Abstract
Orbitofrontal alteration in schizophrenia has not been well characterized, likely due to marked anatomical variability. To investigate the presence of such alterations, we evaluated the sulcogyral pattern of this ‘H-shaped’ sulcus. Fifty patients with schizophrenia (100 hemispheres) and 50 age- and gender-matched control subjects (100 hemispheres) were evaluated using 3D high-spatial resolution MRI. Based on a previous study by Chiavaras and Petrides (2000), the sulcogyral pattern of the ‘H-shaped’ sulcus, which forms the boundaries of major orbitofrontal gyri, was classified into three types (Type I, II and III, in order of frequency) within each hemisphere. Chi-square analysis was performed to compare the sulcogyral pattern, and categorical regression was applied to investigate clinical/cognitive associations. The control data replicated the orbitofrontal sulcogyral pattern reported by Chiavaras and Petrides (P = 0.90–0.95), where the distribution was significantly different between the left and right hemisphere (Type I: right>left, Type II, III: left>right, χ2 = 6.41, P = 0.041). For schizophrenics, the distribution differed significantly from controls (χ2 = 11.90, P = 0.003), especially in the right hemisphere (χ2 = 13.67, P = 0.001). Moreover, the asymmetry observed in controls was not present in schizophrenia (χ2 = 0.13, P = 0.94). Specifically, the most frequent Type I expression was decreased and the rarest Type III expression was increased in schizophrenia, relative to controls. Furthermore, patients with Type III expression in any hemisphere evinced poorer socioeconomic status, poorer cognitive function, more severe symptoms and impulsivity, compared to patients without Type III expression. In contrast, patients with Type I in any hemisphere showed better cognitive function and milder symptoms compared to patients without Type I. Structurally, patients with Type III had significantly smaller intra-cranial contents (ICC) volumes than did patients without Type III (t40 = 2.29, P = 0.027). The present study provides evidence of altered distribution of orbitofrontal sulcogyral pattern in schizophrenia, possibly reflecting a neurodevelopmental aberration in schizophrenia. Such altered sulcogyral pattern is unlikely to be due to secondary effects of the illness such as medication. Moreover, the structural association between Type III and small ICC volume, observed in the patient group, may suggest that Type III expression could be part of a systematic neurodevelopmental alteration, given that the small ICC volume could reflect early reduction of cranial growth driven by brain growth. The observed contrasting association of Type III expression with poorer outcome, and that of Type I expression with better outcome, further suggests clinical heterogeneity, and possible differences in treatment responsiveness in schizophrenia.
Keywords: schizophrenia, sulcus, orbitofrontal cortex, magnetic resonance imaging, neurodevelopment
Introduction
Orbitofrontal cortex (OFC) is important for sensory–visceromotor multimodal integration (Ongur and Price, 2000), as well as for emotional processing and hedonic experience (Kringelbach, 2005). It is also likely important in the affective evaluation of reinforcers (rewards and punishers), expectation, motivation, decision-making and goal-directed behaviour (Gottfried et al., 2003; Holland and Gallagher, 2004; Walton et al., 2004). One notable feature of OFC is its enormous individual variability at both the level of cytoarchitecture (especially, granularity) (Ongur and Price, 2000) and gross anatomy (sulcogyral pattern) (Ono et al., 1990; Chiavaras and Petrides, 2000). In terms of social neuroscience, OFC figures importantly in emotions and social behaviour, and individual variability in OFC may be associated with individual differences in personality traits, emotional processing and behaviour.
Of note here, the social deficit consequences of large orbitofrontal pathological lesions have long been known (Harlow, 1848), although the association of more subtle anatomical anomalies of OFC with social behaviour have not been well characterized. Similarly, dating to the seminal work of Bleuler (1911/1950), the social disturbances of schizophrenia have been often elegantly described, but the extent to which they may reflect disease-related neuropathology of the OFC has yet to be established. In the current study, we predict that OFC will be abnormal in schizophrenia as these patients evince sensory integration and emotional processing disturbances, which may, in turn, be manifested in the observed hallucinations, especially for somatic hallucinations, blunted affect, anhedonia, apathy and social dysfunctions in this disorder.
However, previous MR findings from OFC volume studies have been inconsistent, with some reporting smaller OFC volume in schizophrenia compared with controls (Gur et al., 2000; Convit et al., 2001), and others reporting negative findings (Baare et al., 1999; Szeszko et al., 1999; Chemerinski et al., 2002; Rupp et al., 2005). The large individual variability in OFC also makes it difficult to define OFC precisely and consistently for both manual ROI and for voxel-based morphometry (VBM) studies. In fact, the OFC ROI definition has been inconsistent among previous volume studies (Lacerda et al., 2003), and this variability may be one of the major reasons for the inconsistent morphometry findings reported for OFC. Likewise, medication-induced effects may also be a potential confound and are critical to the interpretation of previous volumetric studies, as (typical) antipsychotics have been reported to be associated with grey matter volume reduction (Dorph-Petersen et al., 2005; Lieberman et al., 2005), and mood stabilizers such as lithium and valproate have been reported to increase grey matter volume, due to their neurotrophic effect (Manji et al., 2000).
Given that the sulcogyral pattern of the brain is formed during neurodevelopment (Armstrong et al., 1995) and is genetically determined to some extent (Bartley et al., 1997), the sulcogyral pattern might provide a morphological trait marker to explore morphological alteration, independent of brain tissue volumes, independent of normal or pathological longitudinal changes and independent of confounding factors such as medications and chronic illness. Neurobiologically, the developmental formation of the convolutional sulcogyral pattern, which is termed gyrogenesis, could reflect neuronal migration, local neuronal connection, synaptic development, lamination and formation of cytoarchitecture (Rakic, 1988; Armstrong et al., 1995).
Previously, our group reported temporal lobe sulcogyral pattern anomalies in schizophrenia using MR 3D surface rendering (Kikinis et al., 1994). A number of other studies have utilized the gyrification index (GI) (Zilles et al., 1988), the ratio of the inner and outer cortical surface contours, to estimate the degree of cortical folding. Using this index, Jou and coworkers as well as Kulynch and coworkers (Kulynych et al., 1997; Jou et al., 2005), reported decreased GI (less cortical folding) in the left hemisphere in patients diagnosed with schizophrenia, although Sallet and coworkers have reported decreased GI in both hemispheres (Sallet et al., 2003). However, GI in schizophrenia has also been reported to be increased (more cortical folding) in the right prefrontal region (Vogeley et al., 2000, 2001; Harris et al., 2004a) and in the right temporal lobe (Harris et al., 2004b). More recently, cortical surface morphology (geometry), including cortical thickness, surface area and length of sulcal/gyral curvature, have been evaluated (White et al., 2003). We note here that an essential limitation of methods based on cortical surface morphology, including cortical folding (GI), is that they are not independent of brain tissue volume, and are thus potentially unstable over time and susceptible to confounds affecting brain tissue volume.
Another approach to sulcal morphology is based on measuring the length of a specific sulcus. This method has been used to evaluate the Sylvian fissure (Falkai et al., 1992; DeLisi et al., 1994) and the paracingulate sulcus (Yucel et al., 2002; Le Provost et al., 2003). Interestingly, lack of normal asymmetry in sulcal length is a common feature observed in schizophrenic populations in these previous studies on sulcal length measurement. Taken together, all of these previous sulcogyral pattern studies which have applied different methodologies provide evidence for neurodevelopmental alterations in schizophrenia.
As far as we know, orbitofrontal sulcogyral pattern has not been investigated in schizophrenia. To investigate the presence of morphological alterations of OFC in schizophrenia, we focused on the sulcogyral pattern of the ‘H-shaped’ sulcus, which forms the boundary of four major orbitofrontal gyri including medial, anterior, posterior and lateral orbital gyri (Duvernoy, 1999; Chiavaras and Petrides, 2000). To explore the complexity in OFC anatomy in 50 healthy volunteers (100 hemispheres), Chiavaras and Petrides (2000) focused on continuity among medial, lateral and transverse orbital sulci of this ‘H-shaped’ sulcus, rather than the length of a single sulcus. In the present study, and based on Chiavaras and Petrides' anatomical work, we classified the OFC sulcogyral pattern into three major types (Type I, II and III in order of frequency), and we compared their distribution between schizophrenic patients and matched healthy control subjects. Of particular note, this OFC sulcogyral pattern classification is based on mutual continuity among neighbouring sulci, and thus is independent of brain tissue volume. As such, the OFC sulcogyral patterns may reflect a more reliable and valid neurobiological indicator of regional ‘gyrogenesis’ than cortical surface geometry.
Furthermore, we hypothesized that the difference in OFC sulcogyral pattern may reflect individual variability in cognitive function (such as abstract thinking, decision-making and perceptual organization), psychiatric symptomatology (such as hallucination, psychomotor excitement, disorganized symptom, anhedonia and social deficits) and personality traits (such as impulsivity or apathy).
In order to explore the significance of OFC sulcogyral pattern in terms of neurodevelopment, we focused also on intracranial contents (ICC) volume. After controlling for gender and body size, the magnitude of the adult ICC volume could reflect the early neurodevelopmental phase of cranial growth process, occurring up to 10–13 years of age (Woods et al., 2005). In schizophrenics, the ICC volume has been reported to be smaller compared to non-psychiatric controls (Ward et al., 1996), possibly reflecting early reduction of cranial growth driven by brain parenchymal growth. We hypothesized that a difference in the OFC sulcogyral pattern may be associated with magnitude of the ICC volume as sulcogyral pattern is likely determined during the early neurodevelopmental phase (Armstrong et al., 1995).
To our knowledge, this is the first study reporting the sulcogyral pattern alteration of this ‘H-shaped’ sulcus in any brain-related disorder.
Material and methods
Subjects
Fifty patients with schizophrenia and 50 healthy control subjects participated in this study. Table 1 shows demographic and clinical characteristics of these two groups. All patients were diagnosed with schizophrenia based on the Diagnostic and Statistical Manual of Mental Disorders 4th Edition (DSM-IV) criteria, using information from the Structured Clinical Interview for DSM-III-R (Spitzer et al., 1990b) by trained PhD or MD interviewers. Patients were recruited from the VA Boston Healthcare System, Brockton Division. All patients were receiving antipsychotic medication, with a mean daily dose equivalent to 432.0 ± 185.6 mg of chlorpromazine (Woods, 2003) [typical antipsychotics (8 of the 39 patients, 20.5%), atypical antipsychotics (26/39, 66.7%), or both (5/39, 12.8%)]. The mean age of patients was 40.6 ±10.4 years, their mean age at symptom onset was 21.3 ± 4.6 years and their mean duration of illness was 19.5 ± 11.2 years. Control subjects were recruited through newspaper advertisement and screened using the Structured Clinical Interview (SCID non-patient edition)(Spitzer et al., 1990a) by the same trained interviewers. No control subjects had an Axis-I psychiatric disorder or a first-degree relative with Axis-I psychiatric disorder.
Table 1.
Demographic and clinical characteristics of study groups.
| Variable | Mean (SD) [range] | dfa | t test or χ2 values | p value | |
|---|---|---|---|---|---|
| Schizophrenic patients (n = 50) | Healthy control subjects (n = 50) | ||||
| Age (years) | 40.6 (10.4) [18–57] | 40.8 (9.4) [19–56] | 1, 98 | 0.10 | 0.92 |
| Gender | |||||
| Male/female | 45/5 | 45/5 | 1 | 0.00 | 1.00 |
| Handednessb | 0.78 (0.20) [0.1–1.0] | 0.80 (0.17) [0.4–1.0] | 1, 94 | 0.50 | 0.62 |
| Socioeconomic statusc | |||||
| Subject's own | 3.9 (1.0) | 2.4 (1.1) | 1, 94 | 6.64 | <0.0001** |
| Parental | 3.0 (1.2) | 2.7 (1.2) | 1, 94 | 1.30 | 0.20 |
| Education (school years) | 13.0 (1.8) | 14.9 (2.2) | 1, 95 | 4.92 | <0.0001** |
| MMSE | 28.6 (1.5) | 29.4 (0.8) | 1, 94 | 3.43 | 0.001** |
| WAIS-III Verbal IQ | 93.9 (13.7) | 107.6 (14.8) | 1, 71 | 4.10 | 0.0001** |
| WAIS-III Peformance IQ | 86.2 (11.5) | 106.5 (17.4) | 1, 70 | 5.89 | <0.0001** |
| Symptom onset (years) | 21.3 (4.6), n = 43 | NA | |||
| Duration of illness (years) | 19.5 (11.2), n = 42 | NA | |||
| Antipsychotic medication dosaged | 432.0 (185.6), n = 40 | NA | |||
| PANSS (total score) | 76.8 (23.7), n = 43 | NA | |||
P<0.05,
P<0.01.
MMSE = Mini-Mental State Examination (Folstein et al., 1975); WAIS-III = Wechsler Adult Intelligence Scale—3rd Edition (Wechsler, 1997); IQ = intelligence quotient; PANSS = Positive and Negative Syndrome Scale (Kay et al., 1987); NA = data not applicable.
The degrees of freedom (df) differ among variables owing to missing data for some participants.
Handedness was evaluated using the Edinburgh inventory and right-handedness is above 0.
Higher scores indicate lower socioeconomic status (Hollingshead, 1965).
Chlorpromazine equivalent (mg).
Handedness was assessed using the Edinburgh inventory (Oldfield, 1971). Subjects' own and parental SES were measured by the Hollingshead two-factor index (1 = best, 5 = poorest) (Hollingshead, 1965), which consists of educational and occupational score. As part of a comprehensive neuropsychological battery, subjects from both groups were evaluated using the Wechsler Adult Intelligence Scale (WAIS-III) (Wechsler, 1997) and the WCST (Heaton, 1981), a measure requiring concept formation, abstraction and mental flexibility. Subjects were group matched for age at MRI scan (P = 0.92), gender (P = 1.0), parental SES (P = 0.20), and handedness (P = 0.62) (all right-handed). Patients had poorer SES (P<0.0001) and less education (P<0.0001) and poorer cognitive function than controls, reflecting the debilitating effects of psychosis. The Positive and Negative Syndrome Scale (PANSS) (Kay et al., 1987) was administered to patients in order to evaluate clinical symptoms. To investigate personality traits, the Multidimensional Personality Questionnaire (MPQ) (Tellegen, 1982) was used for both groups. We note that data for subjects recruited prior to the initiation of MPQ are not available. Specifically, almost half of the subjects (23 patients and 28 controls) were recruited prior to use of the MPQ. Moreover, some subjects elected not to participate in some of the measures. Thus as reflected in degrees of freedom indicated in Table 4, the subject sample varied for some of the cognitive and clinical assessments. Using a categorical regression model, we showed that patients' decision to participate in cognitive (F3,46 = 2.21, P = 0.100) or symptom (F3,46 = 1.76, P = 0.168) assessments was not associated with the sulcogyral pattern.
Table 4.
Categorical regression analyses
| Clinical/cognitive measures (dependent variables) | Schizophrenia group | Healthy control group | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ANOVA | Independent variables | β | F | P | ANOVA | Independent variables | β | F | P | |
| Socioeconomic status | ||||||||||
| Subject's own SES | F(3,43)=4.31, P=0.010 | Type I | −0.067 | 0.196 | 0.660 | F(3,45)=1.89, P=0.145 | Type I | 0.254 | 2.098 | 0.154 |
| Type II | −0.074 | 0.174 | 0.678 | Type II | −0.431 | 5.666 | 0.022 | |||
| Type III | 0.494 | 8.112 | 0.007** | Type III | 0.157 | 1.067 | 0.307 | |||
| Parental SES | F(3,43)=1.24, P=0.307 | Type I | −0.048 | 0.084 | 0.773 | F(3,46)=0.26, P=0.854 | Type I | −0.041 | 0.051 | 0.822 |
| Type II | 0.092 | 0.228 | 0.636 | Type II | 0.102 | 0.297 | 0.588 | |||
| Type III | 0.302 | 2.524 | 0.119 | Type III | −0.053 | 0.109 | 0.742 | |||
| Cognition | ||||||||||
| Full-scale IQ (WAIS III) | F(3,41)=2.96, P=0.044 | Type I | 0.201 | 1.515 | 0.225 | F(3,41)=4.01, P=0.014 | Type I | 0.525 | 9.631 | 0.003** |
| Type II | 0.128 | 0.472 | 0.496 | Type II | 0.275 | 2.435 | 0.126 | |||
| Type III | −0.279 | 2.351 | 0.133 | Type III | 0.309 | 4.358 | 0.043* | |||
| Verbal comprehension index (WAIS III) | F(3,34)=5.15, P=0.005 | Type I | 0.311 | 3.224 | 0.081 | F(3,31)=2.20 P=0.108 | Type I | 0.174 | 0.809 | 0.375 |
| Type II | 0.230 | 1.514 | 0.227 | Type II | −0.087 | 0.177 | 0.676 | |||
| Type III | −0.360 | 4.169 | 0.049* | Type III | 0.302 | 2.907 | 0.098 | |||
| Perceptual organization index (WAIS III) | F(3,34)=2.97, P=0.046 | Type I | 0.442 | 5.667 | 0.023* | F(3,30)=3.73, P=0.022 | Type I | 0.546 | 9.086 | 0.005** |
| Type II | 0.269 | 1.791 | 0.190 | Type II | 0.483 | 5.923 | 0.021* | |||
| Type III | −0.066 | 0.121 | 0.731 | Type III | 0.229 | 1.697 | 0.203 | |||
| Working memory index (WAIS III) | F(3,34)=2.03, P=0.129 | Type I | −0.049 | 0.065 | 0.801 | F(3,30)=3.01, P=0.046 | Type I | −0.246 | 1.697 | 0.203 |
| Type II | 0.253 | 1.479 | 0.232 | Type II | 0.441 | 4.648 | 0.039* | |||
| Type III | 0.212 | 1.171 | 0.287 | Type III | 0.479 | 7.455 | 0.010* | |||
| Processing speed index (WAIS III) | F(3,34)=1.71, P=0.184 | Type I | 0.296 | 2.316 | 0.137 | F(3,30)=1.55, P=0.222 | Type I | 0.068 | 0.115 | 0.737 |
| Type II | 0.462 | 4.824 | 0.035 | Type II | 0.045 | 0.043 | 0.837 | |||
| Type III | 0.312 | 2.475 | 0.125 | Type III | 0.328 | 3.109 | 0.088 | |||
| Category completed (WCST) | F(3,29)=1.05, P=0.384 | Type I | 0.116 | 0.336 | 0.567 | F(3,37)=2.10, P=0.117 | Type I | −0.026 | 0.019 | 0.890 |
| Type II | −0.303 | 1.479 | 0.234 | Type II | 0.321 | 2.796 | 0.103 | |||
| Type III | −0.262 | 1.032 | 0.318 | Type III | −0.115 | 0.497 | 0.485 | |||
| Total number incorrect (WCST) | F(3,37)=1.28, P=0.295 | Type I | −0.180 | 1.038 | 0.315 | F(3,38)=4.73, P=0.007 | Type I | 0.263 | 2.411 | 0.129 |
| Type II | 0.223 | 1.050 | 0.312 | Type II | −0.626 | 12.848 | 0.001** | |||
| Type III | −0.038 | 0.031 | 0.861 | Type III | 0.044 | 0.087 | 0.770 | |||
| Perseverative responses (WCST) | F(3,37)=2.21, P=0.103 | Type I | −0.417 | 5.930 | 0.020 | F(3,38)=4.26, P=0.011 | Type I | 0.008 | 0.002 | 0.963 |
| Type II | −0.236 | 1.250 | 0.271 | Type II | −0.286 | 2.621 | 0.114 | |||
| Type III | −0.346 | 2.727 | 0.107 | Type III | 0.349 | 5.391 | 0.026* | |||
| Clinical symptom (PANSS) | ||||||||||
| Total score | F(3,39)=2.59, P=0.067 | Type I | −0.057 | 0.115 | 0.737 | |||||
| Type II | 0.185 | 0.889 | 0.352 | |||||||
| Type III | 0.448 | 5.536 | 0.024 | |||||||
| Negative factor | F(3,37)=2.39, P=0.085 | Type I | −0.302 | 3.069 | 0.088 | |||||
| Type II | 0.166 | 0.617 | 0.437 | |||||||
| Type III | 0.188 | 0.778 | 0.383 | |||||||
| Positive factor | F(3,41)=6.24, P=0.001 | Type I | −0.297 | 4.279 | 0.045* | |||||
| Type II | −0.423 | 5.733 | 0.021* | |||||||
| Type III | 0.392 | 4.918 | 0.032* | |||||||
| Disorganized factor | F(3,41)=5.19, P=0.004 | Type I | −0.017 | 0.013 | 0.908 | |||||
| Type II | 0.551 | 9.028 | 0.005** | |||||||
| Type III | 0.624 | 11.509 | 0.002** | |||||||
| Excited factor | F(3,42)=1.84, P=0.156 | Type I | 0.167 | 1.021 | 0.318 | |||||
| Type II | −0.038 | 0.038 | 0.847 | |||||||
| Type III | 0.306 | 2.466 | 0.124 | |||||||
| Anxiety–depression factor | F(3,41)=1.24, P=.0308 | Type I | −0.283 | 2.734 | 0.106 | |||||
| Type II | 0.003 | 0.000 | 0.988 | |||||||
| Type III | −0.163 | 0.647 | 0.426 | |||||||
| Withdrawal factor | F(3,38)=2.85, P=0.050 | Type I | −0.032 | 0.036 | 0.851 | |||||
| Type II | 0.380 | 3.659 | 0.063 | |||||||
| Type III | 0.528 | 6.963 | 0.012* | |||||||
| Personality (MPQ) | ||||||||||
| Positive emotionality | F(3,23)=0.98, P=0.419 | Type I | 0.254 | 1.019 | 0.323 | F(3,18)=4.22, P=0.020 | Type I | 0.423 | 3.344 | 0.084 |
| Type II | −0.148 | 0.362 | 0.553 | Type II | 0.834 | 11.589 | 0.003** | |||
| Type III | 0.093 | 0.140 | 0.712 | Type III | 0.411 | 4.309 | 0.053 | |||
| Negative emotionality | F(3,23)=2.93, P=0.055 | Type I | −0.024 | 0.011 | 0.917 | F(3,18)=1.28, P=0.312 | Type I | −0.364 | 1.768 | 0.200 |
| Type II | 0.365 | 2.690 | 0.115 | Type II | −0.556 | 3.674 | 0.071 | |||
| Type III | 0.530 | 5.617 | 0.027 | Type III | 0.208 | 0.784 | 0.388 | |||
| Constraint | F(3,23)=5.90, P=0.004 | Type I | 0.021 | 0.011 | 0.916 | F(3,18)=2.64, P=0.081 | Type I | 0.218 | 0.752 | 0.397 |
| Type II | −0.309 | 2.455 | 0.131 | Type II | −0.023 | 0.007 | 0.932 | |||
| Type III | −0.675 | 11.647 | 0.002** | Type III | 0.571 | 7.032 | 0.016 | |||
P<0.05,
P<0.01.
ANOVA=analysis of variance; SES=socioeconomic status; IQ=intelligence quotient; WAIS-III=Wechsler Adult Intelligence Scale—3rd Edition (Wechsler, 1997);WCST=Wisconsin Card SortingTest; PANSS=Positive and Negative Syndrome Scale (Kay et al., 1987); MPQ=Multidimensional Personality Questionnaire (Tellegen, 1982)
This study was approved by the VA Boston Healthcare System, partners, and Harvard Medical School Institutional Review Boards. Written informed consent was obtained from all subjects prior to study participation.
MRI processing
MR images were acquired with a 1.5-tesla General Electric scanner (GE Medical Systems, Milwaukee) at the Brigham and Women's Hospital in Boston. The sequence resulted in contiguous SPGR images (repetition time = 35 ms, echo time = 5 ms, one repetition, 45 degree nutation angle, 24-cm field of view, number of excitations = 1.0, matrix = 256 × 256 [192 phase-encoding steps] × 124). Voxels were 0.9375 × 0.9375 × 1.5 mm. Data were formatted in the coronal plane and analysed as 124 coronal 1.5-mm-thick slices. An anisotropic diffusion filter was applied to reduce noise prior to processing. For consistent identification of the sulcogyral pattern, images were realigned using the line between the anterior and posterior commissures and the midsagittal plane to correct any head tilt, and resampled into isotropic voxels (0.9375 mm3). The ICC volume was derived from the EM atlas segmentation (Bouix et al., 2004; Pohl et al., 2004), and included all grey matter, white matter and CSF volumes above the most inferior axial slice containing cerebellum.
Sulcogyral pattern identification
We based our sulcogyral pattern identification on previous work by Chiavaras and Petrides (2000). These investigators classified the OFC sulcogyral pattern into three types (Type I, II, III) in each hemisphere. This visual classification was based on the continuity of the medial and lateral orbital sulci (MOS, LOS, respectively) (Figs 1 and 2). In Type I, rostral and caudal portions of the LOS were connected, while the MOS were clearly interrupted between rostral and caudal portions of MOS. In Type II, rostral and caudal portions of both the MOS and LOS were connected and continuous MOS and LOS were jointed by the horizontally oriented transverse orbital sulcus (TOS). In Type III, rostral and caudal portions of both MOS and LOS were interrupted. Mutual sulcal connectivity was determined by evaluating several axial slices superior to the most inferior slice where TOS could be observed clearly. To evaluate the sulcogyral pattern precisely and consistently, neighboring sulci including the olfactory sulcus (Olf), intermediate orbital sulcus (IOS), posterior orbital sulcus (POS) and sulcus fragmentosus (Fr) were also identified as landmarks. Of note, Chiavaras and Petrides (2000) reported that IOS was identified in all of 100 observed hemispheres where 19% showed double IOS (medial and lateral IOS). POS was observed in 77%, and Fr was observed in only 10% of the 100 hemispheres.
Fig. 1.
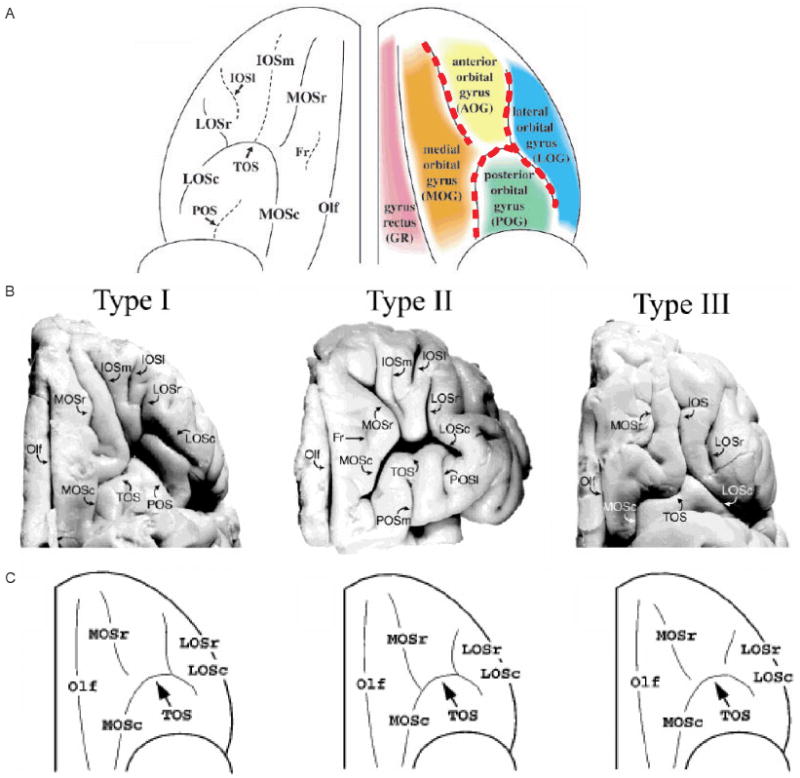
‘H-shaped’ sulcus and its variation in human brain. (A) Schema of orbitofrontal sulci and major gyri.‘H-shaped’ sulcus is traced by red dotted line, dividing orbitofrontal cortex into four gyri of medial, anterior, posterior and lateral orbital gyri. (B) Example of three sulcal pattern. Three main orbitofrontal sulcogyral types are defined based on the continuity of the medial and lateral orbital sulci. Type I expresses most frequently and Type III expresses least frequently in healthy population. (C) Schema of major three types of sulcal patterns of ‘H-shaped’ sulcus. Olf, olfactory sulcus; MOS, medial orbital sulcus (-r: rostral, -c: caudal); TOS, transverse orbital sulcus; LOS, lateral orbital sulcus (-r: rostral, -c: caudal); IOS, intermediate orbital sulcus (-m: medial, -l: lateral); POS, posterior orbital sulcus; Fr, sulcus fragmentosus. Panels A, B, C were adapted and modified from a previous paper by Chiavaras and Petrides (see M. M. Chiavaras and M. Petrides. Orbitofrontal sulci of the human and macaque monkey brain. J Comp Neurol 2000; 422: 35–54; reprinted with permission of Wiley-Liss, Inc., a subsidiary of John Wiley & Sons, Inc.).
Fig. 2.
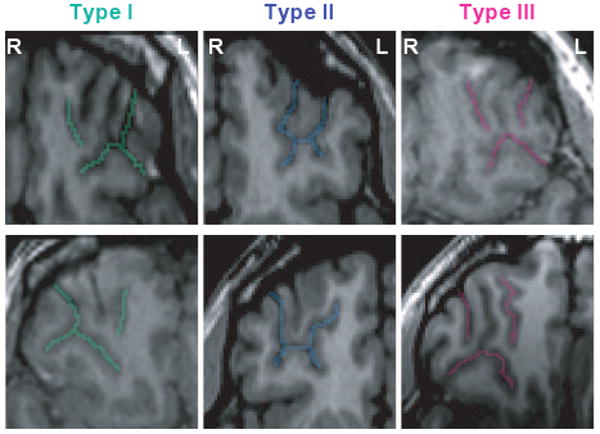
MRI images of major three types of ‘H-shaped’ sulcus. Examples of the major three sulcogyral patterns from six different subjects. On the axial plane of SPGR (spoiled gradient-recalled images), sulci of Type I, II, III are delineated with green, blue and pink colour, respectively. Upper and lower columns demonstrate left and right hemisphere. At this level, olfactory sulcus cannot be observed in most cases. Sulcal continuities of the medial and lateral orbital sulci were determined by evaluating several consecutive axial slices rather than just a single slice. L, left; R, right.
We used a medical image analysis software package [3D slicer, http://www.slicer.org] to provide reliable classification of the OFC sulcogyral pattern and ICC volume measurement.
The sulcogyral pattern classification in each hemisphere of the 100 subjects was done by M.N., blinded to subject group. For assessing interrater reliability, two raters (M.N., T.K.), blinded to diagnoses, independently evaluated the sulcal pattern for 25 random cases. The intraclass correlation coefficients (Cronbach's α) were 0.842 for left hemisphere and 0.836 for right hemisphere.
Statistical analysis
Independent-samples t-tests were performed to assess group differences in demographical data including age, subjects' own SES, parental SES and handedness. A χ2 test was applied to assess group differences in gender frequencies.
To evaluate group difference in sulcogyral pattern distribution, a χ2 test was applied to each hemisphere (n = 50 cases), and also to total number of sulcogyral pattern (n = 100 hemispheres) when collapsed over hemisphere. The sulcogyral pattern distribution observed in healthy controls was entered as the expected number for each sulcogyral type (i.e. Type I, II, III). To specify which type is altered in schizophrenia compared with controls, a χ2 test was also applied to each sulcal type. To evaluate left-right asymmetry in sulcal pattern distribution, a χ2 test was applied within each group (n = 50), entering sulcogyral pattern distribution in one hemisphere as an expected number for the other hemisphere, with the null hypothesis being that sulcogyral pattern is equal distributed in both hemispheres, based on the original paper (Chiavaras and Petrides, 2000) showing asymmetric distribution.
In order to examine the extent to which sulcogyral pattern (a nominal variable) predicted functional outcome in relation to social, cognitive and symptoms in patients with schizophrenia, categorical regression analyses were applied rather than multiple regression analyses. Subjects were classified according to sulcogyral type (e.g., subjects with Type I versus subjects without Type I sulcogyral pattern), and these three nominal variables (Type I, II, III) were entered as independent variables in a single model of categorical regression with each of clinical/cognitive measures entered as a dependent variable within each study group. We note here that contributions of all three sulcogyral patterns to variance in each dependent variable (ordinal or interval variable) were tested in a single model of categorical regression, rather than multiple univariate comparisons, in order to reduce the risk of false positives. When a covariate was needed for an additional analysis, ordinal regression was performed instead of categorical regression by applying a covariate.
For cognitive associations, WAIS-III (full-scale IQ), verbal comprehension index, perceptual organization index, working memory index, processing speed index) and WCST (number of category completed, total number incorrect, perseverative responses) were used as dependent variables. We chose a relatively wide-ranging cognitive assessment, as we intended to use these measures to quantify the relationship of OFC sulcogyral patterns with various aspects of cognitive domains, which have been linked to the integrity of the prefrontal region. In particular, multimodal sensory integration, which may be an important contributor to perceptual organization in WAIS-III, has been associated with orbitofrontal region (Ongur and Price, 2000), as has perseveration evaluated using the WCST (Freedman et al., 1998). For clinical associations, not only PANSS total score, but also six PANSS factors of ‘negative’, ‘positive’, ‘disorganized’, ‘excited’, ‘anxiety–depression’ and ‘withdrawn’ were used as dependent variables (Van den Oord et al., 2006). To investigate the association between OFC sulcogyral pattern and personality trait, three kinds of broad personality traits of the MPQ (Tellegen, 1982) were used as dependent variables: ‘Positive Emotionality’ (Wellbeing, Social Potency and Achievement), ‘Negative Emotionality’ (Stress Reaction, Alienation and Aggression) and ‘Constraint’ (Control, Harm Avoidance and Traditionalism).
To control for gender in correlation analysis between ICC volume and OFC sulcogyral pattern, ICC volume from only male subjects was used for analysis because there were only five female subjects out of 50 subjects in each group. In addition, sometimes a subject had two different sulcogyral patterns in the two hemispheres, and thus we subdivided subjects into with a sulcogyral type and without the type (e.g., subjects with Type I versus subjects without Type I), and compared applying independent sample t-tests. We note that body size information, such as body height and body weight, was not controlled due to lack of data.
Results
Sulcogyral pattern distribution
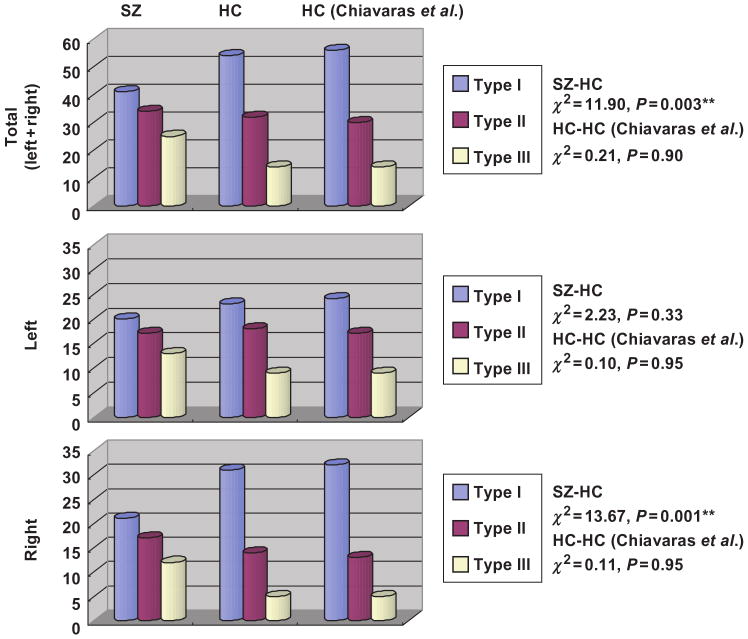
Tables 2 and 3, and Figures 3 and 4 show the OFC sulcogyral pattern distribution observed in each group. In Table 2 and Figure 3, it should be noted that the observed sulcal pattern distribution in the 50 healthy control subjects was almost identical (P = 0.90–0.95) to that reported in healthy population by Chiavaras and Petrides (2000), despite the fact that the current sample of healthy controls is demographically different from the previous study (28 males with mean age of 25.4 ± 5.3, 22 females with mean age of 24.8 ± 5.3). Of particular interest, within the healthy control group, the Type I sulcogyral pattern was more frequently expressed in the right hemisphere, while Type II and III sulcogyral patterns were more frequently expressed in the left hemisphere (χ2 = 6.41, P = 0.041).
Table 2.
Sulcal pattern distribution of the ‘H-shaped’ sulcus in orbitofrontal cortex
| SZ | HC | HC (Chiavaras et al.) | ||||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| Left | ||||||
| Sulcal Type | ||||||
| I | 20 | 40 | 23 | 46 | 24 | 48 |
| II | 17 | 34 | 18 | 36 | 17 | 34 |
| III | 13 | 26 | 9 | 18 | 9 | 18 |
| Total | 50 | 100 | 50 | 100 | 50 | 100 |
| Right | ||||||
| Sulcal Type | ||||||
| I | 21 | 42 | 31 | 62 | 32 | 64 |
| II | 17 | 34 | 14 | 28 | 13 | 26 |
| III | 12 | 24 | 5 | 10 | 5 | 10 |
| Total | 50 | 100 | 50 | 100 | 50 | 100 |
| Total | ||||||
| (Left+Right) | ||||||
| Sulcal type | ||||||
| I | 41 | 41 | 54 | 54 | 56 | 56 |
| II | 34 | 34 | 32 | 32 | 30 | 30 |
| III | 25 | 25 | 14 | 14 | 14 | 14 |
| Total | 100 | 100 | 100 | 100 | 100 | 100 |
SZ = schizophrenia; HC = healthy control. Right-sided column shows results from the previous anatomical study performed by Chiavaras and Petrides (2000).
Table 3.
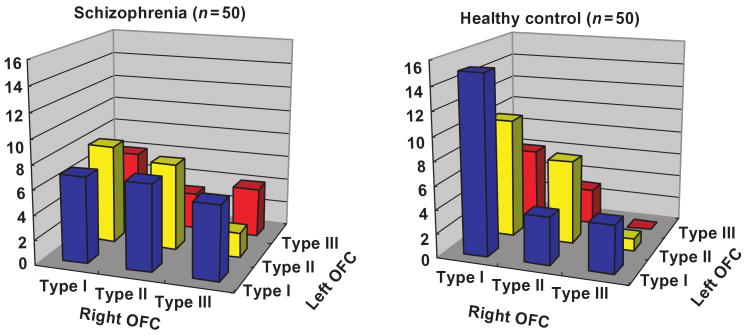
Sulcal pattern distribution (left–right combination)
| Sulcal type | Right | Total | ||||
|---|---|---|---|---|---|---|
| I | II | III | ||||
| SZ | LEFT | I | 7 | 7 | 6 | 20 |
| II | 8 | 7 | 2 | 17 | ||
| III | 6 | 3 | 4 | 13 | ||
| Total | 21 | 17 | 12 | 50 | ||
| Sulcal type | Right | Total | ||||
| I | II | III | ||||
| HC | LEFT | I | 15 | 4 | 4 | 23 |
| II | 10 | 7 | 1 | 18 | ||
| III | 6 | 3 | 0 | 9 | ||
| Total | 31 | 14 | 5 | 50 | ||
SZ = schizophrenia; HC = healthy control.
Fig. 3.
Sulcal pattern distribution of the ‘H-shaped’ sulcus in orbitofrontal cortex. SZ, schizophrenia; HC, healthy control. Right-sided column shows results from the previous anatomical study performed by Chiavaras and Petrides (2000).
Fig. 4.
Sulcal pattern distribution (left and right combination).
In contrast, the schizophrenia group exhibited a quite different distribution of OFC sulcogyral pattern. The most infrequent pattern of Type III was expressed in the schizophrenia group with almost a two-fold increase over the healthy control group (14% versus 25%). A χ2 analysis revealed that the sulcogyral pattern distribution in the schizophrenia group was significantly different from that of the healthy control group, in the right hemisphere (χ2 = 13.67, P = 0.001), and bilateral (left+right) hemispheres (χ2 = 11.90, P = 0.003), but not significant in the left hemisphere alone (χ2 = 2.23, P = 0.33). Within the right hemisphere, Types I and III showed group differences (Type I: χ2 = 8.49, P = 0.004, Type III: χ2 = 10.89, P = 0.001), but there was no significance for Type II (χ2 = 0.89, P = 0.35), indicating that expression was decreased for Type I and increased for Type III in the schizophrenia group. Within the left hemisphere, there were no group differences (Type I: χ2 = 0.73, P = 0.40, Type II: χ2 = 0.09, P = 0.77, Type III: χ2 = 2.17, P = 0.14). When hemisphere was collapsed, Type I and III showed group differences (Type I: χ2 = 6.80, P = 0.009, Type III: χ2 = 10.05, P = 0.002), but there was no significance for Type II (χ2 = 0.18, P = 0.67), indicating the same tendency of decreased Type I expression and increased Type III expression for the patient group.
Moreover, the asymmetrical distribution observed in the healthy control group (χ2 = 6.41, P = 0.041) was not present in the schizophrenia group (χ2 = 0.13, P = 0.94). Table 3 and Figure 4 show the left–right combination of the three sulcal patterns within each group. Note that in the healthy control group (left/right) combinations of [Type I/Type I] and [Type II/Type I] were frequently observed in 25 out of 50 control subjects (50%), while these two common combinations were observed in only 15 patients with schizophrenia (30%). In contrast, the schizophrenia group exhibited Type III-related combinations more frequently than did the healthy controls. Especially, combinations of [Type III/Type III] observed in four schizophrenic patients was never observed in any of the 50 healthy control subjects.
In terms of odds ratio, subjects with Type III sulcogyral pattern in the right hemisphere showed a 2.84-fold risk for schizophrenia, compared to subjects without a Type III sulcogyral pattern in the right hemisphere, and subjects with Type I sulcogyral pattern in the right hemisphere showed a 0.44-fold morbid risk, compared to subjects without Type I sulcogyral pattern in the right hemisphere. Also, subjects with Type III sulcogyral pattern in any hemisphere showed a 2.05-fold morbid risk, compared to subjects without Type III sulcogyral pattern, and subjects with Type I sulcogyral pattern in any hemisphere showed a 0.59-fold morbid risk, compared to subjects without Type I sulcogyral pattern.
Categorical regression analysis of OFC sulcogyral pattern
Demographic data (Table 4)
The OFC sulcogyral pattern was not associated with subjects' age, gender, handedness, length of illness or chlorpromazine-equivalent antipsychotic dosage.
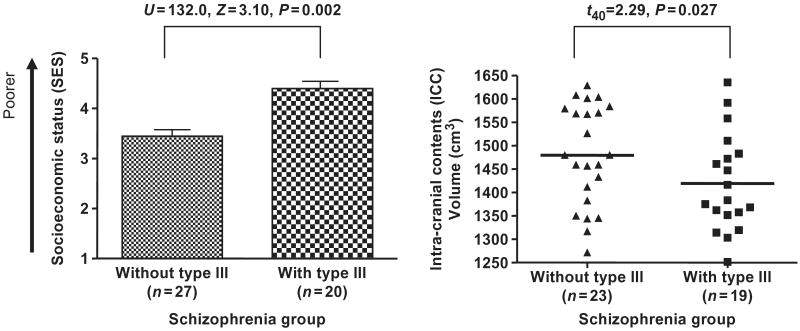
Within the schizophrenia group, Type III sulcogyral pattern in any hemisphere was associated with subjects' own SES (β = 0.49, F = 8.11, P = 0.007), while parental SES was not associated with any sulcogyral type. A Mann–Whitney U test revealed that SES was higher (poorer) in patients with Type III sulcogyral pattern than for patients without Type III sulcogyral pattern (U = 132.0, Z = 3.10, P = 0.002, Fig. 5). Additionally, ordinal regression analysis with parental SES as a covariate revealed that the positive association between Type III sulcogyral pattern and subjects' own SES was still significant (Wald = 8.14, P = 0.004), suggesting that the association was independent of parental SES. Similarly, full-scale IQ (WAIS-III) and total PANSS score were entered as covariates, and the same association with subjects' own SES was observed (Wald = 7.49, P = 0.006), suggesting that the association with social disability was also independent of cognition and clinical symptom severity.
Fig. 5.
Functional and structural association with theType III expression in the patient group. The higher SES indicates poorer socioeconomic status. The volume of the intracranial contents (ICC) was computed from total grey matter, white matter and CSF volumes, i.e. above the most inferior axial slice containing cerebellum.
Cognitive measures (Table 4)
Within the schizophrenia group, Type I sulcogyral pattern (in any hemisphere) was associated with higher scores for the WAIS-III perceptual organization index (β = 0.44, F = 5.67, P = 0.023), and Type III sulcogyral pattern (in any hemisphere) was associated with lower scores in WAIS-III verbal comprehension (β = −0.36, F = 4.17, P = 0.049). Within the healthy control group, Type I sulcogyral pattern was associated with higher WAIS-III full scale IQ score (β = 0.53, F = 9.63, P = 0.003) as well as higher scores for the WAIS-III perceptual organization index (β = 0.55, F = 9.09, P = 0.005). For controls, Type II sulcogyral pattern was associated with higher levels of perceptual organization (β = 0.48, F = 5.92, P = 0.021) and working memory (β = 0.44, F = 4.65, P = 0.039). Of note, Type III sulcogyral pattern in controls was associated with frequent perseverative responses in WCST (β = 0.35, F = 5.39, P = 0.026), although it was also associated with higher scores for both IQ (β = 0.31, F = 4.36, P = 0.043) and for the WAIS-III working memory index (β = 0.48, F = 7.46, P = 0.010).
In order to investigate a specific cognitive association commonly observed across the two study groups with different ranges of IQ, the groups were collapsed covarying total IQ, and then the ordinal regression analysis was applied to WAIS III indices and WCST. Only working memory index in WAIS III showed significant findings in that the Type I sulcogyral pattern in both groups was associated with better performance in working memory compared to subjects without Type I (Wald = 5.50, P = 0.019).
Clinical measures (Table 4)
Within the schizophrenia group, Type III sulcogyral pattern corresponded with increased severity of three PANSS factors: positive factor (β = 0.39, F = 4.92, P = 0.032), disorganized factor (β = 0.62, F = 11.51, P = 0.002), and withdrawn factor (β = 0.53, F = 6.96, P = 0.012). In contrast, Type I corresponded with reduced symptoms ratings for the PANSS positive factor (β = −0.30, F = 4.28, P = 0.045). Type II sulcogyral pattern also corresponded with reduced symptom ratings for the PANSS positive factor (β = −0.42, F = 5.73, P = 0.021), but with increased symptom ratings for the PANSS disorganized factor (β = 0.55, F = 9.03, P = 0.005).
Personality trait (Table 4)
Within the schizophrenia group, Type III expression was negatively associated with the ‘Constraint’ trait (β = −0.68, F = 11.65, P = 0.002), which reflects tendencies to inhibit impulsivity, unconventional behaviour, and risk-taking, at the high end. Although ANOVA for hypothesis testing of model fitting was nearly significant (P = 0.055), Type III expression was positively associated with ‘Negative Emotionality’ trait (β = 0.53, F = 5.62, P = 0.027), which represents tendencies to experience anxiety, aggression and related states of negative engagement.
Within the healthy control group, Type II expression was positively associated with ‘Positive Emotionality’ trait (β=0.83, F=11.59, P=0.003), which represents behavioural and temperamental tendencies to joy, excitement, vigour and generally to states of positive engagement. In contrast to Type III expression in the schizophrenia group, Type III expression in controls was positively associated with ‘Constraint’ tendency (β = 0.57, F=7.03, P = 0.016), although ANOVA for hypothesis testing only showed a trend-level significance (P = 0.081).
Independence of associations
As the patient group showed associations of the OFC sulcogyral pattern with a broad range of functional outcome, we subsequently examined the specificity or independence of the significant associations observed in the initial categorical regression analyses, by applying an ordinal regression model to the significant findings in the initial categorical regression analyses within the patient group. After controlling for all other clinical/cognitive measures showing significant association with the OFC sulcogyral pattern, Type III associations with subjects' own SES (Wald = 7.67, P = 0.006) and ‘withdrawal’ PANSS factor (Wald = 3.91, P = 0.048) were still significant while other associations lost significance. Additionally, although the available data were limited for the MPQ measurement, a negative association between Type III and ‘Constraint’ in MPQ, which reflects Type III–impulsivity association, remained significant (Wald = 4.38, P = 0.036) when controlling for all of the other measures showing significant associations. These additional analyses suggest that Type III–poor social functioning associations are more specific and independent than the other significant associations shown in Table 4.
OFC sulcogyral pattern and intracranial contents volume
ICC volume was significantly smaller in male patients with schizophrenia compared to male controls (SZ: 1460.7 ± 111.8 cm3, HC: 1509.1 ± 103.7 cm, t84 = 2.08, P = 0.040). Since the two study groups had different ranges of ICC volumes, analysis was performed within each group separately. Within the control group, there was no significant difference in ICC volumes between subjects with and without a specific OFC sulcogyral type. However, within schizophrenia group, patients with Type III had significantly smaller ICC volumes than patients without Type III did (t40 = 2.29, P= 0.027, Fig. 5).
Discussion
The present study compared the distribution of OFC sulcogyral patterns in patients with schizophrenia and age-matched control subjects. Similar to a previous study of healthy volunteers, findings from the present study demonstrated substantial stability of the OFC sulcogyral pattern distribution in the current sample of control subjects. That is, controls manifested almost the identical orbitofrontal sulcogyral pattern reported by Chiavaras and Petrides (P = 0.90–0.95), where the distribution was significantly different between the left and right hemisphere (Type I: right>left, Type II, III: left>right, χ2 = 6.41, P = 0.041). This high concordance between two different healthy samples, in their age ranges (mean age: 25 versus 40 years old), suggests the longitudinal stability of the OFC sulcogyral pattern distribution following neurodevelopment.
In contrast, the patient group showed a significantly different distribution of sulcogyral patterns from that of the age-matched control group. First, the patient group did not show the expected asymmetry in the left and right hemispheres that was observed in the healthy control group. That is, whereas healthy controls showed greater right than left asymmetry for Type I expression, and a greater left than right asymmetry for both Type II and Type III expressions, the patients did not. Of further note, the most frequent Type I expression was decreased and the rarest Type III expression was increased in schizophrenia, relative to controls, although the frequency of Type II was almost the same for the two groups. Additionally, within the right hemisphere, subjects with Type III showed a 2.84-fold risk of being categorized in the patient group, compared to those without Type III.
The present study thus provides substantial evidence of altered sulcogyral pattern in orbitofrontal cortex in schizophrenia population. Although longitudinal stability of the sulcogyral pattern should be confirmed in a future study with longitudinal design, the pattern is not likely to change over time following neurodevelopment. Further, while one might argue that longitudinal deterioration in global prefrontal structure might account for changes in the sulcogyral pattern, we think this unlikely as this pattern is set in neurodevelopment and is independent of brain tissue volume changes. We thus interpret findings of altered distribution (increased Type III and decreased Type I) of the sulcogyral pattern in the schizophrenia group as reflecting a possible risk factor or susceptibility to schizophrenia, rather than secondary to the effects of illness. Indeed, in the present cross-sectional dataset, the OFC sulcogyral pattern was not associated with subjects' age at MRI scan, length of the illness, or antipsychotic dosage. Although the sulcogyral pattern of the ‘H-shaped’ sulcus cannot serve as a diagnostic marker of schizophrenia, it could provide a morphological trait marker in the ventral prefrontal cortex, possibly related to a neurodevelopmental variation in the prefrontal paralimbic region.
OFC sulcogyral pattern and outcome
A further question we had is: within the schizophrenia group, does the OFC sulcogyral pattern affect patients' outcomes? We tried to address this question using a categorical regression analysis, which revealed that the least commonly occurring Type III expression in healthy controls was increased in the schizophrenia group was indeed associated with poorer outcome, including poor socioeconomic status, poor cognitive performance and more severe clinical symptoms. In contrast, the most commonly occurring Type I expression in healthy controls, was decreased in the schizophrenia group, and was associated with better outcome, including better cognitive performance and mild clinical symptoms. Even in the control group, Type I expression was associated with better cognitive performance. Type III for the control sample also was associated with perseveration, which is often viewed as indicative of difficulties in switching attentional set. However, the meaning of this association is complicated by other significant correlations with better cognitive performance. Due to the nature of the sulcogyral pattern, which seems to be stable over time following neurodevelopment, observed clinical associations with specific sulco-gyral pattern could reflect the heterogeneity (clinical and biological variability) of schizophrenia, itself, rather than secondary change in the sulcogyral pattern due to environmental factors linked to clinical outcome.
Type III expression in patients with schizophrenia was also strongly associated with poor socioeconomic status, consisting of educational and vocational background. This association is independent of parental socioeconomic status, cognitive function and clinical symptom severity. Therefore, this might suggest that schizophrenic patients with Type III expression have more difficulty in social adjustment than patients without Type III expression. Although the underlying mechanism between brain morphology and social neuroscience should be further investigated, this morphometric marker could be used as a potential clinical marker in the field of psychiatric rehabilitation.
Of further note, within each group, the Type I expression was associated with better cognitive performance, particularly for perceptual organization. In addition, collapsing both groups and covarying full-scale IQ, Type I expression was associated with better performance in working memory index.
For clinical symptoms the results also provided evidence linking Type III expression with poorer outcome and Type I and II expressions with better outcome. Of particular interest, PANSS symptoms that might capture some of the dimensions of the elusive but disabling social disturbance of schizophrenia were more closely associated with Type III expression. These symptoms consisted of passive/apathetic social withdrawal, active social avoidance and emotional withdrawal, which together form a newly introduced ‘withdrawal’ factor (Van den Oord et al., 2006). This factor seems to reflect social deficit more specifically than an overall negative symptom factor. In contrast, Type I and II expressions were associated with milder symptoms in the positive factor.
For total PANSS score, Type III expression was also associated with higher score (β = 0.45, F = 5.54, P = 0.024), although ANOVA for hypothesis testing of model fitting was only nearly significant (P = 0.067). These clinical associations, especially for the positive factor, might at least partly reflect responsiveness to medication treatment, because all of the present patients were chronically treated patients (duration of illness was 19.5 years on average), except for three first-episode patients who were included in the sample. That is, while speculative, the Type III pattern might be related to more treatment-resistance, and the Type I pattern might be related to more treatment-effectiveness.
Although available data in MPQ were limited, Type III expression in the schizophrenia group was negatively associated with ‘Constraint’ and positively associated with ‘Negative Emotionality’, both of which might reflect impulsivity, as predicted. These associations evoke antisocial and disinhibitory personality changes, commonly observed in patients with ventromedial prefrontal damage or degeneration (Cummings, 1993), although it is difficult to differentiate intrinsic personality traits from secondary personality changes due to schizophrenic psychosis. Within controls, Type II was positively associated with the ‘Positive Emotionality’ trait.
Among the significant functional–anatomical associations in the patient group, the following three associations of Type III–poor SES (poor social achievement), Type III–severe ‘withdrawal’ PANSS factor (social withdrawal/avoidance), and Type III–less ‘Constraint’ MPQ trait (impulsivity and risk-taking), were found to be more independent and specific than other significant associations, using ordinal regression analyses. Of particular interest, these three variables are specifically related to social functioning, suggesting that the Type III expression may serve as a trait marker for poor social adjustment in schizophrenic population.
Type III expression was also associated with smaller ICC volume in the schizophrenia group, although body sizes of the two subgroups were unknown. This observation may suggest that Type III expression was part of a systematic alteration in the early phase of neurodevelopment. Since adult ICC volume is quite stable over time, this structural association between Type III expression and smaller ICC volume suggests that the increased expression of Type III is not associated with the secondary effects of the illness, but is associated with neurodevelopment (Woods et al., 2005). Additionally, the lack of normal asymmetric distribution observed in the patient group suggests an alteration in genes that regulate early cortical development, as evidence suggests genetic involvement in human cerebral cortical asymmetry (Sun et al., 2005). Finally, this pattern may also reflect individual difference in ‘gyrogenesis’ within OFC, involved in regional neurobiological properties such as local connectivity and cytoarchitecture (Armstrong et al., 1995; Rakic, 1988).
Schizophrenic patients with Type III may, therefore, represent a subpopulation of schizophrenia, which might be characterized by an early neurodevelopmental aberration together with a more severe clinical picture including social deficit symptoms and poor treatment response, compared to schizophrenic patients without Type III.
Based on these findings, we view the OFC region, a major part of the social brain, as likely involved in many neuropsychiatric disorders, including, in particular, schizophrenia, affective psychosis, obsessive–compulsive disorder, dementia and a broad range of addiction. The OFC sulcogyral pattern classification could be investigated as a common modulator in social functioning in these different clinical entities.
Possible caveats
We note a few limitations in our interpretation of the present results. First, the three categorical sulcogyral patterns were observed across both controls and patients, and we did not include a non-schizophrenic psychosis group to determine the specificity of the findings to schizophrenia. Thus the altered sulcogyral pattern distribution should be regarded as a susceptibility to schizophrenia, but not necessarily as a specific marker for schizophrenia. Second, Type III expression was associated with poorer social functioning in the patient group but not in the control group, suggesting a disease-specific association. We caution, however, that the sample size of controls having Type III is small due to its low expression rate and there is some missing data for the clinical/cognitive measures, thereby inflating the risk of false negatives. For these reasons, we think we should be cautious in concluding group specificity of the poor social functioning–Type III association. Third, interrater reliability of 0.84 for the sulcogyral pattern classification is high, though not a perfect association, thus suggesting perhaps some uncertainty in the classification. In reviewing each case, we note that out of the 50 hemispheres (25 cases), six hemispheres showed a discrepancy between the two raters. More specifically, three out of the six discrepancies were disagreements between Types I and II, and the other three were disagreements between Types I and III. In these controversial hemispheres, the sulcus was disrupted in a few consecutive axial slices and it was connected in a few consecutive axial slices, which made judgement different between the raters. We point out, however, that all of the measures were done by one person (M.N.), and the interrater reliability measures did not change the original determination of Type I, II or III expression. We note that better spatial resolution of MRI data might reduce this kind of ambiguous pattern.
Conclusion
In conclusion, the present study revealed that the orbitofrontal sulcogyral pattern was altered in schizophrenic population, where the most frequently expressed Type I was decreased, and the least frequently expressed Type III was increased in the schizophrenia group, with a lack of normal asymmetrical distribution of the sulcogyral pattern. Furthermore, within the schizophrenia group, Type III expression was associated with poorer socioeconomic status, poorer cognitive function, more severe clinical symptoms (including increased apathy) and impulsivity as reflected in aggressive and reckless personality traits. In contrast, the Type I expression was associated with better cognitive function and milder clinical symptoms. The former was similar to findings in the healthy control group, where the Type I expression was associated with better cognitive function. These findings, taken together, suggest that the orbitofrontal sulcogyral pattern could be used as a morphometric trait marker in the fields of brain research and also clinical neuropsychiatry, and, that for a subset of patients with schizophrenia, Type III expression might also serve as a predictive marker for poorer social ability.
Acknowledgments
This study was supported, in part, by grants from the National Institutes of Health (K02 MH 01110 and R01 MH 50747 to M.E.S., R01 MH 40799 to R.W.M. and an NIH Roadmap for Medical Research Grant U54 EB005149, MES PI on Core 3), the MIND foundation (Albuquerque, NM, R.W.M.), the Welfide Medicinal Research Foundation, Japan (M.N.), and from the Department of Veterans Affairs Merit Awards (M.E.S., R.W.M.), a Research Enhancement Award Program (R.W.M., M.E.S.) and a Middleton Award (R.W.M.) from the Department of Veterans Affairs. Some of the data were presented at annual meetings of Biological Psychiatry, Toronto, Ontario, May 19, 2006, and the Organization of Human Brain Mapping, Florence, Italy, June 11–15, 2006. The authors gratefully acknowledge the administrative support of Marie Fairbanks and Nancy Maxwell, and the research assistant support of Lisa Lucia, BA, Matthew Koskowski, BA and Elizabeth Lewis, BA.
Abbreviations
- ANOVA
analysis of variance
- ICC
intra-cranial contents
- IQ
intelligence quotient
- LOS
lateral orbital sulcus
- MOS
medial orbital sulcus
- MPQ
multidimensional personality questionnaire
- OFC
orbitofrontal cortex
- PANSS
positive and negative syndrome scale
- TOS
transverse orbital sulcus
- WAIS-III
Wechsler Adult Intelligence Scale, 3rd edition
- WCST
Wisconsin card sorting test
References
- Armstrong E, Schleicher A, Omran H, Curtis M, Zilles K. The ontogeny of human gyrification. Cereb Cortex. 1995;5:56–63. doi: 10.1093/cercor/5.1.56. [DOI] [PubMed] [Google Scholar]
- Baare WF, Hulshoff Pol HE, Hijman R, Mali WP, Viergever MA, Kahn RS. Volumetric analysis of frontal lobe regions in schizophrenia: relation to cognitive function and symptomatology. Biol Psychiatry. 1999;45:1597–605. doi: 10.1016/s0006-3223(98)00266-2. [DOI] [PubMed] [Google Scholar]
- Bartley AJ, Jones DW, Weinberger DR. Genetic variability of human brain size and cortical gyral patterns. Brain. 1997;120:257–69. doi: 10.1093/brain/120.2.257. [DOI] [PubMed] [Google Scholar]
- Bleuler E. In: Dementia praecox of the group of schizophrenias. Zinkin J, translator. New York, NY: International Universities Press; 1950. 1911. [Google Scholar]
- Bouix S, Ungar L, Dickey CC, McCarley RW, Shenton ME. Evaluating automatic brain tissue classifiers. International Conference on Medical Image Computing and Computer-Assisted Intervention; St. Malo, France. 2004. pp. 1038–9. [Google Scholar]
- Chemerinski E, Nopoulos PC, Crespo-Facorro B, Andreasen NC, Magnotta V. Morphology of the ventral frontal cortex in schizophrenia: relationship with social dysfunction. Biol Psychiatry. 2002;52:1–8. doi: 10.1016/s0006-3223(01)01363-4. [DOI] [PubMed] [Google Scholar]
- Chiavaras MM, Petrides M. Orbitofrontal sulci of the human and macaque monkey brain. J Comp Neurol. 2000;422:35–54. [PubMed] [Google Scholar]
- Convit A, Wolf OT, de Leon MJ, Patalinjug M, Kandil E, Caraos C, et al. Volumetric analysis of the pre-frontal regions: findings in aging and schizophrenia. Psychiatry Res. 2001;107:61–73. doi: 10.1016/s0925-4927(01)00097-x. [DOI] [PubMed] [Google Scholar]
- Cummings JL. Frontal-subcortical circuits and human behavior. Arch Neurol. 1993;50:873–80. doi: 10.1001/archneur.1993.00540080076020. [DOI] [PubMed] [Google Scholar]
- DeLisi LE, Hoff AL, Neale C, Kushner M. Asymmetries in the superior temporal lobe in male and female first-episode schizophrenic patients: measures of the planum temporale and superior temporal gyrus by MRI. Schizophr Res. 1994;12:19–28. doi: 10.1016/0920-9964(94)90080-9. [DOI] [PubMed] [Google Scholar]
- Dorph-Petersen KA, Pierri JN, Perel JM, Sun Z, Sampson AR, Lewis DA. The influence of chronic exposure to antipsychotic medications on brain size before and after tissue fixation: a comparison of haloperidol and olanzapine in macaque monkeys. Neuropsychopharmacology. 2005;30:1649–61. doi: 10.1038/sj.npp.1300710. [DOI] [PubMed] [Google Scholar]
- Duvernoy HM. The human brain: surface, three-dimensional sectional anatomy with MRI, and blood supply. New York, NY: Springer-Verlag; 1999. [Google Scholar]
- Falkai P, Bogerts B, Greve B, Pfeiffer U, Machus B, Folsch-Reetz B, et al. Loss of sylvian fissure asymmetry in schizophrenia. A quantitative post mortem study. Schizophr Res. 1992;7:23–32. doi: 10.1016/0920-9964(92)90070-l. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Freedman M, Black S, Ebert P, Binns M. Orbitofrontal function, object alternation and perseveration. Cereb Cortex. 1998;8:18–27. doi: 10.1093/cercor/8.1.18. [DOI] [PubMed] [Google Scholar]
- Gottfried JA, O'Doherty J, Dolan RJ. Encoding predictive reward value in human amygdala and orbitofrontal cortex. Science. 2003;301:1104–7. doi: 10.1126/science.1087919. [DOI] [PubMed] [Google Scholar]
- Gur RE, Cowell PE, Latshaw A, Turetsky BI, Grossman RI, Arnold SE, et al. Reduced dorsal and orbital prefrontal gray matter volumes in schizophrenia. Arch Gen Psychiatry. 2000;57:761–8. doi: 10.1001/archpsyc.57.8.761. [DOI] [PubMed] [Google Scholar]
- Harlow JM. Passage of an iron rod through the head. Boston Med Surg J. 1848;39:389–93. [PMC free article] [PubMed] [Google Scholar]
- Harris JM, Whalley H, Yates S, Miller P, Johnstone EC, Lawrie SM. Abnormal cortical folding in high-risk individuals: a predictor of the development of schizophrenia? Biol Psychiatry. 2004a;56:182–9. doi: 10.1016/j.biopsych.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Harris JM, Yates S, Miller P, Best JJ, Johnstone EC, Lawrie SM. Gyrification in first-episode schizophrenia: a morphometric study. Biol Psychiatry. 2004b;55:141–7. doi: 10.1016/s0006-3223(03)00789-3. [DOI] [PubMed] [Google Scholar]
- Heaton RK. Wisconsin Card Sorting Test, Manual. Odessa, FL: Psychological Assessment Resources; 1981. [Google Scholar]
- Holland PC, Gallagher M. Amygdala-frontal interactions and reward expectancy. Curr Opin Neurobiol. 2004;14:148–55. doi: 10.1016/j.conb.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Hollingshead A. Two factor index of social position. New Haven, CT: Yale University Press; 1965. [Google Scholar]
- Jou RJ, Hardan AY, Keshavan MS. Reduced cortical folding in individuals at high risk for schizophrenia: a pilot study. Schizophr Res. 2005;75:309–13. doi: 10.1016/j.schres.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–76. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Kikinis R, Shenton ME, Gerig G, Hokama H, Haimson J, O'Donnell BF, et al. Temporal lobe sulco-gyral pattern anomalies in schizophrenia: an in vivo MR three-dimensional surface rendering study. Neurosci Lett. 1994;182:7–12. doi: 10.1016/0304-3940(94)90192-9. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nat Rev Neurosci. 2005;6:691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- Kulynych JJ, Luevano LF, Jones DW, Weinberger DR. Cortical abnormality in schizophrenia: an in vivo application of the gyrification index. Biol Psychiatry. 1997;41:995–9. doi: 10.1016/S0006-3223(96)00292-2. [DOI] [PubMed] [Google Scholar]
- Lacerda AL, Hardan AY, Yorbik O, Keshavan MS. Measurement of the orbitofrontal cortex: a validation study of a new method. Neuroimage. 2003;19:665–73. doi: 10.1016/s1053-8119(03)00137-x. [DOI] [PubMed] [Google Scholar]
- Le Provost JB, Bartres-Faz D, Paillere-Martinot ML, Artiges E, Pappata S, Recasens C, et al. Paracingulate sulcus morphology in men with early-onset schizophrenia. Br J Psychiatry. 2003;182:228–32. doi: 10.1192/bjp.182.3.228. [DOI] [PubMed] [Google Scholar]
- Lieberman JA, Tollefson GD, Charles C, Zipursky R, Sharma T, Kahn RS, et al. Antipsychotic drug effects on brain morphology in first-episode psychosis. Arch Gen Psychiatry. 2005;62:361–70. doi: 10.1001/archpsyc.62.4.361. [DOI] [PubMed] [Google Scholar]
- Manji HK, Moore GJ, Chen G. Clinical and preclinical evidence for the neurotrophic effects of mood stabilizers: implications for the pathophysiology and treatment of manic-depressive illness. Biol Psychiatry. 2000;48:740–54. doi: 10.1016/s0006-3223(00)00979-3. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Ongur D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex. 2000;10:206–19. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- Ono M, Kubik S, Abernathey CD. Atlas of the cerebral sulci. New York, NY: Thieme Medical Publishers; 1990. [Google Scholar]
- Pohl K, Bouix S, Kikinis R, Grimson WE. Anatomical guided segmentation with non-stationary tissue class distributions in an expectation-maximization framework. IEEE International Symposium on Biomedical Imaging: From Nano to Macro; Arlington, VA, USA. 2004. pp. 81–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P. Specification of cerebral cortical areas. Science. 1988;241:170–6. doi: 10.1126/science.3291116. [DOI] [PubMed] [Google Scholar]
- Rupp CI, Fleischhacker WW, Kemmler G, Kremser C, Bilder RM, Mechtcheriakov S, et al. Olfactory functions and volumetric measures of orbitofrontal and limbic regions in schizophrenia. Schizophr Res. 2005;74:149–61. doi: 10.1016/j.schres.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Sallet PC, Elkis H, Alves TM, Oliveira JR, Sassi E, Campi de Castro C, et al. Reduced cortical folding in schizophrenia: an MRI morphometric study. Am J Psychiatry. 2003;160:1606–13. doi: 10.1176/appi.ajp.160.9.1606. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Williams JBW, Gibbson M, First M. The Structured Clinical Interview for DSM-III-R-Non-Patient Edition (SCID-NP) Washington, DC: American Psychiatric Association; 1990a. [Google Scholar]
- Spitzer RL, Williams JBW, Gibbson M, First M. The Structured Clinical Interview for DSM-III-R (SCID) Washington, DC: American Psychiatric Association; 1990b. [Google Scholar]
- Sun T, Patoine C, Abu-Khalil A, Visvader J, Sum E, Cherry TJ, et al. Early asymmetry of gene transcription in embryonic human left and right cerebral cortex. Science. 2005;308:1794–8. doi: 10.1126/science.1110324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szeszko PR, Bilder RM, Lencz T, Pollack S, Alvir JM, Ashtari M, et al. Investigation of frontal lobe subregions in first-episode schizophrenia. Psychiatry Res. 1999;90:1–15. doi: 10.1016/s0925-4927(99)00002-5. [DOI] [PubMed] [Google Scholar]
- Tellegen A. Brief manual for the Multidimensional Personality Questionnaire. University of Minnesota; Minneapolis: 1982. [Google Scholar]
- Van den Oord EJ, Rujescu D, Robles JR, Giegling I, Birrell C, Bukszar J, et al. Factor structure and external validity of the PANSS revisited. Schizophr Res. 2006;82:213–23. doi: 10.1016/j.schres.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Vogeley K, Schneider-Axmann T, Pfeiffer U, Tepest R, Bayer TA, Bogerts B, et al. Disturbed gyrification of the prefrontal region in male schizophrenic patients: a morphometric postmortem study. Am J Psychiatry. 2000;157:34–9. doi: 10.1176/ajp.157.1.34. [DOI] [PubMed] [Google Scholar]
- Vogeley K, Tepest R, Pfeiffer U, Schneider-Axmann T, Maier W, Honer WG, et al. Right frontal hypergyria differentiation in affected and unaffected siblings from families multiply affected with schizophrenia: a morphometric mri study. Am J Psychiatry. 2001;158:494–6. doi: 10.1176/appi.ajp.158.3.494. [DOI] [PubMed] [Google Scholar]
- Walton ME, Devlin JT, Rushworth MF. Interactions between decision making and performance monitoring within prefrontal cortex. Nat Neurosci. 2004;7:1259–65. doi: 10.1038/nn1339. [DOI] [PubMed] [Google Scholar]
- Ward KE, Friedman L, Wise A, Schulz SC. Meta-analysis of brain and cranial size in schizophrenia. Schizophr Res. 1996;22:197–213. doi: 10.1016/s0920-9964(96)00076-x. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale. 3rd. San Antonio, TX: Psychological Corporation: Harcourt Brace Jovanovich Inc.; 1997. [Google Scholar]
- White T, Andreasen NC, Nopoulos P, Magnotta V. Gyrification abnormalities in childhood- and adolescent-onset schizophrenia. Biol Psychiatry. 2003;54:418–26. doi: 10.1016/s0006-3223(03)00065-9. [DOI] [PubMed] [Google Scholar]
- Woods BT, Ward KE, Johnson EH. Meta-analysis of the time-course of brain volume reduction in schizophrenia: implications for pathogenesis and early treatment. Schizophr Res. 2005;73:221–8. doi: 10.1016/j.schres.2004.05.014. [DOI] [PubMed] [Google Scholar]
- Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64:663–7. doi: 10.4088/jcp.v64n0607. [DOI] [PubMed] [Google Scholar]
- Yucel M, Stuart GW, Maruff P, Wood SJ, Savage GR, Smith DJ, et al. Paracingulate morphologic differences in males with established schizophrenia: a magnetic resonance imaging morphometric study. Biol Psychiatry. 2002;52:15–23. doi: 10.1016/s0006-3223(02)01312-4. [DOI] [PubMed] [Google Scholar]
- Zilles K, Armstrong E, Schleicher A, Kretschmann HJ. The human pattern of gyrification in the cerebral cortex. Anat Embryol (Berl) 1988;179:173–9. doi: 10.1007/BF00304699. [DOI] [PubMed] [Google Scholar]