Abstract
The aim of this work was to study alterations in the extracellular matrix of liver in dogs naturally infected with Leishmania (Leishmania) chagasi that are correlated with clinical aspects and with histological, parasitological and immunological findings. The study was carried out on 30 dogs, 10 uninfected (control group) and 20 infected. The infected animals were further divided into two groups: an asymptomatic group of 10 dogs without clinical signs of the disease; and a symptomatic group of 10 dogs with classical clinical signs. All thirty animals were mongrel dogs of undefined age, obtained from the municipality of Belo Horizonte, MG, metropolitan area. During necropsy, liver fragments were collected and fixed in 10% buffered formaldehyde for histological examination. Paraffined sections of the tissues were stained with haematoxylin–eosin, Gomori’s ammoniacal silver stain for reticular fibres and strepto-avidin peroxidase for immunohistochemical detection of Leishmania amastigotes. Frozen tissue sections were stained by immunofluorescence for fibronectin (FN) and laminin (LN). Liver collagen deposition was significantly greater in the infected than the control animals and differed significantly between the symptomatic and asymptomatic dogs. There was a positive correlation between the parasite load and liver collagen deposition. The increased collagen deposition in infected animal livers may be associated with the parasite burden. Adhesive FN and LN fibres were significantly more highly expressed in the livers of symptomatic than of asymptomatic dogs. Our results demonstrate that canine visceral leishmaniasis causes fibrogenesis in liver, associated with the parasite load and degenerative processes.
Keywords: canine visceral leishmaniasis, extracellular matrix, pathology
Human (HVL) and canine (CVL) visceral leishmaniasis in the New World are caused by Leishmania (Leishmania) chagasi, which is transmitted by the phlebotomine Lutzomyia (Lutzomyia) longipalpis. Leishmania species are digenetic protozoa that alternately parasitize sand fly vectors and mammalian macrophages. The parasites are deposited in the mammalian skin by infected sand flies and must thereafter interact with and overcome a variety of obstacles, including extracellular matrix (ECM) and basement membrane (BM) proteins, to establish infection within macrophage phagolysosomes (Chang et al. 1986; Bandyopadhyay et al. 2003; Kulkarni et al. 2008). In fact, Ghosh et al. (1996) reported the presence of a 67-kDa glycoprotein on the surface of L. donovani that binds to laminin (LN), a major protein of ECM. Detailed characterization revealed that it might act as an adhesin that may constitute the basis for the homing of the parasites to its physiological address (Bandyopadhyay et al. 2001). McGwire et al. (2003) showed that the migration of Leishmania spp. through the ECM in vitro due to the leishmanolysin (metalloprotease), that is able to mediate proteolysis of fibronectin (FN) and collagen type IV.
Visceral leishmaniasis remains a serious public health problem in the world, and dogs (Canis familaris) are the peridomestic reservoir hosts (Anderson et al. 1980; Grimaldi et al. 1989; Tesh 1995). Classical histopathological lesions have been described mainly in organs rich in cells of the mononuclear-phagocytic system such as liver, spleen, lymph nodes, bone marrow, gastrointestinal tract and skin. In general, an intense chronic inflammatory reaction consisting of infiltration by mononuclear cells (macrophages, plasma cells and lymphocytes) is observed in liver and spleen (Tryphonas et al. 1977; Anosa & Idowu 1983; Keenan et al. 1984; Tafuri et al. 1996; Rallis et al. 2005), skin (Ferrer et al. 1988; Tafuri et al. 2001; Solano-Gallego et al. 2004; Giunchetti et al. 2006), bone-marrow (Tafuri et al. 2001; Reis et al. 2006) and lymph nodes (Martinez-Moreno et al. 1993; Lima et al. 2004; Costa et al. 2008).
The ECM consists of fibrous proteins (collagen and elastin), proteoglycans, glycosaminoglycans and structural proteins. The fibrous components may be divided into two chemically distinct systems: elastic and collagen (Montes 1996). The ECM plays an essential role in cell anchorage, migration, division and differentiation, and also in cell death. Furthermore, it participates in tissue fluid dynamics and provides mechanical support for both rigid and elastic tissues (Rodgers & Irving Rodgers 2002). LN and FN, large mosaic proteins of the ECM, are important in the development and maintenance of cellular organization and are key components in several biological processes (Wyler 1987; Beck et al. 1990; Bandyopadhyay et al. 2003; Kulkarni et al. 2008). FN has several functional domains that enable it to interact with cells, heparin, fibrin, collagen and immunoglobulins, and also with parasites (Wyler et al. 1985; Kulkarni et al. 2008). Macrophages can interact with different FN domains via different receptors. This interaction could increase the phagocytic capacities of macrophages and neutrophils as it enhances chemotaxis, phagocyte adherence and phagocytosis (Proctor 1987; Vannier-Santos et al. 1992), or the binding of macrophages to peptide fragments containing the FN interconnecting segment (ICS) domain can decrease the macrophage functions (Korom et al. 1998).
Studies of both Leishmania spp. and Trypanosoma cruzi have provided strong evidence that these protozoan parasites use host FN and LN to bridge their association with host monocytes and macrophages (Wyler 1987; Ghosh et al. 1996;McGwire et al. 2003; Kulkarni et al. 2008). Several glycoproteins including FN, LN and tenascin (TN) are involved in the interaction of the cells with the ECM and thereby influence the hardness of tissue (Bandyopadhyay et al. 2003). FN has been implicated in the assembly of the ECM, and in the interaction of collagens and proteoglycans with the cell surface through integrins and cell migration (Hynes et al., 1992; Ruoslahti 1988;Kulkarni et al. 2008).
In the liver, the interaction between the ECM and cells is essential for normal homeostasis and for the maintenance of lobular architecture; modification of the ECM results in deranged hepatic function. The ECM content of the liver has been shown to undergo quantitative and qualitative changes in hepatic fibrosis and cirrosis (Schuppan 1990; Martinez-Hernandez & Amenta 1995). The ECM components associated with CVL have not been fully characterized. In this work, we report histological and immunohistochemical changes in the hepatic ECM and in collagen fibre and FN expression in dogs naturally infected with Leishmania (Leishmania) chagasi. Animals were obtained from an endemic area of Brazil, the municipality of Belo Horizonte, MG (Southern Brazil).
Materials and methods
Animals
Twenty mongrel dogs of unknown age naturally infected with L. chagasi were identified during an epidemiological survey of CVL carried out by the municipality of Belo Horizonte, MG (Southern Brazil). Enzyme-linked immunosorbent assays (ELISA; optical density >0.100 (cut-off) >1:400) were positive for all infected animals (da Costa-Val et al. 2007). We also analysed serum samples with a commercial kit containing an immunochromatographic strip that uses a recombinant leishmanial antigen k39 and a dominant amastigote antigen of L. chagasi (rK39), which is highly sensitive and specific for Leishmania donovani complex infection, as previously described (Burns et al. 1993;Houghton et al. 1998;Sundar et al. 2002). Sera from all infected dogs were also positive for this test. Another 10 dogs with serological examinations negative for Leishmania were obtained as controls.
Groups and clinical aspects of infected dogs
All infected dogs were clinically classified and divided into three groups as follows. Group 1: symptomatic dogs – 10 animals that exhibited the classic signs of the disease such as lymphadenopathy, cutaneous alterations (alopecia, dry exfoliative dermatitis or ulcers), onychogryphosis, keratoconjunctivitis, weight loss or cachexia and anaemia. Group 2: asymptomatic dogs – 10 apparently healthy animals with no signs of the visceral disease. Group 3: control dogs – 10 uninfected animals with serological and parasitological examinations negative for Leishmania.
Parasitological diagnosis of Leishmania infection
All dogs were anesthetized with 2.5% (1.0 ml/kg) intravenous thiopental. The experimental protocol using dogs was approved by CETEA-UFMG (Brazilian Animal Experimental College, number 106/2004). Touch aspirates of bone marrow were obtained for parasitological diagnosis of infected and control animals. The smears were air-dried and stained with 10% Giemsa. Leishmania amastigotes were detected in all infected animals by light microscopy using oil immersion (×1000 magnification). Control animals were parasitologically negative.
Necropsy and histopathology
The animals were anesthetized with 0.5 ml/kg intravenous thiopental (2.5%) and killed with T-61 (0.3 ml/kg). During necropsy, the livers were weighed, and tissue touch preparations (smears) were obtained from small samples as described for the bone marrow aspirates. Leishmania amastigotes were observed on slides stained with 10% Giemsa. Amastigote forms of Leishmania were observed in all smears of infected animal livers. Other liver fragments were collected for histopathology. These samples were fixed in 10% neutral-buffered formalin and were dehydrated, cleared, embedded in paraffin, cut into 4–5 μm thick sections and stained with haematoxylin and eosin (HE). For collagen studies, all liver fragments were stained with Gomori ammoniacal silver. After silver staining, the fibrillar collagen becomes black.
Histomorphometric analysis
Liver sections stained with Gomori ammoniacal silver were analysed morphometrically to characterize intralobular collagen deposition, excluding perivascular collagen. This analysis was carried out using an Axiolab light microscope (Zeiss) with ×440 resolution. The images were transferred to a computer video screen using software and relayed to a computer-assisted image analysis system (Kontron Elektronic/Carl Zeiss, Oberkochen, Germany). Using a digital pad, the total area occupied by the stained collagen fibres was measured from real images and segmented to generate binary images. The results are expressed in square micrometers (Caliari 1997).
For all infected animals (20), the number of hepatic granulomas was determined by quantification of 20 microscope optic fields using the 40 objective of an Axiolab light microscope (Zeiss).
Immunohistochemical labelling of Leishmania amastigotes and morphometric analysis
The streptavidin–biotin immunohistochemical method was used to detect Leishmania in formalin-fixed paraffin-embedded canine tissues as described by Tafuri and others (Tafuri et al. 2004). Leishmania amastigotes were readily observed within macrophages in fragments of the livers from all naturally infected dogs. For histomorphometric study, 40 randomly chosen images from histological slides of liver tissue fragments were used to assess the number of immunolabelled amastigotes in a Kontron Elektronick/Carl Zeiss image analyzer (KS300 software) as described above, using an Axiolab light microscope (Zeiss) with a ×440 resolution (Caliari 1997)
Immunohistochemical and immunofluorescence characterization of laminin and fibronectin, and morphometric analysis
Frozen sections of liver 3–4 μm thick were embedded in tissue freezing medium (OCT compound-embedding medium for frozen specimens; Miles Laboratories, Elkart, IN, USA) and immediately frozen. Cryosections thick were blocked and fixed in cold acetone for 15 min. For labelling procedures, sections were blocked in PBS containing 0.2% gelatin, 0.1% NaN3 and 0.1% saponin (PGN-saponin), then incubated with monoclonal antibodies and secondary antibodies against the ECM proteins as follows: (i) LN [rabbit anti-human laminin – AHP 420T (AbD Serotec Kidlington, Oxford, UK), diluted 1:400]; (ii) FN [rabbit anti-human fibronectin – F3648 (Sigma-Aldrich Co., Sigma-Aldrich Chemie Gmbh, Munich, Germany); diluted 1:400]; (iii) a secondary antibody, streptoavidin-peroxidase conjugated goat anti-rabbit and mouse (KIT Dako LSAB2 – Cat 0675; Dako Cytomation Inc., Carpinteria, CA, USA), was used for the LN protocol; (iv) a secondary antibody, FITC-conjugated goat anti-rabbit (F9262 – Sigma), diluted 1:100, was used for the FN protocol. All incubations were performed for 40 min using antibodies diluted with PGN-saponin. After three washes in PBS, the slides were mounted in glycerol containing 0.1%p–phenylenediamine (Sigma). The slides were examined on a Bio-Rad 1024 (UV) confocal scanning system (Hercules, CA, USA) coupled to a Zeiss Axiovert 100 microscope, using a 40r 1.2 N.A. PlanApochromatic water immersion objective. Quantitative results were measured in μm2.
Statistical analysis
All collagen staining results were compared between group of dogs by one-way analysis of variance (anova). P< 0.05 was considered significant.
Results
Clinical aspects of the animals
Skin abnormalities are the most usual manifestation of CVL. The most frequent clinical sign was a chronic dry desquamation primarily located on the ears and limbs, followed by onychogryphosis and skin ulcerations. However, a generalized lymphadenopathy was also frequently observed.
Pathological and parasitological findings
Macroscopically, the livers of all naturally infected animals were generally enlarged. However, hepatomegaly was not necessarily found in asymptomatic or symptomatic animals; it varied markedly in parallel with a dark red coloration implying congestion. Histological analysis by HE showed a general chronic inflammatory reaction involving the entire architecture of the liver including the capsule, portal tracts and central veins or perisinusoidal spaces. The portal space showed no fibrosis, but there was mild infiltration with lymphocytes, plasma cells and macrophages parasitized or not (Figure 1a,b). However, a chronic granulomatous inflammatory reaction characterized by numerous intralobular hepatic granuloma formations was found in 95% of the infected animals. The granulomas presented with variable size constituted by macrophages (parasitized or not with amastigotes of L. chagasi), some epithelioid cells, small numbers of lymphocytes, plasma cells and rare neutrophils. In addition, multinucleated giant cells were not observed in these hepatic granulomas (Figure 1c–e). The granulomas were rarely confluent and localized to the sinusoid lumen (total or partial). Kupffer cells showed hyperplasia and hypertrophy, and they were frequently parasitized with amastigotes of Leishmania (Figure 1f).
Figure 1.
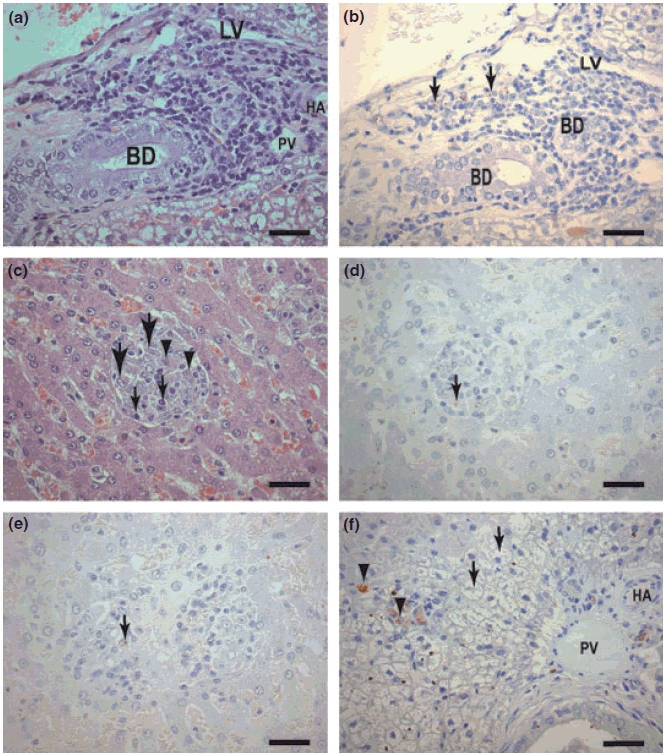
(a–f) Liver sections of dogs naturally infected with L. (L) chagasi. (a,b) Asymptomatic dog: (a) Portal space with a cellular infiltrated of plasma cells lymphocytes and macrophages. HE (Bar = 16 μm). (b) Same field showing immunolabelled amastigotes forms of Leishmania in macrophages (large arrows). Streptoavidin-peroxidase method (Bar = 16 μm). BD, bile duct; LV, lymphatic vessel; HA, hepatic arteriole; (c) Asymptomatic dog. Observe an intralobular granulomas formation comprising macrophages (epithelioid cells) (large arrows), plasma cells (arrowheads) and lymphocytes (small arrows). HE (Bars = 16 μm). (d) Same field showing immunolabelled amastigotes into granulomas macrophages (arrows), Streptoavidin-peroxidase method (Bar = 16 μm). (e) Symptomatic dog: Observe two confluent granulomas in the centre of the figure. Amastigote could be seen (arrow). (f) Symptomatic dog: Kupffer cells intensely parasitized (arrowheads). Note an intense swelling of the hepatocytes (‘balloon cells’) (arrows) (Bar = 16 μm). BD, bile duct; LV, lymphatic vessel; HA, hepatic arteriole; PV, portal vein.
In this work, we have measured the number of granulomas per unit tissue area where asymptomatic dogs showed higher numbers of liver intralobular granuloma than symptomatic ones (P = 0.002 by t-test) (data not shown).
Amastigote forms of Leishmania were observed within the cytoplasmic of the granuloma macrophages and hypertrophied Kupffer cells in HE-stained sections. The result of the hepatic parasitism burden was characterized immunohistochemically and by quantification of Leishmania amastigotes forms in twenty fields at 440× magnification. The parasitism burden showed statistically significant differences between infected groups (P = 0.0032): symptomatic dogs showed a higher parasitism than asymptomatic ones.
None of the hepatic lesions was specific to asymptomatic or symptomatic animals. Thus, although symptomatic animals could harbour more parasites than asymptomatic animals, we were not in general able to distinguish asymptomatic and symptomatic dogs by histological criteria alone.
Extracellular matrix alterations in liver
Evaluation of hepatic reticular fibres by Gomori’s ammoniacal silver
Hepatic reticular fibres in symptomatic dogs were thicker than those in the control group and were mostly found in the portal space region and in sinusoids in the hepatic lobe walls (Figure 2a,b). Fibres were diffusely spread in several directions forming a compact network; some fibres encircled groups of hepatocytes or even a single cell, producing the aspect of cirrhosis called ‘cirrhosis dites monocelular’ (Nattan-Larrier 1918), and some were diffuse, mostly in areas such as that described in the hepatic cirrhosis of Rogers (1908) (Figure 2c,d).
Figure 2.
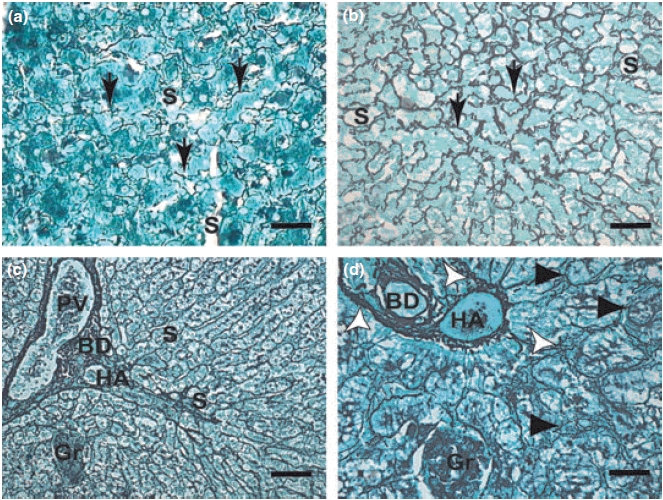
(a–d): Liver sections of control dogs and naturally infected dogs with L. (L) chagasi. (a,b,c.d): (a) Control dog: Higher magnification showing a delicate network of intralobular collagen fibres (reticular fibres) (arrows). Gomori ammoniacal silver-staining (Bars = 16 μm). Note collagen fibres extend through sinusoids. (b) Symptomatic dog: Intralobular fibrosis characterized by collagen fibres extend through sinusoids. Note conspicuous collagen thickening in the space of Disse HE (Bars = 16 μm); (c,d) Symptomatic dog: (c) Lower magnification (panoramic view) showing an intense proliferation of collagen and reticulin fibres detected by ammoniacal silver-staining extend through portal spce (Bars = 32 μm). (d) Detail showing hepatic cells that had become isolated from the sinusoidal blood by the fibropoiesis (arrowheads). Observe dense and coiled fibres (white arrows) extend through sinusoids from the portal tract. Gomori ammoniacal silver-staining (Bars = 16 μm). BD, bile duct; HA, hepatic arteriole; PV, portal vein; S, sinusoids; Gr, granuloma.
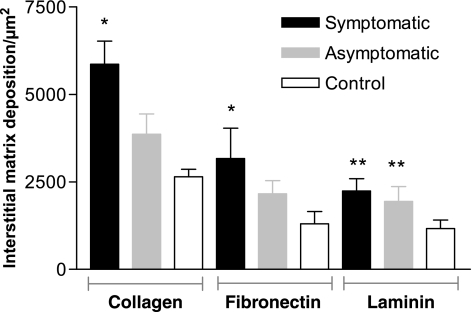
Quantification showed a significantly increased deposition of hepatic collagen in both infected animal groups (symptomatic and asymptomatic) compared with control dogs (P < 0.0001). Symptomatic animals showed more intense fibrilopoiesis than asymptomatic dogs and controls (Figure 3).
Figure 3.
Morphometrical analyses of collagen, fibronectin and laminin deposition in liver of naturally infected dogs with L. chagasi.*Significant difference of asymptomatic and symptomatic collagen and fibronectin liver deposition (P < 0.001).
There was a positive correlation between hepatic collagen deposition and the parasite load in livers. This correlation was highly significant (r = 0.7124, P < 0.0001, Pearson test).
Hepatic laminin and fibronectin expression
Hepatic FN was mostly distributed in the portal and sinusoidal spaces in all the groups studied. Hepatic FN expression was significantly higher in infected animals (symptomatic and asymptomatic) than controls (P < 0.0001) (Figures 3 and 4) and also higher in the symptomatic group than in the asymptomatic and control group dogs (P < 0.001). Similarly, there were statistically significant differences between the groups in hepatic FN deposition. There was a highly significant positive correlation between FN expression and the liver parasite load in livers (r = 0.3868, P < 0.001, Pearson test).
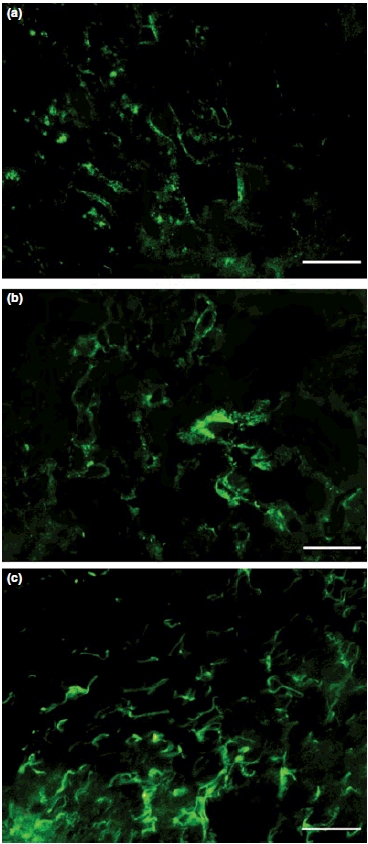
Frozen liver section of control dog (a) and naturally infected dogs (b,c): (a) fibronectin discreet expression in hepatic lobe; (b) asymptomatic dog with fibronectin expression in hepatic lobe; (c) symptomatic dog with exuberant and diffuse fibronectin expression in hepatic lobe. Immunofluorescence technique (Bars = 50 μm).
Infected dogs showed higher LN deposition in their livers than non-infected animals (P<0.001) (Figure 3). Spearman Rank Correlation between parasite load and LN deposition showed a positive correlation (r = 0.4838, P = 0.068). Liver LN was detected discontinuously, mainly in the walls of the central veins and perisinusoidal spaces (Figure 5). In some cases, we observe positive cells into the hepatic intralobular granulomas (Figure 5d).
Figure 5.
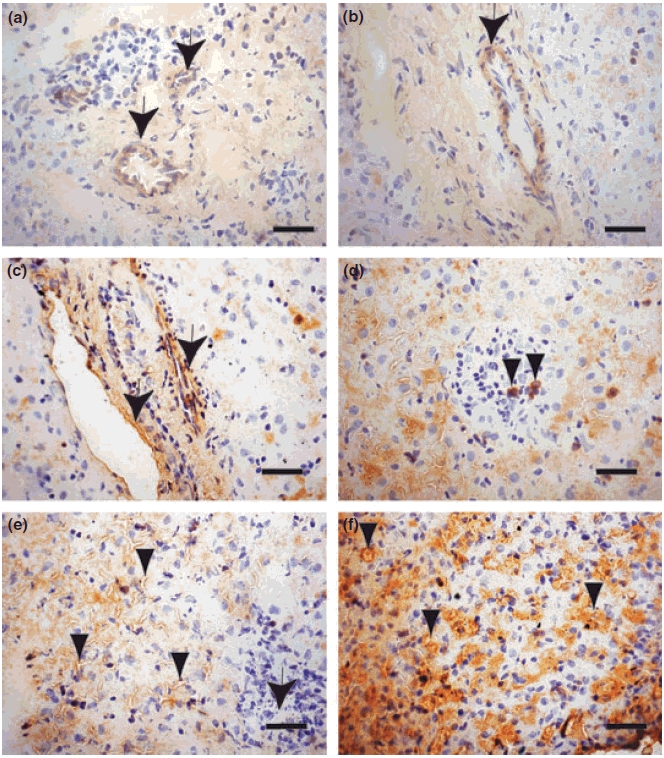
Frozen liver section of control dog and naturally infected dogs (a,b) Control dog: laminin was weakly detected discontinuously, mainly in the walls of the central veins and perisinusoidal spaces (arrows). (c,d) Asymptomatic dog: (c) Presence of laminin around vessels (arrows); (d) an intralobular granuloma with a few positive cells for laminin (arrowheads). (e,f) Symptomatic dog: Diffuse laminin expression around the sinusoids (arrowheads). At the right corner, an intralobular granuloma with amastigotes forms of Leishmania can be observed (arrow), (f) Note strong laminin expression around the sinusoids (arrowheads). Immuno-streptoavidin-peroxidase method (Bars = 16 μm).
Discussion
Canine visceral leishmaniasis is chronic disease of great epidemiological importance; the dog being the main urban reservoir for human disease (Tesh 1995; Ashford 2000; Margonari et al. 2006; Baneth et al. 2008). Dogs that show disease manifest different clinical signs and variable degrees of severity. However, substantial research recently published studies related on the pathogenesis of the canine disease and have recently been intensified with the aim of understanding the mechanisms involved in the formation of lesions and the clinical signals shown by infected animals (Quinnell et al. 2001; Papadogiannakis et al. 2005; Rallis et al. 2005; Reis et al. 2006; Lage et al. 2007; Strauss-Ayali et al. 2007).
A large spectrum of lesions and histological alterations can be observed during the infection of dogs by L. chagasi. In dogs, alterations associated with the skin are common, as described in the literature, particularly dry desquamation (55%) and alopecia (40%). When we consider the skin alterations overall, desquamation, alopecia and ulcerations predominate, in accordance with the findings described by Ferrer et al. (1988); Ciaramella et al. (1997), Lima et al. (2004) and Xavier et al. (2006).
In asymptomatic and symptomatic dog livers, a chronic inflammatory reaction was generally observed, characterized by mononuclear cell infiltration in the portal space and the hepatic parenchyma (lobes). An inflammatory exudate was also observed, and there were particular formations such as hepatic interlobular granulomas. A similar finding has been described in murine visceral leishmaniasis models (Murray 2001), in dogs experimentally infected with L. donovani and L. chagasi (Gonzalez et al. 1988; Oliveira et al. 1993; Tafuri et al. 1996), and in dogs naturally infected with L. chagasi (Tafuri et al. 1996; Sanchez et al. 2004). In this work, we found that the hepatic granulomas were more numerous in asymptomatic infected dogs. According to Lima et al. (2007), in a study with Brazilian naturally infected dogs, these authors showed, by histomorphometrical analysis, asymptomatic animals with higher numbers of granulomas than symptomatic ones. Moreover, they demonstrated that the parasitism decreases while granuloma diameter increases. In the present work, we found higher numbers of granulomas in asymptomatic animals. We could believe that it might be directly related to the lower hepatic parasite load also found in asymptomatic animals.
Collagen fibre deposition, evaluated histochemically by Gomori ammoniacal silver, was statistically significantly greater in symptomatic and asymptomatic dog livers than in controls. Moreover, there was a positive correlation between hepatic collagen deposition in naturally infected dogs and the parasite burden. Infected dogs showed more hepatic collagenogenesis (intralobular fibrosis), probably stimulated by the tissue parasite load as suggested by Bogliolo (1956) and Corbett et al. (1993) in HVL.
Collagen fibres were deposited in the hepatic lobes in all animals, although with varied intensities. New deposition was extremely intense in the livers of symptomatic dogs, the fibres being distributed diffusely and in several directions, but parts of the hepatic lobe parenchyma were not isolated as in other fibrosing hepatopathies (Bogliolo 1956; Andrade & Andrade 1966;Corbett et al. 1993). These collagen fibres showed various thicknesses, being thicker in certain lobular areas than in others. This may indicate that intralobular deposition is a distinct evolutionary phase, the process eventually reaching all the hepatic lobes (Duarte & Corbett 1987). Collagen was also prominent in the hepatic portal space and hepatic capsule, but there was no indication of continuity with the intralobular fibrosis. Melo et al. (2008) described two cases of symptomatic dogs naturally infected with L. chagasi with a peculiar and diffuse intralobular fibrosis compatible with the description by Rogers (1908) (so-called ‘Rogers’ cirrhosis’) in Indian Kala-azar. The fibrosis had developed to link the portal areas and central veins, whereas the silver staining showed a conspicuous thickening of the collagen (reticulin) fibres in the space of Disse. In some areas, hepatic cells were isolated from the sinusoidal blood by fibropoesis. Some aspects of this peculiar histological pattern was also observed in this work as depicted in Figure 2d.
Fibrosis is an important manifestation of several parasitic diseases, but is not irreversible (Corbett et al. 1993). A marked degree of ECM degradation can occur after parasitism is cured. Matrix formation and degeneration are balanced processes dependent on the same cell types. Excess matrix (fibrosis) accumulates when formation exceeds degradation. Chronic inflammation, a common consequence of parasitic infections, is a potent promoter of ECM formation (Andrade 1991). In response to tissue injuries, greatly increased quantities of transforming growth factor (TGF-β), and platelet-derived growth factor (PDGF) are released by macrophages, inducing fibrogenesis by excessive ECM production (Guyot et al. 2006). Corbett et al. (1993) described electron microscopic aspects of this fibrosis in the sinusoids. Thickening in the Space of Disse was due to collagen fibres and an increased extracellular, non-fibrilar, electron-dense matrix. The Ito cells showed signs of increased activity with many fat vacuoles and dilated endoplasmic reticulum containing internal electron-dense material. It is known that stellate hepatic cells (Ito cells, hepatic lipocytes, fat-storing spaces of Disse surrounding the sinusoids) are the predominant source of ECM responsible for hepatic fibrosis. In normal liver, stellate cells store retinoids (vitamin A metabolites) (Li & Friedman 1999; Eng & Friedman 2000) and produce small amounts of extracellular matrix components such as LN and collagen type IV to form the basement membrane (Maher & Bissell 1993). Under conditions of liver injury, stellate cells undergo a transformation (‘activation’) in which they acquire a myofibroblastic phenotype. The activated stellate cell is characterized by its ability to migrate to areas of injury, proliferate, synthesize extracellular matrix (fibrogenesis) and generate contraction force (Friedman 2000; Abath et al. 2006).
Hepatic FN expression was also greater in symptomatic and asymptomatic animals than controls. There were statistical differences between all the groups and a positive correlation between hepatic FN expression in naturally infected animals and the liver parasite load. However, LN deposition was higher in infected dogs, but there was no difference among the different groups of animals with defined clinical status. Thus, the excessive FN and LN expression in infected animal livers reflect the inflammatory and degenerative processes described. Studies have shown that FN is secreted during hepatic regeneration after partial hepatectomy or cirrhogenesis, assuming in these cases an important role in the process of capillarization of the sinusoids (Martinez-Hernandez & Amenta 1995). In addition, Korom et al. (1998) described that peptide fragments produced by the proteolytic degradation of FN can have a dramatic and varied influence on macrophages activation and function. The binding of macrophages to peptide fragments containing the FN interconnecting segment (ICS) domain (Hynes et al. 1985) can decrease macrophage expression of gamma interferon, interleukin 12, monocyte chemoattractant protein 1 (MCP-1) and TGF-β. We have found a strict correlation between the tissue parasite load and alterations in ECM components where FN and LN deposition was higher in infected dogs than controls. Taken all these ideas together we could infer that the deposition of FN and LN might be responsible for the success of the infection.
The fact of the presence of LN positive cells of hepatic granulomas, we could think that these cells might be important for the architecture of the granuloma formation. Moreover, it could be especially important in asymptomatic animals, as we have found more hepatic granuloma formations in these ones. However, we concluded that we need to carry out more studies concerning the fact that asymptomatic dogs could present granulomas better organized than symptomatic dogs. In fact, some authors as Sanchez et al. (2004) described a distinct architecture and immune tissue characterization of hepatic granulomas in asymptomatic and symptomatic naturally infected dogs in Venezuela. The livers of asymptomatic animals showed effective immunity with well-organized granulomas walling off the parasites in an environment of effector T cells expressing CD44low, CD45ROhi, CD44hi, CD45ROlow, MHC class II, CD11c and CD18 integrins. In contrast, symptomatic livers showed a non-organized and non-effective infiltrate composed of T cells and heavily parasitized.
In this work, we characterized fibrosis in the livers of dogs naturally infected with L. chagasi. We also observed a positive correlation between the tissue parasite load, collagen deposition, and FN and LN expression in liver. These ECM alterations might be directly related to the progress of the canine disease.
References
- Figure 4.Abath FG, Morais CN, Montenegro CE, Wynn TA, Montenegro SM. Immunopathogenic mechanisms in schistosomiasis: what can be learnt from human studies? Trends Parasitol. 2006;22:85–91. doi: 10.1016/j.pt.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Anderson DC, Buckner RG, Glenn BL, MacVean DW. Endemic canine leishmaniasis. Vet. Pathol. 1980;17:94–96. doi: 10.1177/030098588001700110. [DOI] [PubMed] [Google Scholar]
- Andrade ZA. Contribution to the study of septal fibrosis of the liver. Int. J. Exp. Pathol. 1991;72:553–562. [PMC free article] [PubMed] [Google Scholar]
- Andrade ZA, Andrade SG. Some new aspects of the kala-azar pathology. (Morphologic study of 13 autopsy cases) Rev. Inst. Med. Trop. Sao Paulo. 1966;8:259–266. [PubMed] [Google Scholar]
- Anosa VO, Idowu AL. The clinico-haematological features and pathology of leishmaniasis in a dog in Nigeria. Zentralbl Veterinarmed B. 1983;30:600–608. doi: 10.1111/j.1439-0450.1983.tb01886.x. [DOI] [PubMed] [Google Scholar]
- Ashford RW. The leishmaniases as emerging and reemerging zoonoses. Int. J. Parasitol. 2000;30:1269–1281. doi: 10.1016/s0020-7519(00)00136-3. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay K, Karmakar S, Ghosh A, Das PK. Role of 67 kDa cell surface laminin binding protein of Leishmania donovani in pathogenesis. J. Biochem. 2001;130:141–148. doi: 10.1093/oxfordjournals.jbchem.a002953. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay K, Karmakar S, Biswas A, Das PK. Membrane orientation of laminin binding protein. Eur. J. Biochem. 2003;270:3806–3813. doi: 10.1046/j.1432-1033.2003.03768.x. [DOI] [PubMed] [Google Scholar]
- Baneth G, Koutinas AF, Solano-Gallego L, Bourdeau P, Ferrer L. Canine leishmaniosis – new concepts and insights on an expanding zoonosis: part one. Trends Parasitol. 2008;24:324–330. doi: 10.1016/j.pt.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Beck K, Hunter I, Engel J. Structure and function of laminin: anatomy of a multidomain glycoprotein. FASEB J. 1990;4:148–160. doi: 10.1096/fasebj.4.2.2404817. [DOI] [PubMed] [Google Scholar]
- Bogliolo L. Nova contribuição ao conhecimento da anatomia patológica da leishmaniose visceral. A propósito de um caso brasileiro e com especial referência à fibrose hepática leishmaniótica. O Hospital. 1956;3:101–164. [Google Scholar]
- Burns JM, Jr, Shreffler WG, Benson DR, Ghalib HW, Badaro R, Reed SG. Molecular characterization of a kinesin-related antigen of Leishmania chagasi that detects specific antibody in African and American visceral leishmaniasis. Proc. Natl. Acad. Sci. USA. 1993;90:775–779. doi: 10.1073/pnas.90.2.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caliari M. Principios de Morfometria Digital. Belo Hrozionte: UFMG; 1997. [Google Scholar]
- Chang KP, Nacy CA, Pearson RD. Intracellular parasitism of macrophages in leishmaniasis: in vitro systems and their applications. Methods Enzymol. 1986;132:603–626. doi: 10.1016/s0076-6879(86)32045-7. [DOI] [PubMed] [Google Scholar]
- Ciaramella P, Oliva G, Luna RD, Gradoni L, Ambrosio R, Cortese L, Scalone A, Persechino A. A retrospective clinical study of canine leishmaniasis in 150 dogs naturally infected by Leishmania infantum. Vet Rec. 1997;141:539–543. doi: 10.1136/vr.141.21.539. [DOI] [PubMed] [Google Scholar]
- Corbett CE, Duarte MI, Bustamante SE. Regression of diffuse intralobular liver fibrosis associated with visceral leishmaniasis. Am. J. Trop. Med. Hyg. 1993;49:616–624. doi: 10.4269/ajtmh.1993.49.616. [DOI] [PubMed] [Google Scholar]
- Costa MM, Lima WG, Figueiredo MM, Michalick MS, Tafuri WL. Cervical, mandibular, and parotid lymph nodes of dogs naturally infected with Leishmania infantum: a histopathologic and immunohistochemistry study and its correlation with facial skin lesions. Vet. Pathol. 2008;45:613–616. doi: 10.1354/vp.45-5-613. [DOI] [PubMed] [Google Scholar]
- da Costa-Val AP, Cavalcanti RR, de Figueiredo Gontijo N, et al. Canine visceral leishmaniasis: relationships between clinical status, humoral immune response, haematology and Lutzomyia (Lutzomyia) longipalpis infectivity. Vet. J. 2007;174:636–643. doi: 10.1016/j.tvjl.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte MI, Corbett CE. Histopathological patterns of the liver involvement in visceral leishmaniasis. Rev. Inst. Med. Trop. Sao Paulo. 1987;29:131–136. doi: 10.1590/s0036-46651987000300003. [DOI] [PubMed] [Google Scholar]
- Eng FJ, Friedman SL. Fibrogenesis I. New insights into hepatic stellate cell activation: the simple becomes complex. Am. J. Physiol. Gastrointest. Liver Physiol. 2000;279:G7–G11. doi: 10.1152/ajpgi.2000.279.1.G7. [DOI] [PubMed] [Google Scholar]
- Ferrer L, Rabanal RM, Domingo M, Ramos JA, Fondevila D. Identification of Leishmania donovani amastigotes in canine tissues by immunoperoxidase staining. Res. Vet. Sci. 1988;44:194–196. [PubMed] [Google Scholar]
- Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J. Biol. Chem. 2000;275:2247–2250. doi: 10.1074/jbc.275.4.2247. [DOI] [PubMed] [Google Scholar]
- Ghosh A, Kole L, Bandyopadhyay K, Sarkar K, Das PK. Evidence of a laminin binding protein on the surface of Leishmania donovani. Biochem. Biophys. Res. Commun. 1996;226:101–106. doi: 10.1006/bbrc.1996.1317. [DOI] [PubMed] [Google Scholar]
- Giunchetti RC, Mayrink W, Genaro O, et al. Relationship between canine visceral leishmaniosis and the Leishmania (Leishmania) chagasi burden in dermal inflammatory foci. J. Comp. Pathol. 2006;135:100–107. doi: 10.1016/j.jcpa.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Gonzalez JL, Rollan E, Novoa C, Castano M. Structural and ultrastructural hepatic changes in experimental canine leishmaniasis. Histol. Histopathol. 1988;3:323–329. [PubMed] [Google Scholar]
- Grimaldi G, Jr, Tesh RB, McMahon-Pratt D. A review of the geographic distribution and epidemiology of leishmaniasis in the New World. Am. J. Trop. Med. Hyg. 1989;41:687–725. doi: 10.4269/ajtmh.1989.41.687. [DOI] [PubMed] [Google Scholar]
- Guyot C, Lepreux S, Combe C, et al. Hepatic fibrosis and cirrhosis: the (myo)fibroblastic cell subpopulations involved. Int. J. Biochem. Cell Biol. 2006;38:135–151. doi: 10.1016/j.biocel.2005.08.021. [DOI] [PubMed] [Google Scholar]
- Houghton RL, Petrescu M, Benson DR, et al. A cloned antigen (recombinant K39) of Leishmania chagasi diagnostic for visceral leishmaniasis in human immunodeficiency virus type 1 patients and a prognostic indicator for monitoring patients undergoing drug therapy. J. Infect. Dis. 1998;177:1339–1344. doi: 10.1086/515289. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Keenan CM, Hendricks LD, Lightner L, Webster HK, Johnson AJ. Visceral leishmaniasis in the German shepherd dog. I. Infection, clinical disease, and clinical pathology. Vet. Pathol. 1984;21:74–79. doi: 10.1177/030098588402100113. [DOI] [PubMed] [Google Scholar]
- Korom S, Hancock WW, Coito AJ, Kupiec-Weglinski JW. Blockade of very late antigen-4 integrin binding to fibronectin in allograft recipients. II. Treatment with connecting segment-1 peptides prevents chronic rejection by attenuating arteriosclerotic development and suppressing intragraft T cell and macrophage activation. Transplantation. 1998;65:854–859. doi: 10.1097/00007890-199803270-00014. [DOI] [PubMed] [Google Scholar]
- Kulkarni MM, Jones EA, McMaster WR, McGwire BS. Fibronectin binding and proteolytic degradation by Leishmania and effects on macrophage activation. Infect. Immun. 2008;76:1738–1747. doi: 10.1128/IAI.01274-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lage RS, Oliveira GC, Busek SU, et al. Analysis of the cytokine profile in spleen cells from dogs naturally infected by Leishmania chagasi. Vet. Immunol. Immunopathol. 2007;115:135–145. doi: 10.1016/j.vetimm.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Li D, Friedman SL. Liver fibrogenesis and the role of hepatic stellate cells: new insights and prospects for therapy. J. Gastroenterol. Hepatol. 1999;14:618–633. doi: 10.1046/j.1440-1746.1999.01928.x. [DOI] [PubMed] [Google Scholar]
- Lima WG, Michalick MS, de Melo MN, Luiz Tafuri W. Canine visceral leishmaniasis: a histopathological study of lymph nodes. Acta Trop. 2004;92:43–53. doi: 10.1016/j.actatropica.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Lima WG, Oliveira PS, Caliari MV, Gonçalves R, Michalick MS, Melo MN, Tafuri WL, Tafuri WL. Histopathological and immunohistochemical study of type 3 complement receptors (CD11b/CD18) in livers and spleens of asymptomatic and symptomatic dogs naturally infected with Leishmania (Leishmania) chagasi. Vet Immunol Immunopathol. 2007;117:129–136. doi: 10.1016/j.vetimm.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Maher JJ, Bissell DM. Cell-matrix interactions in liver. Semin. Cell Biol. 1993;4:189–201. doi: 10.1006/scel.1993.1023. [DOI] [PubMed] [Google Scholar]
- Margonari C, Freitas CR, Ribeiro RC, et al. Epidemiology of visceral leishmaniasis through spatial analysis, in Belo Horizonte municipality, state of Minas Gerais, Brazil. Mem. Inst. Oswaldo Cruz. 2006;101:31–38. doi: 10.1590/s0074-02762006000100007. [DOI] [PubMed] [Google Scholar]
- Martinez-Hernandez A, Amenta PS. The extracellular matrix in hepatic regeneration. FASEB J. 1995;9:1401–1410. doi: 10.1096/fasebj.9.14.7589981. [DOI] [PubMed] [Google Scholar]
- Martinez-Moreno A, Martinez-Cruz MS, Blanco A, Hernandez-Rodriguez S. Immunological and histological study of T- and B-lymphocyte activity in canine visceral leishmaniosis. Vet. Parasitol. 1993;51:49–59. doi: 10.1016/0304-4017(93)90195-s. [DOI] [PubMed] [Google Scholar]
- McGwire BS, Chang KP, Engman DM. Migration through the extracellular matrix by the parasitic protozoan Leishmania is enhanced by surface metalloprotease gp63. Infect. Immun. 2003;71:1008–1010. doi: 10.1128/IAI.71.2.1008-1010.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo F, Amaral M, Oliveira P, et al. Diffuse intralobular liver fibrosis in dogs naturally infected with Leishmania (Leishmania) chagasi. Am. J. Trop. Med. Hyg. 2008;79:198–204. [PubMed] [Google Scholar]
- Montes GS. Structural biology of the fibres of the collagenous and elastic systems. Cell Biol. Int. 1996;20:15–27. doi: 10.1006/cbir.1996.0004. [DOI] [PubMed] [Google Scholar]
- Murray HW. Tissue granuloma structure-function in experimental visceral leishmaniasis. Int. J. Exp. Pathol. 2001;82:249–267. doi: 10.1046/j.1365-2613.2001.00199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nattan-Larrier L. Les cirrhoses hepatiques due au Kalazar. Bull. Acad. Med. 1918;89:402–408. [Google Scholar]
- Oliveira GG, Santoro F, Sadigursky M. The subclinical form of experimental visceral leishmaniasis in dogs. Mem. Inst. Oswaldo Cruz. 1993;88:243–248. doi: 10.1590/s0074-02761993000200011. [DOI] [PubMed] [Google Scholar]
- Papadogiannakis EI, Koutinas AF, Saridomichelakis MN, et al. Cellular immunophenotyping of exfoliative dermatitis in canine leishmaniosis (Leishmania infantum) Vet. Immunol. Immunopathol. 2005;104:227–237. doi: 10.1016/j.vetimm.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Proctor RA. Fibronectin: an enhancer of phagocyte function. Rev. Infect. Dis. 1987;9(Suppl. 4):S412–S419. doi: 10.1093/clinids/9.supplement_4.s412. [DOI] [PubMed] [Google Scholar]
- Quinnell RJ, Courtenay O, Shaw MA, et al. Tissue cytokine responses in canine visceral leishmaniasis. J. Infect. Dis. 2001;183:1421–1424. doi: 10.1086/319869. [DOI] [PubMed] [Google Scholar]
- Rallis T, Day MJ, Saridomichelakis MN, et al. Chronic hepatitis associated with canine leishmaniosis (Leishmania infantum): a clinicopathological study of 26 cases. J. Comp. Pathol. 2005;132:145–152. doi: 10.1016/j.jcpa.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Reis AB, Teixeira-Carvalho A, Giunchetti RC, et al. Phenotypic features of circulating leucocytes as immunological markers for clinical status and bone marrow parasite density in dogs naturally infected by Leishmania chagasi. Clin. Exp. Immunol. 2006;146:303–311. doi: 10.1111/j.1365-2249.2006.03206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers RJ, Irving Rodgers HF. Extracellular matrix of the bovine ovarian membrana granulosa. Mol. Cell. Endocrinol. 2002;191:57–64. doi: 10.1016/s0303-7207(02)00057-6. [DOI] [PubMed] [Google Scholar]
- Rogers L. A peculiar intralobular cirrhosis of the liver produced by the protozoal parasite of Kala-azar. Ann. Trop. Med. a. Parasitol. 1908;2:147–152. [Google Scholar]
- Ruoslahti E. Fibronectin and its receptors. Annu. Rev. Biochem. 1988;57:375–413. doi: 10.1146/annurev.bi.57.070188.002111. [DOI] [PubMed] [Google Scholar]
- Sanchez MA, Diaz NL, Zerpa O, Negron E, Convit J, Tapia FJ. Organ-specific immunity in canine visceral leishmaniasis: analysis of symptomatic and asymptomatic dogs naturally infected with Leishmania chagasi. Am. J. Trop. Med. Hyg. 2004;70:618–624. [PubMed] [Google Scholar]
- Schuppan D. Structure of the extracellular matrix in normal and fibrotic liver: collagens and glycoproteins. Semin. Liver Dis. 1990;10:1–10. doi: 10.1055/s-2008-1040452. [DOI] [PubMed] [Google Scholar]
- Solano-Gallego L, Fernandez-Bellon H, Morell P, et al. Histological and immunohistochemical study of clinically normal skin of Leishmania infantum-infected dogs. J. Comp. Pathol. 2004;130:7–12. doi: 10.1016/s0021-9975(03)00063-x. [DOI] [PubMed] [Google Scholar]
- Strauss-Ayali D, Baneth G, Jaffe CL. Splenic immune responses during canine visceral leishmaniasis. Vet. Res. 2007;38:547–564. doi: 10.1051/vetres:2007015. [DOI] [PubMed] [Google Scholar]
- Sundar S, Pai K, Sahu M, Kumar V, Murray HW. Immunochromatographic strip-test detection of anti-K39 antibody in Indian visceral leishmaniasis. Ann. Trop. Med. Parasitol. 2002;96:19–23. doi: 10.1179/000349802125000466. [DOI] [PubMed] [Google Scholar]
- Tafuri WL, Barbosa AJ, Michalick MS, et al. Histopathology and immunocytochemical study of type 3 and type 4 complement receptors in the liver and spleen of dogs naturally and experimentally infected with Leishmania (Leishmania) chagasi. Rev. Inst. Med. Trop. Sao Paulo. 1996;38:81–89. doi: 10.1590/s0036-46651996000200001. [DOI] [PubMed] [Google Scholar]
- Tafuri WL, de Oliveira MR, Melo MN. Canine visceral leishmaniosis: a remarkable histopathological picture of one case reported from Brazil. Vet. Parasitol. 2001;96:203–212. doi: 10.1016/s0304-4017(00)00436-2. [DOI] [PubMed] [Google Scholar]
- Tafuri WL, Santos RL, Arantes RM, Goncalves R, de Melo MN, Michalick MS. An alternative immunohistochemical method for detecting Leishmania amastigotes in paraffin-embedded canine tissues. J. Immunol. Methods. 2004;292:17–23. doi: 10.1016/j.jim.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Tesh RB. Control of zoonotic visceral leishmaniasis: is it time to change strategies? Am. J. Trop. Med. Hyg. 1995;52:287–292. doi: 10.4269/ajtmh.1995.52.287. [DOI] [PubMed] [Google Scholar]
- Tryphonas L, Zawidzka Z, Bernard MA, Janzen EA. Visceral leishmaniasis in a dog: clinical, hematological and pathological observations. Can. J. Comp. Med. 1977;41:1–12. [PMC free article] [PubMed] [Google Scholar]
- Vannier-Santos MA, Saraiva EM, Martiny A, Neves A, de Souza W. Fibronectin shedding by Leishmania may influence the parasite-macrophage interaction. Eur. J. Cell Biol. 1992;59:389–397. [PubMed] [Google Scholar]
- Wyler DJ. Fibronectin in parasitic diseases. Rev. Infect. Dis. 1987;9(Suppl. 4):S391–S399. doi: 10.1093/clinids/9.supplement_4.s391. [DOI] [PubMed] [Google Scholar]
- Wyler DJ, Sypek JP, McDonald JA. In vitro parasite-monocyte interactions in human leishmaniasis: possible role of fibronectin in parasite attachment. Infect. Immun. 1985;49:305–311. doi: 10.1128/iai.49.2.305-311.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xavier SC, de Andrade HM, Monte SJ, et al. Comparison of paraffin-embedded skin biopsies from different anatomical regions as sampling methods for detection of Leishmania infection in dogs using histological, immunohistochemical and PCR methods. BMC Vet. Res. 2006;2:17. doi: 10.1186/1746-6148-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]