Abstract
Matrix metalloproteinases (MMPs) modulate extracellular matrix turnover, inflammation and immunity. We studied MMP-9 and MMP-2 in experimental paracoccidioidomycosis. At 15 and 120 days after infection (DAI) with virulent Paracoccidioides brasiliensis, MMP-9 was positive by immunohistochemistry in multinucleated giant cells, in mononuclear cells with macrophage and lymphocyte morphologies and also in fungal cells in the lesions of susceptible and resistant mice. Using gelatin zymography, pro- and active MMP-9 and active MMP-2 were detected in all infected mice, but not in controls. Gelatinolytic activity was not observed in P. brasiliensis extracts. Semiquantitative analysis of gelatinolytic activities revealed weak or absent MMP-2 and strong MMP-9 activity in both mouse strains at 15 DAI, declining at 120 DAI. Avirulent P. brasiliensis-infected mice had residual lesions with MMP-9-positive pseudoxantomatous macrophages, but no gelatinase activity at 120 DAI. Our findings demonstrate the induction of MMPs, particularly MMP-9, in experimental paracoccidioidomycosis, suggesting a possible influence in the pattern of granulomas and in fungal dissemination.
Keywords: experimental paracoccidioidomycosis, gelatinases, granulomas, matrix metalloproteinases
Paracoccidioides brasiliensis is a dimorphic fungus which causes paracoccidioidomycosis (PCM), a human systemic mycosis endemic in Latin America (Restrepo 1985). PCM is a granulomatous disease which has two major clinical forms: the chronic and severe, characterized by multifocal, disseminated lesions comprised loose granulomas, and the benign, presenting unifocal, localized lesions constituted of well-formed, compact granulomas (Camargo & Franco 2000).
The omentum is constituted by a loose connective tissue with leucocyte aggregates named milky spots, a coelom-associated lymphomyelopoietic tissue, which represents the main immune tissue of the peritoneal cavity (Lenzi et al. 1996). The omentum milky spot is a major target in intraperitoneal PCM, as observed in studies consistently demonstrating high omentum fungal burden in disseminated disease (Nishikaku & Burger 2003a).
In experimental PCM, after infection with the highly virulent Pb18 P. brasiliensis isolate, susceptible mice (B10.A strain) developed mainly loose granulomas, with reticular fibres around multinucleated giant cells and intense fungal dissemination. On the other hand, resistant mice (A/J strain) presented two lesion types: active, composed by type I collagen capsules surrounding inflammatory foci of polymorphonuclear neutrophils and giant cells circumscribing fungal cells; and residual, containing pseudoxantomatous macrophages and fungal debris (Xidieh et al. 1999). In addition, the presence of extracellular matrix (ECM) components, as well as of the fibrogenic cytokine, transforming growth factor (TGF)-β, was observed in omentum granulomas (Xidieh et al. 1999; Nishikaku & Burger 2003b; Nishikaku et al. 2008).
The balance between synthesis and breakdown of ECM components present in the micromillieu of inflamed tissue involves the participation of matrix-degrading enzymes, such as matrix metalloproteinases (MMPs). MMPs are a family of zinc-dependent enzymes able to degrade ECM proteins and also several inflammatory and immune mediators. Almost all classes of MMPs are secreted by cells as inactive forms, called zymogens or pro-MMPs, and require proteolytic processing to become activated in the extracellular microenvironment (Sternlicht & Werb 2001).
Matrix metalloproteinase-2 and -9 have a broad substrate spectrum, including basal membrane proteins, gelatin and cytokines precursors (Sternlicht & Werb 2001). MMP-2 is constitutively expressed, whereas MMP-9 is weakly expressed in the normal tissue, but strongly induced by several mediators during tissue remodelling and tumour invasion (Stamenkovic 2000). A role of MMPs, particularly of MMP-9 and/or MMP-2, has been suggested in the pathogenesis of several infectious diseases (Chang et al. 1996; Williams et al. 2002; Elkington et al. 2005). However, in P. brasiliensis infection, the involvement of MMPs has been poorly investigated.
Products secreted by pathogens may also contribute to the structural and functional alterations of the infected tissue. They could act as ligands of ECM components, and as proteases, contributing to pathogen adherence and facilitating colonization and dissemination in the tissue. Binding of ECM components to P. brasiliensis yeast cells (Vicentini et al. 1994; Mendes-Giannini et al. 2006) and the presence of gelatinase (Vaz et al. 1994), collagenase (Bedoya-Escobar et al. 1993) and serine protease activities (Puccia et al. 1998) in culture extracts have been shown, but the role of matrix-degrading enzymes produced by P. brasiliensis during host infection remains still unsolved.
Fibrosis is a late event of the granulomatous response, commonly observed in experimental models of PCM (Lenzi et al. 1994) and in patients with severe organ dysfunctions and leading to an incapacitating condition (Montenegro & Franco 1994). In PCM granulomas, intense tissue remodelling (necrosis and fibrosis) could involve the presence of ECM components and also of proteolytic enzymes, such as MMPs. Therefore, comprehension of these phenomena during the development of granulomatous inflammation may help in the establishment of therapies to block or attenuate fibrotic sequelae, which commonly limit the normal function of the affected organs.
In this study, the presence and gelatinolytic activity of MMPs were evaluated in P. brasiliensis-infected mice to determine the participation of these enzymes in the chronic granulomatous response, trying to establish an association with the loose or compact morphology of the lesions and with the control or permissiveness of fungal dissemination.
Material and methods
Paracoccidioides brasiliensis
Yeast forms of P. brasiliensis isolates, Pb18 and Pb265, respectively, highly and slightly virulent to mice (Kashino et al. 1985), were cultivated using Fava Netto’s culture medium, kept at 37 °C and used at the seventh day of culture, which corresponds to the exponential phase of growth.
Infection
Groups of 5–10 female, 8- to 10-week-old B10.A and A/J mice were intraperitoneally (i.p.) infected with 5 × 106 yeasts of Pb18 or Pb265, suspended in 0.5 ml sterile phosphate-buffered saline (PBS, pH 7.2). Mice injected with sterile PBS were used as controls. Mice were housed at the Department of Immunology animal facilities and fed with sterilized food and acidified water. This work was approved by the Ethical Committee for Animal Research of the Biomedical Sciences Institute of São Paulo University, Brazil.
Tissue samples collection
At 15 and 120 days of infection, mice were euthanized and the peripancreatic/perisplenic omentum, the target organ of i.p. P. brasiliensis infection, was collected and used for histological and immunohistochemical procedures and also for gelatin zymography.
Histological and immunohistochemical procedures
The omentum tissue was fixed in Methacarn solution (60% methanol, 30% chloroform and 10% acetic acid) and paraffin-embedded sections were used in immunohistochemical reactions (Nishikaku & Burger 2003b). Briefly, slides were deparaffinized and rehydrated, then incubated with 30% hydrogen peroxide to block endogenous peroxidase. Non-specific protein binding was blocked with normal serum and followed by application of TBS (Tris-buffered saline, pH 7.4; Pierce Chemical Co., Rockford, IL, USA) to block endogenous biotin. Slides were incubated overnight with rabbit polyclonal antibody against mouse MMP-9 (Chemicon International, Temecula, CA, USA) used at dilution 1:100 (PBS/Tween 20). Biotinylated goat anti-rabbit IgG (1:500) (Rockland, Gilbertsville, PA, USA) was applied for 1 h at room temperature, followed by streptavidin-peroxidase (1:50) (Vector Laboratories, Burlingame, CA, USA). The chromogen 3.3′ diaminobenzidine tetrahydrocloride (DAB; Sigma-Aldrich, St. Louis, MO, USA) was used and the sections were then counterstained with Mayer’s haematoxylin and examined using a light microscope (Nikon Eclipse 80i, Japan). Control slides were made with specimens of non-infected mice or without primary antibody, replaced by diluent.
Paracoccidioides brasiliensis culture extracts
The yeast forms of Pb18 and Pb265 were employed for obtention of ‘cell-free antigen’ preparation, a culture extract composed of several preserved cell surface molecules, extensively used for evaluation of immunological responses (Blotta & Camargo 1993). Briefly, fungal cells were cultivated in Fava Netto’s culture medium at 37 °C and used at the seventh day of culture. About 300 mg of cell mass was collected and suspended in 1 ml of PBS, homogenized for 30 s and immediately centrifuged at 16,000 g for 60 s. The supernatant, which contains the exoantigen, was collected, filtered (0.22 μm) and stored at −20 °C until use for gelatin zymography.
Gelatin zymography
Gelatinolytic activities of MMPs in omentum homogenate samples (20 μg of protein) and also of P. brasiliensis (20 μg of protein) were analysed using gelatin zymography. This is a sensitive and quantifiable method which allows visualization and quantitation of proteolytic activity of enzymes, based on the molecular weights of their pro- and active forms after gel electrophoresis (Kleiner & Stetler-Stevenson 1994). As the activation of the pro-MMP occurs after denaturation and renaturation processes, both forms of MMPs can be detected in the samples. In brief, tissue homogenates and fungal extracts were diluted into sample buffer (10% SDS, 4% sucrose, 0.25 m Tris–HCl, pH 6.8, and 0.1% bromophenol blue) and applied under non-reducing conditions in 10% polyacrilamide-SDS gels containing 0.1% gelatin type B (Sigma-Aldrich, St. Louis, MO, USA), using the Mini-PROTEAN™ II system (Bio-Rad Laboratories, Hercules, CA, USA). After electrophoresis at 30 mA (4 °C), gels were washed with 2.5% Triton X-100 at room temperature for 1 h, and incubated with activation buffer (50 mm Tris, 200 mm NaCl, 5 mm CaCl2, 0.02% Brij 35P, pH 7.5) overnight at 37 °C. Gels were stained with 0.1% Coomassie Brilliant Blue, and proteolytic activity was visualized as clear bands indicating areas of gelatin degradation. Molecular weights of the bands were estimated by using prestained SDS-PAGE markers (Fermentas, Glen Burnie, MD, USA) and mouse MMP-9 and MMP-2 standards were used as positive controls (R&D Systems, Minneapolis, MN, USA). To confirm the presence of gelatinolytic activity of MMPs, tissue extracts of infected mice were treated with 20 mm ethylenediaminetetraacetic (EDTA), or 5 mm orto-phenanthroline (Sigma-Aldrich, St. Louis, MO, USA), as MMP inhibitors, or 2 mm phenylmethylsulphonylfluoride (PMSF; Sigma-Aldrich, St. Louis, MO, USA), the inhibitor of serine proteinases (inhibitors applied in the incubation buffer). Semiquantitation of MMP-9 and MMP-2 proteolytic activities and determination of the molecular weight of the bands in the gels were performed by employing the AlphaEase DigiDoc 1000 software, version 3 (Alpha Innotech Corp., San Leandro, CA, USA). The gels were digitally scanned and standardized in images with similar pattern of black and white colours (white, the local enzymatic digestion, and black, the background). The relative integrated optical density value (IDV, sum of all pixel values) after background correction was calculated in each sample in the gel and then, the relative ratio of IDV of pro- and active MMPs’ activity in samples was determined in each gel and expressed as percentage value (%). In each gel, the percentage value (Y) was determined by ratio of IDV of one sample (band) considered as 100% (X, always the sample with more band intensity) in relation to IDV of another sample (band) with equal or minor band intensity (Z). The calculation can be expressed as Y = (Z/X) × 100. Results were expressed as mean ± SEM (standard error of the mean) of percentage of gelatinolytic activity (pro-MMP or active MMP) observed in samples of each experimental group.
Statistical analysis
Two-way analysis of variance (two-way anova), followed by Bonferroni posttests, and one-way analysis of variance (one-way anova), followed by Tukey method for multiple comparisons, were done to evaluate the differences among the groups studied (P< 0.05). Data were analysed using the GraphPad Prism software, version 4 (GraphPad Software, Inc., La Jolla, CA, USA).
Results
MMP-9 immunostaining in omentum granulomas
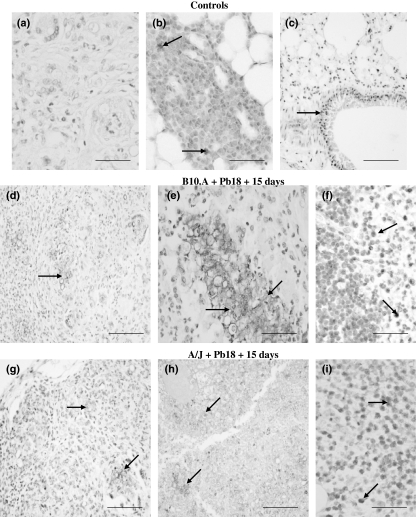
Control reactions were carried out without the application of primary antibody, always resulting in the absence of labelling in the tissue sections (Figure 1a). In non-infected animals, MMP-9 positivity was observed in mononuclear cells present in omentum milky spots (Figure 1b). Histological sections of lungs were used as positive controls (Figure 1c).
Figure 1.
Immunostaining for MMP-9 in omentum granulomatous lesions of B10.A and A/J mice at 15 days after infection with Pb18 Paracoccidioides brasiliensis isolate (DAB staining, counterstained with hematoxylin). Controls: (a) no reaction without primary antibody in omentum of infected mouse (magnification ×400); (b) positive mononuclear cells (arrows) in omentum of non-infected mouse (×400); (c) positive control showing MMP-9 staining in pulmonary epithelial tissue (arrow) (×200); B10.A + Pb18 + 15 days: (d,e) granulomatous foci containing fungi with typical morphology (arrow, ×200) and debris (arrows, ×400) positive to MMP-9 in the centre of loose granulomas; (f) positive mononuclear cells with lymphocyte morphology (arrows) at the periphery of lesion (×400); A/J + Pb18 + 15 days: (g) immunoreactivity to MMP-9 in fungal cells and also in the inflammatory cells in loose granuloma (arrows, ×200); (h) areas of tissue degradation containing MMP-9 positive cellular debris (arrows, ×200); (i) presence of positive mononuclear cells with lymphocyte morphology (arrows) at the periphery of loose granuloma (×400). The results shown are representative of two independent experiments performed with four mice per group. Scale bars: 50 μm (a, b, e, f and i) and 100 μm (c, d, g and h).
Paracoccidioides brasiliensis infection induced an intense granulomatous inflammation in omentum milky spots, which changed in size and morphological aspects. At 15 days after infection (DAI) with Pb18, the granulomas of B10.A showed MMP-9 positivity in fungal cells with preserved (Figure 1d) or much altered (Figure 1e) morphology and also in the inflammatory foci (Figure 1d), localized mainly in the centre of the lesions. In A/J mice, MMP-9 immunostaining was detected in fungi (Figure 1g), markedly in the centre of necrotic lesions, at areas of cellular debris (Figure 1h). Positive mononuclear cells with lymphocyte morphology were detected at the periphery of granulomas (Figure 1f,i), which showed mainly a loose aspect, in both susceptible and resistant mice.
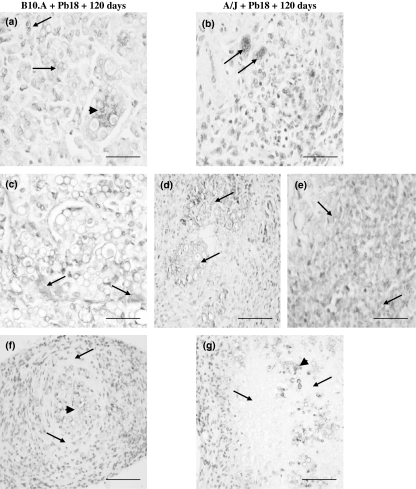
At 120 DAI with Pb18, marked MMP-9 immunostaining was detected in the lesions of both mouse strains, mainly in the areas of tissue degradation, characterized by the presence of several fungal foci. In B10.A, MMP-9 immunoreactivity was localized in fungal cells circumscribed by multinucleated giant cells which were also MMP-9-positive (Figure 2a) within the granulomas. Positive mononuclear cells with lymphocyte morphology were observed at the periphery of the lesions, but only few areas of ECM showed MMP-9 immunoreactivity (Figure 2c). Loose and extensive lesions were the main pattern of granulomas observed in B10.A in the late phase of infection. Weak MMP-9 positive staining was found in fungal foci surrounded by ECM fibres negative for this enzyme (Figure 2f). In A/J, evident MMP-9 staining was detected mainly in giant cells (Figure 2b) localized in the centre of compact granulomas. Intense MMP-9 positivity was observed in areas of tissue degradation (Figure 2d) within the necrotic lesions, whereas only few areas showed ECM positivity at the periphery of granulomas (Figure 2e). As observed in B10.A, weak MMP-9 staining was found in fibrotic lesions (Figure 2g).
Figure 2.
Immunostaining for MMP-9 in omentum granulomatous lesions of B10.A and A/J mice at 120 days after infection with Pb18 Paracoccidioides brasiliensis isolate (DAB staining, counterstained with hematoxylin). B10.A + Pb18 + 120 days: (a) extensive loose granuloma showing several foci of fungal cells (arrows), circumscribed by multinucleated giant cells, both positive to MMP-9 (arrowhead) (×400); (c) immunostained ECM (arrows) near the areas containing fungal foci (×400); (f) fibrotic granuloma with weak positivity in fungal cells (arrowhead) and no staining in the ECM (arrows) (×200); A/J + Pb18 + 120 days: (b) compact granuloma containing MMP-9-positive mononuclear cells and also giant cells (arrows) (×400); (d) positive reaction in the areas of tissue degradation (arrows) inside the necrotic lesion (×200); (e) immunostained ECM (arrows) at the periphery of granuloma (×400); (g) fibrotic granuloma with positive reaction in foci of fungal cells (arrowhead) and no staining in the ECM surrounding the central area of the lesion (×200). The results shown are representative of two independent experiments performed with four mice per group. Scale bars: 50 μm (a, b, c and e) and 100 μm (d, f and g).
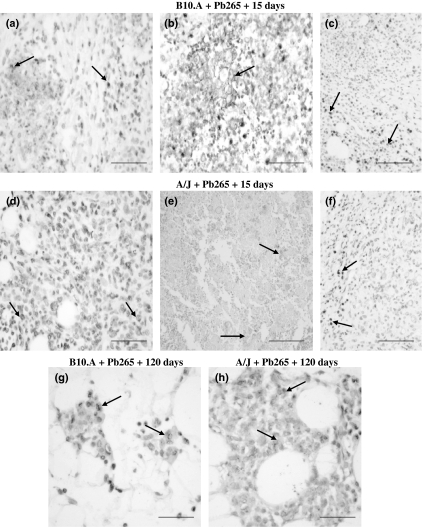
At 15 DAI with Pb265, MMP-9 immunostaining was similar to that observed in granulomas developed at the early phase of Pb18 infection. MMP-9 was observed in mononuclear cells with macrophage morphology (Figure 3a,d) in the centre of loose granulomas of both mouse strains. Positivity to MMP-9 was found in areas of tissue degradation in B10.A and A/J (Figure 3b,e), mainly in necrotic lesions and also in mononuclear cells with lymphocyte morphology localized at the periphery of the lesions (Figure 3c,f). On the contrary, at 120 DAI with Pb265, MMP-9-positive pseudoxantomatous macrophages were found in residual lesions of both mouse strains (Figure 3g,h).
Figure 3.
Immunostaining for MMP-9 in omentum granulomatous lesions of B10.A and A/J mice at 15 and 120 days after infection with Pb265 Paracoccidioides brasiliensis isolate (DAB staining, counterstained with hematoxylin). B10.A + Pb265 + 15 days and A/J + Pb265 + 15 days: (a,d) mononuclear cells with macrophage morphology (arrows, ×400) positive to MMP-9 in loose granulomas; (b,e) necrotic lesions containing positive areas of tissue degradation (arrows, ×400 and ×200); (c,f) positive mononuclear cells with lymphocyte morphology (arrows) at the periphery of loose granulomas (×200). B10.A + Pb265 + 120 days and A/J + Pb265 + 120 days: (g,h) both mouse strains developed residual lesions containing pseudoxantomatous macrophages positive to MMP-9 (arrows) (×400). The results shown are representative of two independent experiments performed with four mice per group. Scale bars: 50 μm (a, b, d, g and h) and 100 μm (c, e and f).
Gelatinolytic activities of MMPs
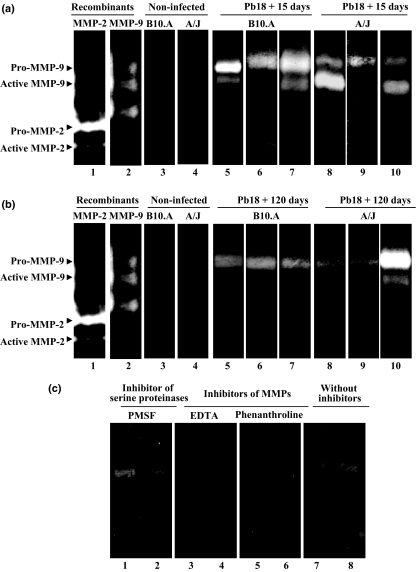
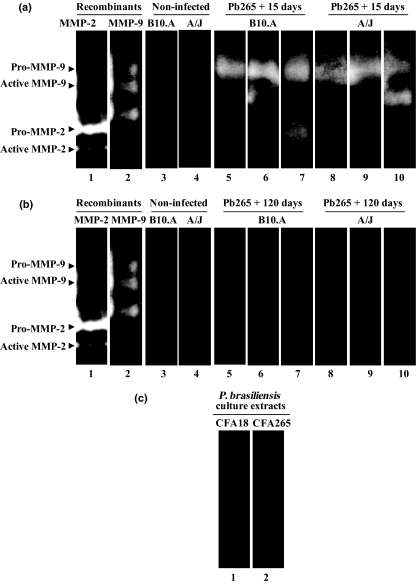
Using prestained SDS-PAGE markers and MMP-9 and MMP-2 standards in zymography, we could estimate the molecular weights of the bands, which were approximately 106 and 95 kDa, corresponding, respectively, to pro- and active MMP-9, and also approximately 68 and 62 kDa, corresponding, respectively, to pro- and active MMP-2 (Figures 4 and 5). Gelatinolytic activity was detected in omentum homogenates, with the presence of three major bands, corresponding to pro- and active MMP-9 and also to active MMP-2 (Figures 4 and 5).
Figure 4.
Gelatin zymography for detection of MMP-9 and MMP-2 activities in omentum tissue extracts of B10.A and A/J mice infected with Pb18 Paracoccidioides brasiliensis isolate. (a and b, lanes 1 and 2) recombinant MMP-2 and MMP-9; (a and b, lanes 3 and 4) control groups of non-infected mice; (a, lanes 5–7) B10.A + Pb18 + 15d; (a, lanes 8–10) A/J + Pb18 + 15d; (b, lanes 5–7) B10.A + Pb18 + 120d; (b, lanes 8–10) A/J + Pb18 + 120d; samples of infected mice treated with serine protease inhibitor PMSF (c, lanes 1 and 2); MMP inhibitors EDTA (c, lanes 3 and 4) or orto-phenanthroline (c, lanes 5 and 6) or in the absence of inhibitors (c, lanes 7 and 8). Pro-MMP-9 (approximately 106 kDa), Active MMP-9 (approximately 95 kDa), Pro-MMP-2 (approximately 68 kDa), Active MMP-2 (approximately 62 kDa). The results shown were obtained from samples of three to six mice per group.
Figure 5.
Gelatin zymography for detection of MMP-9 and MMP-2 activities in omentum tissue extracts of B10.A and A/J mice infected with Pb265 Paracoccidioides brasiliensis isolate (a,b) and of proteases of P. brasiliensis (c). (a and b, lanes 1 and 2) recombinant MMP-2 and MMP-9; (a and b, lanes 3 and 4) control groups of non-infected mice; (a, lanes 5–7) B10.A + Pb265 + 15d; (a, lanes 8–10) A/J + Pb265 + 15d; (b, lanes 5–7) B10.A + Pb265 + 120d; (b, lanes 8–10) A/J + Pb265 + 120d; (c, lane 1) CFA18 and (c, lane 2) CFA265 from P. brasiliensis culture extracts. Pro-MMP-9 (approximately 106 kDa), active MMP-9 (approximately 95 kDa), pro-MMP-2 (approximately 68 kDa) and active MMP-2 (approximately 62 kDa). The results shown were obtained from samples of three to six mice per group.
At 15 DAI with Pb18, although both pro- and active forms of MMP-9 were detected in the two mouse strains, the main form was the pro-MMP-9, which was observed in all samples of infected mice (Figure 4a). At 120 DAI, both pro- and active forms of MMP-9 were detected in the infected animals, with the presence of the proenzyme in all the samples (Figure 4b). Gelatinolytic activity was not found in samples of non-infected mice. The presence of MMP in infected tissue extracts was confirmed by the elimination of the gelatinolytic activities by MMP inhibitors (EDTA and orto-phenanthroline), but not by serine proteinase inhibitor PMSF (Figure 4c). The active form of MMP-2 was observed in B10.A at 15 and 120 DAI with Pb18, but only at the later stage in A/J (Figure 4a,b).
In Pb265 infection, zymography revealed the presence of pro- and active MMP-9 in both mouse strains, with detection of pro-MMP-9 in all the samples and also of active MMP-2 in B10.A at 15 DAI (Figure 5a). On the other hand, MMP-9 and MMP-2 enzymatic activity was undetectable at 120 DAI (Figure 5b) and no gelatinase bands were observed in non-infected mice.
Culture extracts of Pb18 and Pb265 P. brasiliensis isolates showed undetectable gelatinolytic activity, independently of fungal virulence (Figure 5c).
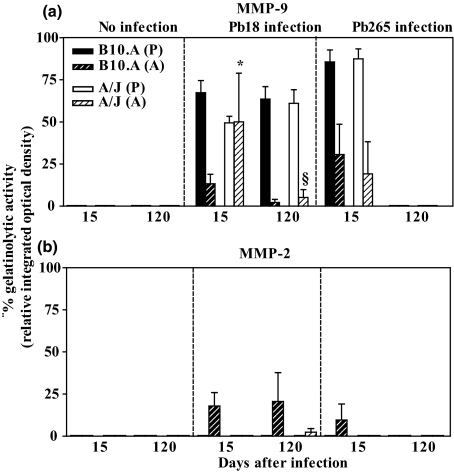
Semiquantitative evaluation was also carried out to estimate the percentage of gelatinolytic activity as revealed by zymography (Figure 6). In accordance with the results shown in Figures 4 and 5, we found the prevalence of the pro-form over the active form of MMP-9 in infected mice and no gelatinolytic activity in control mice. In Pb18 infection, high pro-MMP-9 activity was observed in both mouse strains at 15 and 120 DAI. Regarding active MMP-9, high gelatinolytic activity, similar to that of pro-MMP-9, was detected in A/J, which was significantly higher than in B10.A at 15 DAI (P< 0.05). In contrast, a tenfold decrease of active MMP-9 was observed in A/J at 120 DAI, which was significantly lower than that found at 15 DAI (P< 0.05). Moreover, weak or no enzymatic activity was detected in B10.A at 120 DAI (Figure 6a). In Pb265 infection, high pro-MMP-9 activity was detected in B10.A and A/J mice, whereas low levels of active MMP-9 were observed in both mouse strains at 15 DAI. At 120 DAI, no gelatinolytic activity was observed (Figure 6a). Active MMP-2 gelatinolytic activity was not detected in samples of control mice, but was found in B10.A at 15 DAI and at 120 DAI with Pb18. Only low enzymatic activity could be seen in A/J at 120 DAI (Figure 6b). In Pb265 infection, we observed low active MMP-2 activity in B10.A, and no activity in A/J at 15 DAI and in both mouse strains at 120 DAI (Figure 6b). Paracoccidioides brasiliensis infection with either isolate induced MMP-2 gelatinolytic activity (Figure 6b); however, this activity was weaker in comparison with MMP-9 gelatinolytic activity.
Figure 6.
Semiquantitative analysis of MMP-9 and MMP-2 activity in omentum of non-infected mice and of mice at 15 and 120 DAI with Pb18 or Pb265 Paracoccidioides brasiliensis isolates. (a) Gelatinolytic activity of pro-MMP-9 (P) in B10.A (solid black bars) and A/J mice (solid white bars) and also of active MMP-9 (a) in B10.A (diagonally striped black bars) and A/J mice (diagonally striped white bars) infected with Pb18 or Pb265 and in non-infected mice; (b) Gelatinolytic activity of pro-MMP-2 (P) in B10.A (solid black bars) and A/J mice (solid white bars) and also of active MMP-2 (A) in B10.A (diagonally striped black bars) and A/J (diagonally striped white bars) infected with Pb18 or Pb265 and in non-infected mice. Results were expressed as mean ± SEM of percentage of gelatinolytic activity (relative integrated optical density) observed in samples of each experimental group. The results shown were obtained from three to six mice per group. *P< 0.05 comparing mouse strains (B10.A × A/J), §P< 0.05 comparing 15 and 120 DAI.
Discussion
In this study, immunolocalization of MMP-9 was demonstrated in multinucleated giant cells and also in mononuclear cells with macrophage and lymphocyte morphologies present in granulomas of P. brasiliensis-infected mice. MMP-9 positivity in macrophages (Hsu et al. 2005) and giant cells (González et al. 2002) in granulomas has been described in the literature, suggesting the participation of these cells as main cellular sources of MMPs. Moreover, the ability of these cell populations to produce MMP-9 under different inflammatory and immune processes has been reported (Goetzl et al. 1996; Opdenakker et al. 2001). Therefore, our data suggest that in the PCM granulomas, giant cells and mononuclear cells, probably macrophages and lymphocytes, are cellular sources of MMPs.
In addition to immunohistochemical analysis, which allows the visualization of specific MMPs localization in tissue sections, but does not discriminate between the pro- and active forms of these enzymes, gelatin zymography was used to assess the functional activity of matrix-degrading enzymes. The gelatinolytic activity of MMPs in omentum extracts of susceptible and resistant mice infected with P. brasiliensis was evaluated, and bands corresponding to pro-MMP-9 and active MMP-9 were detected in infected mice, but not in control mice. Regarding MMP-2, only weak activity was observed in infected mice, probably corresponding to basal production, because this enzyme is constitutively secreted in various tissues. In our results, P. brasiliensis infection induces metalloproteinases secretion with gelatinases activity, mainly of MMP-9, and a limited degree of MMP-2, confirming the inducible character of the former and suggesting that this infection elicits limited MMP-2 secretion.
Matrix metalloproteinase-2 and -9 have several substrates, but their function is mainly attributed to their collagenase and gelatinase activities, which result in important roles in ECM turnover and cell migration. They are able to degrade the basement membrane proteins, such as type IV collagen and laminin, which are important to the invasion process of pathogens (Elkington et al. 2005) and of tumour cells (Stamenkovic 2000). These enzymes also regulate the cell behaviour during inflammation, contributing to the cell activation and migration, as well as to activation or inhibition of cytokines (Opdenakker et al. 2001; Sternlicht & Werb 2001).
The role of MMPs in infectious diseases, such as the participation of MMP-9 in mycobacterial infection, contributing to the early bacterial dissemination and to the adequate cellular recruitment to form well-organized granulomas (Taylor et al. 2006), has been described. MMP involvement in fungal infections has been shown in clinical and experimental studies, with special attention to MMP-2 and MMP-9. Secretion of MMP-9 was observed in pulmonary aspergillosis, associated with neutrophil infiltrate and tissue damage (Gibson et al. 2003). High levels of MMP-9 were detected in an experimental model of meningitis and vasculitis induced by Coccidioides immitis infection, suggesting a role of MMPs in the breakdown of the blood–brain barrier (Williams et al. 2002).
We observed weak MMP-9 staining in fungal cells, but no staining in areas of intense deposits of ECM fibres localized in fibrotic lesions. In contrast, MMP-9 was detected in cells and also in the lesions containing tissue degradation, including necrotic zones found mainly in resistant mice, which could indicate the participation of proteolytic enzymes, especially MMP-9. The presence of pro-MMP-9 over active MMP-9 at the later phase of infection with Pb18 suggests a less pronounced MMP proteolytic activation, leading to increased deposition of ECM components in the inflamed tissue. In previous studies, we detected the presence of intense deposits of type I and type III collagens fibres, as well as of the proteoglycan decorin, mainly at later stages of Pb18 infection (Xidieh et al. 1999; Nishikaku & Burger 2003b; Nishikaku et al. 2008), reinforcing the possible dominance of ECM synthesis over its degradation.
In this study, MMP-9 immunostaining was observed in the lesions at 15 and 120 DAI with Pb265, but gelatinase activity of MMP-9 was not found at the later time point. As seen previously, extensive ECM component deposition was observed at 120 DAI with Pb18, delimiting fungal foci in granulomas, whereas residual lesions with scarce deposits of matrix were found at the late phase of Pb265 infection, showing the resolution of the infection (Nishikaku et al. 2008). Thus, predominance of ECM protein synthesis over MMP-9 activation at the chronic stage of P. brasiliensis could be due, in part, to the regulation by endogenous inhibitors of MMPs and/or by other mechanisms which involve profibrotic cytokines (Wynn 2004). In fact, our previous results confirm higher TGF-β positivity in omentum lesions at later phases of P. brasiliensis infection (Nishikaku & Burger 2003b).
In this investigation, the conspicuous presence of MMP-9, found in samples of infected mice, independently of fungal virulence, indicates a major role of MMP-9 in the chronic inflammation induced by P. brasiliensis, in contrast to the weak proteolytic activity of MMP-2.
Tissue invasion by P. brasiliensis during the infection has been attributed in part to secretion of proteases by fungal cells with gelatinase (Vaz et al. 1994) and collagenase activity (Bedoya-Escobar et al. 1993) and also to the ability of serine proteinases to degrade basement membrane components (Puccia et al. 1998). In contrast to these findings, in this study we did not detect gelatinolytic activity in P. brasiliensis culture extracts, but found MMP-9-positive fungi in the omentum lesions by immunohistochemistry. Using the later technique, no reactions were detected in control sections, demonstrating the specificity of this assay. These findings suggest that P. brasiliensis could be producing enzymes similar to MMP-9 in the host tissue but not in culture extracts. Alternatively, P. brasiliensis yeast cells could present receptors able to recognize and bind to MMP-9 secreted by the host, contributing to the ECM turnover or to the fungal dissemination into the tissue. It has been reported that proteolytic enzymes secreted by bacteria activate host pro-MMPs, and this would indicate an important role of these components in immunopathology (Elkington et al. 2005). Although no proteolytic activity of P. brasiliensis had been detected in our in vitro assays, the occurrence of a MMP-9-like enzyme in P. brasiliensis yeast cells or of MMP-9-binding receptors during host infection cannot be excluded.
Taken together, our findings demonstrate for the first time the presence and the gelatinolytic activity of MMPs, particularly of MMP-9, in P. brasiliensis infection, suggesting a possible influence in the organization pattern of the granulomatous lesions and also in fungal dissemination.
Acknowledgments
The authors gratefully acknowledge Dr Danielle P. Cavalcante (Imperial College London, UK) and Dr Sílvia C. Alfieri (Department of Parasitology, Institute of Biomedical Sciences – University of São Paulo, Brazil) for their help with the zymography technique and also Dr Telma M.T. Zorn and Dr Sebastian A. San-Martin (Department of Cell and Developmental Biology, Institute of Biomedical Sciences – University of São Paulo, Brazil) for their help with the immunohistochemical reactions and Célia R. P. Pizzo for technical assistance.
Financial support
This work was supported by grants from Conselho Nacional para o Desenvolvimento Científico e Tecnológico (CNPq, 307492/2006–0), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, grants numbers: 00/10647–1, 06/60091–6).
References
- Bedoya-Escobar VI, Naranjo-Mesa MS, Restrepo-Moreno A. Detection of proteolytic enzymes released by the dimorphic fungus Paracoccidioides brasiliensis. J. Med. Vet. Mycol. 1993;31:299–304. [Google Scholar]
- Blotta MHSL, Camargo ZP. Immunological response to cell-free antigens of Paracoccidioides brasiliensis: relationship with clinical forms of paracoccidioidomycosis. J. Clin. Microbiol. 1993;31:671–676. doi: 10.1128/jcm.31.3.671-676.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camargo ZP, Franco MF. Current knowledge on pathogenesis and immunodiagnosis of paracoccidioidomycosis. Rev. Iberoam. Micol. 2000;17:41–48. [PubMed] [Google Scholar]
- Chang JC, Wysocki A, Tchou-Wong KM, Moskowitz N, Zhang Y, Rom WN. Effect of Mycobacterium tuberculosis and its components on macrophages and the release of matrix metalloproteinases. Thorax. 1996;51:306–311. doi: 10.1136/thx.51.3.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkington PTG, O′Kane CM, Friedland JS. The paradox of matrix metalloproteinases in infectious disease. Clin. Exp. Immunol. 2005;142:12–20. doi: 10.1111/j.1365-2249.2005.02840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson PG, Wark PA, Simpson JL, et al. Induced sputum IL-8 gene expression, neutrophil influx and MMP-9 allergic bronchopulmonary aspergillosis. Eur. Respir. J. 2003;21:582–588. doi: 10.1183/09031936.03.00001803. [DOI] [PubMed] [Google Scholar]
- Goetzl EJ, Banda MJ, Leppert D. Matrix metalloproteinases in immunity. J. Immunol. 1996;156:1–4. [PubMed] [Google Scholar]
- González AA, Segura AM, Horiba K, et al. Matrix metalloproteinases and their tissue inhibitors in the lesions of cardiac and pulmonary sarcoidosis: an immunohistochemical study. Hum. Pathol. 2002;33:1158–1164. doi: 10.1053/hupa.2002.129423. [DOI] [PubMed] [Google Scholar]
- Hsu LS, Lee HH, Chen KM, Chou HL, Lai SC. Matrix metalloproteinase-2 and -9 in the granulomatous fibrosis of rats infected with Angiostrongylus cantonensis. Ann. Trop. Med. Parasitol. 2005;99:61–70. doi: 10.1179/136485905X19919. [DOI] [PubMed] [Google Scholar]
- Kashino SS, Calich VLG, Burger E, Singer-Vermes LM. In vivo and in vitro characteristics of six Paracoccidioides brasiliensis strains. Mycopathologia. 1985;92:173–178. doi: 10.1007/BF00437630. [DOI] [PubMed] [Google Scholar]
- Kleiner DE, Stetler-Stevenson WG. Quantitative zymography: detection of picogram quantities of gelatinases. Anal. Biochem. 1994;218:325–329. doi: 10.1006/abio.1994.1186. [DOI] [PubMed] [Google Scholar]
- Lenzi HL, Calich VLG, Miyaji M, Sano A, Nishimura K, Burger E. Fibrosis patterns of lesions developed by athymic and euthymic mice infected with Paracoccidioides brasiliensis. Braz. J. Med. Biol. Res. 1994;27:2301–2308. [PubMed] [Google Scholar]
- Lenzi HL, Oliveira DN, Pelajo-Machado M, Borojevic R, Lenzi JA. Coelom-associated lymphomyeloid tissue (milky spots): site of lymphoid and myeloid cell generation. Braz. J. Med. Biol. Res. 1996;29:19–24. [PubMed] [Google Scholar]
- Mendes-Giannini MJS, Andreotti PF, Vincenzi LR, et al. Binding of extracellular matrix proteins to Paracoccidioides brasiliensis. Microbes Infect. 2006;8:1550–1559. doi: 10.1016/j.micinf.2006.01.012. [DOI] [PubMed] [Google Scholar]
- Montenegro MR, Franco M. Pathology. In: Franco M, Lacaz CS, Restrepo-Moreno A, Del Negro D, editors. Paracoccidioidomycosis. Boca Raton, FL, USA: CRC Press; 1994. pp. 131–150. [Google Scholar]
- Nishikaku AS, Burger E. Evaluation of fungal burden in experimental paracoccidioidomycosis by using the fluorescent dye Blankophor. J. Clin. Microbiol. 2003a;41:3419–3422. doi: 10.1128/JCM.41.7.3419-3422.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikaku AS, Burger E. Immunohistochemical demonstration of transforming growth factor-beta and decorin in paracoccidioidal granulomas. Braz. J. Med. Biol. Res. 2003b;36:1073–1078. doi: 10.1590/s0100-879x2003000800014. [DOI] [PubMed] [Google Scholar]
- Nishikaku AS, Scavone R, Molina RFS, Albe BP, Cunha CS, Burger E. Osteopontin involvement in granuloma formation and in the severity of Paracoccidioides brasiliensis infection. Med. Mycol. 2008;1:1–13. doi: 10.1080/13693780802342537. iFirst article (article DOI 10.1080/13693780802342537. [DOI] [PubMed] [Google Scholar]
- Opdenakker G, Van Den Steen PE, Dubois B, et al. Gelatinase B functions as regulator and effector in leukocyte biology. J. Leukoc. Biol. 2001;69:851–859. [PubMed] [Google Scholar]
- Puccia R, Carmona AK, Gesztesi J-L, Juliano L, Travassos LR. Exocellular proteolytic activity of Paracoccidioides brasiliensis: cleavage of components associated with the basement membrane. Med. Mycol. 1998;36:345–348. [PubMed] [Google Scholar]
- Restrepo A. The ecology of Paracoccidioides brasiliensis: a puzzle still unsolved. J. Med. Vet. Mycol. 1985;23:323–334. [PubMed] [Google Scholar]
- Stamenkovic I. Matrix metalloproteinases in tumor invasion and metastasis. Semin. Cancer Biol. 2000;10:415–433. doi: 10.1006/scbi.2000.0379. [DOI] [PubMed] [Google Scholar]
- Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu. Rev. Cell. Dev. Biol. 2001;17:463–561. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JL, Hattle JM, Dreitz SA, et al. Role for matrix metalloproteinase 9 in granuloma formation during pulmonary Mycobacterium tuberculosis infection. Infect. Immun. 2006;74:6135–6144. doi: 10.1128/IAI.02048-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaz CAC, Mackenzie DWR, Hearn V, et al. Gelatinase activity of exoantigens from virulent and non-virulent isolates of Paracoccidioides brasiliensis. J. Med. Vet. Mycol. 1994;32:65–69. doi: 10.1080/02681219480000091. [DOI] [PubMed] [Google Scholar]
- Vicentini AP, Gesztesi J-L, Franco MF, et al. Binding of Paracoccidioides brasiliensis to laminin through surface glycoprotein gp43 leads to enhancement of fungal pathogenesis. Infect. Immun. 1994;62:1465–1469. doi: 10.1128/iai.62.4.1465-1469.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams PL, Leib SL, Kamberi P, et al. Levels of matrix metalloproteinase-9 within cerebrospinal fluid in a rabbit model of coccidioidal meningitis and vasculitis. J. Infect. Dis. 2002;186:1692–1695. doi: 10.1086/345365. [DOI] [PubMed] [Google Scholar]
- Wynn TA. Fibrotic disease and the Th1/Th2 paradigm. Nat. Rev. Immunol. 2004;4:1–12. doi: 10.1038/nri1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xidieh CF, Lenzi HL, Calich VLG, Burger E. Influence of the genetic background on the pattern of lesions developed by resistant and susceptible mice infected with Paracoccidioides brasiliensis. Med. Microbiol. Immunol. 1999;188:41–49. doi: 10.1007/s004300050103. [DOI] [PubMed] [Google Scholar]