Abstract
OBJECTIVE
We sought to determine whether exosome-like vesicles (ELVs) released from adipose tissue play a role in activation of macrophages and subsequent development of insulin resistance in a mouse model.
RESEARCH DESIGN AND METHODS
ELVs released from adipose tissue were purified by sucrose gradient centrifugation and labeled with green fluorescent dye and then intravenously injected into B6 ob/ob mice (obese model) or B6 mice fed a high-fat diet. The effects of injected ELVs on the activation of macrophages were determined through analysis of activation markers by fluorescence-activated cell sorter and induction of inflammatory cytokines using an ELISA. Glucose tolerance and insulin tolerance were also evaluated. Similarly, B6 mice with different gene knockouts including TLR2, TLR4, MyD88, and Toll-interleukin-1 receptor (TIR) domain–containing adaptor protein inducing interferon-β (TRIF) were also used for testing their responses to the injected ELVs.
RESULTS
ELVs are taken up by peripheral blood monocytes, which then differentiate into activated macrophages with increased secretion of tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6). Injection of obELVs into wild-type C57BL/6 mice results in the development of insulin resistance. When the obELVs were intravenously injected into TLR4 knockout B6 mice, the levels of glucose intolerance and insulin resistance were much lower. RBP4 is enriched in the obELVs. Bone marrow–derived macrophages preincubated with recombinant RBP4 led to attenuation of obELV-mediated induction of IL-6 and TNF-α.
CONCLUSIONS
ELVs released by adipose tissue can act as a mode of communication between adipose tissues and macrophages. The obELV-mediated induction of TNF-α and IL-6 in macrophages and insulin resistance requires the TLR4/TRIF pathway.
Adipose tissue macrophages (ATMs) infiltrate adipose tissue during obesity and contribute to the development of insulin resistance (1–5). In both humans and rodents, accumulation of ATMs in adipose tissue correlates with increasing body weight and with increasing insulin resistance (6). ATMs are a prominent source of proinflammatory cytokines, such as tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6), that can block insulin action in adipocytes, thus providing a potential link between inflammation and insulin resistance (7–12).
The events that lead to the initial activation of macrophages and their migration into adipose tissues are not fully understood. Recent evidence suggests that inflammatory processes induced by nutrient excess can cause systemic insulin resistance via a mechanism involving TLR4 (13–17). Peritoneal macrophages isolated from TLR4 knockout mice have a reduced capacity to produce cytokines in response to lipid-induced activation of inflammation and insulin resistance (14). Moreover, TLR4 deletion partly prevents diet-induced insulin resistance (17).
Several other pathways have been implicated in the development of insulin resistance. Studies of mice and humans have suggested that elevated levels of RBP4 in the serum could play a causal role in insulin resistance. Manipulation of the levels of RBP4 in the serum affects insulin responses. In mice, transgenic overexpression of RBP4 or injection of purified RBP4 protein into wild-type C57BL/6 (B6) mice causes insulin resistance (18); conversely, RBP4 knockout mice exhibit enhanced insulin sensitivity. In humans, the concentration of RBP4 in the serum is elevated in insulin-resistant humans with obesity, type 2 diabetes, and impaired glucose tolerance (18,19). Moreover, the improvement in insulin sensitivity that occurs in response to interventions such as gastric banding surgery is associated with a lowering in the concentration of RBP4 in the serum (18,20).
Exosomes are endosome-derived organelles (50–100 nm) that are actively secreted through an exocytosis pathway. Recent studies have demonstrated that exosomes can mediate intercellular cross-talk under normal and pathological conditions (21,22). Although communication between adipose tissue and immune cells appears to be of importance in the interconnection between obesity and inflammation and the development of diabetes, research into the signals underlying this communication has, for the most part, been limited to analysis of the roles of cytokines and chemokines. The possibility that adipose tissue–derived exosome-like vesicles (ELVs) are involved in this process and act as a mode of systemic communication has not been explored to any great extent.
In the present study, we found that obELVs are released from adipose tissue, are preferentially taken up by peripheral blood monocytes, and stimulate the differentiation of the monocytes into activated macrophages. This finding suggests that the obELVs released by the adipose tissue could act as a mode of communication between adipose tissues and macrophages. Evidence that this interaction contributes to the development of insulin resistance was obtained by administering obELVs into wild-type B6 mice and the induction of the insulin-resistant phenotype in a mouse model.
RESEARCH DESIGN AND METHODS
C57BL/6j (B6) male mice (Jackson Laboratory) were maintained on a high-fat diet (HFD) (60% fat, LabDiet, 5001; Richmond, IN) or a standard rat diet (10% fat) for 3 months starting at 2 months of age. Male B6 ob/ob mice, B6.Cg-Nfkb1tm1Bal/J, and control littermates were purchased from Jackson Laboratories. TLR2, TLR4, MyD88, and Toll-interleukin-1 receptor (TIR) domain–containing adaptor protein inducing interferon-β (TRIF) knockout male mice on a B6 background were provided by Dr. Shizuo Akira (University of Osaka, Japan). All of the animal experiments were performed under protocols approved by the University of Alabama at Birmingham.
ELV preparation and electron microscopy examination.
To isolate the ELVs, visceral adipose tissue of 5-month-old mice was washed with PBS and cut into small pieces <4 mm, transferred to six-well plates containing 3 ml/well of Dulbecco's modified Eagles medium (Invitrogen) supplemented with 50 μg/ml gentamicin and 10% FBS with bovine sera exosomes predepleted using a method as described previously (23), and cultured in a 37°C incubator in an atmosphere of 5% CO2/95% air. The cultured supernatants were used for ELV purification, which was accomplished by differential centrifugation using a previously described method (24). Concentrated ELVs were analyzed using a Hitachi H7000 electron microscope (Electronic Instruments) as previously described (24). Thymus exosomes were isolated from male B6 mice using a method as described previously (25).
Labeling macrophages with fluorescent dyes and macrophage trafficking in vivo.
PKH67 and PKH26 kits were used for labeling bone marrow– derived macrophages (BMDM) according to the manufacturer's instructions (Sigma). BMDMs from 7-day primary cultures of femoral bone marrow from 6- to 8-week-old female wild-type mice were generated as described previously (26) and detailed in the supplemental Methods in the online appendix available at http://diabetes.diabetesjournals.org/cgi/content/full/db09-0216/DC1. The trafficking of injected macrophages was monitored by fluorescence-activated cell sorter (FACS) analysis of labeled macrophages in adipose tissue, liver, spleen, and bone marrow. As a means to monitor the proliferation potential of injected macrophages, 24 h before mice were killed, 1 mg of bromodeoxyuridine (BrdU; Sigma), dissolved in 300 μl PBS, was injected intraperitoneally with a second dose 3 h before the mice were killed. The leukocytes were then isolated from each tissue including adipose, liver, spleen, and bone marrow using protocols as described previously (24) and stained with anti-BrdU antibody. Once stained, the leukocytes were analyzed by FACS using a protocol described previously (27).
Glucose tolerance and insulin tolerance test.
Mice were injected intravenously with ELVs (30 μg/mouse) every 3 days for 21 days. After the last injection, glucose tolerance and insulin tolerance tests were performed using a method as described (7) and detailed in the supplemental Methods.
Glucose uptake assay.
C2C12 myocytes were purchased from American Type Culture Collection and cultured in α-minimal essential medium supplemented with 10% FBS. Once 80% confluency was attained, the C2C12 myocyte culture medium was replaced with conditioned media from bone marrow precursor cells that had been pretreated with wild-type (wt)ELVs or obELVs or thymus exosomes (10 μg/ml) for 14 days in the presence of granulocyte monocyte colony–stimulating factor (GM-CSF; 20 ng/ml). The effects of combined medium with or without addition of anti–TNF-α and anti–IL-6 antibodies on the [3H]glucose uptake of C2C12 myocytes was assessed as described (28) and detailed in the supplemental Methods.
Macrophages treated with a recombinant mouse RBP4.
A recombinant mouse RBP4 (R&D Systems, catalog number 3476-LC) was purchased and used for stimulating BMDMs (1 × 105/100 μl in RPMI160 medium). Twenty-four hours after the stimulation, the cell culture supernatants were harvested and assayed for TNF-α and IL-6 using an ELISA. To determine the in vivo effects of a mouse RBP4 on the induction of IL-6 and TNF-α, wild-type B6 mice or TLR4 knockout B6 mice were injected intravenously with mouse RBP4 (250 μg/mouse in 200 μl of PBS). Six hours after the injection, serum levels of IL-6 and TNF-α were measured using a standard ELISA. To determine whether the cells pretreated with recombinant mouse RBP4 respond to subsequent ELV RBP4 stimulation, the BMDMs treated with mouse RBP4 were washed with PBS 3 times and then cultured in the presence of wtELVs or obELVs (10 μg/ml) for an additional 24 h. TNF-α and IL-6 in the supernatants was quantified using an ELISA. The details of other methods used for this study have been published by this laboratory and are described in the supplemental Methods.
Statistical analysis.
Statistical differences between groups were determined by ANOVA with multiple comparisons using Fischer's post hoc analysis. The Student's t test was used for comparisons when only two parameters were evaluated. P values < 0.05 were considered significant.
RESULTS
ELVs released from adipose tissue.
Others have demonstrated previously that exosomes are released by 3T3-L1, a precursor adipocyte cell line (29,30). We found that more ELVs are released in 30-min ex vivo adipose tissue cultures of age-matched wild-type (B6) mice fed a HFD over 3 months (HFDELVs, 16.5 ± 1.2 μg/g of adipose weight) and of leptin-deficient (ob/ob) B6 mice (obELVs, 14.4 ± 1.1 μg/g of adipose weight) when compared to adipose tissue cultures of wild-type lean B6 mice (wtELVs, 4.1 ± 1.0 μg/g of adipose weight). The quantity of ELVs released from adipose tissue increased over a period from 30 min to 6 h of ex vivo adipose tissue culture (supplemental Fig. S1A), that is, 16.5–22.2 μg/g HFDELVs; 14.4– 23.2 μg/g obELVs; and 4.1–6.7 μg/g wtELVs. Electron microscopy examination revealed vesicles that measured ∼60–100 nm in diameter and had a cup-shaped morphology (supplemental Fig. S1B). Neither calnexin nor Lamp-1 were detectable when the ELVs were analyzed by immunoblotting (supplemental Fig. S1C), indicating that our ELV preparations were free of contaminating nonexosome membrane proteins (31). Further evidence that the vesicles were exosomes was obtained through analysis of the protein composition using linear ion trap mass spectrometery (LTQ LC/MS) (supplemental Table S1). These analyses indicated a protein composition typical of exosomes derived from other cell types (32–35) (supplemental Table S2). Specific proteins were at undetectable levels in wt-ELVs when compared with protein detected in obELVs and HFDELVs (supplemental Table S1), but in both cases the ELVs contained proteins known to be involved in cell metabolism, membrane trafficking, multiple small GTP-binding proteins, integral membrane proteins, and several class E vacuolar protein sorting proteins. The fatty acid composition of the obELVs was almost exclusively palmitic acid (n = 8, 40.22 ± 3.82%) and stearic acid (52.71 ± 4.42%) (supplemental Table S3). Because there are minor differences in the protein composition of ELVs released from B6 mice fed an HFD in comparison with obELVs (supplemental Table S1), we focused on the B6 obELVs released from adipose tissues at 30-min ex vivo culture for the remainder of study because a larger amount of adipose tissue for isolation of ELVs was available from ob/ob mice of the same age.
ELVs released from the adipose tissue of ob/ob mice activate monocytes.
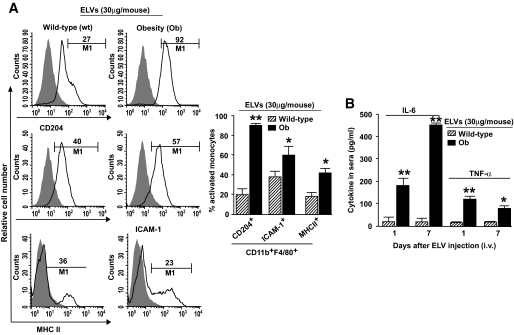
We determine whether obELVs released from adipose tissue are taken up by cells of the immune system. We labeled obELVs with PKH67 dye before injecting them intravenously into B6 mice fed an HFD or standard diet for 3 months starting at 2 months of age. FACS analysis of single-cell suspensions of several tissues that had been harvested 24 h after the injection of obELVs indicated that more than 1.1% of total blood cells or liver leukocytes had taken up obELVs. Approximately 80% of the cells having taken up obELVs were CD11bF4/80+ monocytes (supplemental Fig. S2A and B), but no B cells, T cells, nor natural killer cells had taken up obELVs (data not shown). This result is unlikely because of PKH67 dye leakage from obELVs to the monocytes as no PKH67- positive macrophages were detected in the tissues of mice having been injected intravenously with free PKH67 dye (data not shown). FACS analysis of the gated PKH67+ cells isolated from peripheral blood further indicated that the monocytes (CD11b+F4/80+PKH67+) that had taken up the obELVs expressed higher levels of the monocyte receptors ICAM-1 (intracellular adhesion molecule-1), CD204, and MHCII (major histocompatibility complex II) than did monocytes that had taken up ELVs isolated from lean, wild-type B6 mice (wtELVs) (Fig. 1A), although both wtELVs and obELVs were taken up by the monocytes with equal efficiency (data not shown). In addition, at days 1 and 7 after injection of the ELVs there were higher levels of the proinflammatory cytokines IL-6 and TNF-α in the sera of the mice that had been injected with obELVs than in the sera of B6 mice that had been injected with wtELVs (Fig. 1B). Analysis of ELISA results indicated that the injection of ELVs does not induce the host to generate antibodies against the injected ELVs (data not shown), implying that induction of inflammatory cytokines may not lead to further activation of adaptive immune responses. The experiments as described above were also repeated in B6 mice fed a standard rat diet, and similar results were obtained (data not shown), suggesting that HFD preconditioning is not required for obELV-mediated activation of monocytes.
FIG. 1.
Adipose obELVs activate macrophages. A: Wild-type B6 mice fed an HFD for 3 months starting at 2 months of age were injected intravenously with the PKH67+-labeled obELVs or wtELVs (30 μg/mouse). Twenty-four hours after the injection, CD11b+F4/80+PKH67+ cells from peripheral blood were analyzed for the presence of CD204, ICAM-1, and MHCII markers. Results were pooled from five independent experiments (n = 5 mice/experiment) and are presented as the means ± SE, *P < 0.05, **P < 0.01. B: Mice treated as described above at day 1 and 7, peripheral blood was collected at 4 h after the ELV injections, and the serum concentration of IL-6 and TNF-α were determined using a standard ELISA, *P < 0.05, **P < 0.01 (n = 5 mice per group). i.v., intravenous.
ObELV-mediated activation of macrophages impairs glucose uptake and the insulin response of myocytes in vitro.
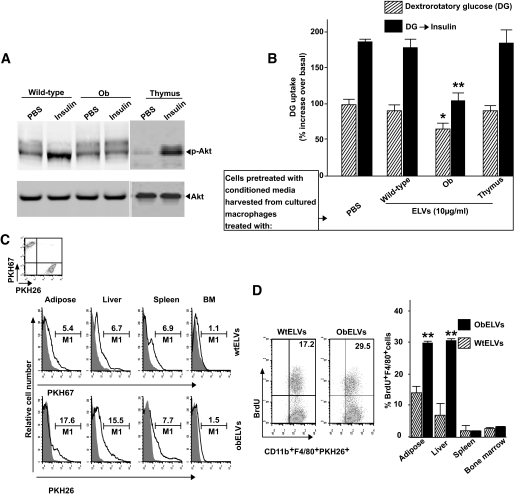
To determine whether the obELV-activated macrophages produce factors that affect insulin sensitivity, we determined the effect of conditioned medium harvested from bone marrow precursor cells that had been pretreated with obELVs for 14 days on glucose uptake and the insulin response of myocytes. The conditioned medium was harvested from 14-day cultures of bone marrow cells that had been pretreated with obELVs or wtELVs and cultured in the presence or absence of GM-CSF. In the course of these experiments, we noted that on day 4 after the addition of the ELVs to the day 0 cultured bone marrow precursor cells that the bone marrow precursors exhibited differentiation into macrophages instead of dendritic cells and that the differentiation into macrophages occurred when the dendritic cell differentiation factor GM-CSF had been added to the day 0 cultures (supplemental Fig. S3A). This differentiation did not occur when wtELVs (supplemental Fig. S3A) or thymus exosomes (supplemental Fig. S3D) were added to the day 0 bone marrow precursor cultures. These obELV-stimulated macrophages continued to proliferate until day 14 (supplemental Fig. S3B), even in the absence of growth factors. These activated macrophages secreted higher quantities of macrophage colony-stimulating factor, IL-6, and TNF-α into the culture supernatants than did the bone marrow precursors that had been stimulated with wtELVs (supplemental Fig. S3C) or thymus exosomes (supplemental Fig. S3E). Furthermore, elevated obELV concentrations were associated with the promotion of bone marrow precursor differentiation (supplemental Fig. S4A), proliferation (supplemental Fig. S4B), and induction of IL-6 (supplemental Fig. S4C).
Upon addition of the conditioned medium to the myocyte cultures, the levels of phosphorylation of Akt were lower in the myocytes cultured with the conditioned medium harvested from macrophages pretreated with obELVs than in the myocytes cultured with conditioned medium from wtELVs (Fig. 2A). Furthermore, basal and insulin-stimulated transport of glucose was inhibited in myocytes treated with obELV-conditioned medium, indicating that insulin function is also impaired (Fig. 2B). The conditioned medium–induced impairment of insulin responses was not observed when bone marrow precursors were stimulated with thymus exosomes (Fig. 2B), suggesting that obELV-mediated impairment of insulin responses in myocytes is adipose tissue exosome specific.
FIG. 2.
Adipose obELVs promote the differentiation and proliferation of BMDMs and impair activation of the insulin signaling pathway in vitro and macrophage infiltration into adipose tissues in vivo. C2C12 cells at 80% confluency were cultured for 24 h in the presence of conditioned medium harvested from 14-day cultures of bone marrow cells that had been pretreated with obELVs, wtELVs, or thymus exosomes (10 μg/ml) at day 0 of the culture and cultured in the presence or absence of GM-CSF. After a 3-h starvation, the C2C12 cells were stimulated with insulin (100 nmol/l) for 20 min and either lysed for Western blot analysis of phosphorylated Akt (A) or used for glucose uptake testing (B). Results presented are representative of a minimum of three experiments (A) or data are the means ± SE of three experiments with two replicates of each (B). *P < 0.05; **P < 0.01. C: B6 mice fed an HFD were intravenously injected with a mixture (1:1) of 4 × 106 of BMDMsPKH26+ that had been preincubated with obELVs with BMDMsPKH67+ that had been preincubated with wtELVs. The percentage of injected macrophages infiltrating adipose tissue, liver, spleen, and bone marrow were determined by FACS analysis of PKH67 and PKH26 14 days after the injection. D: The PKH67+ or PKH26+ cells were gated and analyzed for CD11b+F4/80+. The proliferation of injected fluorescent dye–labeled CD11b+F4/80+ macrophages was then determined by FACS analysis of BrdU+ cells in the adipose tissue, liver, spleen, and bone marrow. A representative graph of FACS analysis of macrophage infiltration in adipose tissue is shown and the data represent the means ± SE from five mice from each group. **P < 0.01. BM, bone marrow.
In addition, in agreement with the data published by other groups (36–38), addition of anti–TNF-α and anti–IL-6 neutralizing antibodies to the conditioned medium harvested from the obELV-treated wild-type bone marrow precursor cells led to a partial reversal of the impaired responses (supplemental Fig. S5).
To assess the homing of the macrophages that had taken up the ELVs, we injected B6 mice fed an HFD over 3 months with a mixture of PKH26-labeled BMDMs that had been preincubated with obELVs (BMDMsPKH26+) with PKH67- labeled BMDMs that had been preincubated with wtELVs (BMDMsPKH67+). FACS analysis of the tissues harvested 14 days after the BMDMsPKH26+ or BMDMsPKH67+ injections revealed that the number of PKH26+ macrophages were remarkably higher in adipose tissue and liver but not in the spleen and bone marrow (Fig. 2C). Analysis of the proliferation of the infiltrating macrophages (CD11b+F4/80+PKH26+) using a BrdU incorporation assay suggested that the macrophages that had been pretreated with obELVs proliferated faster than those pretreated with wtELVs (Fig. 2D). Preferential homing to and faster proliferation of macrophages prepulsed with obELVs, but not wtELVs, in adipose and liver tissue was also observed in the same-aged B6 ob/ob mice (data not shown).
ObELV-induced activation of macrophages is dependent on the TLR4 pathway.
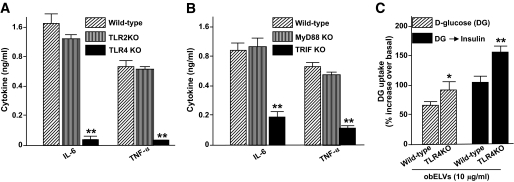
The TLR pathway has been shown to play a role in the development of obesity (14); therefore, we repeated the above studies using BMDMs from TLR2 knockout, TLR4 knockout, and B6 wild-type mice. ELISA analysis of supernatants obtained from the obELV-stimulated BMDMs show there were much higher levels of IL-6 and TNF-α in the culture medium of the obELV-treated BMDMs from TLR2 knockout and wild-type B6 mice than the obELV-treated BMDMs from TLR4 knockout mice (Fig. 3A). FACS analysis of the cells indicated that the obELV-induced expression of CD204 was also lower in the obELV-treated BMDMs from the TLR4 knockout mice than the obELV-treated BMDMs from the wild-type B6 mice and the TLR2 knockout mice (supplemental Fig. S6). Similar results were obtained when ICAM and MHCII expression were analyzed by FACS (data not shown). Together, these data suggest that obELVs are capable of utilizing TLR4 signaling to induce a macrophage inflammatory response. To further substantiate the involvement of the TLR4 signaling pathway, we sought to determine if knockout of either MyD88 or TRIF affected the response, as both these molecules can be used as adaptors for the TLR4 signaling pathway (39–42). Knockout of TRIF, but not MyD88, substantially inhibited the obELV-mediated induction of IL-6 and TNF-α (Fig. 3B), suggesting that this response is TRIF dependent.
FIG. 3.
The TLR4/TRIF pathway plays a role in adipose obELV-mediated macrophage activation and the impairment of the insulin response. A: BMDMs from wild-type B6, TLR2 knockout, or TLR4 knockout mice were treated with obELVs (10 μg/ml), and the quantity of IL-6 and TNF-α in 24 h culture supernatants was measured using a standard ELISA. B: BMDMs from wild-type B6, MyD88, or TRIF mice were treated with obELVs (10 μg/ml) at 0 h of the culture, and the quantity of IL-6 and TNF-α in the 24 h culture supernatants was determined as done in Fig. 3A, **P < 0.01. C: The C2C12 cells were cultured for 24 h in the presence of conditioned medium harvested at 24 h after addition of obELV (10 μg/ml) to BMDMs from TLR4 knockout B6 mice or wild-type B6 mice. Glucose uptake experiments were conducted as described in Fig. 2B, *P < 0.05, **P < 0.01. Data are the means ± SE of three experiments with two replicates (A–C). KO, knockout.
In addition, the combined medium harvested at 24 h after addition of obELV (10 μg/ml) to BMDMs from TLR4 knockout B6 mice led to better glucose uptake or insulin response of myocytes than from wild-type B6 mice (Fig. 3C).
ObELV RBP4 plays a role in the adipose obELV-mediated, TLR4-dependent induction of macrophage IL-6 and TNF-α.
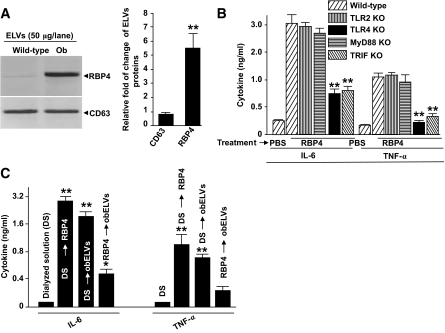
RBP4 knockout mice display enhanced insulin sensitivity (18). We found that RBP4 is present in ELVs isolated from the adipose tissue of B6 ob/ob mice and in significantly higher amounts than wtELVs (Fig. 4A) isolated from age-matched mice. The addition of recombinant RBP4 (rRBP4) alone to BMDMs from wild-type B6 mice induced the production of IL-6 and TNF-α in a concentration-dependent manner (supplemental Fig. S7A). This RBP4-induced production of IL-6 and TNF-α was dependent on TLR4 because there was less induction of either cytokine when TLR4 knockout macrophages were used in experiments (Fig. 4B). This result was also supported by in vivo data indicating that 6 h after intravenous injection of recombinant RBP4 (250 μg/mouse) in TLR4 knockout B6 mice, a reduced induction of serum TNF-α and IL-6 had occurred (supplemental Fig. S7B). Nuclear factor (NF)-κB activation has been shown to play a role in TLR4-driven inflammatory responses. This RBP4-induced production of IL-6 and TNF-α was also attenuated when BMDMs from NF-κB p50 knockout mice were used (supplemental Fig. S7C). Collectively, these data suggest that RBP4-mediated induction of TNF-α and IL-6 is regulated through TLR4/NF-κB pathway.
FIG. 4.
ObELV RBP4 induces the production of macrophage proinflammatory cytokines via activation of the TLR4/TRIF pathway. A: Fifty micrograms of obELVs and wtELVs were lysed in protein lysis buffer, and each lysate was resolved by PAGE in a 10% SDS gel for Western blot analysis. The signal intensity of each protein was quantified using an Odyssey infrared imaging system (LI-COR). The ratios of signal intensity of each protein in obELVs:wtELVs were calculated and plotted (bar graphs). The data are presented as means of three independent experiments. B: BMDMs from wild-type B6 mice or B6 mice with different gene knockouts were treated with 5 μg/ml of the RBP4. The quantity of IL-6 and TNF-α produced was determined from the supernatants of 24-h cultures. The results represent the means ± SE of triplicate cultures. C: BMDMs from wild-type B6 mice were treated with a mouse RBP4 (5 μg/ml) or dialyzed solution as a control for 24 h. Cells were washed with PBS three times and cultured in the presence of obELVs (10 μg/ml) for an additional 24 h. The supernatants were harvested and IL-6 and TNF-α quantified by ELISA. Data represent the means ± SE of five replicate wells, *P < 0.05, **P < 0.01.
To address the possibility that ELV RBP4 utilizes a different pathway to stimulate TNF-α and IL-6 production by BMDMs than does free RBP4, we used a competitive assay. The BMDMs were pretreated 24 h before the addition of obELVs with mouse RBP4 or its dialyzed buffer as a control. ELISA analysis of the supernatants confirmed that obELVs or RBP4 alone could induce the production of TNF-α and IL-6; however, pretreatment of the BMDMs with RBP4 attenuated the ability of the adipose obELVs to induce the production of TNF-α and IL-6 (Fig. 4C), suggesting that RBP4 may compete with the same pathway as obELVs to induce TNF-α and IL-6.
ELVs from the adipose tissue of ob/ob mice induce insulin resistance in mice.
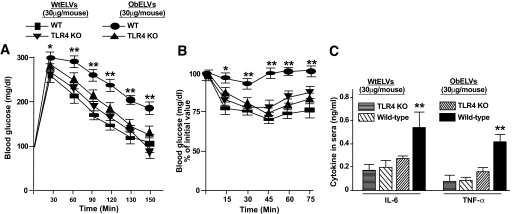
To test the effects of ELVs in vivo, B6 and TLR4 knockout mice were injected intravenously (30 μg/mouse) every 3 days for 3 weeks with ELVs released from the adipose tissue of lean wild-type mice or ob/ob mice. Glucose uptake, insulin response, and serum levels of TNF-α and IL-6 were determined after the last injection. Remarkably, the injection of ELVs released from the adipose tissue of ob/ob mice, but not wild-type mice, led to the development of glucose intolerance (Fig. 5A) and insulin resistance in wild-type mice (Fig. 5B). Moreover, the levels of glucose intolerance (Fig. 5A), insulin resistance (Fig. 5B), and serum TNF-α and IL-6 (Fig. 5C) were much lower in the TLR4 knockout mice that were treated with the obELVs than the wtELVs. Thus, ELVs released from the adipose tissue of ob/ob mice substantially enhance the development of insulin resistance and impair glucose tolerance and induction of inflammatory cytokines in a TLR4-dependent manner.
FIG. 5.
Injection of obELVs leads to the intolerance of glucose uptake, insulin resistance, and induction of inflammatory cytokines of mice. A and B: Wild-type B6 mice or TLR4 knockout of B6 mice (n = 10) were injected intravenously with obELVs or wtELVs (30 μg/mouse in 200 μl of PBS) every 3 days for 21 days. One day after the last injection (day 22), mice were fasted either overnight before receiving an intraperitoneal injection of 2 mg of dextrose/g body wt for glucose tolerance testing (A) or fasted for 4 h before receiving recombinant human insulin (1 unit/kg i.p.) for insulin responsiveness testing (B). Blood samples were taken at the indicated times (n = 10). Data are means ± SE, *P < 0.05, **P < 0.01. In addition, serum TNF-α and IL-6 was also quantified using a standard ELISA at 4 h after the last injection (C). Data are means ± SE, **P < 0.01. KO, knockout.
DISCUSSION
In this study, we found that ELVs released from adipose tissue of ob/ob mice induce macrophage activation in a TLR4-dependent manner and that the RBP4 that is incorporated in these ELVs plays a role in the induction of macrophage activation. Several independent lines of evidence support these conclusions. The exposure of wild-type macrophages to obELVs resulted in an increased production of the proinflammatory cytokines IL-6 and TNF-α, enhanced the migration of macrophages into adipose tissue and the liver, and promoted the development of insulin resistance. In contrast, the intravenous injection of obELVs into TLR4 knockout mice did not result in the development of insulin resistance, and treatment of TLR4 knockout macrophages with obELVs did not enhance the production of IL-6 or TNF-α. Adipose obELV RBP4 protein can induce the production of macrophage IL-6 and TNF-α in a TLR4-dependent manner.
Using an ex vivo adipose tissue culture approach, we provide evidence that ELVs are secreted from adipose tissue. Unlike the data for the exosomes released from in vitro cultured cells, the data generated using the exosomes released from adipose tissue as demonstrated in this study are more relevant to what may take place in an in vivo obesity mouse model. The released exosomes are predicted to have both local and systemic effects. The exosomes could be taken up by resident macrophages leading to their activation in adipose tissue. Because of resident macrophage activation, more monocytes/macrophages may be recruited into adipose tissue to further augmented inflammatory responses with more ELVs released from adipose tissue as the size of adipocytes increased. This increase in adipose cell size is one of the major features of obesity and may contribute to an increased amount of ELVs circulating in the peripheral blood. Therefore, activated monocytes circulating in the peripheral blood could be further expanded by these ELVs circulated in the peripheral blood.
Although the factors driving monocytes/macrophages to preferentially infiltrate adipose tissue and liver are not clear, this study provides strong evidence supporting the idea that the macrophage TLR4-mediated pathway plays a role in obELV-mediated activation of macrophages. A physiological role of TLR4 in insulin resistance was demonstrated previously in a model in which TLR4 knockout mice were fed an HFD (13,14,17). We found that the obELV-mediated induction of IL-6 and TNF-α occurs through activation of the TLR4 pathway of macrophages. Our data further suggest that the exosome-mediated induction of IL-6 and TNF-α of macrophages is dependent on the TLR4/TRIF pathway. This is consistent with recent data suggesting that signaling through the TRIF-mediated pathway can activate NF-κB leading to the production of IL-6 and TNF-α (40,42–44). The preferential signaling through the TLR4/TRIF pathway rather than the TLR4/MyD88 pathway is of particular interest with respect to the potential mechanisms by which the obELVs may act to stimulate the macrophages. It has been reported that the TLR4-TRIF pathway is activated in endosomes, whereas the TLR4-MyD88 pathway is activated at the plasma membrane level (41,45). The biogenesis of exosomes is considered to be initiated in endosomes, and it is conceivable that the proteins contained in exosomes may preferentially home to the endosome compartment of the cells where they are taken up and that this may influence the effects of the exosomes on the cells in terms of the type of response they elicit and the magnitude of the response. Currently, it is unknown whether the activation of the TLR4/TRIF pathway and the induction of IL-6 and TNF-α by obELVs is dependent on obELV RBP4. A neutralizing anti-RBP4 antibody to block obELV RBP4–mediated induction of IL-6 and TNF-α would further confirm the results presented in Fig. 4C. We have been unable to develop a satisfactory neutralizing anti-RBP4 antibody, and there is no commercially available anti-RBP4 antibody for use in neutralization experiments at the present time.
Finally, we propose a hypothetical model in which obesity may cause dysregulation of the adipose exosome protein and/or fatty acid sorting machinery, resulting in the unregulated sorting of certain proteins/lipids into exosomes. Factors, such as inflammatory cytokines that are high-risk indicators for developing obesity and insulin resistance, may play a direct role in dysregulation of the cell sorting machinery. Because of the dysregulation, the exosomes released by the adipose tissue in obese mice contain proinflammatory favorable proteins. These exosomes are capable of a potent stimulatory effect locally and at some distance from the adipose tissue, which could mediate both the obesity-associated inflammatory responses and the development of insulin resistance. We propose that the contribution of adipose ELVs to obesity-associated insulin resistance is likely multifactorial. It is conceivable that if a single protein could be responsible for certain biological effects without regulating other effects, the formation of exosomes as a complex to execute their immune regulatory function would seem to waste host energy to assemble.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grant nos. RO1-CA-116092, RO1-CA-107181, R01-AT-004294 (H.-G.Z.), University of Alabama at Birmingham Diabetes Research and Training Center (P60 DK07626), and RO1-DK-038765 (Principal investigator, Dr. Timothy Garvey); Birmingham Veterans Administration Medical Center Merit Review Grants (H.-G.Z.); and a grant from the Susan G. Komen Breast Cancer Foundation.
No potential conflicts of interest relevant to this article were reported.
We thank Dr. Fiona Hunter for critical review of the manuscript and Dr. Jerald Ainsworth for editorial assistance.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Nakamachi T, Nomiyama T, Gizard F, et al. : PPARα agonists suppress osteopontin expression in macrophages and decrease plasma levels in patients with type 2 diabetes. Diabetes 2007;56:1662–1670 [DOI] [PubMed] [Google Scholar]
- 2.Weisberg SP, Hunter D, Huber R, et al. : CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest 2006;116:115–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Varma V, Yao-Borengasser A, Rasouli N, et al. : Human visfatin expression: relationship to insulin sensitivity, intramyocellular lipids, and inflammation. J Clin Endocrinol Metab 2007;92:666–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liang CP, Han S, Okamoto H, et al. : Increased CD36 protein as a response to defective insulin signaling in macrophages. J Clin Invest 2004;113:764–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morrison CD, Huypens P, Stewart LK, et al. : Implications of crosstalk between leptin and insulin signaling during the development of diet-induced obesity. Biochim Biophys Acta 2008;1792:409–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu H, Barnes GT, Yang Q, et al. : Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 2003;112:1821–1830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arkan MC, Hevener AL, Greten FR, et al. : IKK-β links inflammation to obesity-induced insulin resistance. Nat Med 2005;11:191–198 [DOI] [PubMed] [Google Scholar]
- 8.Cawthorn WP, Sethi JK: TNF-α and adipocyte biology. FEBS Lett 2008;582:117–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Taeye BM, Novitskaya T, McGuinness OP, et al. : Macrophage TNF-α contributes to insulin resistance and hepatic steatosis in diet-induced obesity. Am J Physiol Endocrinol Metab 2007;293:E713–E725 [DOI] [PubMed] [Google Scholar]
- 10.Hoene M, Weigert C: The role of interleukin-6 in insulin resistance, body fat distribution and energy balance. Obes Rev 2008;9:20–29 [DOI] [PubMed] [Google Scholar]
- 11.Klover PJ, Clementi AH, Mooney RA: Interleukin-6 depletion selectively improves hepatic insulin action in obesity. Endocrinology 2005;146:3417–3427 [DOI] [PubMed] [Google Scholar]
- 12.Lumeng CN, Deyoung SM, Saltiel AR: Macrophages block insulin action in adipocytes by altering expression of signaling and glucose transport proteins. Am J Physiol Endocrinol Metab 2007;292:E166–E174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim F, Pham M, Luttrell I, et al. : Toll-like receptor-4 mediates vascular inflammation and insulin resistance in diet-induced obesity. Circ Res 2007;100:1589–1596 [DOI] [PubMed] [Google Scholar]
- 14.Shi H, Kokoeva MV, Inouye K, et al. : TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest 2006;116:3015–3025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song MJ, Kim KH, Yoon JM, et al. : Activation of Toll-like receptor 4 is associated with insulin resistance in adipocytes. Biochem Biophys Res Commun 2006;346:739–745 [DOI] [PubMed] [Google Scholar]
- 16.Suganami T, Mieda T, Itoh M, et al. : Attenuation of obesity-induced adipose tissue inflammation in C3H/HeJ mice carrying a Toll-like receptor 4 mutation. Biochem Biophys Res Commun 2007;354:45–49 [DOI] [PubMed] [Google Scholar]
- 17.Tsukumo DM, Carvalho-Filho MA, Carvalheira JB, et al. : Loss-of-function mutation in Toll-like receptor 4 prevents diet-induced obesity and insulin resistance. Diabetes 2007;56:1986–1998 [DOI] [PubMed] [Google Scholar]
- 18.Yang Q, Graham TE, Mody N, et al. : Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature 2005;436:356–362 [DOI] [PubMed] [Google Scholar]
- 19.Graham TE, Yang Q, Bluher M, et al. : Retinol-binding protein 4 and insulin resistance in lean, obese, and diabetic subjects. N Engl J Med 2006;354:2552–2563 [DOI] [PubMed] [Google Scholar]
- 20.Haider DG, Schindler K, Prager G, et al. : Serum retinol-binding protein 4 is reduced after weight loss in morbidly obese subjects. J Clin Endocrinol Metab 2007;92:1168–1171 [DOI] [PubMed] [Google Scholar]
- 21.Looze C, Yui D, Leung L, et al. : Proteomic profiling of human plasma exosomes identifies PPARγ as an exosome-associated protein. Biochem Biophys Res Commun 2009;378:433–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Valadi H, Ekstrom K, Bossios A, et al. : Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 2007;9:654–659 [DOI] [PubMed] [Google Scholar]
- 23.Xiang X, Poliakov A, Liu C, et al. : Induction of myeloid-derived suppressor cells by tumor exosomes. Int J Cancer 2009;124:2621–2633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu C, Yu S, Zinn K, et al. : Murine mammary carcinoma exosomes promote tumor growth by suppression of NK cell function. J Immunol 2006;176:1375–1385 [DOI] [PubMed] [Google Scholar]
- 25.Wang GJ, Liu Y, Qin A, et al. : Thymus exosomes-like particles induce regulatory T cells. J Immunol 2008;181:5242–5248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu S, Liu C, Su K, et al. : Tumor exosomes inhibit differentiation of bone marrow dendritic cells. J Immunol 2007;178:6867–6875 [DOI] [PubMed] [Google Scholar]
- 27.Li L, Hsu HC, Grizzle WE, et al. : Cellular mechanism of thymic involution. Scand J Immunol 2003;57:410–422 [DOI] [PubMed] [Google Scholar]
- 28.Ruan H, Pownall HJ: Overexpression of 1-acyl-glycerol-3-phosphate acyltransferase-α enhances lipid storage in cellular models of adipose tissue and skeletal muscle. Diabetes 2001;50:233–240 [DOI] [PubMed] [Google Scholar]
- 29.Aoki N, Jin-no S, Nakagawa Y, et al. : Identification and characterization of microvesicles secreted by 3T3–L1 adipocytes: redox- and hormone-dependent induction of milk fat globule-epidermal growth factor 8-associated microvesicles. Endocrinology 2007;148:3850–3862 [DOI] [PubMed] [Google Scholar]
- 30.Lancaster GI, Febbraio MA: Exosome-dependent trafficking of HSP70: a novel secretory pathway for cellular stress proteins. J Biol Chem 2005;280:23349–23355 [DOI] [PubMed] [Google Scholar]
- 31.Escola JM, Kleijmeer MJ, Stoorvogel W, et al. : Selective enrichment of tetraspan proteins on the internal vesicles of multivesicular endosomes and on exosomes secreted by human B-lymphocytes. J Biol Chem 1998;273:20121–20127 [DOI] [PubMed] [Google Scholar]
- 32.Pisitkun T, Shen RF, Knepper MA: Identification and proteomic profiling of exosomes in human urine. Proc Natl Acad Sci U S A 2004;101:13368–13373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Skokos D, Botros HG, Demeure C, et al. : Mast cell-derived exosomes induce phenotypic and functional maturation of dendritic cells and elicit specific immune responses in vivo. J Immunol 2003;170:3037–3045 [DOI] [PubMed] [Google Scholar]
- 34.Thery C, Boussac M, Veron P, et al. : Proteomic analysis of dendritic cell-derived exosomes: a secreted subcellular compartment distinct from apoptotic vesicles. J Immunol 2001;166:7309–7318 [DOI] [PubMed] [Google Scholar]
- 35.Wubbolts R, Leckie RS, Veenhuizen PT, et al. : Proteomic and biochemical analyses of human B cell-derived exosomes. Potential implications for their function and multivesicular body formation. J Biol Chem 2003;278:10963–10972 [DOI] [PubMed] [Google Scholar]
- 36.Yuen DY, Dwyer RM, Matthews VB, et al. : Interleukin-6 attenuates insulin-mediated increases in endothelial cell signaling but augments skeletal muscle insulin action via differential effects on tumor necrosis factor-α expression. Diabetes 2009;58:1086–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Uysal KT, Wiesbrock SM, Marino MW, et al. : Protection from obesity-induced insulin resistance in mice lacking TNF-α function. Nature 1997;389:610–614 [DOI] [PubMed] [Google Scholar]
- 38.Hofmann C, Lorenz K, Braithwaite SS, et al. : Altered gene expression for tumor necrosis factor-α and its receptors during drug and dietary modulation of insulin resistance. Endocrinology 1994;134:264–270 [DOI] [PubMed] [Google Scholar]
- 39.Biswas SK, Bist P, Dhillon MK, et al. : Role for MyD88-independent, TRIF pathway in lipid A/TLR4-induced endotoxin tolerance. J Immunol 2007;179:4083–4092 [DOI] [PubMed] [Google Scholar]
- 40.Johnson AC, Li X, Pearlman E: MyD88 functions as a negative regulator of TLR3/TRIF-induced corneal inflammation by inhibiting activation of c-Jun N-terminal kinase. J Biol Chem 2008;283:3988–3996 [DOI] [PubMed] [Google Scholar]
- 41.Kagan JC, Su T, Horng T, et al. : TRAM couples endocytosis of Toll-like receptor 4 to the induction of interferon-β. Nat Immunol 2008;9:361–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamamoto M, Sato S, Hemmi H, et al. : Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science 2003;301:640–643 [DOI] [PubMed] [Google Scholar]
- 43.Cusson-Hermance N, Khurana S, Lee TH, et al. : Rip1 mediates the Trif-dependent toll-like receptor 3- and 4-induced NF-{κ}B activation but does not contribute to interferon regulatory factor 3 activation. J Biol Chem 2005;280:36560–36566 [DOI] [PubMed] [Google Scholar]
- 44.Jeyaseelan S, Young SK, Fessler MB, et al. : Toll/IL-1 receptor domain-containing adaptor inducing IFN-β (TRIF)-mediated signaling contributes to innate immune responses in the lung during Escherichia coli pneumonia. J Immunol 2007;178:3153–3160 [DOI] [PubMed] [Google Scholar]
- 45.Suzuki M, Sugimoto Y, Ohsaki Y, et al. : Endosomal accumulation of Toll-like receptor 4 causes constitutive secretion of cytokines and activation of signal transducers and activators of transcription in Niemann-Pick disease type C (NPC) fibroblasts: a potential basis for glial cell activation in the NPC brain. J Neurosci 2007;27:1879–1891 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.