Abstract
The PI3K/Akt/mTOR signaling pathway is critical for cellular growth and survival in skeletal muscle, and is activated in response to growth factors such as insulin-like growth factor-I (IGF-I). We found that in C2C12 myoblasts, deficiency of PI3K p110 catalytic subunits or Akt isoforms had distinct effects on phosphorylation of mTOR and p70S6K. siRNA-mediated knockdown of PI3K p110α, p110β, and simultaneous knockdown of p110α and p110β resulted in increased basal and IGF-I-stimulated phosphorylation of mTOR S2448 and p70S6K T389; however, phosphorylation of S6 was reduced in p110β-deficient cells, possibly due to reductions in total S6 protein. We found that IGF-I-stimulated Akt1 activity was enhanced in Akt2- or Akt3-deficient cells, and that knockdown of individual Akt isoforms increased mTOR/p70S6K activation in an isoform-specific fashion. Conversely, levels of IGF-I-stimulated p70S6K phosphorylation in cells simultaneously deficient in both Akt1 and Akt3 were increased beyond those seen with loss of any single Akt isoform, suggesting an alternate, Akt-independent mechanism that activates mTOR/p70S6K. Our results collectively suggest that mTOR/p70S6K is activated in a PI3K/Akt-dependent manner, but that in the absence of p110α or Akt, alternate pathway(s) may mediate activation of mTOR/p70S6K in C2C12 myoblasts.
Keywords: PI3K, Akt, mTOR, S6K, myoblast
Introduction
Cellular growth, survival, and energy homeostasis are mediated by a number of intracellular signaling cascades; one important pathway is the phosphoinositide 3-kinase/Akt/mammalian target of rapamycin (PI3K/Akt/mTOR) pathway. PI3K is recruited to the cell membrane upon growth factor stimulation and catalyzes the production of phosphatidylinositol (3,4,5)-trisphosphate [PI(3,4,5)P3] from phosphatidylinositol (4,5)-bisphosphate [PI(4,5)P2], allowing for binding and activation of downstream effector molecules (1-4). PI3K Class IA catalytic p110α and p110β subunits are ubiquitous; p110α plays a dominant role in growth factor-induced PI(3,4,5)P3 production (5, 6), while p110β mediates G-protein-coupled receptor signal transduction (7), may maintain a pool of PI(3,4,5)P3 (6, 8), and possesses a non-catalytic, scaffolding function (8-10). Although previous research has identified these general actions of PI3K in several cell types, the contribution of different PI3K p110 catalytic subunits on mTOR-mediated processes in myoblasts is less clear.
Secondary lipid messenger production by PI3K promotes membrane recruitment of AGC kinases such as Akt, whose actions support cellular growth and survival (1, 11-13). In mammals, three Akt isoforms have been identified; Akt1−/− mice have small body size, Akt2−/− mice have a diabetic phenotype, and Akt3−/− mice have reduced brain size (14-17). There is also mounting evidence that redundant as well as overlapping functions exists between isoforms (18-21); however, little is known regarding isoform-specific Akt regulation of mTOR.
The mTOR/ regulatory associated protein of mTOR (Raptor) complex (mTORC1) mediates numerous intracellular processes, including protein synthesis and survival, through actions on target molecules including the 70 kDa ribosomal S6 kinase (p70S6K) (22). Activation of mTOR is regulated in part by Akt through phosphorylation of proline-rich Akt substrate of 40 kDa (PRAS40) (23) and tuberous sclerosis complex 2 (TSC2) (24), thus placing Akt upstream of mTOR. However, when mTOR is associated with rapamycin-insensitive companion of mTOR (Rictor), a complex referred to as mTORC2, PI3K-mediated activation of mTORC2 can phosphorylate Akt at the hydrophobic motif (Serine 473 in Akt1), thus placing mTOR upstream of Akt (25-27). mTOR-activated p70S6K phosphorylates ribosomal protein S6 and directly phosphorylates mTOR at Serine 2448 (28, 29), although the biological significance of mTOR S2448 phosphorylation is not fully understood. Despite our current knowledge of these regulatory events, there still remain significant questions with respect to upstream regulation of Akt and mTOR. Mapping the complexity of the multiple forward and feedback loops in Akt/mTOR/p70S6K pathways is essential to gain a greater understanding of these processes.
We report here that C2C12 myoblasts deficient in Class IA PI3K p110 isoforms, particularly p110β, show increases in phosphorylation of mTOR and p70S6K. Additionally, we show that knockdown of Akt2 or Akt3 results in hyper-activation of Akt1 in response to IGF-I stimulation. We also found that, despite being downstream of Akt, levels of phosphorylated p70S6K were actually increased under conditions of Akt isoform deficiency. Finally, we show that activation of mTOR/p70S6K can still occur despite reductions in Akt, suggesting the existence of an Akt-independent mechanism that activates mTOR/p70S6K in myoblasts.
Methods
Materials
C2C12 murine myoblasts originally purchased from ATCC (Manassas, VA) were a kind gift from Dr. John C. Lee of The University of Texas Health Science Center at San Antonio. Bovine serum albumin (fraction V) was purchased from Sigma (St. Loius, MO). rhIGF-I was purchased form Austral Biologicals (San Ramon, CA). Primary antibodies directed against p110β and GAPDH and HRP-linked secondary antibodies were purchased from Santa Cruz (Santa Cruz, CA); all other antibodies, as well as Rapamycin, were obtained from Cell Signaling Technologies (Danvers, MA).
Cell culture conditions and siRNA transfections
C2C12 myoblasts were maintained in high-glucose DMEM containing 10% FBS and antibiotics (growth medium), with medium being replenished after 24-hours. Forty-eight hours after initial seeding cells were ~95% confluent and experiments were conducted under these conditions (in growth medium). Pre-designed Silencer Select siRNAs for mouse p110α (ID# s71604), p110β (ID# s93108), Akt1 ( ID# s62215), Akt2 (ID# s62219), Akt3 (ID# s76463), and negative controls (non-targeting siRNA ID# 4390843) were purchased from Ambion Inc. (Austin, TX). Cells (1.8×10^5/well in 6-well culture dish) were reverse-transfected with double-stranded siRNA in antibiotic-free DMEM plus 10% FBS using Lipofectamine 2000 according to manufacturer's instructions (Invitrogen). Twenty-four hours after reverse transfection, medium was changed to DMEM with 10% FBS containing antibiotics. RNA was isolated 48-hours after transfections using RNA-STAT (Tel-test, Friendswood, TX), quantified, and the level of silencing determined by real-time PCR as described later in this section.
RNA Isolation, cDNA synthesis, and real-time PCR
Conversion of total RNA to cDNA was accomplished using the High-Capacity cDNA Archive Kit (P/N 4322171; Applied Biosystems, Foster City, CA). Briefly, 2 μg total RNA was reverse transcribed using random primers for the following incubation times: 25° C for 10 minutes, then 37° C for 2 hours. cDNA samples were stored at -80° C until use. TaqMan-MGB p110α (Mm00435673_m1), p110β (Mm00659576_m1), Akt1 (Mm00437443_m1), Akt2 (Mm00545827_m1), Akt3 (Mm00442194_m1), and B2M (Mm00437762_m1) probe and primers were purchased from Applied Biosystems as “Gene Expression Assays.” The real-time PCR reaction was performed within an ABI 7500 thermal cycler. The fluorescence of 3 to 15 cycles was set up as background. Data was collected at the annealing step of each cycle, and the threshold cycle (Ct) for each sample calculated by determining the point at which the fluorescence exceeded the threshold limit. The standard curve was calculated automatically via software by plotting the Ct values against each standard of known concentration and calculation of the linear regression line of this curve. Serially diluted amounts of cDNA were used to establish standard curves. All samples were run in duplicate.
Protein extraction, immunoprecipitation, and Western immunoblot
Protein extraction was preformed exactly as described previously (21). Protein concentrations were determined by the method of Bradford (30). For Western blotting, equal amounts of cell lysate proteins (typically 25μg) were electrophoresed through 4 – 20% NuPage gels (Invitrogen). Proteins were transferred to PVDF membranes (Millipore Corp., Bedford MA). Membranes were incubated for 1 h in 5% dry milk solution in Tris-buffered saline plus 0.5% Tween-20 (TBST) and then incubated with the appropriate primary antibody at an appropriate dilution overnight in 5% BSA in TBST. Membranes were washed three times in TBST followed by incubation with the appropriate secondary antibody and again washed three times. Membranes were incubated with enhanced chemiluminescence reagents (Thermo Fisher, Rockford, Il) and exposed to film.
In Vitro kinase assay
Immune complex-kinase assays were performed by a protocol from an assay kit purchased from Cell Signaling technologies. Cell lysates (200 μg) were incubated with anti-Akt1, anti-Akt2, or anti-Akt3 antibodies overnight at 4°C in cell lysis buffer. Immune complexes were then rotated for 2 – 4 h at 4°C with protein A-conjugated agarose beads (20 μl of 50% slurry/reaction). Complexes were washed twice in cell lysis buffer and twice in kinase buffer (25 mM Tris-HCl (pH 7.5), 5 mM beta-glycerophosphate, 2 mM dithiothreitol, 0.1 mM Na3VO4, 10 mM MgCl2). After resuspension in kinase assay buffer containing ATP and a GST-GSK-3 α/β fusion protein (residues surrounding GSK-3 α/β Ser21/9 (CGPKGPGRRGRRRTSSFAEG)) the reaction was allowed to proceed at 30°C for 30 min. After the reaction was stopped by addition of concentrated SDS-PAGE loading buffer, samples were separated by SDS-PAGE and transferred to PVDF membranes as described above. Immunoblotting was performed using primary antibodies to phospho-GSK-3α/β provided in the kinase assay kit, followed by addition of HRP-conjugated secondary antibodies, detection by ECL, and exposure to X-ray film. Results were quantitated by densitometry.
Statistics
Data are presented as means ± S.E.M. Statistics were performed using oneway ANOVA with Dunnett's test a posteriori, as described in figure legends. Densiometric analysis was performed using Image J 1.60 (NIH). A P-value < 0.05 was considered significant.
Results
Deficiency of p110 isoforms is associated with increased mTOR/p70S6K activation
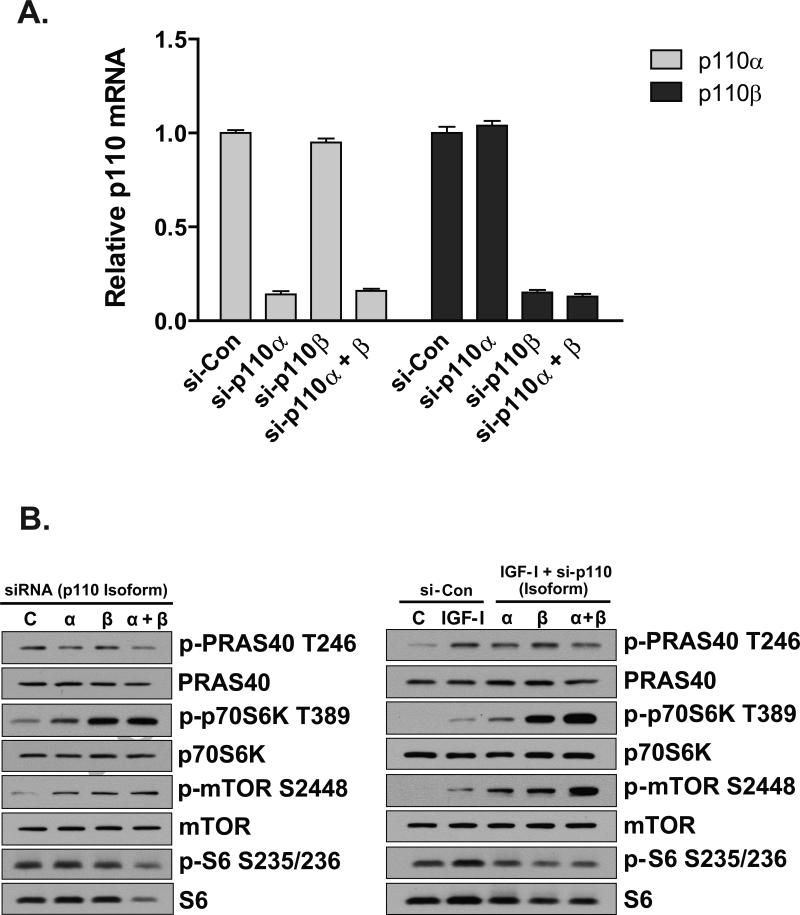
To establish efficacy of siRNA-mediated knockdown of p110α and p110β, cells were transfected with non-targeting control siRNA (si-Con), siRNA against p110α (si-p110α), siRNA against p110β (si-p110β), or siRNA against p110α and p110β simultaneously (p110α + p110β). Figure 1A demonstrates that siRNA-mediated knockdown of p110α and p110β was ~85%.
Figure 1.
Knockdown of p110α and p110β stimulate AMPK and mTOR/p70S6K phosphorylation. (A) Efficacy of RNAi-mediated knockdown of p110α and p110β was determined by real-time PCR using B2M as a reference gene. Cells were reverse-transfected with 10nM negative control siRNA (si-Con) or 5nM siRNA against p110α (si-p110α) plus 5nM si-Con, 5nM p110β (si-p110β) plus 5nM si-Con, or simultaneous transfection (co-transfection) with 5nM p110α and 5nM p110β (si-p110α \ β). Cells were maintained until harvest as described in Materials and Methods. Values are expressed relative to si-Con which was arbitrarily set at 1.0 for p110α and p110β. Columns are averages of independent duplicate determinations and error bars indicate S.E.M. (B) Cells were reverse-transfected and maintained as described in (A). Forty-eight hours after transfection, cells were harvested (left column) or treated with 125ng/ml IGF-I for 30-minutes and then harvested for protein lysates (right column). Western blotting was performed using antibodies indicated to the right of the respective images. Blots are representative of two independent experiments.
To determine the effects of p110 isoform deficiency on signaling molecules involved in protein synthesis and energy regulation, cells were grown for 48 hours and harvested (Figure 1B, left), or grown for 48 hours and then treated with 125 ng/ml IGF-I for 30 minutes (Figure 1B, right), followed by analysis by Western blot. Phosphorylation of PRAS40 at the Akt-directed site (T246) was reduced under conditions of p110α deficiency, suggesting that Akt is activated primarily through p110α. However, phosphorylation of p70S6K at T389 and mTOR at S2448 were increased in p110α-, p110β-, and p110α + p110β-deficient cells (Figure 1B). IGF-I-induced phosphorylation of S6 at S235/236 was reduced in p110α and p110β-deficient cells, possibly the result of decreased total S6 protein (Figure 1B). Together, these data suggest increased activation of components of the protein translational machinery under conditions of decreased PI3K catalytic subunit availability.
Akt1 and Akt3 are activated by IGF-I in myoblasts, and reduction of Akt2 or Akt3 causes hyper-activation of Akt1
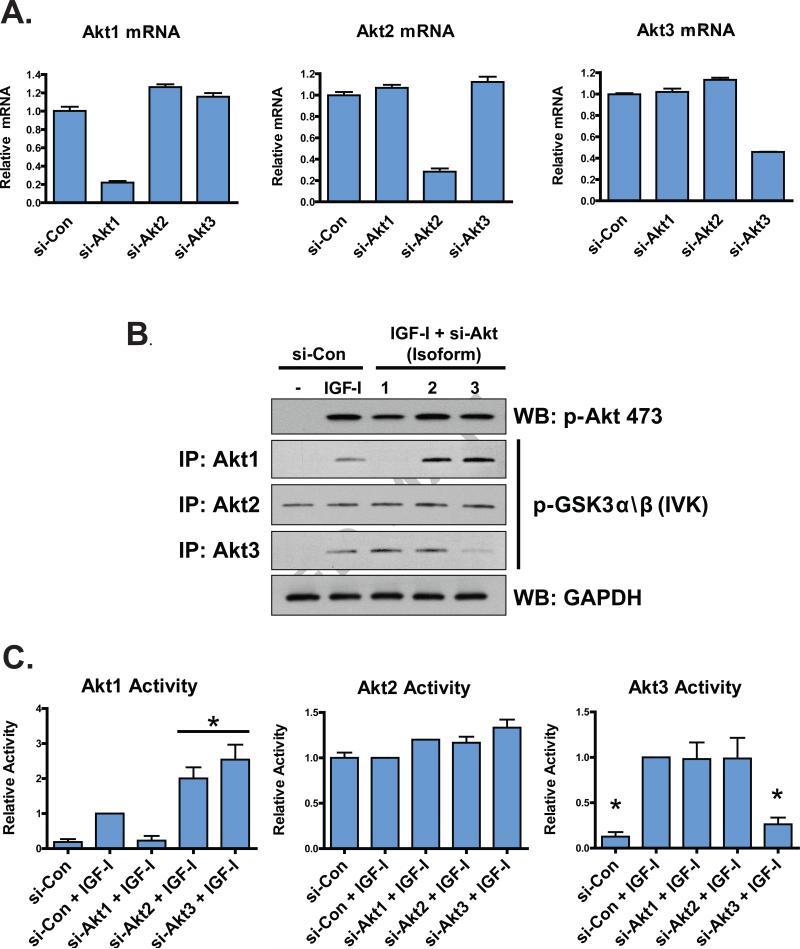
Akt is a principal mediator of PI3K-mediated cell growth and survival, and recent evidence suggests that individual Akt isoforms can exhibit both overlapping and distinct functions (31). We examined the activation of Akt to determine whether loss of one isoform results in increased activation of another in C2C12 cells. siRNA-mediated knockdown of Akt1, Akt2, and Akt3 was effective (Figure 2A), and IGF-I promoted the activation of Akt1 and Akt3, but not Akt2; furthermore, under conditions of Akt2 and Akt3 deficiency, the activation of Akt1 in response to IGF-I was greater than in cells expressing normal levels of Akt2 and Akt3 (P < 0.05, Figure 2B and 2C). These data suggest that IGF-I-stimulated Akt1 activity is hyper-activated under conditions of diminished Akt2 and Akt3, but neither Akt2 nor Akt3 activation is hyper-stimulated by IGF-I under conditions of diminished Akt1.
Figure 2.
Efficacy of si-RNA-mediated knockdown of Akt isoforms and IGF-I-stimulated Akt activation. (A) Efficacy of RNAi-mediated knockdown of Akt isoforms was determined by real-time PCR normalizing to invariant B2M as a reference gene. Cells were reverse-transfected with 5 nM negative control siRNA (si-Con), 5 nM siRNA against Akt1 (si-Akt1), 5 nM siRNA against Akt2 (si-Akt2) or 5 nM siRNA against Akt3 (si-Akt3). Cells were maintained until harvest as described in Materials and Methods. Values are expressed relative to si-Con for each isoform, which was arbitrarily set at 1.0. Columns represent averages of two independent duplicate experiments and error bars indicate S.E.M. (B) Activation of Akt isoforms was determined by immunoprecipitation with isoform-specific antibodies followed by in vitro kinase assays (IVK) using a GSTGSK3α/β fusion protein as substrate. Western blotting was performed on at least 3 independent experiments to detect levels of phosphorylated GSK3α/β, and the intensities of the bands normalized to GAPDH for each specific run. Phosphorylation of Akt S473 is also shown. “WB” – Western blot without in vitro kinase assay performed. (C) Quantification of Akt isoform activation from (B). Asterisks indicate significant differences (P < 0.05) from IGF-I-treated cells also treated with negative control siRNA as measured by oneway ANOVA followed by Dunnet's test. A horizontal bar spans multiple treatments that statistically differed from IGF-I-treated control cells. Error bars indicate S.E.M.
Increased phosphorylation of mTOR/p70S6K in response to Akt knockdown
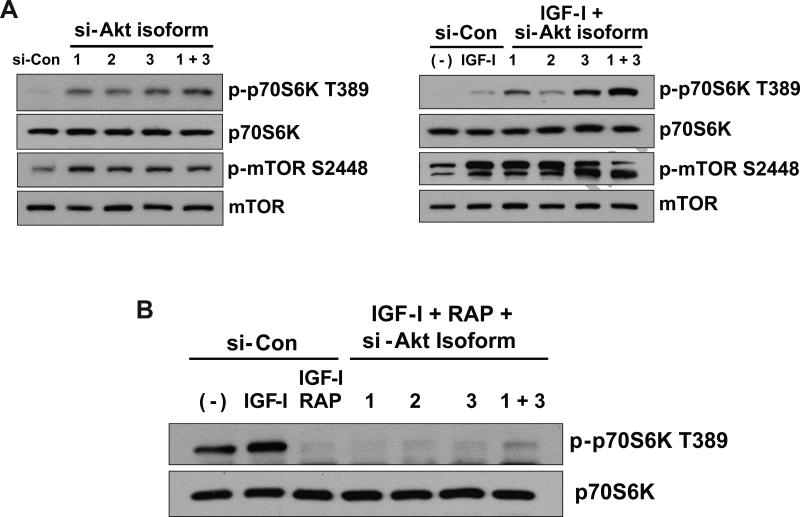
Akt is a principal molecule involved in PI3K-mediated mTOR activation (22). To identify the Akt isoforms involved in mTOR activation, cells deficient in Akt isoforms were examined, and levels of phosphorylated p70S6K at T389 were used as a determinant of mTOR activation. Deficiency of Akt isoforms resulted in increased basal and IGF-I-stimulated phospho-p70S6K T389 and phospho-mTOR S2448 in an Akt isoform-specific fashion (Figure 3A). Akt1-deficient cells had greater basal and IGF-I-stimulated p70S6K phosphorylation than cells treated with control siRNA, and Akt3-deficient cells had greater basal and IGF-I-stimulated p70S6K phosphorylation than Akt1-deficient cells. Both unstimulated and IGF-I-treated cells deficient in both Akt1 and Akt3 showed levels of phospho-p70S6K greater than those seen with deficiency of either Akt1 or Akt3 alone (Figure 3A). Pre-treatment with 1 nM Rapamycin inhibited p70S6K T389 phosphorylation (Figure 3B), suggesting that the increases in p70S6K T389 phosphorylation seen in Akt isoform-deficient cells may be mediated through mTOR. Moreover, in IGF-I- and rapamycin-treated cells simultaneously deficient in Akt1 and Akt3, p70S6K phosphorylation was not completely eliminated (Figure 3B), further suggesting an Akt-independent activation of mTOR/p70S6K, as well as rapamycin-insensitive activation of p70S6K under conditions of low Akt abundance in myoblasts.
Figure 3.
Regulation of mTOR and p70S6K under conditions of Akt isoform deficiency. (A) Reverse-transfections using siRNA directed against Akt isoforms were performed with the following siRNA concentrations: 10 nM negative control siRNA (si-Con), 5 nM siRNA against Akt1 (si-Akt1) plus 5 nM si-Con; 5 nM siRNA against Akt2 (si-Akt2) plus 5 nM si-Con; 5 nM siRNA against Akt3 (si-Akt3) plus 5 nM si-Con; or 5 nM si-Akt1 + 5 nM si-Akt3. After 48-hours, cells were either harvested (Left panel), or treated with 16.6 nM IGF-I for 30-minutes and then harvested (Right panel). Levels of total and phosphorylated mTOR and p70S6K were determined using antibodies indicated to the right of the images. Blots are representative of two independent experiments. (B) Reverse-transfections using siRNA directed against Akt isoforms were performed as described in (A). After 48-hours, the indicated cells were pre-treated with 1 nM Rapamycin for 1-hour followed by 125 ng/ml IGF-I for 30-minutes. Cells were harvested and Western blots performed using antibodies indicated at the right of the images.
Discussion
Our observations that phosphorylation of p70S6K T389, and mTOR S2448 are increased in cells deficient in p110α, p110β, and p110α + p110β may reflect a compensatory response to a deficit in growth/translational paths. In fact, it has recently been shown that both p110α and p110β contribute to S-phase entry (32). In this study, p70S6K phosphorylation was elevated in cells deficient in p110α, p110β, and p110α + p110β, concomitant with decreased phosphorylation of S6. This latter decrease was associated with reduced levels of total S6, particularly in cells simultaneously deficient in p110α and p110β, suggesting a role for PI3K in mediating levels of S6. Current models suggest that Akt positively regulates mTOR/p70S6K action by acting on mTOR-inhibitory molecules PRAS40 and TSC2 (22, 24, 33, 34), and previous work from our laboratory has established that PI3K p110α is the primary PI3K catalytic isoform involved in IGF-I-stimulated Akt activation in C2C12 cells (Matheny and Adamo, manuscript in press). Thus, the increased mTOR/p70S6K activation in p110α-deficient cells observed in this study suggests that Akt-independent mechanisms may be responsible. We speculate that, under normal conditions, p110α mediates mTOR/p70S6K activation through Akt; however, loss of p110α attenuates Akt activation, to which the cell responds by activating a yet unidentified pathway(s) that enhance activation of mTOR/p70S6K.
Increased mTOR/p70S6K activation in p110β-deficient cells may be secondary to increased p110α/Akt activation due to p110β deficiency; indeed, higher levels of p-Akt S473 were seen in p110β -/- MEFs in response to insulin stimulation (8), or in p110β-deficient C2C12 myoblasts in response to IGF-I (Matheny and Adamo, manuscript in press). However, the results from this study cannot rule out contribution from Akt-independent mechanism(s) in activating mTOR/p70S6K in p110β-deficient cells. Overall, these observations suggest that p110α and/or p110β deficiency is associated with increased activation of mTOR/p70S6K, which may represent a compensatory mechanism to promote growth and survival under conditions of reduced isoform-specific PI3K signaling.
Knockdown of Akt isoforms was associated with increased IGF-I-stimulated phosphorylation of p70S6K T389 in an Akt isoform-specific- and rapamycin-sensitive manner, suggesting the involvement of mTORC1 (Figure 3). The increases in IGF-I-stimulated p70S6K phosphorylation seen in Akt2- and Akt3-deficient cells may be explained by hyper-activation Akt1 (Figure 2). However, simultaneous knockdown of Akt1 and Akt3 actually resulted in greater increases in p70S6K T389 phosphorylation (Figure 3A, Right), an effect not fully abolished by rapamycin (Figure 3B), suggesting that phosphorylation of p70S6K T389 can occur independent of Akt and mTORC1 under conditions of greatly diminished Akt activation. Taken together, these data suggest the possibility that isoform-specific compensatory activation of Akt, as well as Akt-independent actions, may positively regulate mTOR and p70S6K under these conditions in myoblasts. The observation that mTOR/p70S6K activation was increased in p110α-deficient cells (Figure 1B), and Akt activation is significantly impaired in p110α-deficient cells (Matheny and Adamo, manuscript in press), suggests that decreases in Akt activation, whether through p110α deficiency or Akt deficiency, may provide feedback to an Akt-independent mechanism that activates mTOR/p70S6K. Indeed, recent reports suggest the involvement of PKC (35), nutrients (36), or downstream mediators of Thyroid Stimulating Hormone in Akt-independent activation of mTOR (37). That loss of specific Akt isoforms is associated with differential activation of mTOR/p70S6K, suggests that isoform-specific feedback may exist.
In summary, our results highlight the role of PI3K p110α and p110β deficiency on increased mTOR/p70S6K activation in C2C12 cells. Furthermore, our findings demonstrate that mTOR/p70S6K signaling is enhanced in cells deficient in Akt isoforms. Finally, our results suggest that a PI3K p110α/Akt pathway may regulate mTOR/p70S6K activation in C2C12 myoblasts, but that Akt-independent mechanisms may exist that activate mTOR/p70S6K when activation of Akt is reduced.
Acknowledgement
The authors wish to thank Dr. John C. Lee of the Department of Biochemistry, University of Texas Health Science Center at San Antonio, for many helpful discussions and technical support during the preparation of this work.
This work was supported by NIA grant R01AG026012 to MLA. RWM was supported by NIA training grant T32 AG021890-08.
Abbreviations
- PI3K
phosphoinositide 3-kinase
- mTOR
mammalian target of rapamycin
- p70S6K
70 kDa ribosomal protein S6 kinase
- IGF-I
insulin-like growth factor-I
- PRAS40
proline-rich Akt substrate of 40 kDa
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–74. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vanhaesebroeck B, Waterfield MD. Signaling by distinct classes of phosphoinositide 3-kinases. Exp Cell Res. 1999;253:239–54. doi: 10.1006/excr.1999.4701. [DOI] [PubMed] [Google Scholar]
- 3.Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PR, Reese CB, Cohen P. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr Biol. 1997;7:261–9. doi: 10.1016/s0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- 4.Hirsch E, Costa C, Ciraolo E. Phosphoinositide 3-kinases as a common platform for multi-hormone signaling. J Endocrinol. 2007;194:243–56. doi: 10.1677/JOE-07-0097. [DOI] [PubMed] [Google Scholar]
- 5.Foukas LC, Claret M, Pearce W, Okkenhaug K, Meek S, Peskett E, Sancho S, Smith AJ, Withers DJ, Vanhaesebroeck B. Critical role for the p110alpha phosphoinositide-3-OH kinase in growth and metabolic regulation. Nature. 2006;441:366–70. doi: 10.1038/nature04694. [DOI] [PubMed] [Google Scholar]
- 6.Knight ZA, Gonzalez B, Feldman ME, Zunder ER, Goldenberg DD, Williams O, Loewith R, Stokoe D, Balla A, Toth B, Balla T, Weiss WA, Williams RL, Shokat KM. A pharmacological map of the PI3-K family defines a role for p110alpha in insulin signaling. Cell. 2006;125:733–47. doi: 10.1016/j.cell.2006.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guillermet-Guibert J, Bjorklof K, Salpekar A, Gonella C, Ramadani F, Bilancio A, Meek S, Smith AJ, Okkenhaug K, Vanhaesebroeck B. The p110beta isoform of phosphoinositide 3-kinase signals downstream of G protein-coupled receptors and is functionally redundant with p110gamma. Proc Natl Acad Sci U S A. 2008;105:8292–7. doi: 10.1073/pnas.0707761105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jia S, Liu Z, Zhang S, Liu P, Zhang L, Lee SH, Zhang J, Signoretti S, Loda M, Roberts TM, Zhao JJ. Essential roles of PI(3)K-p110beta in cell growth, metabolism and tumorigenesis. Nature. 2008;454:776–9. doi: 10.1038/nature07091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marques M, Kumar A, Poveda AM, Zuluaga S, Hernandez C, Jackson S, Pasero P, Carrera AC. Specific function of phosphoinositide 3-kinase beta in the control of DNA replication. Proc Natl Acad Sci U S A. 2009;106:7525–30. doi: 10.1073/pnas.0812000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ciraolo E, Iezzi M, Marone R, Marengo S, Curcio C, Costa C, Azzolino O, Gonella C, Rubinetto C, Wu H, Dastru W, Martin EL, Silengo L, Altruda F, Turco E, Lanzetti L, Musiani P, Ruckle T, Rommel C, Backer JM, Forni G, Wymann MP, Hirsch E. Phosphoinositide 3-kinase p110beta activity: key role in metabolism and mammary gland cancer but not development. Sci Signal. 2008;1:ra3. doi: 10.1126/scisignal.1161577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–68. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 12.Kim AH, Khursigara G, Sun X, Franke TF, Chao MV. Akt phosphorylates and negatively regulates apoptosis signal-regulating kinase 1. Mol Cell Biol. 2001;21:893–901. doi: 10.1128/MCB.21.3.893-901.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–41. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 14.Dummler B, Hemmings BA. Physiological roles of PKB/Akt isoforms in development and disease. Biochem Soc Trans. 2007;35:231–5. doi: 10.1042/BST0350231. [DOI] [PubMed] [Google Scholar]
- 15.Cho H, Thorvaldsen JL, Chu Q, Feng F, Birnbaum MJ. Akt1/PKBalpha is required for normal growth but dispensable for maintenance of glucose homeostasis in mice. J Biol Chem. 2001;276:38349–52. doi: 10.1074/jbc.C100462200. [DOI] [PubMed] [Google Scholar]
- 16.Cho H, Mu J, Kim JK, Thorvaldsen JL, Chu Q, Crenshaw EB, 3rd, Kaestner KH, Bartolomei MS, Shulman GI, Birnbaum MJ. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta). Science. 2001;292:1728–31. doi: 10.1126/science.292.5522.1728. [DOI] [PubMed] [Google Scholar]
- 17.Easton RM, Cho H, Roovers K, Shineman DW, Mizrahi M, Forman MS, Lee VM, Szabolcs M, de Jong R, Oltersdorf T, Ludwig T, Efstratiadis A, Birnbaum MJ. Role for Akt3/protein kinase Bgamma in attainment of normal brain size. Mol Cell Biol. 2005;25:1869–78. doi: 10.1128/MCB.25.5.1869-1878.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu X, Shi Y, Birnbaum MJ, Ye K, De Jong R, Oltersdorf T, Giranda VL, Luo Y. Quantitative analysis of anti-apoptotic function of Akt in Akt1 and Akt2 double knock-out mouse embryonic fibroblast cells under normal and stressed conditions. J Biol Chem. 2006;281:31380–8. doi: 10.1074/jbc.M606603200. [DOI] [PubMed] [Google Scholar]
- 19.Sale EM, Hodgkinson CP, Jones NP, Sale GJ. A new strategy for studying protein kinase B and its three isoforms. Role of protein kinase B in phosphorylating glycogen synthase kinase-3, tuberin, WNK1, and ATP citrate lyase. Biochemistry. 2006;45:213–23. doi: 10.1021/bi050287i. [DOI] [PubMed] [Google Scholar]
- 20.Chin YR, Toker A. Function of Akt/PKB signaling to cell motility, invasion and the tumor stroma in cancer. Cell Signal. 2009;21:470–6. doi: 10.1016/j.cellsig.2008.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matheny RW, Jr., Adamo ML. Role of Akt isoforms in IGF-I-mediated signaling and survival in myoblasts. Biochem Biophys Res Commun. 2009 doi: 10.1016/j.bbrc.2009.08.101. DOI: 10.1016/j.bbrc.2009.08.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pende M. mTOR, Akt, S6 kinases and the control of skeletal muscle growth. Bull Cancer. 2006;93:E39–43. [PubMed] [Google Scholar]
- 23.Kovacina KS, Park GY, Bae SS, Guzzetta AW, Schaefer E, Birnbaum MJ, Roth RA. Identification of a proline-rich Akt substrate as a 14-3-3 binding partner. J Biol Chem. 2003;278:10189–94. doi: 10.1074/jbc.M210837200. [DOI] [PubMed] [Google Scholar]
- 24.Inoki K, Li Y, Zhu T, Wu J, Guan KL. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol. 2002;4:648–57. doi: 10.1038/ncb839. [DOI] [PubMed] [Google Scholar]
- 25.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 26.Alessi DR, Pearce LR, Garcia-Martinez JM. New insights into mTOR signaling: mTORC2 and beyond. Sci Signal. 2009;2:pe27. doi: 10.1126/scisignal.267pe27. [DOI] [PubMed] [Google Scholar]
- 27.Guertin DA, Sabatini DM. The pharmacology of mTOR inhibition. Sci Signal. 2009;2:pe24. doi: 10.1126/scisignal.267pe24. [DOI] [PubMed] [Google Scholar]
- 28.Chiang GG, Abraham RT. Phosphorylation of mammalian target of rapamycin (mTOR) at Ser-2448 is mediated by p70S6 kinase. J Biol Chem. 2005;280:25485–90. doi: 10.1074/jbc.M501707200. [DOI] [PubMed] [Google Scholar]
- 29.Holz MK, Blenis J. Identification of S6 kinase 1 as a novel mammalian target of rapamycin (mTOR)-phosphorylating kinase. J Biol Chem. 2005;280:26089–93. doi: 10.1074/jbc.M504045200. [DOI] [PubMed] [Google Scholar]
- 30.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 31.Brognard J, Sierecki E, Gao T, Newton AC. PHLPP and a second isoform, PHLPP2, differentially attenuate the amplitude of Akt signaling by regulating distinct Akt isoforms. Mol Cell. 2007;25:917–31. doi: 10.1016/j.molcel.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 32.Marques M, Kumar A, Cortes I, Gonzalez-Garcia A, Hernandez C, Moreno-Ortiz MC, Carrera AC. Phosphoinositide 3-kinases p110alpha and p110beta regulate cell cycle entry, exhibiting distinct activation kinetics in G1 phase. Mol Cell Biol. 2008;28:2803–14. doi: 10.1128/MCB.01786-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sancak Y, Thoreen CC, Peterson TR, Lindquist RA, Kang SA, Spooner E, Carr SA, Sabatini DM. PRAS40 is an insulin-regulated inhibitor of the mTORC1 protein kinase. Mol Cell. 2007;25:903–15. doi: 10.1016/j.molcel.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 34.Vander Haar E, Lee SI, Bandhakavi S, Griffin TJ, Kim DH. Insulin signalling to mTOR mediated by the Akt/PKB substrate PRAS40. Nat Cell Biol. 2007;9:316–23. doi: 10.1038/ncb1547. [DOI] [PubMed] [Google Scholar]
- 35.Fan QW, Cheng C, Knight ZA, Haas-Kogan D, Stokoe D, James CD, McCormick F, Shokat KM, Weiss WA. EGFR signals to mTOR through PKC and independently of Akt in glioma. Sci Signal. 2009;2:ra4. doi: 10.1126/scisignal.2000014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wlodarski P, Kasprzycka M, Liu X, Marzec M, Robertson ES, Slupianek A, Wasik MA. Activation of mammalian target of rapamycin in transformed B lymphocytes is nutrient dependent but independent of Akt, mitogen-activated protein kinase/extracellular signal-regulated kinase kinase, insulin growth factor-I, and serum. Cancer Res. 2005;65:7800–8. doi: 10.1158/0008-5472.CAN-04-4180. [DOI] [PubMed] [Google Scholar]
- 37.Brewer C, Yeager N, Di Cristofano A. Thyroid-stimulating hormone initiated proliferative signals converge in vivo on the mTOR kinase without activating AKT. Cancer Res. 2007;67:8002–6. doi: 10.1158/0008-5472.CAN-07-2471. [DOI] [PubMed] [Google Scholar]