Pharmingen), anti-human CD8-PerCP (BD Biosciences), and Mamu-A*01 tetrameric complexes refolded in the presence of a specific peptide and conjugated to allophycocyanin-labeled streptavidin (Beckman Coulter). Gag181–189 CM9 (CTPYDINQM)-specific or Tat28–35 SL8 (STPESANL)-specific tetramer was used.
After 30 min of incubation in the dark at room temperature, cells were washed with 1% FCS in PBS and fixed with 1% paraformaldehyde in PBS (pH 7.4). Samples were analyzed on a FACSCalibur (BD Biosciences), and the data are presented as percentage of tetramer+ cells in all CD3+CD8+ lymphocytes.
Tryptophan and kynurenine concentration measurement by HPLC
Detection of tryptophan and kynurenine was performed on plasma collected from animals by HPLC as previously described (36).
Intracellular cytokine (ICC) staining
ICC staining was performed using the anti-TNF-α, -IFN γ, and -IL-2 Abs. A total of 106 fresh PBMC in 1 ml of complete medium were incubated for 1 h at 37°C in the absence or presence of a pool of Gag, Nef, Tat, Rev, Pol, or Env peptides (1 µg/ml) and in the presence of CD28 and CD49d (1 µg/ml each) in a 5-ml round-bottom tube. After addition of 10µg/ml brefeldin A (Sigma-Aldrich), cells were incubated for 5 h at 37°C and processed for surface and ICC staining. Briefly, cells were washed with 1% FCS in PBS, surface stained for 20 min with CD8β-PE (Immunotech) and CD4-PerCP (BD Biosciences), washed again, and fixed and permeabilized with Cytofix/Cytoperm (BD Pharmingen) for 20 min at 4°C in the dark. Following two further washes in Perm/Wash buffer (BD Pharmingen), cells were intracellularly stained with FITC-conjugated anti-IL-2 and allophycocyanin- conjugated anti-IFN-γ or allophycocyanin-conjugated-TNF-α (BD Pharmingen), incubated for 20 min at 37°C, fixed with 1% paraformaldehyde in PBS, and analyzed on a FACSCalibur.
Immunohistochemistry and immunofluorescent Ab labeling of tissue sections
Tissue was fixed in 4% paraformaldehyde (Electron Microscopy Sciences) and embedded in paraffin. All slides were stained using the Autostainer (DakoCytomation). TBS with Tween 20 (DakoCytomation) was used for all washes and Ab diluent (DakoCytomation), 5% BSA/TBS, or 2% horse serum/TBS was used for all mAb dilutions. The primary Abs used included polyclonal anti-CD3 rabbit serum (DakoCytomation), mAb anti-CD4 mouse serum (clone IF6; Vector Laboratories), polyclonal anti-FoxP3 rabbit serum (Abcam), polyclonal anti-CD8 rabbit serum (DBS), and polyclonal anti-Ki67 rabbit serum (Lab Vision). For all primary Abs, slides were subjected to an Ag-retrieval step consisting of incubation in AR10 (Biogenex) for 2 min at 123°C in the Digital Decloaking Chamber (Biocare Medical) followed by cooling to 90°C before rinsing in running water and a final buffer rinse. Primary Abs were replaced by normal rabbit IgG (Zymed Laboratories) or mouse IgG (DakoCytomation) and included with each staining series as the negative control. For double-label immunofluorescence, primary Abs were diluted in BSA/monkey serum/TBS. Nonspecific binding sites were blocked with 10% goat serum and Tween 20 in PBS (Background Eraser; Biocare Medical). Binding of the primary Abs was detected simultaneously using Alexa Fluor 488-labeled polyclonal goat anti-rabbit IgG (Molecular Probes) and Alexa Fluor 568-labeled polyclonal goat anti-mouse IgG (Molecular Probes) for 1 h. Nonspecific binding sites were further blocked by the addition of a 30-min incubation in 5% BSA/TBS between the first primary Ab incubation and detection reagents, and the addition of a second primary Ab and detection reagents. All slides were coverslipped using Prolong Gold with 4′,6-diamidino-2-phenylindole dihydrochloride hydrate (DAPI; Molecular Probes) to stain nuclei. All the control experiments gave appropriate results with minimal nonspecific staining (data not shown).
Slides were visualized with epifluorescent illumination using a Zeiss Axioplan 2 microscope (Carl Zeiss) and appropriate filters. Digital images were captured and analyzed by using a Zeiss Axiocam System and Openlab software (Inprovision).
Quantitative image analysis
The number of positive mAb-labeled cells/mm2 of tissue sample was quantified using a Zeiss AxioCam HRc digital camera mounted on a Zeiss microscope (Carl Zeiss) fitted with a ×40 plan neofluar objective and a polarizing filter cube with appropriate filters (Omega Optical). Digital images were captured with Openlab software. One section per tissue with representative histomorphologic components (cortex, paracortex, follicles, medulla for lymph nodes; and epithelium, lamina propria and muscularis mucosa for rectum) was analyzed. Five high-power (×40) microscope fields of the T cell-rich zone (paracortex) per lymph node section and five high-power fields of lamina propria from rectal sections were randomly chosen and captured digitally with the system described above. Each captured field includes an area of ~0.04 mm2. Only clearly positive cells with distinctly labeled nuclei (DAPI) and bright staining were considered positive. Individual positive cells in the five captured high-power microscope fields of the immunohistochemical-stained tissue sections were counted manually by a single observer. The numbers of positive cells are presented as cells/mm2. All samples were analyzed by an operator who was blinded to the experimental design described in Fig. 1.
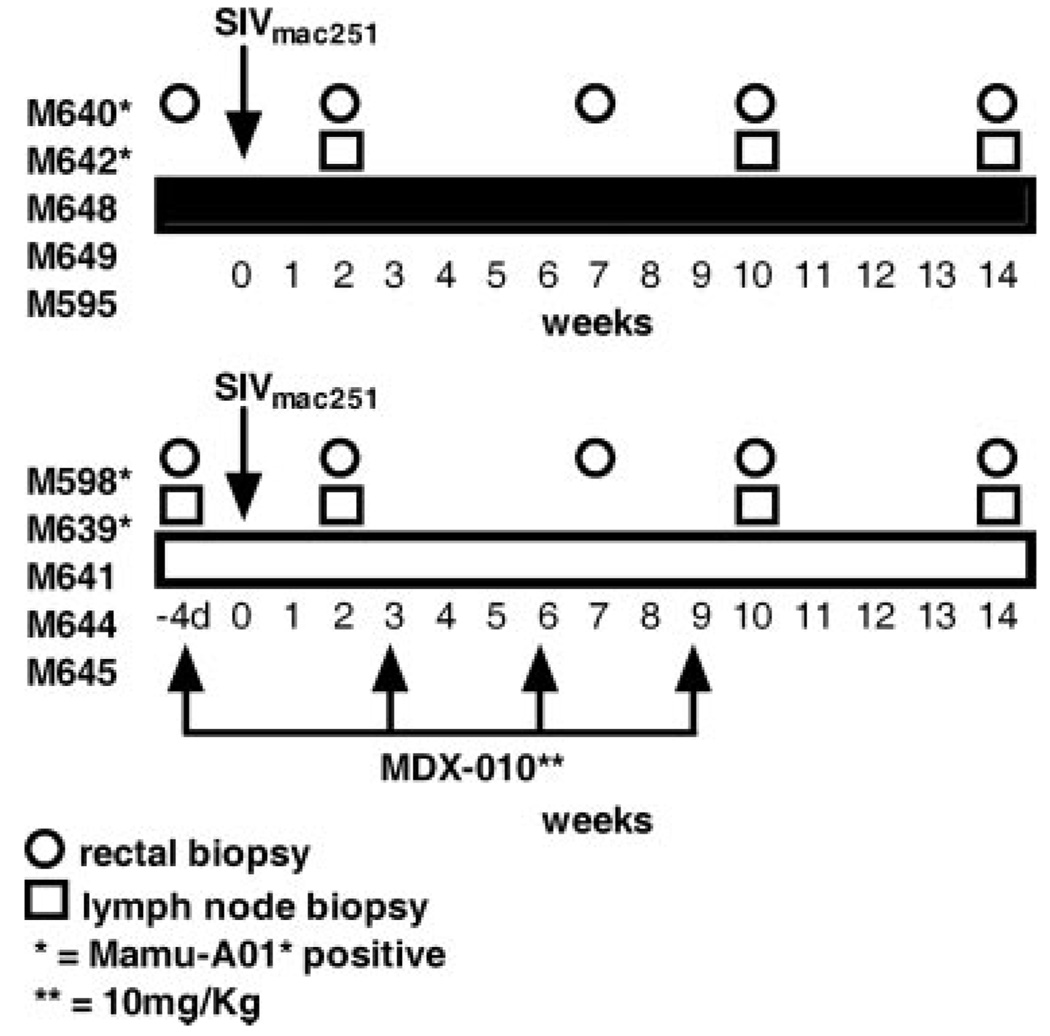
FIGURE 1.
SIV primary infection: study design and tissue collection. All macaques were infected with SIVmac251 by the intrarectal route, as previously described (31). Macaques were treated or not at the times indicated with MDX-010. The time of rectal or lymph node biopsy is indicated with symbols: *, Mamu-A*01+ macaques. The MDX-010 Ab was given i.v. at 10 mg/kg/dose; d, days.
Statistical analysis
Statistical analyses for mRNA expression, tryptophan and kynurenine ratio, and lymph node viral load were performed using SPSS 13.0 software. Differences between groups were assessed by nonparametric Mann-Whitney U test. Differences before and after treatment within the same group were assessed using the Wilcoxon test. All p values shown in the text and figures are two tailed.
Results
CTLA-4 blockade increases T cell activation in early SIV infection
Ten macaques were enrolled in the first study (Fig. 1). The animals were divided into two groups of five animals each and were all exposed intrarectally to SIVmac251, as previously described (31). Five animals received four consecutive inoculations, 3 wk apart at a dose of 10 mg/kg MDX-010, a blocking Ab against human CTLA-4. The first inoculation of Ab was given 4 days before challenge exposure to SIVmac251 to allow for adequate tissue distribution of the Abs. This dose of MDX-010 was chosen because of its ascertained biological effect in macaques (23). To assess the effect of treatment not only in blood but also at mucosal sites, we obtained blood, rectal biopsies, and axillary and inguinal lymph node biopsies at the times indicated in Fig. 1.
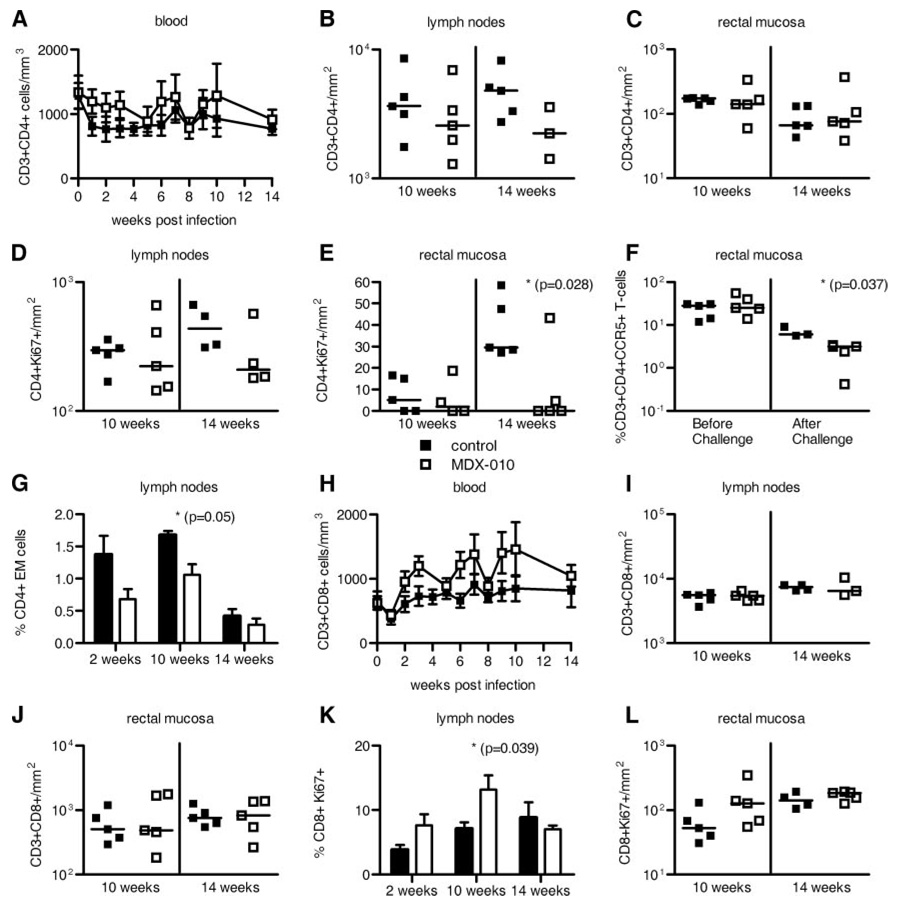
CTLA-4 expression is constitutive on Treg and is induced transiently on CD4+ and CD8+ T cells upon T cell activation. Because activation of CD4+ T cells could provide more targets for SIV infection and increased viral replication, we quantified the total number of T cells, and of T cells that entered the cell cycle (expressing Ki67) in blood and tissues over time. The total number of CD3+CD4+ T cells did not change significantly over time in blood, lymph nodes, or rectal mucosa of the treated macaques (Fig. 2, A–C). However, there was a significant decrease in the absolute number of CD4+Ki67+ T cells in rectal mucosa (p = 0.028) but not in lymph nodes by week 14 (Fig. 2, D and E), suggesting that the loss of CD4+ T cells occurs faster in MDX-010-treated macaques. The fact that CD4+CCR5+ T cells, the targets for SIV infection, were also significantly decreased (p = 0.037) at mucosal sites in the treated group (Fig. 2F), and that CD4+ effector cells were maintained at higher numbers in the tissues of the untreated macaques (p = 0.05) (Fig. 2G) argues in favor of a more pronounced loss of CD4+ T cells due to direct viral cytopathic effect. Likewise, anti-CTLA-4 treatment did not result in a significant change in the absolute number of CD8+ T cells in blood (Fig. 2H), lymph nodes, and rectal mucosa (Fig. 2, I and J). Quantitation of the total number or frequency of activated CD8+Ki67+ T cells in intact tissues by immunohistochemistry or by analysis of singlecell suspension revealed a significant increase in lymph nodes by week 10 (p = 0.039), and, to a lesser extent, at mucosal sites (Fig. 2, K and L). Altogether, these results indicate that CTLA-4 blockade induced cell cycle entry of both CD4+ and CD8+ T cell subsets, but that the appropriate enumeration of activated CD4+ T cells may be precluded because they rapidly become infected and consequently lost.
FIGURE 2.
MDX-010 treatment increases T cell activation and exacerbates CCR5+CD4+ T cell loss in tissues. Mean values (±SE) of the absolute CD3+CD4+ T cells/mm3 in blood (A). Total number of CD3+CD4+ T cells/mm2 in lymph nodes (B) and rectal mucosa (C). Total number of CD4+Ki67+ T cell numbers/mm2 in lymph nodes (D) and rectal mucosa (E). F, Frequency of CCR5+CD4+ T cells in rectal mucosa before and after challenge exposure to SIVmac251. For the after challenge analysis, the geometric mean of the frequency of CCR5+CD4+ T cells at weeks 7–14 is shown for each animal. The bars represent the mean value in each group. G, Mean value (+SE) of the frequency of effect or memory CD4+ T cells in lymph nodes of animals over time. Absolute number of CD3+CD8+ T cells/mm3 in blood (H) or CD3+CD8+ T cells/mm2 in lymph nodes (I) and rectal mucosa (J). Percentage (mean value + SE) of CD8+Ki67+ T cells in lymph nodes (K) and absolute number of CD8+Ki67+ T cells/mm2 in rectal mucosa (L). All the data in B–E and H–J and L were obtained by immunohistochemistry counting of the number of CD4+ or CD8+ T cells/mm2 of tissues. Lymph nodes were either axillary or inguinal. Horizontal bars in the scatter dot plots correspond to the median.
CTLA-4 blockade increases systemic and mucosal virus levels but not virus-specific immune responses
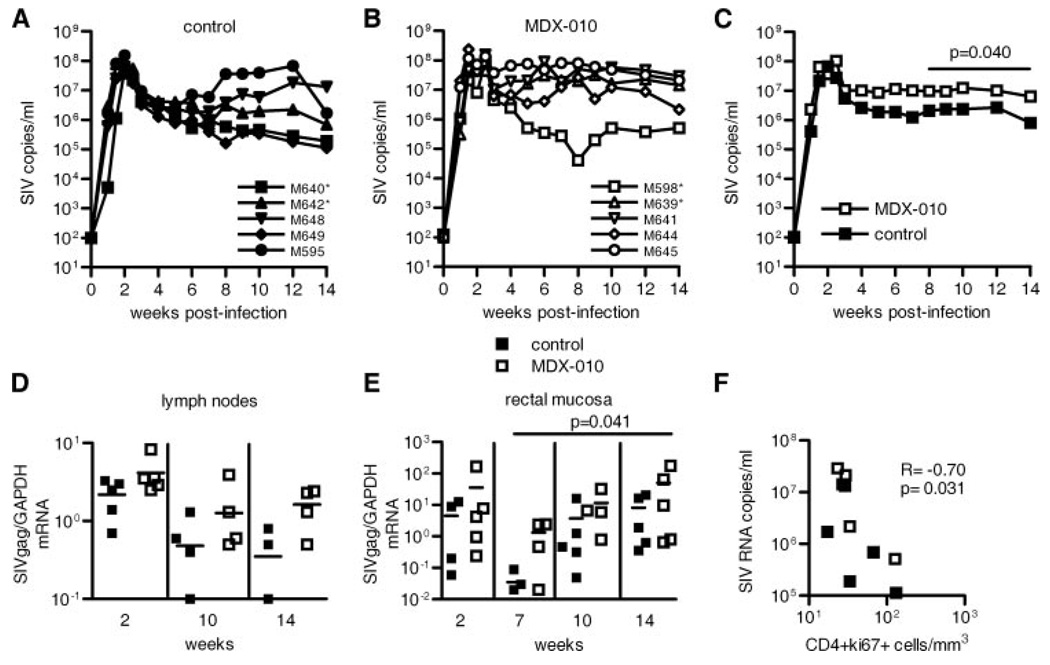
To evaluate the effect of MDX-010 treatment on viral replication, we measured virus RNA levels not only in plasma but also in lymph nodes and rectal biopsies of all animals. Both groups mounted similar plasma virus levels during the first 3 wk of infection (Fig. 3, A and B). However, the group treated with MDX-010 had significantly higher levels of plasma virus in the interval between weeks 8 and 14 (p = 0.04) (Fig. 3C), but surprisingly this difference did not reach significance in lymph nodes (Fig. 3D). In contrast, virus RNA levels were higher in rectal biopsies of the treated macaques (p = 0.041), implying an association between increased T cell activation and virus levels (Fig. 3E). Indeed, we found a negative correlation between the number of activated CD4+ T cells in blood and plasma virus levels (Fig. 3F), further supporting the notion that activated T cells may become infected and die.
FIGURE 3.
Systemic and mucosal virus levels. A and B, Plasma SIV RNA level over time for each macaque in the control and MDX-010 groups, respectively. *, Mamu-A*01+. C, Geometric mean values of virus levels in the two groups. Statistical analysis by the Wei-Johnson test counting weeks 9, 10, 12, and 14 demonstrated significantly higher viremia in macaques treated with MDX-010 (p = 0.032). Similarly, from weeks 8 to 14, the difference between the two groups was significant (p= 0.040), as indicated in the figure. D and E, SIV Gag RNA levels normalized on GAPDH in RNA of lymph nodes (D) and rectal mucosa (E) of control animals (■) and MDX-010-treated macaques (□). Statistical analysis was performed using the repeated measures ANOVA. Horizontal bars in the scatter dot plots correspond to the median. F, Correlative analysis between the number of activated CD4+ T cells in blood and plasma virus levels at week 14 postinfection.
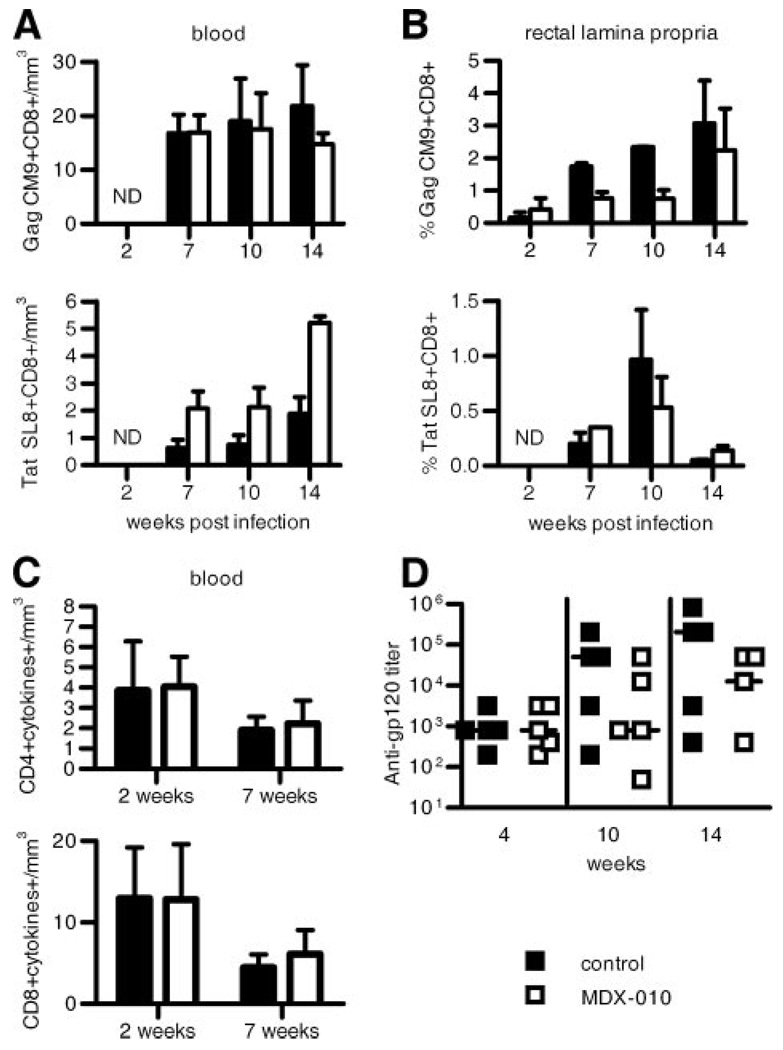
To assess whether CTLA-4 blockade modified virus-specific immune responses, we measured CD8+ T cell responses using the Gag CM9 and Tat SL8 tetramers (37) in Mamu-A*01+ macaques. In addition, we measured the ability of blood and lymph node mononuclear cells of all animals to produce IFN-γ, TNF-α, and IL-2 following stimulation with the entire Gag peptide pool by ICC staining. No differences in the frequency of tetramer+ Gag- or Tat-specific T cells were found in blood, lymph nodes, or rectal mucosa of the treated or untreated macaques (Fig. 4, A and B, and data not shown for lymph nodes). Similarly, no difference in the ability of CD4+ or CD8+ T cells to produce cytokines following Gag stimulation was found in blood or lymph nodes of the animals in the two groups (Fig. 4C and data not shown for lymph nodes). Lastly, no difference in serum Ab titers to the SIV Env protein was found between the two groups (Fig. 4D).
FIGURE 4.
Virus-specific immune responses in blood and tissues. A and B, Mean values (+SE) of the absolute number of CM9+ or SL8+tetramer+ T cells/mm3 of blood (A) and of the frequency of these responses in rectal mucosa (B) following exposure to SIVmac251 in treated and untreated macaques. C, Total number of CD4+ and CD8+ T cells producing IFN-γ, TNF-α, and IL-2 following in vitro stimulation with the entire SIV Gag peptide pool in the blood of macaques during primary infection. D, Titers of serum-binding Abs to gp120 measured by ELISA. Horizontal bars in the scatter dot plot correspond to the median.
CTLA-4 blockade increases IDO expression and activity and decreases the number of FoxP3+ T cells at mucosal sites
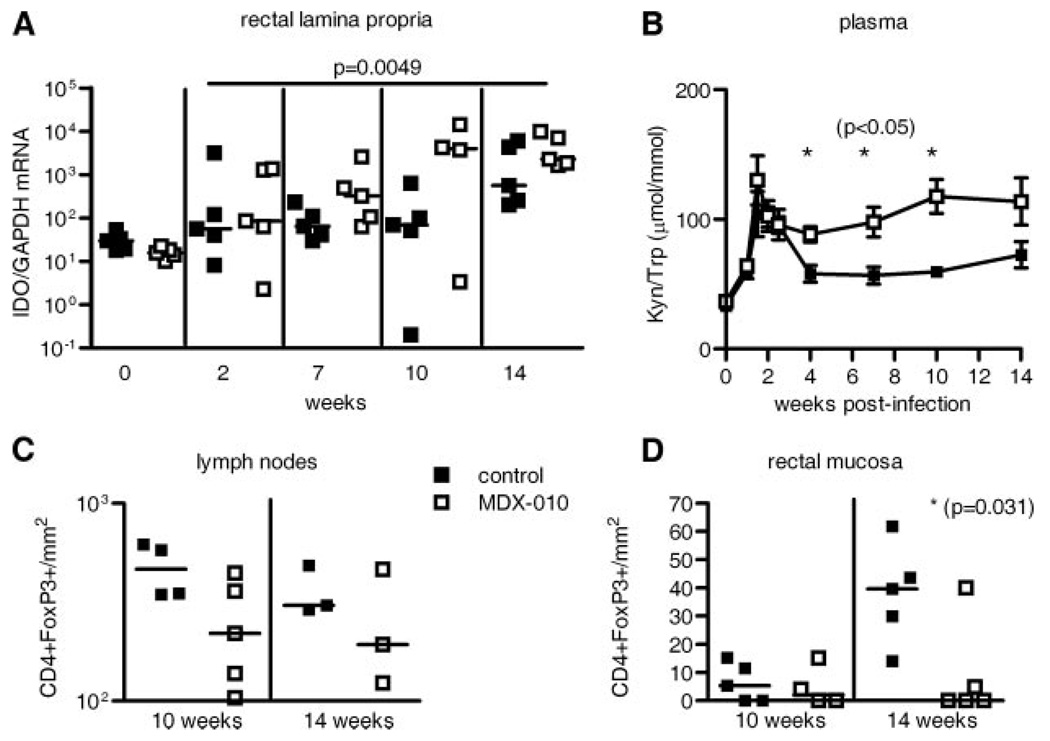
IDO, an enzyme that converts tryptophan into kynurenine and halts T cell proliferation, is expressed at high level in tissues of HIV/SIV-infected hosts (19, 20, 38). Because IDO is induced by the engagement of CTLA-4 expressed on Treg or activated T cells to the CD80 or CD86 molecules on the surface of APC (15), analysis of IDO level provided an opportunity to assess whether the high level of IDO in SIV infection could be lowered by CTLA-4 blockade. CTLA-4 blockade was associated with a significant increase rather than a decrease in IDO expression in rectal tissues following all treatments (p = 0.0049) (Fig. 5A), and, accordingly, the overall rate of IDO activity, measured as a ratio between plasma concentration of kynurenine to tryptophan, was also significantly increased in MDX-010-treated macaques (p = 0.05 at 4, 8, and 10 wk) (Fig. 5B).
FIGURE 5.
Increased IDO expression and activity and decreased FoxP3+CD4+ T cells in MDX-010-treated macaques. A, IDO RNA levels were measured by RT-PCR in rectal mucosa. Horizontal bars in the smaller plot correspond to the median. B, The kynure-nine-to-tryptophan ratio was measured in plasma at the times indicated. *, Significant (p = 0.05) differences in the two groups. The data are expressed as mean value (±SE). Total number of CD4+FoxP3+ T cells/mm2 in lymph nodes (C) and rectal mucosa (D).
Of note, quantitation of the total number of CD4+FoxP3+ T cells in tissues by immunohistochemistry revealed a decrease rather than increase in lymph nodes and rectal mucosa (p = 0.031) of the MDX-010-treated macaques (Fig. 5, C and D). This finding may be a consequence of the overall decrease in the total number of CD4+ T cells in these compartments due to increased viral replication (see Fig. 2 and 3) and do not directly address Treg as CD4+FoxP3+ T cells could be either Treg or activated T cells. Considered together, these data suggest that the increase in IDO is likely a consequence of increased levels of virus, which can directly induce IDO expression in different APC (39, 40). The decrease in CD4+FoxP3+ T cells in MDX-010-treated animals may be the result of enhanced cytopathic effects of viral replication, as Treg have been shown to be rapidly depleted from the intestinal lamina propria of SIV-infected pigtailed macaques during acute infection with a rate similar to that of CCR5+ CD4+ T cells, suggestive of a common underlying mechanism of depletion (29).
CTLA-4 blockade reduces responsiveness to ART and abrogates the virological benefit of vaccination
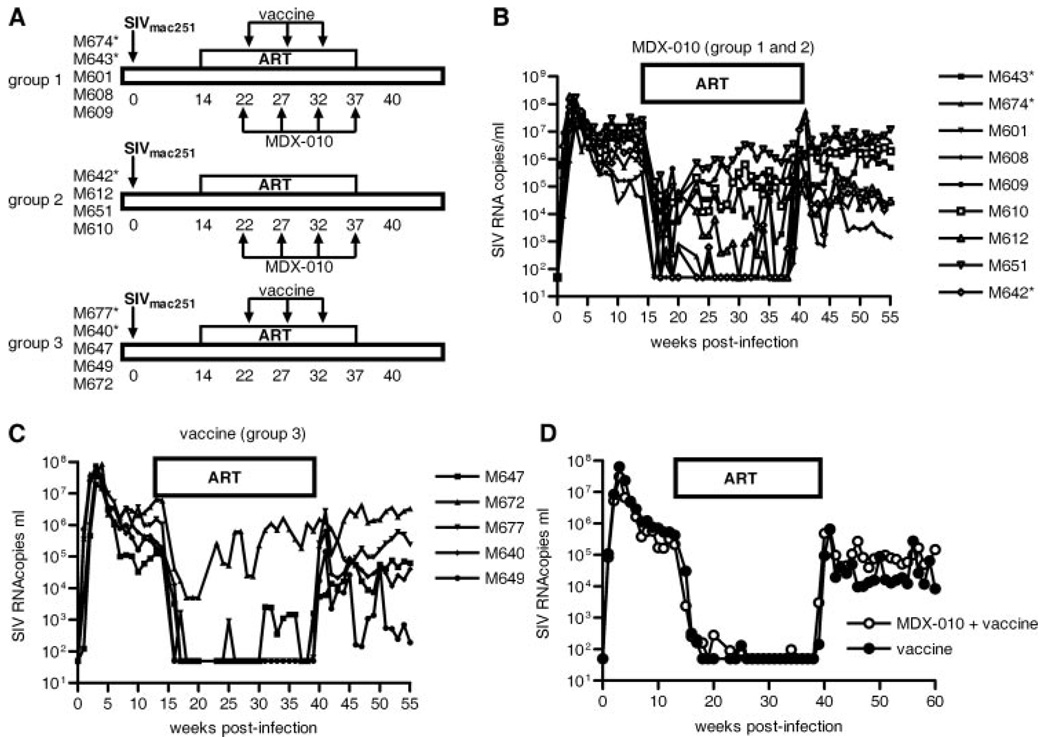
During SIV infection, primary virus-induced immune activation may confound a possible therapeutic effect of CTLA-4 blockade. Indeed, a short treatment with MDX-010 of SIV-infected macaques treated with ART resulted in increased immune activation but was associated with a decrease rather than an increase in viral replication in tissues (23). Therefore, we investigated the safety of a protracted treatment with MDX-010 in macaques and whether, in the presence of ART, this treatment could provide an immunological and virological benefit. Furthermore, because the inhibitory effect of CTLA-4 is more evident during secondary rather than primary immune response (41), we combined treatment of MDX-010 with vaccination. We designed a study whereby macaques were infected intrarectally with SIVmac251 and treated with ART at week 14 after infection (Fig. 6A). Two groups of macaques were treated with four inoculations of MDX-010 5 wk apart at the same dose used in primary infection (10 mg/kg). In addition, one of those groups also received modified vaccinia Ankara-based vaccines expressing the structural and regulatory genes of SIV (Fig. 6A) 4 days after the first three inoculations of MDX-010. As a control, one group was vaccinated in an identical manner in the absence of MDX-010 Ab treatment (Fig. 6A). Vaccination of SIV infected macaques with this vaccine modality usually results in a decrease of viral set point, when compared with the pre-ART level, by ~1 log in virus plasma level after ART is suspended (31, 42, 43). We found that infected macaques treated with MDX-010 (groups 1 and 2) predominantly did not respond to ART (Fig. 6B) in contrast to those in group 3, which predominantly responded (Fig. 6C). Furthermore, when the vaccine effect was evaluated in macaques that effectively responded to therapy (three in the MDX- 010-treated group and four in the vaccine-only group), we found that vaccination alone decreased the set point by ~1 log, as expected (Fig. 6D). However, when vaccination was combined with MDX-010, the reduction of virus level following suspension of ART became less evident (Fig. 6D). These results suggested that CTLA-4 blockade may have increased T cell activation in the presence of ART and decreased ART responsiveness. Indeed, similar to our previous report (23), MDX-010 treatment of chronically infected macaques increased Ki67 on CD4+ and CD8+ T cells in blood and lymph nodes (data not shown), even in the presence of ART.
FIGURE 6.
MDX-010 decreases ART responsiveness and abrogates the vaccine effect in chronically infected macaques. A, Schematic representation of the second study design and animal groups. MDX-010 was given at weeks 22, 27, 32, and 37 at a dose of 10 mg/kg. Vaccination was performed with two inoculations of modified vaccinia Ankara-expressing Gag, Pol, Env, and retanef (56) 4 days after the administration of the Ab in each case. *, Mamu-A*01+. Plasma virus levels during the study in all the MDX-010-treated macaques (groups 1 and 2) (B) and in macaques vaccinated only (C). *, Mamu-A*01+. D, Plasma virus levels in macaques M674, M601, M608 from group 1 and M677, M640, M647, and M649 from group 3. These animals were selected to evaluate the vaccine effect as they maintained adequate suppression of viral replication during ART treatment.
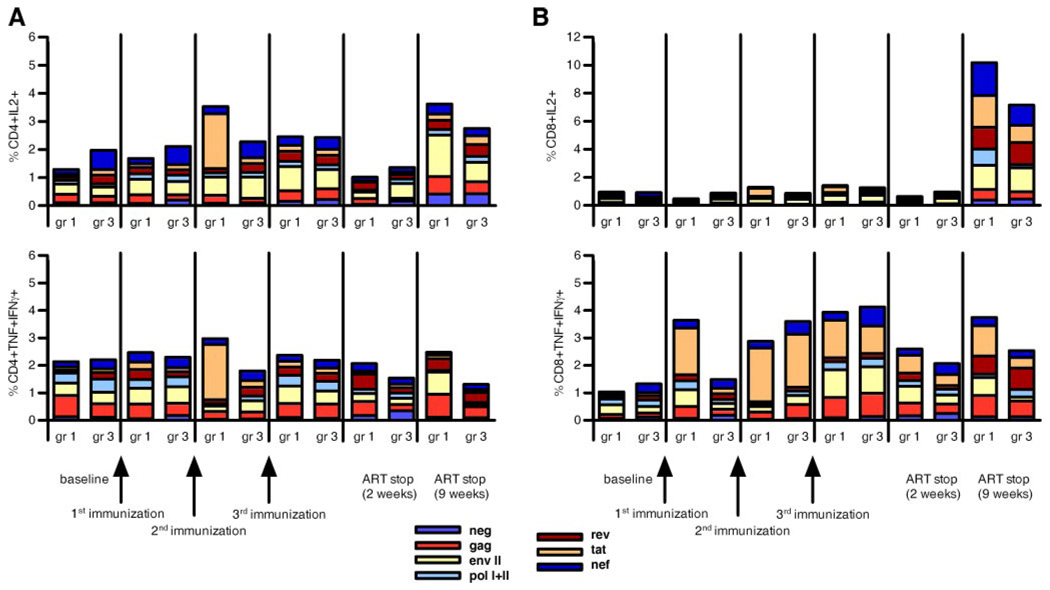
A detailed analysis of vaccine-induced immune response by ICC staining using nearly the entire SIV proteome demonstrated that MDX-010 did not significantly augment vaccine-induced CD4+ or CD8+ T cell responses in blood (Fig. 7), as observed in our previous study (23) as well as in primary SIVmac251 infection (see Fig. 4).
FIGURE 7.
Virus-specific immune responses induced by vaccination. CD4+ and CD8+ T cell responses (A and B, respectively) measured as IL-2 or TNF-α and IFN-γ production following vaccination and ART suspension were evaluated in the same macaques used in Fig. 6D whose viremia remained suppressed during ART. These animals were selected to limit the confounding factor of overt viral replication on the vaccine immunogenicity. In these experiments, almost the entire SIV proteome was used as stimulus.
Discussion
Treg have been measured in HIV/SIV infection using either phenotypic markers or RNA expression of molecules constitutively up-regulated in Treg, such as FoxP3 and CTLA-4. These reports disagree as to whether there is an increase or a decrease in blood and tissues of HIV/SIVmac251-infected rhesus macaques (17, 18, 20–28, 44, 45). However, when the suppressive function of CD4+CD25+ T cells on HIV/SIV-specific immune responses is measured in vitro, there is agreement in both HIV and SIV infection that these cells attenuate both CD4+ and CD8+ virus-specific immune responses (17, 23, 46). Because Treg constitutively express the immune regulatory CTLA-4 molecule (47), and because CTLA-4 is also rapidly expressed by newly activated T cells, providing an efficient negative feedback to activation (14), we directly assessed the effect of CTLA-4 blockade in SIV infection. The expected outcome was uncertain, as inhibition of the suppressive activity of Treg could increase immune response and ameliorate the virological outcome or, alternatively, increase immune activation and provide more cellular targets for the virus.
Our study demonstrates that CTLA-4 blockade significantly increases viral replication and CD4+ T cell loss, particularly at mucosal sites, and does not significantly restore virus-specific immune responses. The increase in T cell activation was documented by several methods, including quantitating the absolute number of CD8+ T cells in tissues by immunohistochemistry. In the case of activated CD4+ T cells, we observed a significant decrease in their absolute number in tissues, concomitant with a significant increase in viral replication in the same locale, suggesting that inhibition of CTLA-4 likely exacerbates viral replication and CD4+ T cell loss. Paradoxically, CTLA-4 blockade increased the tissue expression and activity of IDO, an enzyme induced by the engagement of B7 by CTLA-4 (48, 49) and, directly, by exposure of APC to HIV virions (39, 40). IDO activity is strictly associated with activation of the immune system and can be induced by cytokines such as types I and II IFN (50, 51). HIV is also able to directly induce IDO expression in vitro (39); therefore, it is possible that the increase in IDO expression and activity observed in the infected macaques treated with MDX-010 is due to the high level of viral replication. An alternative explanation, however, could be that the anti-CTLA-4 Ab may work as an agonist in vivo and increase Treg activity.
Importantly, in this macaque model, even when viral replication is suppressed by ART, protracted treatment with MDX-010 resulted in increased T cell activation and an apparent diminished responsiveness to ART, and, again, no increase in the SIV-specific virus-induced or vaccine-induced immune response was observed. A caveat in this animal model, however, is that ART treatment of macaques does not include protease inhibitors that greatly contribute to suppression of viral replication in HIV-1-infected individuals. Therefore, these results may not be directly applicable to patients treated with optimal ART regimens. Nevertheless, the evidence that we provide supports the notion that, by decreasing the threshold of T cell activation, particularly when viral replication is not fully controlled, CTLA-4 blockade in vivo may provide more target cells to the virus.
In this study, we have not directly addressed the role of Treg “per se” in SIV infection, because CTLA-4 is also expressed on activated T cells. However, treatment with MDX-010 in humans is frequently associated with manifestation of autoimmunity, including erythematosus rash, generation of autoantibodies, colitis, hypophysitis, and uveitis, suggesting its ability to break tolerance to T cell Ags (52). Of notice, MDX-010 treatment affected T cell activation and viral replication, particularly at mucosal sites, where Treg are likely to maintain a state of controlled inflammation, given the continual exposure to alimentary and bacterial Ags (53).
We believe that the fact that CTLA-4 blockade in SIVmac251− infected macaques did not result in an increase of immune response elicited by viral infection or vaccination in blood and tissues suggest a predominant role for immune activation, particularly at mucosal sites, and a limited contribution of Treg to the suppression of immune responses in vivo. The study presented here therefore complements work of others demonstrating that the low level of immune activation in Sooty may be related to the lack of progression to AIDS, despite the high levels of SIV replication (13).
The direct demonstration in our work that immune activation, particularly at mucosal sites, increases both local and systemic viral replication underscores the importance of paying attention to the gastrointestinal immune system in AIDS pathogenesis, as also proposed by others (54), and highlights the usefulness of macaque models for dissecting underlying pathogenic mechanisms of disease induction and for the development of therapeutic intervention for HIV (55).
Acknowledgments
We thank Steven Snodgrass for editorial assistance; Claire Chougnet, Jan Andersson, and Jakob Nilsson for helpful discussion; and Phillip Markham, Jim Treece, Deborah Weiss, Eun Lee, and Sharon Orndorff at Advanced BioScience Laboratories for help with the animal study.
Footnotes
This work was supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health.
Abbreviations used in this paper: Treg, regulatory T cell; ART, antiretroviral therapy; NASBA, nucleic acid sequence-based amplification; ICC, intracellular cytokine.
Disclosures
Israel Lowy is an employee of Medarex, Bloomsbury, NJ, whose product, MDX-010, was used in this manuscript.
References
- 1.Mattapallil JJ, Douek DC, Hill B, Nishimura Y, Martin M, Roederer M. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature. 2005;434:1093–1097. doi: 10.1038/nature03501. [DOI] [PubMed] [Google Scholar]
- 2.Mehandru S, Poles MA, Tenner-Racz K, Horowitz A, Hurley A, Hogan C, Boden D, Racz P, Markowitz M. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J. Exp. Med. 2004;200:761–770. doi: 10.1084/jem.20041196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mehandru S, Poles MA, Tenner-Racz K, Manuelli V, Jean-Pierre P, Lopez P, Shet A, Low A, Mohri H, Boden D, et al. Mechanisms of gastrointestinal CD4+ T-cell depletion during acute and early human immunodeficiency virus type 1 infection. J. Virol. 2007;81:599–612. doi: 10.1128/JVI.01739-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smit-McBride Z, Mattapallil JJ, McChesney M, Ferrick D, Dandekar S. Gastrointestinal T lymphocytes retain high potential for cytokine responses but have severe CD4+ T-cell depletion at all stages of simian immunodeficiency virus infection compared to peripheral lymphocytes. J. Virol. 1998;72:6646–6656. doi: 10.1128/jvi.72.8.6646-6656.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Veazey RS, DeMaria M, Chalifoux LV, Shvetz DE, Pauley DR, Knight HL, Rosenzweig M, Johnson RP, Desrosiers RC, Lackner AA. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science. 1998;280:427–431. doi: 10.1126/science.280.5362.427. [DOI] [PubMed] [Google Scholar]
- 6.Perelson AS, Neumann AU, Markowitz M, Leonard JM, Ho DD. HIV-1 dynamics in vivo: virion clearance rate, infected cell life-span, and viral generation time. Science. 1996;271:1582–1586. doi: 10.1126/science.271.5255.1582. [DOI] [PubMed] [Google Scholar]
- 7.Ho DD, Neumann AU, Perelson AS, Chen W, Leonard JM, Markowitz M. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature. 1995;373:123–126. doi: 10.1038/373123a0. [DOI] [PubMed] [Google Scholar]
- 8.Grossman Z, Meier-Schellersheim M, Paul WE, Picker LJ. Pathogenesis of HIV infection: what the virus spares is as important as what it destroys. Nat. Med. 2006;12:289–295. doi: 10.1038/nm1380. [DOI] [PubMed] [Google Scholar]
- 9.Li Q, Duan L, Estes JD, Ma ZM, Rourke T, Wang Y, Reilly C, Carlis J, Miller CJ, Haase AT. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature. 2005;434:1148–1152. doi: 10.1038/nature03513. [DOI] [PubMed] [Google Scholar]
- 10.Gordon SN, Klatt NR, Bosinger SE, Brenchley JM, Milush JM, Engram JC, Dunham RM, Paiardini M, Klucking S, Danesh A, et al. Severe depletion of mucosal CD4+ T cells in AIDS-free simian immunodeficiency virus-infected sooty mangabeys. J. Immunol. 2007;179:3026–3034. doi: 10.4049/jimmunol.179.5.3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Milush JM, Reeves JD, Gordon SN, Zhou D, Muthukumar A, Kosub DA, Chacko E, Giavedoni LD, Ibegbu CC, Cole KS, et al. Virally induced CD4+ T cell depletion is not sufficient to induce AIDS in a natural host. J. Immunol. 2007;179:3047–3056. doi: 10.4049/jimmunol.179.5.3047. [DOI] [PubMed] [Google Scholar]
- 12.Pandrea IV, Gautam R, Ribeiro RM, Brenchley JM, Butler IF, Pattison M, Rasmussen T, Marx PA, Silvestri G, Lackner AA, et al. Acute loss of intestinal CD4+ T cells is not predictive of simian immunodeficiency virus virulence. J. Immunol. 2007;179:3035–3046. doi: 10.4049/jimmunol.179.5.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Silvestri G, Paiardini M, Pandrea I, Lederman MM, Sodora DL. Understanding the benign nature of SIV infection in natural hosts. J. Clin. Invest. 2007;117:3148–3154. doi: 10.1172/JCI33034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sperling AI, Bluestone JA. The complexities of T-cell co-stimulation: CD28 and beyond. Immunol. Rev. 1996;153:155–182. doi: 10.1111/j.1600-065x.1996.tb00924.x. [DOI] [PubMed] [Google Scholar]
- 15.Miyara M, Sakaguchi S. Natural regulatory T cells: mechanisms of suppression. Trends Mol. Med. 2007;13:108–116. doi: 10.1016/j.molmed.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 16.Boasso A, Vaccari M, Nilsson J, Shearer GM, Andersson J, Cecchinato V, Chougnet C, Franchini G. Do regulatory T-cells play a role in AIDS pathogenesis? AIDS Rev. 2006;8:141–147. [PubMed] [Google Scholar]
- 17.Aandahl EM, Michaelsson J, Moretto WJ, Hecht FM, Nixon DF. Human CD4+ CD25+ regulatory T cells control T-cell responses to human immunodeficiency virus and cytomegalovirus antigens. J. Virol. 2004;78:2454–2459. doi: 10.1128/JVI.78.5.2454-2459.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Apoil PA, Puissant B, Roubinet F, Abbal M, Massip P, Blancher A. FOXP3 mRNA levels are decreased in peripheral blood CD4+ lymphocytes from HIV-positive patients. J. Acquir. Immune. Defic. Syndr. 2005;39:381–385. doi: 10.1097/01.qai.0000169662.30783.2d. [DOI] [PubMed] [Google Scholar]
- 19.Boasso A, Vaccari M, Hryniewicz A, Fuchs D, Nacsa J, Cecchinato V, Andersson J, Franchini G, Shearer GM, Chougnet C. Regulatory T cell markers, indoleamine (2,3)-dioxygenase and virus levels in spleen and gut during progressive SIV infection. J. Virol. 2007;81:11593–11603. doi: 10.1128/JVI.00760-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boasso A, Shearer GM. How does indoleamine 2,3-dioxygenase contribute to HIV-mediated immune dysregulation. Curr. Drug Metab. 2007;8:217–223. doi: 10.2174/138920007780362527. [DOI] [PubMed] [Google Scholar]
- 21.Epple HJ, Loddenkemper C, Kunkel D, Troger H, Maul J, Moos V, Berg E, Ullrich R, Schulzke JD, Stein H. Mucosal but not peripheral FOXP3+ regulatory T cells are highly increased in untreated HIV infection and normalize after suppressive HAART. Blood. 2006;108:3072–3078. doi: 10.1182/blood-2006-04-016923. [DOI] [PubMed] [Google Scholar]
- 22.Estes JD, Li Q, Reynolds MR, Wietgrefe S, Duan L, Schacker T, Picker LJ, Watkins DI, Lifson JD, Reilly C, et al. Premature induction of an immunosuppressive regulatory T cell response during acute simian immunodeficiency virus infection. J. Infect. Dis. 2006;193:703–712. doi: 10.1086/500368. [DOI] [PubMed] [Google Scholar]
- 23.Hryniewicz A, Boasso A, Edghill-Smith Y, Vaccari M, Fuchs D, Venzon D, Nacsa J, Betts MR, Tsai WP, Heraud JM, et al. CTLA-4 blockade decreases TGF-β, indoleamine 2,3- dioxygenase, and viral RNA expression in tissues of SIVmac251-infected macaques. Blood. 2006;108:3834–3842. doi: 10.1182/blood-2006-04-010637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kinter AL, Hennessey M, Bell A, Kern S, Lin Y, Daucher M, Planta M, McGlaughlin M, Jackson R, Ziegler SF, Fauci AS. CD25+CD4+ regulatory T cells from the peripheral blood of asymptomatic HIV-infected individuals regulate CD4+ and CD8+ HIV-specific T cell immune responses in vitro and are associated with favorable clinical markers of disease status. J. Exp. Med. 2004;200:331–343. doi: 10.1084/jem.20032069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nilsson JA, Boasso A, Velilla PA, Zhang R, Vaccari M, Franchini G, Shearer GM, Andersson J, Chougnet C. HIV-1 driven regulatory T cell accumulation in lymphoid tissues is associated with disease progression in HIV/AIDS. Blood. 2006;108:3808–3817. doi: 10.1182/blood-2006-05-021576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nixon DF, Aandahl EM, Michaelsson J. CD4+CD25+ regulatory T cells in HIV infection. Microbes Infect. 2005;7:1063–1065. doi: 10.1016/j.micinf.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 27.Tsunemi S, Iwasaki T, Imado T, Higasa S, Kakishita E, Shirasaka T, Sano H. Relationship of CD4+CD25+ regulatory T cells to immune status in HIV-infected patients. AIDS. 2005;19:879–886. doi: 10.1097/01.aids.0000171401.23243.56. [DOI] [PubMed] [Google Scholar]
- 28.Weiss L, Donkova-Petrini V, Caccavelli L, Balbo M, Carbonneil C, Levy Y. Human immunodeficiency virus-driven expansion of CD4+CD25+ regulatory T cells, which suppress HIV-specific CD4 T-cell responses in HIV-infected patients. Blood. 2004;104:3249–3256. doi: 10.1182/blood-2004-01-0365. [DOI] [PubMed] [Google Scholar]
- 29.Chase AJ, Sedaghat AR, German JR, Gama L, Zink MC, Clements JE, Siliciano RF. Severe depletion of CD4+ CD25+ regulatory T cells from the intestinal lamina propria but not peripheral blood or lymph nodes during acute SIV infection. J. Virol. 2007;81:12748–12757. doi: 10.1128/JVI.00841-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keler TE, Halk E, Vitale L, O’Neill T, Blanset D, Lee S, Srinivasan M, Graziano RF, Davis T, Lonberg N, Korman A. Activity and safety of CTLA-4 blockade combined with vaccines in cynomolgus macaques. J. Immunol. 2003;171:6251–6259. doi: 10.4049/jimmunol.171.11.6251. [DOI] [PubMed] [Google Scholar]
- 31.Hryniewicz A, Price DA, Moniuszko M, Boasso A, Edghill-Spano Y, West SM, Venzon D, Vaccari M, Tsai WP, Tryniszewska E, et al. Interleukin-15 but not interleukin-7 abrogates vaccine-induced decrease in virus level in simian immunodeficiency virus mac251-infected macaques. J. Immunol. 2007;178:3492–3504. doi: 10.4049/jimmunol.178.6.3492. [DOI] [PubMed] [Google Scholar]
- 32.Romano JW, Shurtliff RN, Dobratz E, Gibson A, Hickman K, Markham P, Pal R. Quantitative evaluation of simian immunodeficiency virus infection using NASBA technology. J. Virol. Methods. 2000;86:61–70. doi: 10.1016/s0166-0934(99)00184-6. [DOI] [PubMed] [Google Scholar]
- 33.Malkevitch NV, Patterson LJ, Aldrich MK, Wu Y, Venzon D, Florese RH, Kalyanaraman VS, Pal R, Lee EM, Zhao J, et al. Durable protection of rhesus macaques immunized with a replicating adenovirus-SIV multigene prime/protein boost vaccine regimen against a second SIVmac251 rectal challenge: role of SIV-specific CD8+ T cell responses. Virology. 2006;353:83–98. doi: 10.1016/j.virol.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 34.Vaccari M, Trindade CJ, Venzon D, Zanetti M, Franchini G. Vaccine-induced CD8+ central memory T cells in protection from simian AIDS. J. Immunol. 2005;175:3502–3507. doi: 10.4049/jimmunol.175.6.3502. [DOI] [PubMed] [Google Scholar]
- 35.Knapp LA, Lehmann E, Piekarczyk MS, Urvater JA, Watkins DI. A high frequency of Mamu-A*01 in the rhesus macaque detected by PCR-SSP and direct sequencing. Tissue Antigens. 1997;50:657–661. doi: 10.1111/j.1399-0039.1997.tb02927.x. [DOI] [PubMed] [Google Scholar]
- 36.Widner B, Werner ER, Schennach H, Wachter H, Fuchs D. Simultaneous measurement of serum tryptophan and kynurenine by HPLC. Clin. Chem. 1997;43:2424–2426. [PubMed] [Google Scholar]
- 37.Mothe BR, Horton H, Carter DK, Allen TM, Liebl ME, Skinner P, Vogel TU, Fuenger S, Vielhuber K, Rehrauer W, et al. Dominance of CD8 responses specific for epitopes bound by a single major histocompatibility complex class I molecule during the acute phase of viral infection. J. Virol. 2002;76:875–884. doi: 10.1128/JVI.76.2.875-884.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schroecksnadel K, Zangerle R, Bellmann-Weiler R, Garimorth K, Weiss G, Fuchs D. Indoleamine-2, 3-dioxygenase and other interferon-γ-mediated pathways in patients with human immunodeficiency virus infection. Curr. Drug Metab. 2007;8:225–236. doi: 10.2174/138920007780362608. [DOI] [PubMed] [Google Scholar]
- 39.Boasso A, Herbeuval JP, Hardy AW, Anderson SA, Dolan MJ, Fuchs D, Shearer GM. HIV inhibits CD4+ T-cell proliferation by inducing indoleamine 2,3-dioxygenase in plasmacytoid dendritic cells. Blood. 2007;109:3351–3359. doi: 10.1182/blood-2006-07-034785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grant RS, Naif H, Thuruthyil SJ, Nasr N, Littlejohn T, Takikawa O, Kapoor V. Induction of indoleamine 2,3-dioxygenase in primary human macrophages by HIV-1. Redox. Rep. 2000;5:105–107. doi: 10.1179/135100000101535366. [DOI] [PubMed] [Google Scholar]
- 41.Chambers CA, Sullivan TJ, Truong T, Allison JP. Secondary but not primary T cell responses are enhanced in CTLA-4-deficient CD8+ T cells. Eur. J. Immunol. 1998;28:3137–3143. doi: 10.1002/(SICI)1521-4141(199810)28:10<3137::AID-IMMU3137>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 42.Hel Z, Venzon D, Poudyal M, Tsai W-P, Giuliani L, Woodward R, Chougnet C, Shearer GM, Altman JD, Watkins DI, et al. Viremia control following antiretroviral treatment and therapeutic immunization during primary SIV251 infection of macaques. Nat. Med. 2000;6:1140–1146. doi: 10.1038/80481. [DOI] [PubMed] [Google Scholar]
- 43.Tryniszewska E, Nacsa J, Lewis MG, Silver P, Montefiori D, Venzon D, Hel Z, Parks RW, Moniuszko M, Tartaglia J, et al. Vaccination of macaques with long-standing SIVmac251 infection lowers the viral set point after cessation of antiretroviral therapy. J. Immunol. 2002;169:5347–5357. doi: 10.4049/jimmunol.169.9.5347. [DOI] [PubMed] [Google Scholar]
- 44.Kornfeld C, Ploquin MJ, Pandrea I, Faye A, Onanga R, Apetrei C, Poaty-Mavoungou V, Rouquet P, Estaquier J, Mortara L, et al. Antiinflammatory profiles during primary SIV infection in African green monkeys are associated with protection against AIDS. J. Clin. Invest. 2005;115:1082–1091. doi: 10.1172/JCI23006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oswald-Richter K, Grill SM, Shariat N, Leelawong M, Sundrud MS, Haas DW, Unutmaz D. HIV infection of naturally occurring and genetically reprogrammed human regulatory T-cells. PLoS Biol. 2004;2:E198. doi: 10.1371/journal.pbio.0020198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pereira LE, Villinger F, Onlamoon N, Bryan P, Cardona A, Pattanapanysat K, Mori K, Hagen S, Picker L, Ansari AA. Simian immunodeficiency virus (SIV) infection influences the level and function of regulatory T cells in SIV-infected rhesus macaques but not SIV-infected sooty mangabeys. J. Virol. 2007;81:4445–4456. doi: 10.1128/JVI.00026-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Egen JG, Kuhns MS, Allison JP. CTLA-4: new insights into its biological function and use in tumor immunotherapy. Nat. Immunol. 2002;3:611–618. doi: 10.1038/ni0702-611. [DOI] [PubMed] [Google Scholar]
- 48.Fallarino F, Grohmann U, Hwang KW, Orabona C, Vacca C, Bianchi R, Belladonna ML, Fioretti MC, Alegre ML, Puccetti P. Modulation of tryptophan catabolism by regulatory T cells. Nat. Immunol. 2003;4:1206–1212. doi: 10.1038/ni1003. [DOI] [PubMed] [Google Scholar]
- 49.Grohmann U, Orabona C, Fallarino F, Vacca C, Calcinaro F, Falorni A, Candeloro P, Belladonna ML, Bianchi R, Fioretti MC, Puccetti P. CTLA-4-Ig regulates tryptophan catabolism in vivo. Nat. Immunol. 2002;3:1097–1101. doi: 10.1038/ni846. [DOI] [PubMed] [Google Scholar]
- 50.Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat. Rev. Immunol. 2004;4:762–774. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- 51.Schrocksnadel K, Wirleitner B, Winkler C, Fuchs D. Monitoring tryptophan metabolism in chronic immune activation. Clin. Chim. Acta. 2006;364:82–90. doi: 10.1016/j.cca.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 52.Peggs KS, Quezada SA, Korman AJ, Allison JP. Principles and use of anti-CTLA4 antibody in human cancer immunotherapy. Curr. Opin. Immunol. 2006;18:206–213. doi: 10.1016/j.coi.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 53.Makita S, Kanai T, Nemoto Y, Totsuka T, Okamoto R, Tsuchiya K, Yamamoto M, Kiyono H, Watanabe M. Intestinal lamina propria retaining CD4+CD25+ regulatory T cells is a suppressive site of intestinal inflammation. J. Immunol. 2007;178:4937–4946. doi: 10.4049/jimmunol.178.8.4937. [DOI] [PubMed] [Google Scholar]
- 54.Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, Kazzaz Z, Bornstein E, Lambotte O, Altmann D, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat. Med. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 55.Kaufmann DE, Kavanagh DG, Pereyra F, Zaunders JJ, Mackey EW, Miura T, Palmer S, Brockman M, Rathod A, Piechocka-Trocha A, et al. Upregulation of CTLA-4 by HIV-specific CD4+ T cells correlates with disease progression and defines a reversible immune dysfunction. Nat. Immunol. 2007;8:1246–1254. doi: 10.1038/ni1515. [DOI] [PubMed] [Google Scholar]
- 56.Hel Z, Johnson JM, Tryniszewska E, Tsai WP, Harrod R, Fullen J, Tartaglia J, Franchini G. A novel chimeric Rev, Tat, and Nef (Retanef) antigen as a component of an SIV/HIV vaccine. Vaccine. 2002;20:3171–3186. doi: 10.1016/s0264-410x(02)00258-x. [DOI] [PubMed] [Google Scholar]