Abstract
The Ts65Dn mouse shares many phenotypic characteristics of human Down syndrome. Here, we report that otitis media, characterized by effusion in the middle ear and hearing loss, was prevalent in Ts65Dn mice. Of the 53 Ts65Dn mice tested, 81.1% had high auditory-evoked brainstem response (ABR) thresholds for at least one of the stimulus frequencies (click, 8 kHz, 16 kHz and 32 kHz), in at least one ear. The ABR thresholds were variable and showed no tendency toward increase with age, from 2 to 7 months of age. Observation of pathology in mice, aged 3–4 months, revealed middle ear effusion in 11 of 15 Ts65Dn mice examined, but only in two of 11 wild-type mice. The effusion in each mouse varied substantially in volume and inflammatory cell content. The middle ear mucosae were generally thickened and goblet cells were distributed with higher density in the epithelium of the middle ear cavity of Ts65Dn mice as compared with those of wild-type controls. Bacteria of pathogenic importance to humans also were identified in the Ts65Dn mice. This is the first report of otitis media in the Ts65Dn mouse as a model characteristic of human Down syndrome.
Keywords: auditory brainstem response, Down syndrome, mouse, otitis media, Ts65Dn
Down syndrome is one of the most common serious congenital disorders, affecting one in 800 human births (Roizen & Patterson 2003). In Down syndrome, a meiotic non-disjunction gives individuals an extra copy of chromosome 21 (Chr 21) to form a trisomy, which changes gene dosage, disrupts development in subtle ways and leads to a wide range of characteristic modifications. People with Down syndrome are prone to a number of health problems, including hearing loss (Määttäet al. 2006). It has been reported that about two-thirds of individuals with Down syndrome have a hearing deficiency (Balkany et al. 1979; Dahle & McCollister 1986; Roizen et al. 1993), which can be conductive, sensorineural or of mixed mechanism (Kanamori et al. 2000; Roizen & Patterson 2003). Genetic mouse models provide the most informative approach to understanding the genotype–phenotype relationship in Down syndrome. The most commonly used model of Down syndrome is the Ts65Dn mouse, in which the Ts65Dn trisomy is derived from the reciprocal translocation T(16;17)65Dn and the Ts65Dn chromosome contains more than 80% of the human Chr 21 homologues (Akeson et al. 2001). Although the Ts65Dn mouse shares many phenotypic characteristics of humans affected by Down syndrome, including behavioural and cognitive alterations (Balkany et al. 1979; Hunter et al. 2003; Davisson 2005; Patterson & Costa 2005; Rueda et al. 2008), little is known regarding the hearing property of this mouse model. In this article, we report the prevalent phenomenon of hearing loss in Ts65Dn mice along with evidence of otitis media caused by identifiable pathogens (also seen in human), signs of inflammation and structural damage to the ears, all of which are strikingly similar to the characteristics observed in the otitis media of humans affected by Down syndrome.
Materials and methods
Mice
Ts65Dn mice were developed at The Jackson Laboratory by an induced Robertsonian translocation of Mmu16C3.3-4 to the centromere of Mmu17A2 (Davisson et al. 1990). B6EiC3Sn a/A-Ts(1716)65Dn founder female mice were mated to B6Ei × C3Sn F1 male mice to establish a breeding colony. Trisomic females produced in the colony were mated to F1 euploid males to maintain the inbred backcross necessary for maintenance of T65Dn marker chromosome transmission. For this study, the Ts65Dn colonies were maintained either at The Jackson Laboratory (TJL) animal research facility or at Case Western Reserve University (CWRU) Wolstein Animal Facility. Animals were housed with their littermates until a week before experimental tests and then singly housed after that. Part of the mice was cytogenetically genotyped (according to methods described in Davisson et al. 1990); the remaining mice were genotyped by the quantitative polymerase chain reaction (qPCR) method (Liu et al. 2003). All experimental animal protocols were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of either CWRU or TJL. A total of 53 male Ts65Dn (Ts) mice and 36 male age-matched wild-type (euploid) littermate mice spanning from 2 to 7 months of age were used in experiments.
ABR thresholds
The 53 Ts65Dn and 36 wild-type mice, aged 2 to 7 months, were anaesthetized with avertin (0.5 mg/g mouse mass) by intraperitoneal injection. Auditory-evoked brainstem response (ABR) was utilized to determine the condition of the middle and inner ears of each mouse. A computer-aided evoked potential system (Intelligent Hearing Systems) was used to test the mouse ABR thresholds as previously described (Zheng et al. 1999).
Histological analysis of middle and inner ears
Histological analyses of the middle and inner ears were performed following the methods described previously (Johnson et al. 2003). Briefly, middle and inner ears from Ts65Dn mice and wild-type mice were dissected, perfused with Bouin’s fixative, immersed in the same for 48 h, decalcified with Cal-EX solution for 6 h and embedded in paraffin. Sections (7 μm) were cut, mounted on glass slides and counterstained in haematoxylin/eosin (H&E). Goblet cells, whose sole function is to secrete mucus, were identified by Mayer’s Mucicarmine staining method following the protocol provided by Electron Microscopy Sciences (Catalog #26320).
Bacterial culture and identification
Mice were anaesthetized and ABR thresholds were determined as described above to predict the basic condition of the middle and inner ears of each mouse. Then the mice were euthanized by CO2 asphyxiation. The bullae were removed and the middle ears were isolated under sterile conditions. After microscopic examination (Leica S6D Stereozoom Microscope, Ontario, NY, USA), the middle ears were washed with sterile PBS (phosphate buffered saline). One hundred microlitres of the PBS lavage were inoculated onto a BBL™ Trypticase™ Soy Agar plate with 5% sheep blood (TSA II, Fisher, Pittsburgh, PA, USA) and the plates were incubated at 37 °C with 5% CO2 for 18 h. The colonies were then sorted, counted and subjected to further identification. Staphylococci were identified by standard microbiologic features, with S. aureus differentiated from coagulase-negative Staphylococci by a positive tube coagulase test. Streptococci were identified biochemically using API 20 Strep test strips (BioMerieux, Durham, NC, USA). Gram-negative bacilli were identified biochemically using API 20E or API 20NE test strips (BioMerieux).
Statistical methods
The t-test was used for comparing the means of ABR thresholds (Zheng et al. 1999). Data of semi-quantitative assessment of the ear pathology were analysed by chi-square test. A value of P< 0.05 was considered significant.
Results
ABR thresholds in the Ts65Dn mice indicated time-stable hearing loss in a majority of mice
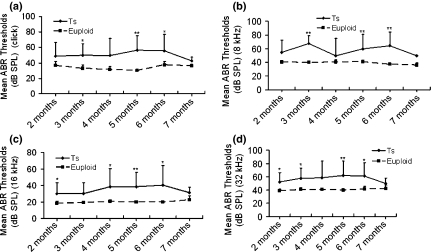
ABR tests were carried out and analysed in all the mice to show the features of the hearing problems in Ts65Dn mice. The ABR threshold values above 55 (for click stimuli), 40 (for 8 kHz), 35 (for 16 kHz) or 60 (for 32 kHz) decibel sound pressure levels (dB SPL) were considered to be hearing impaired (Zheng et al. 1999). (i) Of the 53 Ts65Dn mice observed, 81.1% (43/53) had high ABR thresholds for at least one of the stimulus frequencies (click, 8 kHz, 16 kHz and 32 kHz) in at least one ear, with 73.6% (39/53) for the right ears and 75.5% (40/53) for the left ears. (ii) Comparisons of ABR thresholds in the right ears were made between wild-type and Ts65Dn mice, and the results showed that the mean ABR thresholds at any of the stimulus frequencies in the Ts65Dn mice (n = 53) were significantly higher than those of the wild-type controls (n = 36) (P< 0.001, respectively) (Figure 1). (iii) A time-course observation of the ABR thresholds in the right ears of Ts65Dn and wild-type mice is shown in Figure 2, in which we divided the mice in each group into six sub-groups by approximately 1-month increments in age. The results showed that the mean ABR thresholds of Ts65Dn mice in each sub-group (n = 8, 8, 9, 15, 7 and 6 respectively) were higher than those of wild-type mice (n = 5, 8, 7, 5, 6 and 5 respectively) at the different time points, at all stimulus frequencies (with significance indicated by asterisks), but the overall tendency towards elevation was stable at any stimulus frequency. The ABR thresholds in the left ears showed the same trend as described above (data not shown).
Figure 1.
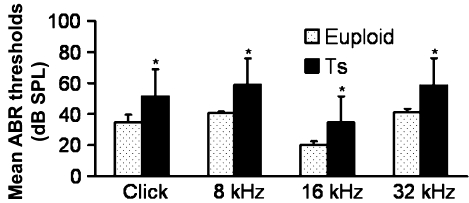
Comparison of the mean ABR thresholds of right ears from wild-type (euploid) mice and from Ts65Dn (Ts) mice, at ages ranging from 2 to 7 months. The results showed that the mean ABR thresholds to click stimuli and pure-tone stimulus (8 kHz, 16 kHz or 32 kHz) in the Ts65Dn mice (n = 53) were significantly higher than those of the wild-type control (n = 36). Error bars indicate standard deviation from the mean of each group. *P< 0.001.
Figure 2.
A time-course observation of the ABR thresholds in the right ears of Ts65Dn (Ts) mice and wild-type (euploid) mice. For Ts65Dn mice of ages from 2 to 7 months (n = 8, 8, 9, 15, 7 and 6, respectively, in each sub-group), the individual ABR thresholds at all stimulus frequencies of click (a), 8 kHz (b), 16 kHz (c) and 32 kHz (d) were variable, but the overall tendency was relatively stable at each of the stimulus frequencies, compared with the ABR thresholds of the wild-type mice (n = 5, 8, 7, 5, 6 and 5, respectively, in each sub-group). The mean ABR thresholds in the Ts65Dn mice were about 15 dB SPL higher than the mean thresholds of wild-type mice at all stimulus frequencies measured, with significance indicated by asterisks (*P< 0.05; **P< 0.01). Error bars indicate standard deviation from the mean at each time point, for each mouse group.
Otitis media correlated with ABR threshold elevation in Ts65Dn mice
Fifteen Ts65Dn mice and 11 wild-type mice, aged 3 to 4 months, were randomly chosen to be screened for otitis media. Of the 15 Ts65Dn mice observed, 11 had effusion in the middle ear space (five in both ears and six in only one ear), although the effusion volume varied. Figures 3 and 4 show representative images of the following histological characteristics of Ts65Dn mouse ears. The effusion content also was quite variable with respect to inflammatory cells, which were mainly composed of polymorphonuclear cells. There also was fibrous proliferation in the middle ear space in some mice. The middle ear mucosae of Ts65Dn mice generally became thickened. In the Ts65Dn mice, goblet cells were found at higher density among other cells in the epithelium of the middle ear cavity, especially in the areas where epithelial cell proliferation occurred. By contrast, control mice exhibited few goblet cells in comparable sections of the mucosae of the middle ear cavity (Figure 4). The density of goblet cells in the middle ear mucosae in mice of both groups was semi-quantitatively evaluated and shown in Table 1 (Goblet Cells Column). It was concluded that the scored rate (13/45) of the goblet cells in the middle ear mucosae of Ts65Dn mice was significantly higher than that (2/33) of the wild-type mice (P< 0.05, chi-square test). In six Ts65Dn mice with effusion in the middle ears, there was effusion or serous labyrinthitis at the basal turns of the cochleae in the inner ears (Figure 3g,i). Strikingly, of the 11 Ts65Dn mice displaying middle ear effusion, all showed correspondingly high ABR thresholds for at least one of the stimulus frequencies (click, 8 kHz, 16 kHz and 32 kHz) in one or both ears. The four Ts65Dn mice that showed no effusion in the middle ears showed correspondingly normal ABR thresholds. Only two of the 11 wild-type mice had any effusion in one middle ear and the amount of effusion was small. Semi-quantitative analysis based on scoring of the pathology in each mouse ear revealed that the degree of inflammation in ears of Ts65Dn mice was significantly greater than the inflammation in ears of wild-type mice (P< 0.01) (Table 1).
Figure 3.
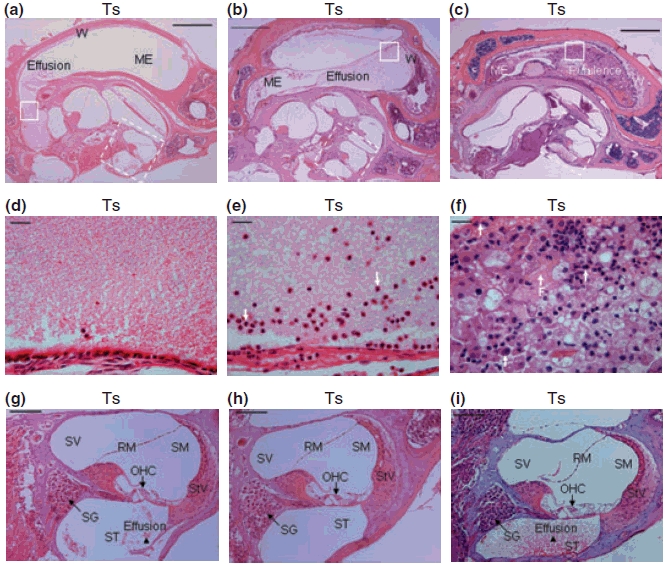
Representative histological images of inflammation in the right middle and inner ears in three different Ts65Dn (Ts) mice. (a), (b) and (c) Middle ear (ME) inflammation with effusion in the middle ear space. The walls (W) of the middle ears became thickened, in sample (c) especially. (d–f) Enlarged images from comparable areas (indicated by squares in solid line) in (a), (b) and (c), respectively, to show the effusion content in the middle ears, with a few inflammatory cells (serous) in (d), modest inflammatory cells (mucoid) in (e) and a large amount of inflammatory cells (purulent) and fibrous proliferation (arrow F) in (f). Polymorphonuclear cells were indicated by arrows in (e) and (f). (g–i) Enlarged images of the cochleae (indicated by squares in broken line) from (a), (b) and (c) respectively. In the mice with middle ear effusion, the cochleae also were involved, especially in scala tympani (ST), revealing serous labyrinthitis as indicated by arrowheads. SV, scala vestibuli; SM, scala media; RM, Reissner’s membrane; StV, stria vascularis; SG, spiral ganglion; OHC, outer hair cell. Scale bars = 500 μm in panels a–c, 20 μm in d–f and 100 μm in panels g–i.
Figure 4.
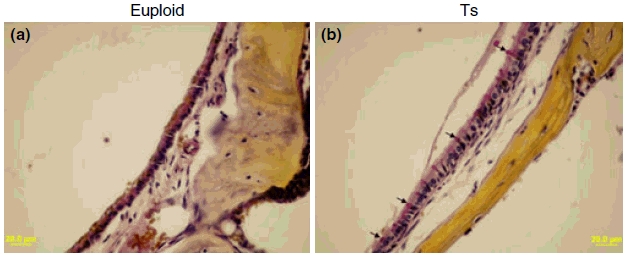
Representative figures to visualize goblet cells in the middle ear mucosae of Ts65Dn (Ts) mice and wild-type controls (euploid). Few goblet cells could be found in the middle ear mucosa in the control mouse (a). By contrast, the goblet cells were present at high density among other cells in the epithelium of the middle ear cavity of Ts65Dn mouse [(b) typical goblet cells are indicated by arrows]. Goblet cells have a distinctly polarized morphology in which the nucleus stains black in colour at the cell bas, and the mucus stains a deep rose colour in the middle and apical portions of the cell. Scale bars = 20 μm.
Table 1.
| Mouse ID | Genotype | Effusion | Inflammatory cells | Tissue debris | Tissue proliferation | Goblet cells | Inner ear effusion |
|---|---|---|---|---|---|---|---|
| Dn1 | Ts | ++ | ++ | ++ | ++ | + | + |
| Dn2 | Ts | +++ | +++ | +++ | +++ | + | +++ |
| Dn3 | Ts | + | + | + | + | + | ++ |
| Dn4 | Ts | − | − | + | − | − | − |
| Dn5 | Ts | ++ | − | + | + | +++ | ++ |
| Dn6 | Ts | +++ | ++ | +++ | + | ++ | − |
| Dn7 | Ts | + | + | + | + | + | − |
| Dn8 | Ts | − | + | + | + | − | − |
| Dn9 | Ts | ++ | − | + | + | + | − |
| Dn10 | Ts | + | − | + | + | + | − |
| Dn11 | Ts | − | − | − | − | − | − |
| Dn12 | Ts | − | − | − | + | − | − |
| Dn13 | Ts | ++ | ++ | ++ | + | + | + |
| Dn14 | Ts | + | + | + | − | − | − |
| Dn15 | Ts | ++ | ++ | + | ++ | + | + |
| DnC1 | Euploid | − | − | + | − | − | − |
| DnC2 | Euploid | − | − | + | − | − | − |
| DnC3 | Euploid | − | − | − | − | − | − |
| DnC4 | Euploid | − | − | − | − | − | − |
| DnC5 | Euploid | + | − | − | − | − | − |
| DnC6 | Euploid | − | − | − | − | − | − |
| DnC7 | Euploid | − | − | − | − | + | − |
| DnC8 | Euploid | − | − | − | − | − | − |
| DnC9 | Euploid | + | + | + | − | + | + |
| DnC10 | Euploid | − | − | − | − | − | − |
| DnC11 | Euploid | − | − | − | − | − | − |
To show degree of pathological alteration in the middle ears, the symbols (−, +, ++ and +++) were used, with ‘−’ indicating no pathology and small to great degree of pathology indicated by +, ++, or +++, respectively, regarding middle ear effusion, inflammatory cells, tissue debris, tissue proliferation, goblet cells or inner ear effusion. Both ears were observed in each mouse and only the one with more severe alteration was evaluated. Numerical scores were assigned to allow for semi-quantitative analysis of pathology (scoring 1 point for each +; for a total maximum possible score of 18 per mouse). The total scored rate (93/270) of the six indicators in Ts65Dn (Ts) mice were significantly higher than that (9/198) of the wild-type mice (euploid) (P< 0.01, chi-square test).
Mice from 94 to 117 days of age were used.
Different bacterial species isolated from middle ears of Ts65Dn mice than from wild-type mice
Four Ts65Dn mice with middle ear effusion and four age-matched wild-type mice (5 months old) were used for middle ear bacterial culture and identification. One ear from each mouse was tested. The profiles of bacteria were different between the two mouse groups. From the middle ears of Ts65Dn mice, three types of bacteria were identified based on morphology and Gram-staining. The bacteria from each type were further determined by biochemical methods to be coagulase-negative Staphylococcus (Staph.), Burkholderia cepacia and Bordetella avium. By contrast, the first two species of bacteria were not found in ears of wild-type mice, but Klebsiella oxytoca and Streptococcus viridans (S. viridans) group or Bordetella avium were identified in each euploid mouse (Table 2).
Table 2.
Bacteria isolated from middle ears of Ts65Dn and control mice
| Mouse genotype | Number of samples | Mean colonies per plate | Bacterial isolate identification |
|---|---|---|---|
| Ts65Dn | 2 | 100 25 | Bordetella avium Coagulase-negative Staph. |
| 2 | 175 50 | Bordetella avium Burkholderia cepacia | |
| Euploid | 3 | 25 50 | Klebsiella oxytoca Streptococcus viridans group |
| 1 | 100 | Bordetella avium |
Discussion
Characterization of hearing loss in Ts65Dn mice as determined by ABR thresholds
ABR has been used extensively for assessment of mouse inner ear function and also offers a valid and simple physiologic test of mouse middle ear inflammation (MacArthur et al. 2006). In this study, ABR tests were carried out in Ts65Dn and wild-type mice to screen for hearing disorders. Overall, most (81.1%) of the Ts65Dn mice observed had high ABR thresholds in at least one of the stimulus frequencies (click, 8 kHz, 16 kHz and 32 kHz) in at least one ear. The ABR thresholds in the Ts65Dn mice were significantly higher than those of the wild-type mice of the same age and the mean ABR thresholds in the Ts65Dn mice were about 15 dB SPL higher on average at all stimulus frequencies than those of the wild-type group (Figures 1 and 2). Furthermore, the results obtained in Ts65Dn mice were quite similar to those reported in humans with Down syndrome. An early study showed that the ABR thresholds of infants with Down syndrome were 10 to 25 dB SPL higher than ABR thresholds reported for normally developing infants (Werner et al. 1996). A recent investigation showed that a high rate of hearing impairment (78% by ears) in a Chinese school-aged sample of children with Down syndrome was noted (McPherson et al. 2007). Therefore, the Ts65Dn mouse may be a valid model for hearing research on human Down syndrome.
Importantly, the ABR thresholds obtained in the Ts65Dn mice were characterized by variability in the mouse population with respect to degree of increment and by an overall tendency toward threshold elevation that was relatively stable in this mouse population during a time-course observation (2 to 7 months). These findings suggest the Ts65Dn mouse is a potential model for at least one type (conductive, see below) of hearing loss in Down syndrome.
Otitis media is prevalent in Ts65Dn mice and is correlated with conductive hearing loss
Identification and treatment of hearing loss is an important part of medical management in individuals with Down syndrome, in which middle ear anomalies or dysfunction are more frequently observed than in the general population (Balkany et al. 1979; Strome & Strome 1992; Maroudias et al. 1994; Blaser et al. 2006; Shott 2006). Middle ear attributes in humans with Down syndrome include residual mesenchyme, stapes abnormality, large facial canal dehiscence and otitis media (Bilgin et al. 1996). Pneumo-otoscopy and acoustic impedance measures were performed on 38 children (mean age, 3.1 years) with Down syndrome. Results indicated that more than 60% of the series demonstrated otoscopic and acoustic impedance evidence of middle ear effusion (Schwartz & Schwartz 1978). The chronic otitis media with effusion found in patients with Down syndrome is generally considered to be secondary to depressed immune function and altered craniofacial dimensions (McLean et al. 2003).
The Ts65Dn mice have altered craniofacial dimensions as well (Hill et al. 2007). Moreover, we found a prevalence of otitis media (11/15) with effusion in the middle ear space in the Ts65Dn mice. The increased frequency of goblet cells in the middle ear epithelium may promote mucin production and secretion. Mucins are important components of the mucociliary transport system in the middle ear and Eustachian tube. Excessive mucin production overwhelms normal mucociliary clearance mechanisms under inflammatory conditions, resulting in a fluid-filled middle-ear cavity in human (Kubba et al. 2000). Because the contents of the effusion in the mouse middle ear space were quite variable, ranging from a few inflammatory cells to a large quantity of inflammatory cells, the source of the effusion may be complicated (or multifactorial). The prevalence of otitis media in Ts65Dn mice may be secondary to depressed immune function and/or defective Eustachian tube structure as in humans, but further study is needed to test this conjecture. Furthermore, the otitis media and pathology noted in our histological examinations correlated well with ABR threshold data from the Ts65Dn and wild-type mice, including several Ts65Dn mice that were normal both histologically and with respect to ABR threshold. Our finding of a correlation between increased ABR thresholds and otitis media in TsDn65 mice indicates that otitis media may be one important factor involved in the hearing loss.
Bacterial flora of Ts65Dn mice with hearing loss differs from the flora of wild-type, normal hearing control mice
Bacteria and their breakdown products are the most inflammatory stimuli known for otitis media with effusion in human (Kubba et al. 2000). In the present investigation, coagulase-negative Staph. and Burkholderia cepacia were specifically isolated from the middle ears of Ts65Dn mice, but not from ears of wild-type mice. Bordetella avium were present in the middle ears of both mouse groups. Coagulase-negative Staph. is part of the indigenous flora of human skin and mucous membranes and is sometimes isolated in large numbers from diverse ophthalmic conditions like chronic blepharitis, purulent conjunctivitis and suppurative keratitis (Ryan & Ray 2004). However, the potentially important role of coagulase-negative Staph. as a pathogen and its increasing incidence have recently been recognized. Coagulase-negative Staph. is now a major cause of nosocomial and health-care related infections (Piette & Verschraegen 2009). A guinea pig animal model showed that coagulase-negative Staph. was pathogenic and its L-forms have a crucial role in the tendency toward secretory otitis media (Göksu et al. 1996). Burkholderia cepacia is an important human pathogen, which most often causes pneumonia in immunocompromised individuals with underlying lung disease (such as cystic fibrosis or chronic granulomatous disease) (Mahenthiralingam et al. 2005). The bacterium also was isolated from ear swabs, infected tissue and purulent middle ear fluid on five occasions over a 6-month period in a patient with a cholesteatoma secondary to chronic suppurative otitis media (Hobson et al. 1995). Additionally, a bacterial strain with typical biochemical reactions of Bordetella avium was isolated in mixed culture from an ear swab of a patient suffering from chronic otitis media (Dorittke et al. 1995), indicating the bacterium can be an opportunistic pathogen in human OM. These species of bacteria may also be pathogenic to mice or may cause opportunistic infections in mice under certain conditions. The presence of these bacterial species may be one of the important factors, which are responsible for the inflammatory effusion that we observed in Ts65Dn mouse middle ears. Of course, other factors that may lead to otitis media and were not identified in this study may need further investigation.
Klebsiella oxytoca and Streptococcus viridans were isolated only from the wild-type mice in this study. However, a recent report showed gram-negative Klebsiella bacteria present in material aspirated from the middle ear of spontaneous chronic otitis media in toll-like receptor 4-deficient C3H/HeJ mice (MacArthur et al. 2008). The bacterium is regarded as an opportunistic pathogen capable of inducing pathological lesions in different rodent species as well (Bleich et al. 2008). Streptococcus viridans may be part of the normal flora in mouse ears, as our unpublished data showed that these bacteria were isolated from middle ears of several single-gene mutant mouse strains and their background colonies.
Sensorineural factors in hearing loss in Ts65Dn mice
Children affected by Down syndrome display various sensory deficits during the first year of life (Chen & Fang 2005). Inner ear dysplasia is common in Down syndrome and inner ear structures are universally hypoplastic (Blaser et al. 2006). To characterize the inner ear defects of patients with Down syndrome, several groups have studied temporal bones from patients with Down syndrome. Important anatomic features in individuals with Down syndrome included overall cochlear lengths that were notably shorter than those of the control group (Igarashi et al. 1977) and spiral ganglion cell populations that were notably less than in controls (Bilgin et al. 1996). In our study, we analysed H&E-stained sections of inner ears from 15 Ts65Dn mice, aged 3 to 4 months. Although effusion was seen in the scala tympani at the basal turn in six mice, we failed to find loss of inner or outer hair cells (OHC), reduction in the stria vascularis (StV) or degeneration of the spiral ganglion (SG) in serial sections of Ts65Dn mouse cochleae (Figure 3g,h), indicating less structural abnormality in the cochleae of Ts65Dn mice than has been described in humans. However, serous labyrinthitis, which may be caused by inflammatory mediators entering the inner ear through the round window (that is closed off from the middle ear by the round window membrane), may also contribute to the hearing loss in Ts65Dn mice that have otitis media with effusion.
It has been concluded that the incidence of conductive hearing loss resulting from middle ear dysfunction decreases with age and that sensorineural hearing loss incidence increases with age in patients with Down syndrome (Krmpotic-Nemanic 1970; Brooks et al. 1972; Keiser et al. 1981; Buchanan 1990; Hassmann et al. 1998). The lack of age-related hearing loss progression shown in the ABR time-course experiment with these mice indicates that sensorineural hearing loss may not be an important factor in Ts65Dn mice.
In conclusion, we have shown that the Ts65Dn mouse provides an excellent model of otitis media associated with Down syndrome. Moreover, the individual variability observed in the Ts65Dn mouse population may provide a valuable control in future explorations of this model. As such, this mouse will allow us to determine causal relationships between the multiple features of otitis media of Down syndrome and to propose an optimal approach to managing or minimizing hearing loss in these individuals.
Acknowledgments
This work was supported by NIH NIDCD grants R01DC007392 (QYZ), R01DC008165 (JZL) and NICHD contract N01HD73265 (MTD). We wish to thank Dr. Michael Jacobs in the Department of Pathology of Case Western Reserve University for his help in the bacterial identification and Drs. James E. Arnold and Cindy Benedict-Alderfer for reviewing this MS.
References
- Akeson EC, Lambert JP, Narayanswami S, Gardiner K, Bechtel LJ, Davisson MT. Ts65Dn – localization of the translocation breakpoint and trisomic gene content in a mouse model for Down’s syndrome. Cytogenet. Cell Genet. 2001;93:270–276. doi: 10.1159/000056997. [DOI] [PubMed] [Google Scholar]
- Balkany TJ, Downs MP, Jafek BW, Krajicek MJ. Hearing loss in Down’s syndrome. A treatable handicap more common than generally recognized. Clin. Pediatr. 1979;18:116–118. doi: 10.1177/000992287901800207. [DOI] [PubMed] [Google Scholar]
- Bilgin H, Kasemsuwan L, Schachern PA, Paparella MM, Le CT. Temporal bone study of Down’s syndrome. Arch. Otolaryngol. Head Neck Surg. 1996;122:271–275. doi: 10.1001/archotol.1996.01890150049009. [DOI] [PubMed] [Google Scholar]
- Blaser S, Propst EJ, Martin D, et al. Inner ear dysplasia is common in children with Down syndrome (trisomy 21) Laryngoscope. 2006;116:2113–2119. doi: 10.1097/01.mlg.0000245034.77640.4f. [DOI] [PubMed] [Google Scholar]
- Bleich A, Kirsch P, Sahly H, et al. Klebsiella oxytoca: opportunistic infections in laboratory rodents. Lab. Anim. 2008;42:369–375. doi: 10.1258/la.2007.06026e. [DOI] [PubMed] [Google Scholar]
- Brooks DN, Wooley H, Kanjilal GC. Hearing loss and middle ear disorders in patients with Down’s syndrome (Mongolism) J. Ment. Defic. Res. 1972;16:21–29. doi: 10.1111/j.1365-2788.1972.tb01567.x. [DOI] [PubMed] [Google Scholar]
- Buchanan LH. Early onset of presbyacusis in Down syndrome. Scand. Audiol. 1990;19:103–110. doi: 10.3109/01050399009070760. [DOI] [PubMed] [Google Scholar]
- Chen YJ, Fang PC. Sensory evoked potentials in infants with Down syndrome. Acta Paediatr. 2005;94:1615–1618. doi: 10.1080/08035250500252609. [DOI] [PubMed] [Google Scholar]
- Dahle AJ, McCollister FD. Hearing and otologic disorders in children with Down syndrome. Am. J. Ment. Defic. 1986;90:636–642. [PubMed] [Google Scholar]
- Davisson MT. Mouse models of Down syndrome. Drug Discov Today Dis Models. 2005;2:103–109. [Google Scholar]
- Davisson MT, Schmidt C, Akeson EC. Segmental trisomy of murine chromosome 16: a new model system for studying Down syndrome. Prog. Clin. Biol. Res. 1990;360:263–280. [PubMed] [Google Scholar]
- Dorittke C, Vandamme P, Hinz KH, Schemken-Birk EM, Wirsing von König CH. Isolation of a Bordetella avium-like organism from a human specimen. Eur. J. Clin. Microbiol. Infect. Dis. 1995;14:451–454. doi: 10.1007/BF02114904. [DOI] [PubMed] [Google Scholar]
- Göksu N, Ataoğlu H, Kemaloğlu YK, Ataoğlu O, Ozsökmen D, Akyildiz N. Experimental otitis media induced by coagulase negative staphylococcus and its L-forms. Int. J. Pediatr. Otorhinolaryngol. 1996;37:201–216. doi: 10.1016/0165-5876(96)01361-4. [DOI] [PubMed] [Google Scholar]
- Hassmann E, Skotnicka B, Midro AT, Musiatowicz M. Distortion products otoacoustic emissions in diagnosis of hearing loss in Down syndrome. Int. J. Pediatr. Otorhinolaryngol. 1998;45:199–206. doi: 10.1016/s0165-5876(98)00106-2. [DOI] [PubMed] [Google Scholar]
- Hill CA, Reeves RH, Richtsmeier JT. Effects of aneuploidy on skull growth in a mouse model of Down syndrome. J. Anat. 2007;210:394–405. doi: 10.1111/j.1469-7580.2007.00705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobson R, Gould I, Govan J. Burkholderia (Pseudomonas) cepacia as a cause of brain abscesses secondary to chronic suppurative otitis media. Eur. J. Clin. Microbiol. Infect. Dis. 1995;14:908–911. doi: 10.1007/BF01691499. [DOI] [PubMed] [Google Scholar]
- Hunter CL, Bimonte HA, Granholm AC. Behavioral comparison of 4 and 6 month-old Ts65Dn mice: age-related impairments in working and reference memory. Behav. Brain Res. 2003;138:121–131. doi: 10.1016/s0166-4328(02)00275-9. [DOI] [PubMed] [Google Scholar]
- Igarashi M, Takahashi M, Alford BR, Johnson PE. Inner ear morphology in Down’s syndrome. Acta Otolaryngol. 1977;83:175–181. doi: 10.3109/00016487709128830. [DOI] [PubMed] [Google Scholar]
- Johnson KR, Gagnon LH, Webb LS, et al. Mouse models of USH1C and DFNB18: phenotypic and molecular analyses of two new spontaneous mutations of the Ush1c gene. Hum. Mol. Genet. 2003;23:3075–3086. doi: 10.1093/hmg/ddg332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanamori G, Witter M, Brown J, Williams-Smith L. Otolaryngologic manifestations of Down syndrome. Otolaryngol. Clin. North Am. 2000;33:1285–1292. doi: 10.1016/s0030-6665(05)70281-4. [DOI] [PubMed] [Google Scholar]
- Keiser H, Montague J, Wold D, Maune S, Pattison D. Hearing loss of Down syndrome adults. Am. J. Ment. Defic. 1981;85:467–472. [PubMed] [Google Scholar]
- Krmpotic-Nemanic J. Down’s syndrome and presbyacousis. Lancet. 1970;2:670–671. doi: 10.1016/s0140-6736(70)91447-9. [DOI] [PubMed] [Google Scholar]
- Kubba H, Pearson JP, Birchall JP. The aetiology of otitis media with effusion: a review. Clin. Otolaryngol. Allied Sci. 2000;25:181–194. doi: 10.1046/j.1365-2273.2000.00350.x. [DOI] [PubMed] [Google Scholar]
- Liu DP, Schmidt C, Billings T, Davisson MT. Quantitative PCR genotyping assay for the Ts65Dn mouse model of Down syndrome. BioTechniques. 2003;35:1170–1174. doi: 10.2144/03356st02. [DOI] [PubMed] [Google Scholar]
- Määttä T, Kaski M, Taanila A, Keinänen-Kiukaanniemi S, Iivanainen M. Sensory impairments and health concerns related to the degree of intellectual disability in people with Down’s syndrome. Downs Syndr. Res. Pract. 2006;11:78–83. doi: 10.3104/reports.317. [DOI] [PubMed] [Google Scholar]
- MacArthur CJ, Hefeneider SH, Kempton JB, Parrish SK, McCoy SL, Trune DR. Evaluation of the mouse model for acute otitis media. Hear. Res. 2006;219:12–23. doi: 10.1016/j.heares.2006.05.012. [DOI] [PubMed] [Google Scholar]
- MacArthur CJ, Pillers DA, Pang J, Degagne JM, Kempton JB, Trune DR. Gram-negative pathogen Klebsiella oxytoca is associated with spontaneous chronic otitis media in Toll-like receptor 4-deficient C3H/H3J mice. Acta Otolaryngol. 2008;128:132–138. doi: 10.1080/00016480701387124. [DOI] [PubMed] [Google Scholar]
- Mahenthiralingam E, Urban TA, Goldberg JB. The multifarious, multireplicon Burkholderia cepacia complex. Nat. Rev. Microbiol. 2005;3:144–156. doi: 10.1038/nrmicro1085. [DOI] [PubMed] [Google Scholar]
- Maroudias N, Economides J, Christodoulou P, Helidonis E. A study on the otoscopical and audiological findings in patients with Down’s syndrome in Greece. Int. J. Pediatr. Otorhinolaryngol. 1994;29:43–49. doi: 10.1016/0165-5876(94)90107-4. [DOI] [PubMed] [Google Scholar]
- McLean L, MacCormick J, Robb I, Carpenter B, Pothos M. Cilia ultrastructure in children with Down syndrome. J. Otolaryngol. 2003;32:379–383. doi: 10.2310/7070.2003.13923. [DOI] [PubMed] [Google Scholar]
- McPherson B, Lai SP, Leung KK, Ng IH. Hearing loss in Chinese school children with Down syndrome. Int. J. Pediatr. Otorhinolaryngol. 2007;71:1905–1915. doi: 10.1016/j.ijporl.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Patterson D, Costa AC. Down syndrome and genetics – a case of linked histories. Nat. Rev. Genet. 2005;6:137–147. doi: 10.1038/nrg1525. [DOI] [PubMed] [Google Scholar]
- Piette A, Verschraegen G. Role of coagulase-negative staphylococci in human disease. Vet. Microbiol. 2009;134:45–54. doi: 10.1016/j.vetmic.2008.09.009. [DOI] [PubMed] [Google Scholar]
- Roizen NJ, Patterson D. Down’s syndrome. Lancet. 2003;361:1281–1289. doi: 10.1016/S0140-6736(03)12987-X. [DOI] [PubMed] [Google Scholar]
- Roizen NJ, Wolters C, Nicol T, Blondis TA. Hearing loss in children with Down syndrome. J. Pediatr. 1993;123:S9–S12. doi: 10.1016/s0022-3476(05)81588-4. [DOI] [PubMed] [Google Scholar]
- Rueda N, Flórez J, Martínez-Cué C. Chronic pentylenetetrazole but not donepezil treatment rescues spatial cognition in Ts65Dn mice, a model for Down syndrome. Neurosci. Lett. 2008;433:22–27. doi: 10.1016/j.neulet.2007.12.039. [DOI] [PubMed] [Google Scholar]
- Ryan KJ, Ray CG. Sherris Medical Microbiology. 4th edn. New York: McGraw Hill; 2004. pp. 293–294. [Google Scholar]
- Schwartz DM, Schwartz RH. Acoustic impedance and otoscopic findings in young children with Down’s syndrome. Arch. Otolaryngol. 1978;104:652–656. doi: 10.1001/archotol.1978.00790110042011. [DOI] [PubMed] [Google Scholar]
- Shott SR. Down syndrome: common otolaryngologic manifestations. Am. J. Med. Genet. C. Semin. Med. Genet. 2006;142C:131–140. doi: 10.1002/ajmg.c.30095. [DOI] [PubMed] [Google Scholar]
- Strome SE, Strome M. Down syndrome: an otolaryngologic perspective. J. Otolaryngol. 1992;21:394–397. [PubMed] [Google Scholar]
- Werner LA, Mancl LR, Folsom RC. Preliminary observations on the development of auditory sensitivity in infants with Down syndrome. Ear Hear. 1996;17:455–468. doi: 10.1097/00003446-199612000-00002. [DOI] [PubMed] [Google Scholar]
- Zheng QY, Johnson KR, Erway LC. Assessment of hearing in 80 inbred strains of mice by ABR threshold analyses. Hear. Res. 1999;130:94–107. doi: 10.1016/s0378-5955(99)00003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]