SUMMARY
Effector T cell differentiation requires the simultaneous integration of multiple, and sometimes opposing, cytokine signals. We demonstrate that mTOR plays a role in dictating the outcome of T cell fate. mTOR deficient T cells display normal activation and IL-2 production upon initial stimulation. However, such cells fail to differentiate into Th1, Th2 or Th17 effector T cells under skewing conditions. The inability to differentiate is associated with a decrease in STAT activation and failure to upregulate lineage specific transcription factors. Under all normally activating conditions, T cells lacking mTOR differentiate into Foxp3+ regulatory cells. This differentiation is associated with hyperactive Smad3 activation in the absence of exogenous TGF-β. Surprisingly, T cells in which TORC1 activity has been selectively deleted do not divert to a regulatory T cell pathway, revealing an unappreciated role for TORC2 signaling in preventing the generation of regulatory T cells. Overall our studies suggest that differential TORC1 and TORC2 signaling regulate decisions between effector and regulatory T cell lineage commitment.
INTRODUCTION
The fate of a naïve T cell upon TCR engagement is dictated by the environmental cues it receives from the inflammatory milieu. For example, cytokines within the inflammatory microenvironment play a critical role in promoting specific effector lineage commitment (Bettelli et al., 2007). Through the activation of specific STAT pathways, lineage specific transcription factors are induced, fostering differentiation into distinct effector lineages (Murphy and Reiner, 2002). Previously, such fates were thought to be confined to Th1 and Th2 lineage commitment (Weaver et al., 2006). Naïve T cells activated in the presence of IFN-γ and IL-12 developed into Th1 effectors while T cells stimulated in the presence of IL-4 were fated to become Th2 effector cells. More recently, however, it has also been shown that T cells stimulated in the presence of IL-6 and TGF-β differentiate into an IL-17 producing effector population termed Th17. In addition, naïve T cells stimulated in the presence of high concentrations of TGF-β are capable of differentiating into Foxp3+ regulatory T cells (Chen et al., 2003). In vitro, differentiation into these T cell subsets is easily achieved through the precise addition of cytokines and cytokine-neutralizing antibodies. However, in vivo, T cells must integrate and respond simultaneously to a broad array of diverse and even opposing signals.
We have been studying the role of mTOR as an important signaling molecule in translating environmental cues into specific types of T cell responses (Powell, 2006). Specifically, inhibition of mTOR in Th1 effector cells by rapamycin promotes T cell tolerance, even in the presence of costimulation (Zheng et al., 2007). In yeast and mammalian cells, TOR integrates environmental cues, including amino acid and nutrient availability, energy stores, and growth factor signaling, and subsequently directs cell growth and proliferation (Hay and Sonenberg, 2004). By analogy, in T cells we hypothesize that mTOR integrates environmental cues and dictates the outcome of antigen recognition. mTOR signaling proceeds via two complexes: TOR Complex 1 (TORC1) and TORC2 (Guertin et al., 2006). TORC1 contains Rheb (a small GTPase), the regulatory-associated protein of mTOR (raptor), G-protein β-subunit-like protein (GβL), the proline-rich PKB/Akt substrate 40 kDa (PRAS40), and is rapamycin sensitive. TORC1 activation leads to the phosphorylation and activation of, among other targets, S6K1, and is thought to be associated with ribosome biogenesis, autophagy and protein translation (Sabatini, 2006). TORC2 contains, in addition to mTOR and GβL, the rapamycin-insensitive companion of mTOR (rictor) and mammalian stress-activated protein kinase interacting protein-1 (mSin1) (Guertin et al., 2006). Interestingly, even though Akt activation is upstream of mTOR signaling, the specific phosphorylation of Akt at serine 473 is in fact a downstream target of TORC2. Also, while it was initially thought that rapamycin exclusively inhibited mTORC1 activation, it has become clear that it can also inhibit TORC2 (Zeng et al., 2007).
In order to explore the potential role of mTOR in integrating cytokine signaling and regulating T effector lineage commitment we generated mice in which mTOR is specifically deleted in T cells. In this report we demonstrate that mTOR activation is necessary for Th1, Th2, and Th17 effector T cell differentiation, and even under fully activating conditions T cells lacking mTOR differentiate into Foxp3+ regulatory cells. Surprisingly, by selectively deleting TORC1 activity in T cells we are able to demonstrate a previously unappreciated role for TORC2 signaling in regulating Foxp3+ T cells.
RESULTS
Generation of mTOR deficient T cells
Because mTOR null mice are early embryonic lethal, we sought to conditionally delete mTOR gene expression in T cells. Mice carrying a floxed mTOR gene were crossed to mice harboring CD4-Cre in order to delete the gene in DP (CD4+CD8+) and CD4/CD8 SP T lymphocytes. In this report, mTORfl/fl mice will be referred to as “wild-type”, while mTORfl/fl; CD4-Cre mice will be referred to as “T-mTOR-/-.” In T-mTOR-/- mice, the mTOR gene was successfully deleted in CD4+ and CD8+ cells (Fig. 1A). As such, mTOR protein fails to be expressed in CD4+ T cells (Fig. 1B). The selective expression of Cre recombinase in CD4+ T cell precursors is supported by the fact that the mTOR gene is present in non-T cells (Fig. 1A). In spite of the central role of mTOR in regulating cell growth and proliferation, the absence of mTOR had a minimal effect on T cell development as determined by flow cytometric analysis of thymocytes from wt and T-mTOR-/- mice. In the thymus, there was a consistent increase in the CD4:CD8 ratio in the T-mTOR-/- mice (3:1 vs. 9:1) (Fig. 1C). However, the CD4:CD8 ratios in the spleen, peripheral blood, and lymph nodes, were similar and normal between the wt and T-mTOR-/- mice (Fig. 1D, Supplemental Figs. S1-S3). B and T cell percentages were equivalent between the wt and T-mTOR-/- mice in peripheral blood and lymph nodes, but a slight decrease in CD3+ cells is seen in the spleen (Supplemental Figs. S1-S3). A role for Akt and mTOR in the development of regulatory T cells has been described (Haxhinasto et al., 2008; Sauer et al., 2008). However, at baseline, deletion of mTOR did not alter the development of “natural” CD4+CD25+Foxp3+ regulatory T cells (Figs. 1C & D). It is of note that in our model (which employs CD4-Cre) mTOR is deleted during the double positive (DP) stage of T cell development. Thus our observations suggest that deleting mTOR at this stage of T cell development does not result in overt perturbations in CD4+, CD8+, and regulatory T cells.
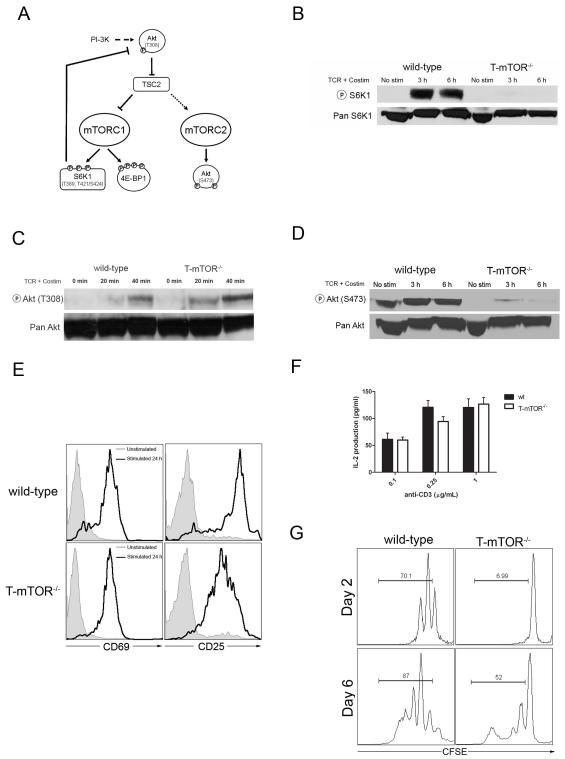
Figure 1.
Phenotypic analysis of conditional mTOR knockout mice. (A) PCR excision assay of cells from wt or T-mTOR-/- mice. “In” refers to amplification of primers confirming the presence of the gene. “Out” refers to amplification of primers confirming excision of the gene. (B) Immunoblot (IB) of mTOR in sorted populations. Raptor is included as a loading control. (C) FACS analysis of thymocytes harvested from mice. Foxp3 vs. CD25 is gated on CD4+ cells. (D) FACS analysis of splenocytes. Foxp3 vs. CD25 is gated on CD4+ cells. All data are representative of at least three independent experiments.
Normal TCR-induced activation and IL-2 secretion in the absence of TORC1 and TORC2 signaling
mTOR signaling occurs via two complexes, TORC1 and TORC2 (Figure 2A). To determine whether T cells from the T-mTOR-/- mice lacked TORC1 activity, T cells from wt and T-mTOR-/- mice were stimulated with anti-CD3 and anti-CD28 and S6K1 phosphorylation was determined by immunoblot analysis (McMahon et al., 2002). There was very little S6K1 phosphorylation in stimulated mTOR deficient T cells, indicating a lack of TORC1 activity, concomitant with mTOR deletion (Fig. 2B). Akt activation is an important upstream inducer of mTOR (Hay and Sonenberg, 2004). Phosphorylation of Akt on threonine 308 was slightly enhanced above wild-type levels (Fig 2C). This observation confirms that Akt activation upstream of mTOR is intact. Furthermore, the enhanced activation of Akt is consistent with the absence of a TORC1-mediated negative feedback loop (Fig. 2A) (Sabatini, 2006). To interrogate TORC2 activity we measured the level of Akt phosphorylation at serine 473, a known substrate of TORC2 signaling. Like S6K1, there was very little TORC2-dependent Akt phosphorylation in the mTOR deficient T cells (Fig. 2D). Thus we have established that while TCR-induced signaling proximal to mTOR is intact, the deletion of mTOR resulted in the inhibition of both TORC1 and TORC2 activity.
Figure 2.
Characterization of T cell function in mTOR deficient T cells. (A) mTOR signaling proceeds through two complexes. (B) IB of phospho-S6K1 from CD4+ T cells stimulated with immobilized anti-CD3 (1μg/mL) and anti-CD28 (2μg/mL) for the indicated times. (C) IB of phospho-Akt (T308), as in B. (D) IB of phospho-Akt (S473), as in B. (E) Activation markers upon stimulation. Cells were gated on CD4 after 24 hours of mock or anti-CD3 stimulation. (F) IL-2 production from initial stimulation of mTOR deficient T cells. Data are pooled from three independent experiments. Error bars indicate S.D. (G) Proliferation analysis of mTOR deficient T cells by CFSE dilution. Plots are gated on CD4+ T cells. Gates indicate cells that have undergone at least one division. All data are representative of at least three independent experiments.
We next wanted to determine the functional consequences of TCR-induced signaling in T-mTOR-/- T cells. Isolated CD4 cells were mock stimulated or stimulated with anti-CD3 and irradiated APCs overnight. Levels of CD69 (Very Early Activation Antigen) and CD25 (IL-2Rα) were examined by FACS. Both wt and T-mTOR-/- CD4 cells upregulated activation markers appropriately (Fig. 2E). Concomitant with activation markers, both wt and T-mTOR-/- T cells produced equivalent amounts of IL-2 upon anti-CD3 stimulation (Fig. 2F). These observations indicate that the TCR-induced signaling cascade remains intact in mTOR deficient T cells and that the ability of naïve T-mTOR-/- T cells to initially become activated is not affected by their lack of mTOR.
mTOR has been shown to play a role in promoting cell cycle progression (Wiederrecht et al., 1995). To determine if the absence of mTOR affected proliferative responses, T cells from wt and T-mTOR-/- mice were labeled with CFSE, stimulated with anti-CD3 and APCs, and evaluated by FACS. T cells from the T-mTOR-/- mice proliferated less than CD4+ T cells from wt mice (Fig. 2G). Consistent with this decrease in proliferation is a failure to upregulate cyclin D3 (Supplemental Fig. S4). However, proliferation of the CD4+ T cells from the T-mTOR-/- mice was not abolished, indicating that mTOR is not strictly necessary for proliferation (Colombetti et al., 2006). After six days of activation, 52% of the T-mTOR-/- cells proliferated compared with 87% of the Wt T cells. Furthermore, there is no evidence of increased death in the activated mTOR-/- T cells as indicated by trypan blue exclusion (data not shown).
mTOR is necessary for Th1 and Th2 effector T cell differentiation
In light of the multiple inputs that dictate effector differentiation, we hypothesized that mTOR might integrate these signals in T cells and subsequently promote T cell lineage commitment. T cells from wt and T-mTOR-/- mice were activated under Th1 (in the presence of IFN-γ, IL-12, and anti-IL-4) or Th2 (in the presence of IL-4 and anti-IFN-γ) skewing conditions for five days and then rechallenged with anti-CD3 and anti-CD28. Upon rechallenge, wt T cells cultured in Th1 skewing conditions produce IFN-γ and fail to produce IL-4 (Fig. 3A). Likewise the wt T cells cultured under Th2 skewing conditions produce IL-4 upon rechallenge and fail to produce IFN-γ (Fig. 3B). In contrast, T cells from T-mTOR-/- mice fail to differentiate into effector Th1 or Th2 cells, regardless of skewing conditions. Thus, although naïve T cells from the T-mTOR-/- mice produce IL-2 levels similar to T cells from wt mice, in the absence of mTOR, T cells fail to differentiate into effector cells under skewing conditions in vitro.
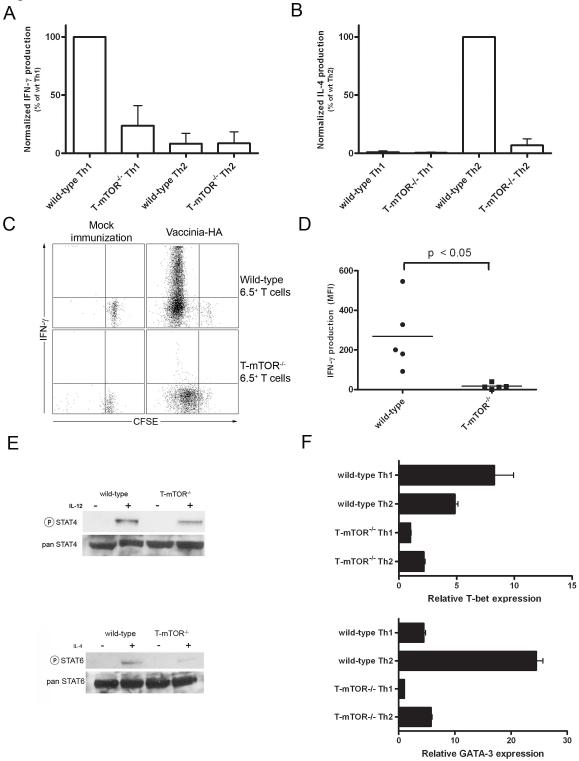
Figure 3.
mTOR deficient T cells fail to differentiate into Th1 or Th2 effector cells. (A) IFN-γ production was measured by ELISA. Total lymphocytes were isolated from mice and stimulated with anti-CD3 in vitro and rested 5 d in the presence of Th1 or Th2 skewing conditions. T cells were isolated by magnetic separation and then restimulated with anti-CD3 and anti-CD28 for 24 h. Data are pooled from three independent experiments and are represented as percent IFN-γ activity relative to wt Th1. (B) As in A, but for IL-4 (percentages are relative to wt Th2). (C) Proliferation and IFN-γ production of donor cells was measured by intracellular cytokine staining 5 days post adoptive transfer. Production = mean fluorescence multiplied by the percent CFSElo. (D) IFN-γ production compiled from multiple mice as in C. Data are representative of three independent experiments. (E) Previously activated T cells were stimulated with IL-12 (5 ng/mL) or IL-4 (5 ng/mL) for 30 mins and blotted for phospho-STAT4 or STAT6, respectively. (F) T-bet and GATA-3 expression from skewed Th1 or Th2 cells by real-time PCR. Values are normalized to 18s rRNA levels and scaled to T-mTOR-/- Th1 conditions. Error bars indicate S.D. Data are representative of five independent experiments.
Next we wanted to determine if mTOR was required for effector T cell differentiation in vivo using Vaccinia virus, a strong inducer of an anti-viral Th1 response. To this end, we bred our mTOR floxed lines to mice carrying a transgenic T cell receptor specific for HA peptide (clone 6.5). These HA-specific, CFSE-labeled wild-type and mTOR deficient CD4+ T cells were adoptively transferred into B10.D2 host mice that were immunized one day prior with Vaccinia virus expressing the HA antigen. Five days post-transfer the mice were sacrificed and spleens were harvested. The HA-specific donor T cells were then rechallenged in vitro with high-dose peptide and APCs and examined by intracellular cytokine staining (Fig. 3C). Donor T cells from host mice that were not immunized did not proliferate, nor did they produce cytokines. Wild-type T cells from immunized mice proliferated in vivo and produced copious amounts of IFN-γ upon rechallenge (Figs. 3C & 3D). mTOR deficient T cells from immunized host mice did proliferate in response to viral infection, albeit to a lesser degree. However, these cells failed to produce IFN-γ upon rechallenge. Importantly, this was the case even for the mTOR deficient T cells that under went several rounds of division. The fact that T cells lacking mTOR went through multiple rounds of proliferation yet failed to produce IFN-γ indicates that the lack of Th1 differentiation is not merely secondary to decreased proliferation. In addition, upon rechallenge the virally-activated mTOR deficient CD4+ T cells produced much less IL-2 and TNF-α compared to T cells from wt mice (Supplemental Figs. S5 & S6). Thus, in the absence of mTOR, Vaccinia-specific T cells fail to differentiate into Th1 effector cells in vivo.
Cytokines promote lineage commitment in part by STAT signaling which mediates subsequent upregulation of lineage specific transcription factors. Critical for effector T cell lineage commitment is appropriate STAT activation in response to specific cytokines. For example, Th1 differentiation is dependent upon IL-12-induced STAT4 activation while Th2 differentiation is promoted by IL-4-induced STAT6 activation. Thus we wanted to determine if mTOR played a role in promoting STAT signaling. Previously activated wt and mTOR deficient T cells were stimulated with either IL-12 or IL-4 for 30 minutes and examined for STAT phosphorylation. IL-12-induced phosphorylation of STAT4 was diminished in the T-mTOR-/- T cells (Fig. 3E). Likewise, IL-4-induced STAT6 phosphorylation was decreased in the T-mTOR-/- T cells. Of note, both the IL-12 and IL-4 receptors are appropriately expressed on T-mTOR-/- T cells (data not shown). Thus, in the absence of mTOR the ability of cytokines to direct effector lineage commitment is mitigated, in part, at the level of STAT activation.
Effector T cell lineage commitment is promoted by the upregulation of lineage specific transcription factors (Murphy and Reiner, 2002). T-bet and GATA-3 play critical roles in promoting Th1 and Th2 differentiation, respectively. We wanted to determine if mTOR was involved in regulating the expression of these transcription factors. Wild-type and mTOR deficient T cells were stimulated under Th1 and Th2 skewing conditions and then interrogated for T-bet and GATA-3 expression by real time PCR. Wild-type T cells demonstrated increased T-bet expression in Th1 cells compared to Th2 cells. GATA-3 was expressed in wild-type Th2 but not Th1 cells (Fig. 3F). However, neither T-bet nor GATA-3 was upregulated in the mTOR deficient T cells under skewing conditions. These observations suggest that mTOR activation is required for the upregulation of Th1 and Th2 lineage specific transcription factors, consistent with decreased STAT4 and STAT6 activation noted above.
mTOR is required for Th17 effector lineage commitment
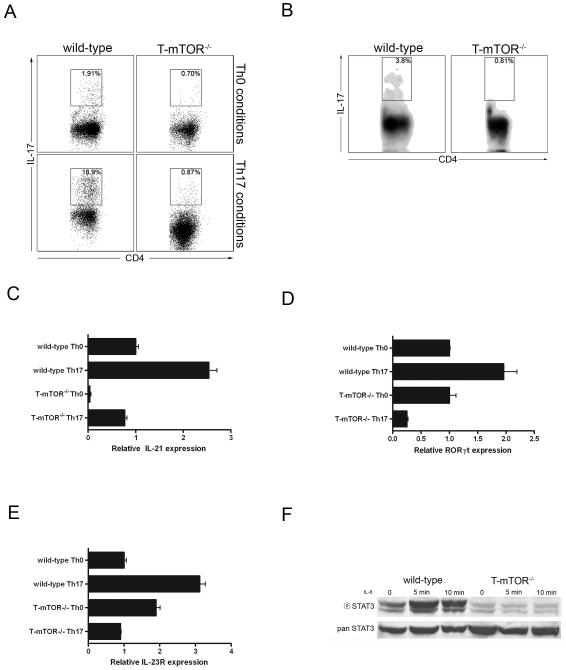
Having established that mTOR deficient T cells fail to differentiate into Th1 and Th2 effector T cells, we next wanted to determine if mTOR also played a role in promoting Th17 effector cells. T cells from wt and T-mTOR-/- mice were activated with anti-CD3 and cultured in IL-7 alone or TGF-β + IL-6 for 5 days, restimulated, and examined by intracellular cytokine staining for IL-17 production. Compared to wt cells cultured in media alone, wt T cells cultured in IL-6 + TGF-β differentiated into Th17 cells (Fig. 4A). Alternatively, the T cells from the T-mTOR-/- mice failed to differentiate into Th17 cells under skewing conditions. Following our in vitro findings, we next examined whether mTOR deficient cells could differentiate to Th17 in vivo. Th17 cells are found at relatively high frequencies in the gut mucosa (Denning et al., 2007). Peyer’s patches were isolated and stimulated with PMA/ionomycin to assay for IL-17 production by intracellular cytokine staining. While 3.8% of the wt cells are IL-17+, only 0.81% of cells from T-mTOR-/- mice produced IL-17 (Fig. 4B). These data suggest that mTOR activation plays a role in Th17 lineage commitment in vivo.
Figure 4.
mTOR deficient T cells fail to differentiate to Th17 effector cells. (A) FACS analysis of Th17 skewed T cells. T cells were activated in the presence or absence of Th17 skewing conditions and restimulated in vitro. (B) Th17 FACS analysis of Peyer’s patch (PP) lymphocytes. PPs were isolated from wt or T-mTOR-/- mice and stimulated with PMA/ionomycin for 5 h. (C) IL-21 expression in nonbiasing or Th17 skewing conditions by real-time PCR analysis. Data are normalized to 18s rRNA and scaled to wt nonbiasing conditions. Error bars indicate SD. (D) As in C, but for the Th17-associated transcription factor ROR-γt. (E) As in C, but for IL-23R. (F) Previously activated T cells were stimulated with IL-6 (5ng/mL) for 5 or 10 mins and blotted for phospho-STAT3. All data are representative of at least three independent experiments.
Better characterization of the genetic program determining the initial differentiation and subsequent expansion of Th17 cells has been elucidated (Ivanov et al., 2007). Upon initial stimulation with TGF-β and IL-6, T cells begin to express IL-21, which acts in an autocrine fashion to potentiate the Th17 phenotype through STAT3. This results in the expression of the retinoic-acid-related orphan receptor γ (RORγt), a transcription factor implicated in the maintenance of the Th17 program. Th17 cells further stabilize their phenotype by upregulating the IL-23R. To examine if there was a block in any of the steps of this process, we stimulated mTOR deficient T cells in Th17 biasing conditions and analyzed the expression of these genes by real-time PCR. When cultured under IL-17 skewing conditions, T cells from wt mice upregulated the expression of IL-21, IL-23R, and ROR-γt. However, mTOR-deficient T cells fail to express any of these genes above wild-type Th0 levels (Figs. 4C, D, & E). Th17 lineage commitment is also dependent upon the activation of STAT3. Since T cells lacking mTOR failed to upregulate the STAT3 dependent genes IL-21 and ROR-γt, we sought to determine whether STAT3 phosphorylation was intact in the absence of mTOR. Previously activated T cells from wt and T-mTOR-/- mice were stimulated with IL-6 to induce STAT3 phosphorylation. While wild type T cells display a robust increase in STAT3 phosphorylation at tyrosine 705, T cells lacking mTOR fail to phosphorylate STAT3 (Fig. 4F). Of note, the IL-6 receptor is appropriately expressed on the T-mTOR-/- T cells (data not shown). Thus, in the absence of mTOR, IL-6 fails to activate STAT3 and consequently the genetic programs that promote Th17 lineage commitment fail to be upregulated.
TCR engagement in the absence of mTOR leads to regulatory T cell differentiation
T cells from T-mTOR-/- mice fail to differentiate into effector Th1, Th2 and Th17 T cells both in vitro and in vivo. Nonetheless, naïve mTOR deficient T cells respond initially to antigen by upregulating activation markers and producing IL-2 (Fig 2). Furthermore, mTOR deficient T cells do not show evidence of increased cell death (data not shown). Therefore, since the T-mTOR-/- cells failed to differentiate into known effector subsets, we sought to determine the phenotype of these cells after antigen recognition. We activated wt and T-mTOR-/- T cells with anti-CD3 and analyzed the percentage of CD4+CD25+Foxp3+ T cells 5 days later. Initially, the percentages of CD4+CD25+Foxp3+ T cells in the wt and the T-mTOR-/- mice were equivalent at 5.92% and 6.07% respectively (Fig. 5A, top panels). However, after stimulation, the percentage of CD4+CD25+Foxp3+ T cells in the wt cultures was only 3.65% while the percentage of CD25+Foxp3+ cells in the T-mTOR-/- cultures expanded to 19.0% (Fig. 5A, bottom panels).
Figure 5.
mTOR deficient T cells differentiate down a regulatory pathway. (A) FACS analysis of CD4+ T cells from wt or T-mTOR-/- mice pre-stimulation and 5 d post-stimulation. Inlaid panel shows GITR expression measured by FACS. The CD25+Foxp3+ fraction appears as a solid line, the Foxp3- fraction is shaded grey. (B) In vitro suppression assay with CD25+ fraction of mTOR deficient T cells. Proliferation of responder cells was measured by CFSE dilution 72 h post stimulation. (C) As in A, but some cultures were supplemented with IL-2. (D) T cells were isolated from wt or T-mTOR-/- mice, CFSE labeled, and stimulated with anti-CD3 (107 per timepoint). Cultures were then supplemented with IL-2 and IL-7. On day 2, 4, 6, and 8 post stimulation cells were removed from culture, counted, and restimulated with anti-CD3 (5μg/mL) and anti-CD28 (2μg/mL), then interrogated by FACS for IFN-γ production and Foxp3 expression. Plots are representative of day 6 cultures from this experiment. IFN-γ+ gates were drawn using unstimulated controls. (E) Treg upregulation occurs in vitro due to an increase in Foxp3+ cells rather than death of effector cells. Data from D were compiled from three experiments and absolute numbers were calculated. (F) Adoptive transfers were performed as in Figure 3C. Donor cells were interrogated by differential Thy1 expression (FACS). (G) Wt B10.D2 host mice (Thy1.2+) were infected with Vac-HA and after 24 h wt or T-mTOR-/- 6.5+ T cells (Thy1.1/Thy1.2) were adoptively transferred. One day later, mice received a second transfer of 6.5+ CFSE-labeled naïve wt responder T cells (Thy1.1+). Splenocytes were harvested and stimulated with peptide and tested for cytokine production. Plots are gated on responders by Thy1.1 homozygosity. (H) IFN-γ production compiled from multiple mice as in E. Data are representative of three independent experiments. (I) Foxp3+ expression of the transferred wt and mTOR deficient (Thy1.1/Thy1.2) population from G.
The phenotype and function of these Foxp3+ T cells derived from T-mTOR-/- mice were examined. The Foxp3+ T cells from the T-mTOR-/- mice expressed levels of Foxp3 similar to wild-type nTreg as determined by MFI on FACS. Furthermore, the Foxp3+ T cells derived from the T-mTOR-/- mice expressed GITR at similar levels as Foxp3+ cells from wild-type mice (Fig. 5A, inlaid panels). To determine the function of these Foxp3+ cells, these cells were tested in an in vitro suppression assay. Expanded mTOR deficient T cells were enriched for CD25+ cells by magnetic separation and co-cultured with CFSE labeled naïve 6.5+ responder T cells, irradiated APCs, and their cognate peptide. The responder cells receiving TCR stimulation in the presence of the mTOR deficient regulatory T cells demonstrated decreased proliferation, indicating the Foxp3+ T cells from the mTOR deficient cultures display potent suppressor activity (Fig. 5B).
Interestingly, while IL-2 clearly plays an important role in activating T cells and enhancing T cell mediated immunity, it also plays an essential role in the induction of regulatory cells (Lohr et al., 2006). Since mTOR is downstream of IL-2 receptor signaling, we wanted to examine the effect of IL-2 on regulatory T cell generation in mTOR deficient T cells. T cells from either wt or T-mTOR-/- mice were activated with anti-CD3 and cultured in IL-7 alone or IL-7 + IL-2 for 5 days and then examined by FACS for CD4+CD25+Foxp3+ T cells. In the absence of mTOR, a normally “activating” in vitro stimulation led to the generation of 25.9% Foxp3+ regulatory T cells compared to 1.72% in wt T cells (Fig. 5C). Culture of the wt T cells with IL-2 failed to increase the percentage of regulatory T cells (0.76%). However, the addition of IL-2 to mTOR deficient T cells led to an expansion of Foxp3+ cells to 50.8%. These findings suggest that the ability of IL-2 to promote regulatory T cell differentiation is independent of IL-2-induced mTOR activation. Thus, under normally activating conditions that would typically induce Th1 responses in vitro, T cell activation in the absence of mTOR signaling results in the generation of functional Foxp3+ regulatory T cells.
One possible explanation for our findings is that mTOR ablation causes the selective death of effector cells leading to an increase in the percentage of regulatory T cells but not an increase in their absolute numbers. For example, initially it was thought that expression of cognate peptide in the thymus induced Treg differentiation. Subsequently, it was shown that while expression of peptide in the thymus led to an increase in the percentage of Foxp3+ T cells, this increase was due to deletion of CD4+ Foxp3- cells. That is, there was no increase in the absolute number of Foxp3+ cells (Apostolou et al., 2002; van Santen et al., 2004). To examine this possibility in our system, T cells were harvested from wt and T-mTOR-/- mice, labeled with CFSE, and stimulated in vitro overnight. The cells were then in IL-2 and IL-7 to promote Treg generation. The cultures were examined daily for the total number of T cells as well as CFSE dilution and Foxp3 expression. By day 6, we observed that the wt T cells proliferated and had differentiated into IFN-γ producing cells (Fig. 5D). However, the T-mTOR- cells proliferated less, did not differentiate into IFN-γ producing cells, and instead became Foxp3+ cells. Importantly, while the absolute number of Foxp3+ T cells remained constant in the wild-type cultures, T-mTOR-/- cultures showed an increase in the number of these cells (Fig. 5E). The increase in absolute numbers of regulatory T cells supports the notion that T cell activation in the absence of mTOR promotes the generation of such cells. In vitro, T cells stimulated in the absence of mTOR signaling differentiate down a regulatory T cell pathway.
We then tested whether T-mTOR-/- cells would differentiate into regulatory cells when activated by the strongly Th1 polarizing Vaccinia virus infection in vivo. Consistent with our in vitro findings, the response to Vaccinia virus in vivo also led to the development of CD4+CD25+Foxp3+ regulatory T cells (Fig. 5F). We next tested the suppressive capabilities of mTOR-deficient T cells that convert into Tregs in vivo. Host B10.D2 (Thy1.2+) mice were infected with Vaccinia-HA, and one-day post infection either wild-type or T-mTOR-/- CD4+ Thy1.1+/Thy1.2+ T cells were adoptively transferred. One day later, CFSE labeled wild-type 6.5+ Thy1.1+ T cells were transferred and would be examined as “responders.” Five days after transfer of responder cells, splenocytes were challenged with high-dose HA peptide and IFN-γ production was examined by intracellular cytokine staining. The T cells that were adoptively transferred along with the naïve mTOR-deficient T cells produced less IFN-γ in response to Vaccinia infection when compared to the responder cells adoptively transferred along with the naïve wt cells (Fig. 5G and 5H). Furthermore, when the transferred cells from T-mTOR-/- were examined for regulatory T cell markers, a clear conversion takes place (Fig. 5I). This indicates that, in vivo, in response to the strong Th1-inducing stimulus of Vaccinia infection, mTOR deficient T cells convert into regulatory T cells with potent suppressive capabilities. The ability of the adoptively transferred naïve T-mTOR-/- cells to suppress in vivo suggests that indeed functional regulatory T cells are being generated. That is, in vivo activation of T cells from T-mTOR-/- mice by Vaccinia does not merely lead to an increase in the percentage of regulatory cells due to the selective death of effector cells, but rather the generation of regulatory T cells that functionally suppress.
mTOR-deficient T cells are hypersensitive to TGF-β
Because TGF-β is such a potent skewing agent of antigen-induced regulatory T cells, one possible explanation for our findings was that cells from T-mTOR-/- mice produce higher amounts of TGF-β. To examine this, splenocytes from wt or T-mTOR-/- mice were place in media alone or stimulated with anti-CD3 overnight and examined for TGF-β production by ELISA. For both the wt and T-mTOR-/- cultures, there was a slight increase in TGF-β expression upon activation (Fig. 6A). However, the levels of TGF-β were equivalent, indicating that the development of regulatory cells upon stimulation of the T-mTOR-/- cultures is not the result of increased levels of TGF-β.
Figure 6.
TORC1 deficient T cells fail to become regulatory T cells (A) TGF-β production in splenocytes from wt and mTOR deficient T cell cultures as measured by ELISA. Data are pooled from 7 experiments. Error bars indicate S.D. (B) mTOR deficient cells have hyperphosphorylated Smad3. Previously activated cells were stimulated with TGF-β (5ng/mL) for 30 min and probed (IB) for phospho-Smad3. (C) TGF-β is necessary for mTOR-deficiency mediated Treg induction. Cells were stimulated as in 5A in the presence or absence of TGF-β neutralizing antibodies. (D) Rheb deficiency selectively inhibits TORC1 activity. Previously activated T-Rheb-/- T cells were stimulated in vitro with anti-CD3 and anti-CD28 for the indicated times and probed for mTOR substrates by IB. Pan Akt is included as a loading control. (E) Rheb-deficient T cells do not spontaneously convert into Tregs. wt, T-Rheb-/- and T-mTOR-/- cells were stimulated with anti-CD3 and rested in IL-7 supplemented media for 5 days and interrogated for Treg markers. (F) Rheb deficient T cells can be skewed to Tregs. wt and T-Rheb-/- cells were stimulated in the presence or absence of Treg skewing conditions and analyzed after 5 days. Cells were then interrogated for Treg markers by FACS. All data are representative of at least three independent experiments.
Smad3 plays a critical role in promoting regulatory T cell differentiation. TGF-β signaling activates Smad3 which, along with TCR induced NF-AT, contributes to the induction of Foxp3 by promoting acetylation at the Foxp3 enhancer (Tone et al., 2008). To examine Smad3 activation in mTOR deficient T cells, previously activated wild-type and T-mTOR-/- T cells were mock stimulated or stimulated with TGF-β and examined by immunoblot for Smad3 activation. At baseline, the mTOR deficient T cells displayed robust phosphorylation of Smad3 and this phosphorylation increased with the addition of TGF-β (Fig. 6B). In contrast, the levels of Smad3 phosphorylation in the wt T cells were very low and they increased modestly upon stimulation with this dose of TGF-β.
TGF-β is a potent stimulus for Smad3 activation. However, we knew that there was not excessive TGF-β produced in the cultured cells from T-mTOR-/- mice. Thus, we wanted to determine if the little TGF-β that was present in these cultures was driving differentiation to regulatory T cells. Wild-type and T-mTOR-/- cells were stimulated overnight in the presence or absence of TGF-β neutralizing antibodies. Cells were washed the next day and rested in IL-7 as well as additional neutralizing antibodies. After a 5 day rest, the cells were examined for Treg markers. While 23.6% of the untreated T-mTOR-/- T cells were Foxp3+, CD4 cells, the addition of neutralizing antibodies to TGF-β reduced the generation of Foxp3+ cells to 14.6% (Fig 6C). These observations suggest that the TGF-β produced in a normally activating inflammatory milieu contributes to the induction of Foxp3+ regulatory cells in the absence of mTOR activation.
TORC1 deficient T cells fail to become regulatory T cells
Rapamycin is believed to directly affect the TORC1 signaling complex. Because rapamycin promotes the generation of regulatory T cells in vitro, the ablation of TORC1 should yield regulatory T cell differentiation. However, it is becoming increasingly apparent that rapamycin can also affect the assembly and signaling of TORC2 (depending on cell type and length of exposure) (Sabatini, 2006). Since mTOR-deficient T cells delete both TORC1 and TORC2 signaling we sought to create a mouse whereby TORC1 signaling was selectively inhibited in T cells.
Rheb is a critical positive regulator of mTORC1 signaling (Avruch et al., 2006). Therefore we bred Rheb floxed mice (Xiao et al, manuscript in preparation) to CD4-Cre mice to generate a conditional Rheb deletion in T cells (Rhebfl/fl; CD4-Cre, referred to as T-Rheb-/-). Similar to the T-mTOR-/- mice, we detected no difference in T cell subsets in the lymph node, spleen and blood (data not shown; the full characterization of the T-Rheb-/- mice will be reported in a separate manuscript). Also, thymic development was normal and no differences in the CD4:CD8 ratio were detected (data not shown). T cells from wt and T-Rheb-/- mice were stimulated with anti-CD3 and anti-CD28 in vitro and interrogated for phospho-S6K1 (TORC1 activation) and phospho-Akt (serine 473) (TORC2 activation) (Fig. 6D). Upon stimulation the T-Rheb-/- T cells failed to phosphorylate S6K1. However there was intact (and slightly elevated) TORC2 activity as determined by Akt phosphorylation of serine 473. These observations are consistent with the ability of TORC1 to inhibit TORC2 signaling through a negative feedback loop.
T cells from wild-type, T-Rheb-/-, and T-mTOR-/- mice were stimulated with anti-CD3 for 24 hours, and rested in IL-7-supplemented media for 5 days. The cells were then harvested and analyzed by FACS. As expected the wt T cells fail to differentiate into regulatory cells and the T-mTOR-/- cells are induced to express Foxp3 (Fig. 6E). However, in spite of the fact that the T-Rheb-/- cells lack TORC1 activation they fail to induce Foxp3. Recent studies have shown that T cells expressing a constitutively active Akt fail to upregulate Foxp3 (Haxhinasto et al., 2008) Since the T-Rheb-/- cells demonstrated slightly elevated Akt activation, we determined if these cells were capable of becoming Foxp3+ cells. Naïve wild-type and T-Rheb-/- T cells were activated in the presence or absence of TGF-β. After five days, the cells were examined for Treg markers. In the presence of TGF-β, the T-Rheb-/- cells were induced to express Foxp3 (Fig. 6F). Thus, even though T-Rheb-/- cells fail to become Tregs under activating conditions, they differentiate into inducible Tregs when activated in the presence of TGF-β.
DISCUSSION
There is much plasticity with regard to the development of distinct T effector or regulatory lineages upon antigen recognition (Weaver et al., 2006). In addition to Th1 or Th2 type CD4+ T cells, a third effector subset of T cells termed Th17 can be produced through the presence of TGF-β and IL-6. However, stimulation in the presence of TGF-β without IL-6 causes CD4+CD25- T cells to develop into Foxp3+ regulatory cells. Our data demonstrate that, by regulating cytokine signals and the subsequent expression of lineage specific transcription factors, mTOR plays a role in determining antigen-induced fate with regard to the development of effector versus regulatory T cells. That is, mTOR activation is essential for the development of effector T cell lineage commitment. Under strongly activating skewing conditions for Th1, Th2, and Th17 development, in the absence of mTOR, T cells differentiated down a regulatory pathway. Thus, our data support a new paradigm whereby the default pathway for TCR engagement is toward regulatory cells, and that accessory signals leading to the activation of mTOR are required to redirect antigen recognition toward active immunity.
In yeast and other primitive eukaryotic cells, TOR senses the environment and instructs cellular processes (Hay and Sonenberg, 2004). In an environment lacking amino acids, energy, and nutrients, TOR is inhibited and protein synthesis curtailed. By analogy, our data suggest an important role for mTOR in integrating environmental cues in the form of cytokine stimulation. In the absence of mTOR, cytokines such as IL-12, IFN-γ and IL-4 fail to promote the upregulation of lineage specific transcription factors, in part due to a decrease in appropriate STAT activation. In this fashion our data reveal a role for mTOR in facilitating STAT activation in response to cytokines in lymphocytes. This was particularly so in the case of IL-6 induced STAT3 activation, that was markedly absent in the mTOR deficient T cells. At this time it is unclear whether mTOR plays a direct role in STAT phosphorylation. However, our findings are consistent with recent observations in tumor cells and in IL-6-induced insulin resistance implicating a role for mTOR in STAT3 activation (Kim et al., 2008; Zhou et al., 2007). Thus mTOR plays the simultaneous role of inducing specific effector differentiation by promoting STAT activation and inhibiting Smad3 phosphorylation.
While mTOR deficient T cells had decreased responses to IL-12, IFN-γ, IL-4 and IL-6, interestingly, exogenous IL-2 hastened the development of Foxp3+ T cells from the mTOR deficient cultures. IL-2 was first identified as “T cell growth factor” and in fact IL-2 receptor signaling leads to mTOR activation (Abraham and Wiederrecht, 1996). However, recent data clearly demonstrate an important role for IL-2 in maintaining tolerance (Lohr et al., 2006). Both IL-2 and IL-2 receptor null mice seem paradoxically to have autoimmune phenotypes (Sadlack et al., 1995). It is believed that the increased autoimmunity in these mice is due the critical role that IL-2 plays in promoting the generation of regulatory T cells. In this context, our data serve to dissect IL-2R signaling into mTOR dependent (activating) and mTOR independent (regulatory) pathways.
Upstream of mTOR, a signaling cascade from PI3K/AKT leads to the inactivation of TSC2 and subsequently the activation of mTOR. Downstream, mTOR signaling occurs via two distinct complexes, mTORC1 and mTORC2 (Fig. 2A). Haxhinasto et al. have been able to link Akt activation and the regulation of Foxp3 (Haxhinasto et al., 2008). In this work, the forced overexpression of a constitutively active Akt led to a decrease in expression of Foxp3 target genes. The addition of rapamycin abrogated this affect and it was concluded that Akt was acting upstream of mTOR. Likewise, based on observations using PTEN null cells and pharmacologic inhibitors of PI3-kinase, Akt and mTOR, Sauer et al. propose that the PI-3K/Akt/mTOR axis negatively regulates Foxp3 expression (Sauer et al., 2008). Our data employing Rheb null T cells (in which TORC1 is selectively eliminated) shed new light on this pathway and reveal a heretofore unappreciated role for TORC2 in regulating Foxp3 expression. Our data support a model whereby mTOR signaling via TORC2-induced activation of Akt plays a role in negatively regulating Foxp3 expression as opposed to Akt inhibiting Foxp3 expression through the exclusive activation of TORC1. Notably, TORC2 inhibition appears to be necessary for the upregulation of Foxp3; however, it is unclear if it is sufficient.
While the generation of inducible regulatory T cells has been shown in the context of suboptimal TCR engagement, inadequate costimulation, and exogenous TGF-β, in our system regulatory T cells are generated despite strong TCR engagement, full costimulation, and exogenous TGF-β (Chen et al., 2003; Kretschmer et al., 2005). While a similar low level of TGF-β production was observed for both wt and T-mTOR-/- T cells, our data support an increased sensitivity of mTOR-deficient cells to such modest TGF-β stimulation. First, the addition of neutralizing antibodies to TGF-β diminished the generation of regulatory T cells. Second, Smad3, a downstream component of TGF-β signaling that is involved in Foxp3 transcription, is hyperactive in the mTOR deficient T cells. It has been previously shown that Akt activation can inhibit TGF-β mediated phosphorylation of Smad3 (Song et al., 2006). Thus mechanistically, we propose that in the absence of mTOR, TORC2 mediated activation of Akt is absent, resulting in hyperphosphorylation of Smad3. Taken as a whole, we propose that in the inflammatory milieu, T cells receive multiple and opposing environmental cues, and that the resulting level of mTOR activation influences the outcome of antigen recognition. For example, in the setting of an inflammatory response without concurrent mTOR activation, even small amounts of TGF-β would lead to regulatory T cell lineage commitment. Recently, it has been shown that TGF-β can antagonize RORγt function and thus promote regulatory T cell development over Th17 effector differentiation (Zhou et al., 2008). Our data suggest that this balance between effector and regulatory lineage commitment extends to all effector cells (not just Th17), and is controlled, at least in part, by mTOR.
As a result of the critical role of mTOR in development, mTOR null mice are early embryonic lethal (Gangloff et al., 2004). Since in our model mTOR is deleted relatively late in T cell development (at the double positive stage) our data do not address the role of mTOR in earlier aspects of T cell development. Nonetheless, in light of the important role of mTOR in multiple cellular functions, it was somewhat surprising that it was dispensable for T cell homeostasis and proliferation. Such findings suggest that the function of mTOR is more important under conditions of activation or stress. mTOR-independent pathways must regulate metabolism, survival and proliferation during T cell development and homeostasis. A potential mechanism to explain this is expression of Pim kinases that have been shown to regulate T cell activation and survival through mTOR independent pathways (Fox et al., 2005). Along these lines it has been proposed that Pim2 kinase is a Foxp3 target gene and thus upregulated in regulatory T cells (Basu et al., 2008). Such alternative pathways provide a potential explanation for the “lack of” effect of mTOR deletion on T cell homeostasis and survival. Likewise, naïve T cells do not require mTOR for initial TCR-mediated cytokine production. However, such antigen recognition in the absence of mTOR induces a subsequent genetic program that prevents effector T cell differentiation and promotes adaptive regulatory T cell lineage commitment.
Finally, our genetic approach unequivocally identifies mTOR and its downstream effectors as potential pharmacologic targets for the prevention of effector T cell development and promoting tolerance. Previously, we have been able to demonstrate the ability of rapamycin but not cyclosporine A (which inhibits TCR signaling) to promote long term bone marrow chimerism in the absence of long term immunosupression (Powell et al., 2005). Along these lines recently it has been shown that both cyclosporine A and rapamycin inhibit IL-17 production in vitro but cyclosporine A inhibits while rapamycin promotes the generation of regulatory T cells (Kopf et al., 2007). Given the role of mTOR in promoting T effector generation, we propose that optimal immunosuppressive regimens would allow for TCR engagement, while simultaneously inhibiting cytokine production (e.g. costimulatory blockade) and inhibiting mTOR activation.
EXPERIMENTAL PROCEDURES
Mice
All mouse work was done in accordance with the Animal Care and Use Committee guidelines for Johns Hopkins University. Animals were housed in pathogen-free conditions prior to manipulations. mTORfl/fl mice were generated in the lab of S. C. Kozma and G. Thomas by selecting the original mTORneo ES cell clone (Gangloff et al., 2004) for loss of the neo cassette following transient transfection with a Cre expressing plasmid. The floxed mTOR (mTORfl/fl) mouse line was bred to B10.D2 mice carrying a CD4-Cre transgene and a TCR transgene specific for HA peptide (clone 6.5) (a gift from C. Drake, Johns Hopkins University, Baltimore, MD). Genotyping on tail DNA was performed as previously described(Safford et al., 2005). An amplification of ∼500bp by primers AC11 (5′-TTCATTCCCTTGAAAGCCAGTCTCACC) and AC14 (5′-TCATTACCTTCTCATCAGCCAGCAGTT) indicates the presence of a floxed mTOR allele. An amplification of ∼250bp indicates a wt mTOR allele. Amplification of primers AC11 and AC16 (5′-TTCATTCCCTTGAAAGCCAGTCTCACC) ∼550bp indicates an excised mTOR gene (as in Fig. 1A). PCR was used to genotype tail DNA for the presence of CD4-Cre (Forward 5′-CGATGCAACGAGTGATGAGG, Reverse 5′-CGCATAACCAGTGAAACAGC). Rhebfl/fl mice were generated by P. F. Worley (Xiao et al, manuscript in preparation). Genotyping on tail DNA was performed using primers RF1 (5′-GCCCAGAACATCTGTTCCAT) and RR1 (5′-GGTACCCACAACCTGACACC). 653 bp indicates a wild-type allele while 850 bp indicates a floxed allele.
FACS analysis
All plots shown are on populations that were gated on lymphocytes by forward and side scatter. All antibodies (except Foxp3) for FACS were purchased from BD Biosciences. The antibody and staining kit for Foxp3 was purchased from eBioscience (Foxp3-FITC, FJK-16s) (San Diego, CA).
Cell isolations
CD4+ T cells were isolated using the MACS CD4+ T cell Isolation Kit (Miltenyi Biotec, Auburn, CA), according to the manufacturer’s instructions. All isolations were performed using LS columns or the autoMACS cell separator. Where indicated, cells were labeled with CFSE in suspension at 10 × 106/mL with 5μM CFSE (BD Bioscience) for 15 minutes. The reaction was quenched with 2% FBS in PBS.
Cell stimulations
Cells were stimulated with anti-CD3 (2C11, BD Biosciences) and HA class II peptide (Peptide Core Facility, Johns Hopkins University) as indicated. Antigen-presenting cells were generated by isolating splenocytes from B10.D2 (syngeneic) mice. T cell depletion was performed by staining with anti-CD3-biotin and anti-biotin microbeads (MACS separation) (Miltenyi Biotec), according to the manufacturer’s instructions. Cells used as APCs were irradiated with 3000 rads in compliance with Radiation Safety at Johns Hopkins University. Cells skewed to Th1 conditions were cultured in media supplemented with mIL-12 (10ng/ml, Peprotech) and anti-IL-4 (10μg/mL, 11B11, NCI-Frederick Repository), with additional IL-2-supplemented media being added after 48 h. Cells skewed to Th2 were supplemented with mIL-4 (10ng/ml, Peprotech), anti-IL-12 (10μg/ml, Invitrogen), and anti-IFN-γ (10μg/ml, XMG1.2, a gift from D. Pardoll, Johns Hopkins University, Baltimore, MD), with additional IL-2 supplementation added after 48 h. Cells skewed to Th17 were supplemented with TGF-β (10ng/ml) and mIL-6 (10ng/ml) (Peprotech). TH17 cells were also isolated from Peyer’s patches by manual dissection, straining cells through a cell strainer, and stimulating all cells with PMA and ionomycin in the presence of GolgiStop.
Real-time PCR
Real-time PCR was performed essentially as described (Safford et al., 2005). Real time PCR used primers and probes obtained from Applied Biosystems (Foster City, CA) specific for T-bet (Tbx21, Mm00450960_m1), GATA-3 (Mm00484683_m1), IL-21 (Mm00517640_m1), and IL-23R (Mm00519942_m1) RORγt was probed as previously described (Harris et al., 2007).
Immunoblotting
Immunoblots were performed essentially as described(Safford et al., 2005). Primary antibodies were used against mTOR, phospho-S6K1(p70S6K) (T421/S424), pan S6K1 (p70S6K), phospho-Akt (T308), phospho-STAT3 (Y705), phospho-STAT6 (Y641), pan STAT3, pan STAT6 phospho-Smad3 (S423/S425), (Cell Signaling Technology, Danvers, MA), AKT1/2, pan Smad3, cyclin D3 (Santa Cruz, Santa Cruz, CA), phospho-Akt (S473) (Upstate, Chicago, IL), phospho-STAT4 (Y693) and pan STAT4 (Abcam, Cambridge, MA)
Detection of cytokines
Supernatants were removed and diluted for cytokine detection. Cytokines were detected using ELISA kits from eBioscience (IL-2, IL-4, IFN-γ, TGF-β) according to the manufacturer’s instructions.
In vitro suppression
CD4+CD25+ cells were isolated from enriched CD4+ 5-day T-mTOR-/- cultures by CD25+ positive selection (Miltenyi Biotec). Naïve 6.5+ T-cells were isolated from splenocytes using the CD4+ T cell isolation kit (negative selection) (Miltenyi Biotec) and CFSE labeled. T cell depleted, irradiated, syngeneic splenocytes were used as APCs to present HA peptide. Responder cells were interrogated for proliferation by FACS analysis via differential Thy expression.
Adoptive transfers
Host mice (B10.D2, Thy1.2) were injected i.p. with 1 × 106 PFU Vac-HA (a gift from C. Drake, Johns Hopkins University). 2 × 106 wt or mTOR deficient CD4+ T cells were injected i.v. 1 d post immunization. Splenocytes were harvested 5 d post-transfer and subjected to ex vivo rechallenge with HA peptide (100 μg/mL) in the presence of GolgiStop (BD Biosciences). 6 h post stimulation cells were harvested and stained using the BD Biosciences Cytofix/Cytoperm kit.
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank the members of the Powell and Drake Labs and Drs. Drew Pardoll, Jun Liu, and George Thomas for their helpful suggestions and reagent contributions. In addition, we would like to thank Dr. Ronald Schwartz with whom this line of inquiry was initiated. This work was supported by NIH grants R01CA098109 and R01CA14227.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abraham RT, Wiederrecht GJ. Immunopharmacology of rapamycin. Annu Rev Immunol. 1996;14:483–510. doi: 10.1146/annurev.immunol.14.1.483. [DOI] [PubMed] [Google Scholar]
- Apostolou I, Sarukhan A, Klein L, von Boehmer H. Origin of regulatory T cells with known specificity for antigen. Nat Immunol. 2002;3:756–763. doi: 10.1038/ni816. [DOI] [PubMed] [Google Scholar]
- Avruch J, Hara K, Lin Y, Liu M, Long X, Ortiz-Vega S, Yonezawa K. Insulin and amino-acid regulation of mTOR signaling and kinase activity through the Rheb GTPase. Oncogene. 2006;25:6361–6372. doi: 10.1038/sj.onc.1209882. [DOI] [PubMed] [Google Scholar]
- Basu S, Golovina T, Mikheeva T, June CH, Riley JL. Cutting edge: Foxp3- mediated induction of pim 2 allows human T regulatory cells to preferentially expand in rapamycin. J Immunol. 2008;180:5794–5798. doi: 10.4049/jimmunol.180.9.5794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettelli E, Korn T, Kuchroo VK. Th17: the third member of the effector T cell trilogy. Curr Opin Immunol. 2007 doi: 10.1016/j.coi.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombetti S, Basso V, Mueller DL, Mondino A. Prolonged TCR/CD28 engagement drives IL-2-independent T cell clonal expansion through signaling mediated by the mammalian target of rapamycin. J Immunol. 2006;176:2730–2738. doi: 10.4049/jimmunol.176.5.2730. [DOI] [PubMed] [Google Scholar]
- Denning TL, Wang YC, Patel SR, Williams IR, Pulendran B. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17- producing T cell responses. Nat Immunol. 2007;8:1086–1094. doi: 10.1038/ni1511. [DOI] [PubMed] [Google Scholar]
- Fox CJ, Hammerman PS, Thompson CB. The Pim kinases control rapamycin- resistant T cell survival and activation. J Exp Med. 2005;201:259–266. doi: 10.1084/jem.20042020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangloff YG, Mueller M, Dann SG, Svoboda P, Sticker M, Spetz JF, Um SH, Brown EJ, Cereghini S, Thomas G, Kozma SC. Disruption of the mouse mTOR gene leads to early postimplantation lethality and prohibits embryonic stem cell development. Mol Cell Biol. 2004;24:9508–9516. doi: 10.1128/MCB.24.21.9508-9516.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guertin DA, Stevens DM, Thoreen CC, Burds AA, Kalaany NY, Moffat J, Brown M, Fitzgerald KJ, Sabatini DM. Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCalpha, but not S6K1. Dev Cell. 2006;11:859–871. doi: 10.1016/j.devcel.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Harris TJ, Grosso JF, Yen HR, Xin H, Kortylewski M, Albesiano E, Hipkiss EL, Getnet D, Goldberg MV, Maris CH, et al. Cutting edge: An in vivo requirement for STAT3 signaling in TH17 development and TH17-dependent autoimmunity. J Immunol. 2007;179:4313–4317. doi: 10.4049/jimmunol.179.7.4313. [DOI] [PubMed] [Google Scholar]
- Haxhinasto S, Mathis D, Benoist C. The AKT-mTOR axis regulates de novo differentiation of CD4+Foxp3+ cells. J Exp Med. 2008 doi: 10.1084/jem.20071477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926–1945. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- Ivanov II, Zhou L, Littman DR. Transcriptional regulation of Th17 cell differentiation. Semin Immunol. 2007 doi: 10.1016/j.smim.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Kim JE, Liu HY, Cao W, Chen J. Regulation of interleukin-6- induced hepatic insulin resistance by mammalian target of rapamycin through the STAT3- SOCS3 pathway. J Biol Chem. 2008;283:708–715. doi: 10.1074/jbc.M708568200. [DOI] [PubMed] [Google Scholar]
- Kopf H, de la Rosa GM, Howard OM, Chen X. Rapamycin inhibits differentiation of Th17 cells and promotes generation of FoxP3+ T regulatory cells. Int Immunopharmacol. 2007;7:1819–1824. doi: 10.1016/j.intimp.2007.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretschmer K, Apostolou I, Hawiger D, Khazaie K, Nussenzweig MC, von Boehmer H. Inducing and expanding regulatory T cell populations by foreign antigen. Nat Immunol. 2005;6:1219–1227. doi: 10.1038/ni1265. [DOI] [PubMed] [Google Scholar]
- Lohr J, Knoechel B, Abbas AK. Regulatory T cells in the periphery. Immunol Rev. 2006;212:149–162. doi: 10.1111/j.0105-2896.2006.00414.x. [DOI] [PubMed] [Google Scholar]
- McMahon LP, Choi KM, Lin TA, Abraham RT, Lawrence JC., Jr. The rapamycin-binding domain governs substrate selectivity by the mammalian target of rapamycin. Mol Cell Biol. 2002;22:7428–7438. doi: 10.1128/MCB.22.21.7428-7438.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy KM, Reiner SL. The lineage decisions of helper T cells. Nat Rev Immunol. 2002;2:933–944. doi: 10.1038/nri954. [DOI] [PubMed] [Google Scholar]
- Powell JD. The induction and maintenance of T cell anergy. Clin Immunol. 2006;120:239–246. doi: 10.1016/j.clim.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Powell JD, Fitzhugh C, Kang EM, Hsieh M, Schwartz RH, Tisdale JF. Low-Dose Radiation Plus Rapamycin Promotes Long-Term Bone Marrow Chimerism. Transplantation. 2005;80:1541–1545. doi: 10.1097/01.tp.0000185299.72295.90. [DOI] [PubMed] [Google Scholar]
- Sabatini DM. mTOR and cancer: insights into a complex relationship. Nat Rev Cancer. 2006;6:729–734. doi: 10.1038/nrc1974. [DOI] [PubMed] [Google Scholar]
- Sadlack B, Lohler J, Schorle H, Klebb G, Haber H, Sickel E, Noelle RJ, Horak I. Generalized autoimmune disease in interleukin-2-deficient mice is triggered by an uncontrolled activation and proliferation of CD4+ T cells. Eur J Immunol. 1995;25:3053–3059. doi: 10.1002/eji.1830251111. [DOI] [PubMed] [Google Scholar]
- Safford M, Collins S, Lutz MA, Allen A, Huang CT, Kowalski J, Blackford A, Horton MR, Drake C, Schwartz RH, Powell JD. Egr-2 and Egr-3 are negative regulators of T cell activation. Nat Immunol. 2005;6:472–480. doi: 10.1038/ni1193. [DOI] [PubMed] [Google Scholar]
- Sauer S, Bruno L, Hertweck A, Finlay D, Leleu M, Spivakov M, Knight ZA, Cobb BS, Cantrell D, O’Connor E, et al. T cell receptor signaling controls Foxp3 expression via PI3K, Akt, and mTOR. Proc Natl Acad Sci U S A. 2008 doi: 10.1073/pnas.0800928105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song K, Wang H, Krebs TL, Danielpour D. Novel roles of Akt and mTOR in suppressing TGF-beta/ALK5-mediated Smad3 activation. Embo J. 2006;25:58–69. doi: 10.1038/sj.emboj.7600917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tone Y, Furuuchi K, Kojima Y, Tykocinski ML, Greene MI, Tone M. Smad3 and NFAT cooperate to induce Foxp3 expression through its enhancer. Nat Immunol. 2008;9:194–202. doi: 10.1038/ni1549. [DOI] [PubMed] [Google Scholar]
- van Santen HM, Benoist C, Mathis D. Number of T reg cells that differentiate does not increase upon encounter of agonist ligand on thymic epithelial cells. J Exp Med. 2004;200:1221–1230. doi: 10.1084/jem.20041022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver CT, Harrington LE, Mangan PR, Gavrieli M, Murphy KM. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity. 2006;24:677–688. doi: 10.1016/j.immuni.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Wiederrecht GJ, Sabers CJ, Brunn GJ, Martin MM, Dumont FJ, Abraham RT. Mechanism of action of rapamycin: new insights into the regulation of G1-phase progression in eukaryotic cells. Prog Cell Cycle Res. 1995;1:53–71. doi: 10.1007/978-1-4615-1809-9_5. [DOI] [PubMed] [Google Scholar]
- Zeng Z, Sarbassov dos D, Samudio IJ, Yee KW, Munsell MF, Ellen Jackson C, Giles FJ, Sabatini DM, Andreeff M, Konopleva M. Rapamycin derivatives reduce mTORC2 signaling and inhibit AKT activation in AML. Blood. 2007;109:3509–3512. doi: 10.1182/blood-2006-06-030833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Collins SL, Lutz MA, Allen AN, Kole TP, Zarek PE, Powell JD. A Role for Mammalian Target of Rapamycin in Regulating T Cell Activation versus Anergy. J Immunol. 2007;178:2163–2170. doi: 10.4049/jimmunol.178.4.2163. [DOI] [PubMed] [Google Scholar]
- Zhou J, Wulfkuhle J, Zhang H, Gu P, Yang Y, Deng J, Margolick JB, Liotta LA, Petricoin E, 3rd, Zhang Y. Activation of the PTEN/mTOR/STAT3 pathway in breast cancer stem-like cells is required for viability and maintenance. Proc Natl Acad Sci U S A. 2007;104:16158–16163. doi: 10.1073/pnas.0702596104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Lopes JE, Chong MM, Ivanov II, Min R, Victora GD, Shen Y, Du J, Rubtsov YP, Rudensky AY, et al. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature. 2008;453:236–240. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.