Abstract
OBJECTIVE
Medium-chain fatty acids (MCFAs) have been reported to be less obesogenic than long-chain fatty acids (LCFAs); however, relatively little is known regarding their effect on insulin action. Here, we examined the tissue-specific effects of MCFAs on lipid metabolism and insulin action.
RESEARCH DESIGN AND METHODS
C57BL6/J mice and Wistar rats were fed either a low-fat control diet or high-fat diets rich in MCFAs or LCFAs for 4–5 weeks, and markers of mitochondrial oxidative capacity, lipid levels, and insulin action were measured.
RESULTS
Mice fed the MCFA diet displayed reduced adiposity and better glucose tolerance than LCFA-fed animals. In skeletal muscle, triglyceride levels were increased by the LCFA diet (77%, P < 0.01) but remained at low-fat diet control levels in the MCFA-fed animals. The LCFA diet increased (20–50%, P < 0.05) markers of mitochondrial metabolism in muscle compared with low-fat diet–fed controls; however; the increase in oxidative capacity was substantially greater in MCFA-fed animals (50–140% versus low-fat–fed controls, P < 0.01). The MCFA diet induced a greater accumulation of liver triglycerides than the LCFA diet, likely due to an upregulation of several lipogenic enzymes. In rats, isocaloric feeding of MCFA or LCFA high-fat diets induced hepatic insulin resistance to a similar degree; however, insulin action was preserved at the level of low-fat diet–fed controls in muscle and adipose from MCFA-fed animals.
CONCLUSIONS
MCFAs reduce adiposity and preserve insulin action in muscle and adipose, despite inducing steatosis and insulin resistance in the liver. Dietary supplementation with MCFAs may therefore be beneficial for preventing obesity and peripheral insulin resistance.
Insulin resistance, defined as an impaired ability of insulin to regulate carbohydrate and lipid metabolism in target tissues, is one of the major metabolic defects of obesity and type 2 diabetes. It is closely linked with excess lipid deposition in nonadipose tissues, particularly skeletal muscle and liver, and several mechanisms have been proposed describing how lipid metabolites antagonize insulin action (1,2). Although the precise factors that cause inappropriate lipid accumulation are still not completely resolved, a number of studies have suggested that reduced mitochondrial capacity for lipid oxidation, particularly in skeletal muscle, may lead to partitioning of fatty acids into lipid storage pathways and a subsequent deterioration in insulin sensitivity (1,3).
Given the close link between lipid accumulation and reduced insulin action, one of the primary experimental paradigms for investigating the etiology of insulin resistance is high-fat feeding (e.g., 45–60% of calories) in rodents. Many studies have demonstrated that consumption of a diet high in long-chain fatty acids (LCFAs) induces widespread insulin resistance in muscle, liver, and adipose tissue of both rats and mice (4–7). Under these conditions of excess LCFA availability, however, we (8) and others (9) have demonstrated that mitochondrial content and fatty acid oxidative capacity are actually increased in muscle, suggesting that there is a compensatory response to increase fatty acid utilization pathways, which is insufficient to prevent lipid overload and insulin resistance. Indeed, we have recently shown that acute overexpression of carnitine palmitoyltransferase (CPT)-1 in muscle increases fatty acid oxidative capacity above that induced by high-fat feeding alone, and this partially protects against lipid-induced insulin resistance (10).
While high-fat diets containing most classes of LCFAs (e.g., saturated, monounsaturated, and omega-6) lead to obesity and insulin resistance (4,6,11), an interesting group of fatty acids that have been suggested to have antiobesity potential are medium-chain (C8–12) fatty acids (MCFAs) (12,13). Studies in humans and rodents have shown that MCFAs induce higher energy expenditure and fatty acid oxidation compared with LCFAs, and this is associated with lower adipose mass (14–17). Compared with LCFAs, however, less is known regarding the effect of MCFAs on insulin sensitivity. In rats, high-fat diets rich in MCFAs have been reported to be less deleterious for glucose and insulin tolerance compared with LCFAs (11,18,19). A limited number of studies in humans have also suggested that MCFAs may not have detrimental effects on insulin action (20,21). Whether specific tissues are involved in the favorable effects of MCFAs on insulin action is currently unclear, particularly as several studies have shown that MCFAs induce hepatic steatosis (11,17), which would be expected to have a negative impact on insulin sensitivity in this tissue. Therefore, our aim in this study was to investigate the tissue-specific effects of high-fat diets containing MCFAs on lipid metabolism and insulin action.
RESEARCH DESIGN AND METHODS
Eight-week-old male C57BL6/J mice and male Wistar rats were purchased from the Animal Resources Centre (Perth, Australia). The animals were kept in a temperature-controlled room (22 ± 1°C) on a 12-h light/dark cycle with free access to water. Mice and rats were fed ad libitum for 1 week on a standard low-fat laboratory diet (low fat; 8% calories from fat, 21% calories from protein, 71% calories from carbohydrate; Gordon's Specialty Stock Feeds, Yanderra, NSW, Australia) and were then randomly allocated to remain on the low-fat diet or to receive a high-fat diet enriched with either LCFAs from lard or MCFAs from hydrogenated coconut oil. The dietary fatty acid composition was determined as described below and is presented in Table 1. For mice, the high-fat diets were based on rodent diet no. D12451 (containing 45% of calories from fat) (Research Diets, New Brunswick, NJ), and animals were fed ad libitum for a period of 5 weeks. For the rat studies, animals were pair-fed LCFA and MCFA high-fat diets (59% of calories from fat) as previously described (6). All experiments were carried out with the approval of the Garvan Institute/St. Vincent's Hospital Animal Experimentation Ethics Committee, following guidelines issued by the National Health and Medical Research Council of Australia.
TABLE 1.
Fatty acid composition of the low-fat and high-fat diets
| Fatty acids | Low-fat diet | MCFAs | LCFAs |
|---|---|---|---|
| 8:0 | 0 | 7.3 | 0 |
| 10:0 | 0 | 5.7 | 0 |
| 12:0 | 0 | 36.7 | 0 |
| 14:0 | 0.6 | 16.5 | 1.3 |
| 16:0 | 13.1 | 9.8 | 24.6 |
| 18:0 | 4.0 | 10.5 | 15.3 |
| 18:1 (n-9) | 38.7 | 3.0 | 32.1 |
| 18:2 (n-6) | 31.7 | 9.8 | 21.7 |
| 18:3 (n-3) | 6.7 | 0.3 | 0.9 |
Data are the percentage of total fatty acids. Fatty acid composition was determined by gas chromatography.
Determination of body composition and energy expenditure.
Fat and lean body mass were measured in mice using dual-energy X-ray absorptiometry (Lunar PIXImus2 mouse densitometer; GE Healthcare) in accordance with the manufacturer's instructions. Oxygen consumption rate (Vo2) of individual mice was measured using an eight-chamber indirect calorimeter (Oxymax series; Columbus Instruments, Columbus, OH) as previously described (8).
In vivo glucose metabolism.
Glucose tolerance tests (2 g/kg glucose i.p.) were performed in overnight-fasted mice. Blood samples were obtained from the tail tip at the indicated times, and glucose levels were measured using a glucometer (Accu-Check, Roche, NSW, Australia). For euglycemic-hyperinsulinemic clamps in rats (insulin infusion 0.25 units · kg−1 · h−1), double jugular cannulae were implanted 7 days prior to experiments, and animals (5 h fasted) were studied over 2 h in the conscious state as previously described (22).
Fatty acid composition, triglycerides, and insulin levels.
Lipids were extracted from tissues and diets by standard methods (23). For tissue lipid extracts, neutral lipids were separated from phospholipids by solid-phase extraction on Waters Sep-Pak silica columns (Milford, MA). Lipid fractions were transmethylated (24), and fatty acid methyl esters were separated by gas-liquid chromatography on a Shimadzu 17A gas chromatograph (NSW, Australia) with a Restek FAMEWAX capillary column (Bellefonte, PA). Plasma, muscle, and liver triglyceride contents were determined using a colorimetric assay kit (Triglycerides GPO-PAP; Roche Diagnostics, Indianapolis, IN) as previously described (22). Plasma insulin was determined by radioimmunoassay using a rat-specific kit (Linco Research, St. Charles, MO).
Enzyme activity measurements.
Muscle and liver samples were homogenized 1:19 (wt/vol) in 50 mmol/l Tris-HCl, 1 mmol/l EDTA, and 0.1% Triton X-100, pH 7.2, using a Polytron instrument (Kinematica, Littau-Lucerne, Switzerland) and were subjected to three freeze-thaw cycles. Citrate synthase (CS), β-hydroxyacyl CoA dehydrogenase (HAD), and medium-chain acyl-CoA dehydrogenase (MCAD) were determined at 30°C as described previously (8) using a Spectra Max 250-microplate spectrophotometer (Molecular Devices, Sunnyvale, CA).
Immunoblotting.
Muscle and liver samples were resuspended in radioimmunoprecipitation buffer (65 mmol/l Tris [pH 7.4], 150 mmol/l NaCl, 1% nonidet NP-40, 0.5% sodium deoxy-cholate, and 0.1% SDS), supplemented with protease and phosphatase inhibitors (10 μg/ml phenylmethylsulfonyl fluoride, 10 μg/ml aprotinin and 10 μg/ml leupeptin, 1 mmol/l Na3VO4, and 10 mmol/l NaF) and solubilized for 2 h at 4°C. Equal amounts of tissue lysates (10–20 μg protein) were resolved by SDS-PAGE and immunoblotted with antibodies against peroxisome proliferator–activated receptor (PPAR)-γ coactivator (PGC)-1α from Chemicon International (Temecula, CA); muscle and liver carnitine palmitoyltransferase-1 (CPT-1) from Alpha Diagnostic International (San Antonio, TX); uncoupling protein 3 (UCP3) from Affinity Bioreagents (Golden, CO); fatty acid synthase (FAS), stearoyl-CoA desaturase (SCD-1), and acetyl-CoA carboxylase (ACC) from Cell Signaling (Beverly, MA); cytochrome oxidase (complex IV) subunit 1 from Invitrogen (Victoria, Australia); and an antibody cocktail that recognizes several subunits of the mitochondrial respiratory chain (MS601) from Mitosciences (Eugene, OR). Immunolabeled bands were quantitated by densitometry.
Statistical analyses.
Data are presented as means ± SE. One-way ANOVA with Fisher's protected least-significant difference post hoc test was used to assess statistical significance between groups. Differences at P < 0.05 were considered to be statistically significant.
RESULTS
Body composition, glucose tolerance, and tissue triglyceride levels in mice.
At the completion of the 5-week feeding regime, body mass was not different among mice fed the low-fat, MCFA, and LCFA diets (Table 2). Whole-body adiposity measured by dual-energy X-ray absorptiometry scanning was not significantly different between MCFA-fed mice and low-fat–fed controls but was substantially increased (P < 0.01) in LCFA-fed mice (Table 2). The reduced level of adipose accumulation in MCFA-fed mice compared with LCFA-fed mice appeared to be primarily due to an increased energy expenditure (Vo2) in MCFA-fed animals (MCFA 3.64 ± 1.3 vs. LCFA 3.34 ± 0.8 ml O2 · g−1 · h−1, n = 7, P < 0.05), as although the caloric intake was increased (P < 0.01) in both fat-fed groups compared with low-fat controls (11.5 ± 0.5 kcal/day, n = 6), no difference was observed between the MCFA and LCFA diets (MCFA 14.2 ± 0.1 vs. LCFA 14.6 ± 0.4 kcal/day, n = 5–6).
TABLE 2.
Body mass, fat pad mass, and tissue triglyceride levels in mice
| Low-fat diet | MCFAs | LCFAs | |
|---|---|---|---|
| Body mass (g) | 28.2 ± 0.5 | 28.7 ± 0.6 | 29.1 ± 1.0 |
| Fat mass (%) | 14.7 ± 0.6 | 17.3 ± 1.7 | 25.6 ± 1.9*† |
| Muscle triglycerides (μmol/g) | 11.1 ± 0.8 | 13.0 ± 2.8 | 19.7 ± 1.9*‡ |
| Liver triglycerides (μmol/g) | 8.5 ± 0.7 | 49.7 ± 4.4* | 20.1 ± 2.7*† |
Data are means ± SE for n = 6–11 animals. Fat mass (%) was determined by dual-energy X-ray absorptiometry scanning.
*P < 0.01 vs. low fat;
†P < 0.01 vs. MCFA;
‡P < 0.05 vs. MCFA.
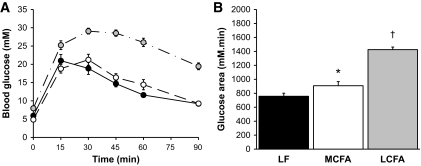
To determine the effect of the MCFA and LCFA diets on whole-body glucose metabolism, we examined glucose clearance during an intraperitoneal glucose tolerance test (Fig. 1). Mice fed the LCFA diet displayed a substantial impairment in glucose tolerance compared with low-fat–fed controls, as represented by the 88% (P < 0.01) increase in the glucose area under the curve (AUC). Animals fed the MCFA diet exhibited a much milder impairment in glucose tolerance (19% increase in area under the curve [P < 0.05] compared with low-fat–fed controls) (Fig. 1). Given the established link between excess intracellular lipid and insulin resistance (2), we examined tissue triglyceride levels to determine whether the difference in glucose tolerance in response to MCFAs and LCFAs may have been linked to a differential effect of the high-fat diets on liver and muscle lipid levels (Table 2). Muscle triglyceride content was not different between low-fat–fed controls and MCFA-fed mice but was significantly elevated in muscle from the LCFA-fed mice compared with both other groups (Table 2). In contrast to this, liver triglycerides were elevated by ∼2.5-fold (P < 0.01) in LCFA-fed animals compared with low-fat–fed controls (Table 2), while MCFA-fed animals displayed liver triglyceride levels that were significantly higher (P < 0.01) than both low-fat–fed controls and LCFA-fed mice.
FIG. 1.
Glucose tolerance test in overnight-fasted low-fat (LF) (●), MCFA (○)-, and LCFA ( )-fed mice. A: Blood glucose levels after an intraperitoneal glucose load (2 g/kg). B: Incremental areas under the curve as an indicator of glucose clearance. Data represent the means ± SE of 5–11 mice. *P < 0.01 vs. low fat; †P < 0.01 vs. low fat and MCFAs.
)-fed mice. A: Blood glucose levels after an intraperitoneal glucose load (2 g/kg). B: Incremental areas under the curve as an indicator of glucose clearance. Data represent the means ± SE of 5–11 mice. *P < 0.01 vs. low fat; †P < 0.01 vs. low fat and MCFAs.
Markers of mitochondrial metabolism and lipogenesis.
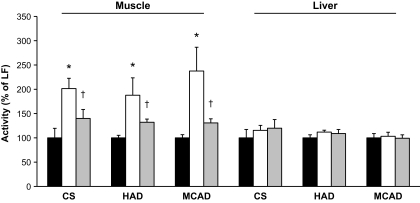
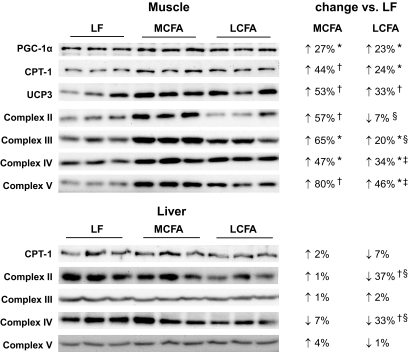
Mitochondria are a major site for lipid oxidation, and, therefore, we examined several markers of mitochondrial metabolism to determine whether the contrasting effects of MCFAs and LCFAs on intracellular lipid levels may be related to differences in fatty acid utilization. In skeletal muscle, we observed a significant upregulation (30–40%) of citrate synthase, β-hydroxyacyl CoA dehydrogenase, and medium-chain acyl-CoA dehydrogenase activity in LCFA-fed mice compared with low-fat–fed control animals (Fig. 2). Interestingly, the MCFA diet induced a much greater increase in muscle oxidative enzyme activity (90–140% higher than low-fat–fed controls) (Fig. 2). We also examined the protein expression of subunits of the mitochondrial respiratory chain, as well as CPT-1, UCP3, and the transcriptional coactivator PGC-1α. Similar to the enzyme activities, increased expression of mitochondrial proteins was observed in muscle of the LCFA-fed mice compared with low-fat–fed controls, with a substantially greater increase seen in the protein levels of respiratory chain subunits, CPT-1, and UCP3 in MCFA-fed animals (Fig. 3). Collectively, these findings suggest a more potent stimulation of mitochondrial biogenic pathways in MCFA-fed mice compared with the LCFA-fed animals; however, this difference was not due to greater PGC-1α expression, as this was increased to a similar extent in both groups (Fig. 3).
FIG. 2.
Oxidative enzyme activity in skeletal muscle and liver from mice fed the low-fat (LF) (■), MCFA (□), and LCFA ( ) diets. Data represent the means ± SE of 5–6 mice. *P < 0.01 vs. low fat and LCFAs; †P < 0.01 vs. low fat.
) diets. Data represent the means ± SE of 5–6 mice. *P < 0.01 vs. low fat and LCFAs; †P < 0.01 vs. low fat.
FIG. 3.
Immunoblots for markers of mitochondrial metabolism and biogenesis in skeletal muscle and liver from mice fed the low-fat (LF), MCFA, and LCFA diets. Equal amounts of muscle lysates (10–20 μg protein) were resolved by SDS-PAGE and immunoblotted with specific antibodies for PGC-1α, CPT-1, UCP3, and mitochondrial respiratory chain subunits. Densitometric analysis (relative to low-fat controls) for n = 6 animals is presented. *P < 0.05; †P < 0.01 vs. low fat; ‡P < 0.05; §P < 0.01 vs. MCFAs.
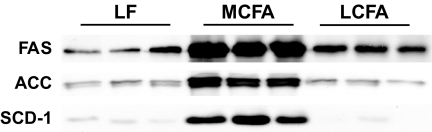
In liver, we observed no difference in the activity of citrate synthase, β-hydroxyacyl CoA dehydrogenase, or medium-chain acyl-CoA dehydrogenase in either the MCFA or LCFA group compared with low-fat–fed controls (Fig. 2). There was no difference between the three dietary groups in the expression of CPT-1 or subunits from complex III and complex V of the respiratory chain; however, LCFA-fed animals displayed a significant reduction in the expression of subunits from complex II and complex IV (Fig. 3). As there was marked hepatic steatosis in MCFA-fed mice, without a consistent decrease in markers of fatty acid oxidative capacity, we also examined the protein expression of several enzymes involved in lipogenesis to determine whether increased flux through lipogenic pathways may be underpinning the elevated triglyceride accumulation in these animals. This appeared to be the case, as compared with low-fat–fed controls, MCFA-fed mice exhibited a 2.4-, 2.5-, and 12-fold increase (P < 0.01, n = 6), respectively, in the protein expression of FAS, ACC, and SCD-1, while relative to low-fat–fed controls, LCFA-fed mice displayed a 43% increase (P < 0.05, n = 6) in FAS expression, with no difference observed for ACC or SCD-1 (Fig. 4).
FIG. 4.
Immunoblots for enzymes involved in lipogenesis in liver from mice fed the low-fat (LF), MCFA, and LCFA diets. Equal amounts of liver lysates (10–20 μg protein) were resolved by SDS-PAGE and immunoblotted with specific antibodies for FAS, ACC, and SCD-1.
Body composition and insulin action in rats.
Overall, our studies in mice showed that MCFAs have differential effects on lipid metabolism in liver and muscle, and to investigate the tissue-specific effect of MCFAs on insulin action, we conducted hyperinsulinemic-euglycemic clamps in rats pair-fed MCFA and LCFA high-fat diets. Following 4 weeks of isocaloric high-fat feeding, rats fed the MCFA diet displayed reduced body weight and lower adiposity compared with the LCFA-fed animals (Table 3). Plasma glucose, insulin, and triglyceride levels were significantly elevated in the LCFA-fed animals compared with the low-fat–fed controls, while MCFA-fed rats displayed only minor elevations in insulin and triglyceride levels (Table 3). Similar to the pattern we observed in the mice, muscle triglyceride levels were not different between animals fed the low-fat and MCFA diets but were increased in the LCFA-fed rats (Table 3). Liver triglyceride levels were also higher in the MCFA-fed animals compared with both the low-fat–fed controls and LCFA-fed rats (Table 3).
TABLE 3.
Body mass, fat pad mass, circulating parameters, and tissue triglyceride levels in rats
| Low-fat diet | MCFAs | LCFAs | |
|---|---|---|---|
| Body mass (g) | 349 ± 7 | 337 ± 6 | 387 ± 5*† |
| Epididymal fat (%) | 1.0 ± 0.1 | 1.3 ± 0.1* | 1.6 ± 0.05*† |
| Retroperitoneal fat (%) | 1.0 ± 0.1 | 1.3 ± 0.1 | 2.1 ± 0.2*† |
| Lumbar fat (%) | 1.5 ± 0.1 | 1.5 ± 0.1 | 2.2 ± 0.1*† |
| Plasma glucose (mmol/l) | 7.2 ± 0.2 | 7.5 ± 0.2 | 8.1 ± 0.2‡ |
| Plasma insulin (mU/l) | 24 ± 2 | 31 ± 3 | 66 ± 5*† |
| Plasma triglycerides (mmol/l) | 0.8 ± 0.1 | 0.9 ± 0.0 | 1.1 ± 0.2‡ |
| Muscle triglycerides (μmol/g) | 1.7 ± 0.1 | 1.9 ± 0.2 | 2.9 ± 0.3*† |
| Liver triglycerides (μmol/g) | 4.8 ± 0.3 | 18.4 ± 0.9* | 14.4 ± 2.1*† |
Data are means ± SE for n = 5–9 animals. Fat pad weights are expressed as a percentage of body mass.
*P < 0.01 vs. low fat;
†P < 0.01 vs. MCFAs;
‡P < 0.05 vs. low fat.
Whole-body insulin sensitivity, measured as the glucose infusion rate (GIR) during a hyperinsulinemic-euglycemic clamp, was 44% (P < 0.01) lower in the LCFA-fed rats compared with low-fat–fed controls, while MCFA-fed animals displayed an 18% (P < 0.01) reduction in GIR compared with low-fat–fed controls (Table 4). The reduced GIR in LCFA-fed rats compared with low-fat–fed controls was the result of both a reduced rate of insulin-stimulated glucose disposal into peripheral tissues (Rd) and a decreased suppression of hepatic glucose output (HGO) (Table 4). In MCFA-fed rats, the effect of insulin to suppress HGO was impaired to a similar degree to the LCFA-fed rats, but strikingly, Rd was not different from low-fat–fed controls. Skeletal muscle is the major peripheral tissue responsible for glucose disposal, and, accordingly, we examined the uptake of 3H-2-deoxyglucose tracer (Rg´) into red and white muscles during the clamp. Compared with low-fat–fed controls, LCFA-fed rats exhibited a reduction (25–40%, P < 0.01) in Rg´ in both red and white muscles (Table 4). Consistent with the results observed for Rd, MCFA-fed animals exhibited similar (red muscle) or even slightly increased (white muscle) Rg´ values compared with low-fat–fed controls (Table 4). In the epididymal adipose depot, we also observed a 45% reduction (P < 0.01) in Rg´ as a result of the LCFA diet, while the MCFA diet preserved insulin action in this tissue (Table 4). Of interest, we analyzed the fatty acid composition of liver, muscle, and epididymal adipose tissue and found that in MCFA-fed animals, MCFAs accounted for ∼20% of the total fatty acids in the neutral lipid fraction in muscle and adipose (i.e., the two tissues that did not develop insulin resistance) but <5% of the total fatty acids in liver, where insulin action was diminished (data not shown). In low-fat– and LCFA-fed animals, MCFAs accounted for <1% of the total fatty acids in all tissues analyzed.
TABLE 4.
Metabolic parameters from hyperinsulinemic-euglycemic clamps in rats
| Low-fat diet | MCFAs | LCFAs | |
|---|---|---|---|
| GIR (mg · kg−1 · min−1) | 38.4 ± 1.6 | 31.4 ± 1.3* | 21.5 ± 1.6*† |
| Rd (mg · kg−1 · min−1) | 36.9 ± 1.3 | 34.3 ± 1.1 | 25.9 ± 1.9*† |
| HGO (mg · kg−1 · min−1) | −1.5 ± 0.63 | 3.0 ± 1.1* | 4.3 ± 0.4*† |
| Rg´ (red quadriceps) | 29.4 ± 2.0 | 26.5 ± 1.8 | 16.9 ± 2.7*† |
| Rg´ (red gastrocnemius) | 29.7 ± 2.8 | 26.8 ± 1.4 | 17.6 ± 0.8*† |
| Rg´ (white gastrocnemius) | 7.1 ± 0.6 | 9.0 ± 0.6* | 5.4 ± 0.4*† |
| Rg´ (epididymal fat) | 1.8 ± 0.2 | 2.2 ± 0.2 | 1.0 ± 0.1*† |
Data are means ± SE for n = 5–7 animals. Plasma levels of glucose and insulin were similar for all groups during the clamp (data not shown).
*P < 0.01 vs. low fat;
†P < 0.01 vs. MCFA. Rg´, insulin-stimulated 3H-2-deoxyglucose uptake in skeletal muscle or adipose tissue during the clamp (μmol · 100 g−1 · min−1).
DISCUSSION
Previous studies examining the effect of different fatty acids on insulin action have reported improved glucose tolerance and insulin tolerance in rodents fed high-fat diets rich in MCFAs compared with LCFAs (11,18,19). Our current study reveals the tissues responsible for the favorable effect of MCFAs on whole-body glucose metabolism, as well as a mechanistic basis for these effects. We have made the intriguing observation that insulin action in skeletal muscle and adipose tissue is preserved at the level of low-fat–fed controls when animals consume a high-fat diet rich in MCFAs. In muscle, the lack of induction of insulin resistance with MCFA high-fat feeding is associated with a substantial increase in mitochondrial oxidative capacity, which is sufficient to prevent lipid accumulation in this tissue. However, the liver of MCFA-fed animals accumulated greater amounts of triglycerides, likely due to upregulation of lipogenic pathways, and as such, hepatic insulin action was reduced after MCFA high-fat feeding.
It is likely that our findings have clinical relevance, as several studies have suggested that MCFAs may be beneficial for insulin action in humans. Eckel et al. (20) showed in a small (n = 3) cohort of subjects with type 2 diabetes that acute treatment with MCFAs (40% fat for 5 days) resulted in a beneficial effect on insulin-stimulated glucose disposal, without consistent effects on insulin-mediated suppression of HGO. Furthermore, a 3-month trial in patients with type 2 diabetes reported improved homeostatic model assessment of insulin resistance in subjects consuming MCFAs compared with LCFAs (21). The above human studies and those in rodents (11,18,19) indicate that MCFAs do not induce insulin resistance to the same degree as LCFAs; however, they have provided limited information regarding the tissue-specific effects of MCFAs on insulin action in vivo and/or a mechanism for any observed beneficial effects. Our current study clearly shows that MCFAs do not induce insulin resistance in either muscle and adipose tissue, and given the fact that muscle is the major tissue for insulin-stimulated glucose disposal (25), the reported favorable effects of MCFAs on whole-body glucose metabolism (11,18,20,21) are probably related to changes in insulin action in muscle. It is worth noting, however, that the daily caloric intake in MCFA-fed mice was ∼25% higher than in low-fat–fed controls, and whether with more prolonged high-fat feeding this elevated energy intake would eventually lead to some metabolic dysfunction in muscle and adipose tissue remains to be determined.
The strong association between lipid accumulation and insulin resistance is well documented (1,2,6), and our findings that MCFAs do not induce lipid accumulation in muscle and concurrently preserve insulin action in this tissue strongly support the above link. We and others have reported that under conditions of increased lipid availability, either through high-fat feeding (with LCFAs), acute lipid infusions, or muscle-specific overexpression of lipoprotein lipase, mitochondrial content and fatty acid oxidative capacity are upregulated in muscle (8,9,26,27). Such a response likely represents an attempt of the muscle to cope with additional fatty acid substrates; however, the fact that lipids still accumulate in muscle in animals under these conditions suggests that the compensatory upregulation of oxidative pathways is unable to deal with the elevated uptake of LCFAs that is observed with such manipulations (26,28). In comparison with LCFAs, however, we have shown that MCFAs induce a substantially greater upregulation of mitochondrial oxidative capacity in muscle, and this appears to be at a sufficient level to prevent the deleterious effects of lipid oversupply on insulin action in this tissue.
The underlying molecular mechanism by which MCFAs induce a more potent upregulation of mitochondrial biogenesis in muscle than LCFAs is currently unclear. We observed a substantial accumulation of MCFAs in the neutral lipid fraction of muscle from MCFA-fed animals, and one major pathway through which fatty acids influence substrate metabolism in muscle is via activation of PPAR, particularly PPARδ. These transcription factors, when activated by fatty acids or other ligands, control genes involved in oxidative and fatty acid metabolism (29). Several studies (30,31) have shown, however, that MCFAs have low binding affinity for PPARs, suggesting that a direct effect of MCFAs on PPAR-dependent transcription is unlikely responsible for the increase in mitochondrial biogenesis. PPARs can also be activated via interaction with the transcriptional coactivator PGC-1α, which is considered a master controller of mitochondrial biogenesis in muscle. We observed similar upregulation of PGC-1α content in muscle with both the MCFA and LCFA diets. However, posttranslational modification (e.g., acetylation) of PGC-1α is known to regulate its activity (32), and whether MCFAs specifically affect this pathway or influence the activity of other transcription factors is currently unknown.
In addition to oxidative metabolism, there are a number of other pathways that influence lipid deposition in tissues, including lipid uptake from the circulation and, for tissues such as the liver, the rate of de novo lipogenesis. With regard to these factors, MCFAs differ from LCFAs in a number of important ways. MCFAs are more readily absorbed into the bloodstream, and, therefore, a greater proportion of these fatty acids reach the liver through the portal vein (33). MCFAs can also enter the mitochondrion for oxidation via CPT-1–independent mechanisms (34). These unusual physical properties are thought to largely explain the increase in energy expenditure and decreased adiposity observed with MCFA-rich diets (i.e., due to enhanced hepatic fatty acid oxidation), particularly in the postprandial period (13). Our novel finding of a very potent upregulation of mitochondrial content in muscle by MCFAs suggests that enhanced flux of substrates through oxidative metabolism in muscle may also contribute to MCFA-induced changes in energy expenditure and adiposity, as well as improved muscle insulin action (current study and 13–15).
The other tissue in which MCFAs were less deleterious than LCFAs for insulin action was adipose tissue. Small adipocytes are more insulin-sensitive than large adipocytes (35,36), and previous studies (16,37) have demonstrated that adipocyte size is reduced with MCFA diets, potentially due to a reduction in adipogenic gene expression (18). It is likely that the preserved insulin sensitivity we observed in adipose following MCFA high-fat feeding is simply a consequence of reduced adipocyte size, although given the fact that MCFAs accumulate significantly in adipose tissue following MCFA high-fat feeding (current study and 18), we cannot rule out a more direct effect of MCFAs on adipocyte function that may be beneficial for insulin action. Furthermore, as adipose tissue secretes a number of adipokines that affect carbohydrate and lipid metabolism in other tissues, it remains to be determined if MCFA-induced changes in adipokine profile (19) partly contribute to the changes in mitochondrial content and insulin action observed in skeletal muscle with the MCFA high-fat diet.
Despite the favorable effects of MCFAs on muscle and adipose metabolism, another important finding was that MCFAs robustly induced insulin resistance in liver and caused a greater degree of hepatic steatosis than LCFAs in both mice and rats. This elevation in liver triglyceride levels did not appear to be due to a decreased capacity for lipid oxidation, as we observed generally similar levels of mitochondrial enzyme activity and protein expression in the different dietary groups. As mentioned above, the entry of MCFAs into mitochondria is less dependent on CPT-1 than LCFAs, and a consequence of accelerated β-oxidation is an excess production of acetyl-CoA. Much of this acetyl-CoA is converted into ketone bodies, which have been reported to be elevated in MCFA-fed animals (17,38). Acetyl-CoA is also a substrate for de novo lipogenesis, and, in line with other reports (39,40), we observed a substantial upregulation of lipogenic enzymes in liver from the MCFA-fed mice, presumably to deal with the excess acetyl-CoA, and this is likely a major contributor to the increased triglyceride levels in these animals. Consistent with this, we only observed a small proportion of MCFAs in the neutral lipid fraction of liver from MCFA-fed animals, suggesting metabolism of these fatty acids through lipogenic pathways.
There is controversy in the literature regarding the effects of MCFAs on liver triglycerides. In rodents, a number of studies (11,17) have reported increased liver triglyceride levels with MCFA feeding, while others (16) show no difference between high-fat diets containing MCFAs or LCFAs. Interestingly, one recent study (41) in rats suggested that liver triglyceride content is significantly lower with a diet containing only MCFAs compared with LCFAs, but this effect was diminished in the presence of LCFAs. In humans, a number of studies have reported that MCFAs do not have adverse effects on liver lipid levels (42,43); however, inconsistent findings have been reported regarding the effect of MCFA on circulating lipid parameters (21,44,45). It is possible that methodological differences may underlie many of these seemingly disparate findings, such as the dietary fat content and composition, the length of dietary intervention, and the composition of other constituents of the diet (e.g., carbohydrates and protein).
In summary, our study shows that high-fat diets containing MCFAs have divergent effects on tissue-specific insulin sensitivity, inducing insulin resistance to a similar degree as LCFAs in liver while preserving insulin action at the level of low-fat–fed controls in muscle and adipose tissue. The preservation of muscle insulin action by MCFAs is associated with a potent stimulation of mitochondrial biogenesis, which appears to be sufficient to prevent lipid accumulation in this tissue. Given that the total amount of dietary fat used in the current studies is relatively high (i.e., 45–60% of energy), it will be important to determine in future studies the amount of dietary MCFAs (both in absolute terms and relative to dietary LCFAs) required for beneficial effects on energy metabolism and insulin action and whether this amount of dietary MCFAs avoids liver lipid accumulation. In this regard, some human studies (21,46,47) have reported positive effects on energy expenditure and body composition with relatively low dietary doses of MCFAs. Additionally, as some antidiabetes therapies (e.g., metformin) are known to exert the majority of their insulin-sensitizing effects via their actions in the liver (48), it will be of interest to determine whether MCFA supplementation in conjunction with such agents results in beneficial effects on insulin action in multiple insulin target tissues.
Acknowledgments
This work was supported by grants from the Diabetes Australia Research Trust (to N.T.) and the National Health and Medical Research Council of Australia (NHMRC) (to J.Y. and E.W.K.). L.E.W. is supported by a University of New South Wales Postgraduate Award. N.T. is supported by a Career Development Award and E.W.K. and G.J.C. by Research Fellowships from the NHMRC.
No potential conflicts of interest relevant to this article were reported.
Parts of this work were presented in abstract form at the 68th American Diabetes Association Scientific Sessions, San Francisco, California, 6–10 June 2008.
The technical assistance of Haiyan Li is acknowledged. We thank Dr. Carsten Schmitz-Peiffer and Dr. Lei Zhang for their helpful discussions. We also thank the Biological Testing Facility at the Garvan Institute for assistance with animal care.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Savage DB, Petersen KF, Shulman GI: Disordered lipid metabolism and the pathogenesis of insulin resistance. Physiol Rev 2007;87:507–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kraegen EW, Cooney GJ: Free fatty acids and skeletal muscle insulin resistance. Curr Opin Lipidol 2008;19:235–241 [DOI] [PubMed] [Google Scholar]
- 3.Turner N, Heilbronn LK: Is mitochondrial dysfunction a cause of insulin resistance? Trends Endocrinol Metab 2008;19:324–330 [DOI] [PubMed] [Google Scholar]
- 4.Storlien LH, Jenkins AB, Chisholm DJ, Pascoe WS, Khouri S, Kraegen EW: Influence of dietary fat composition on development of insulin resistance in rats: relationship to muscle triglyceride and omega-3 fatty acids in muscle phospholipid. Diabetes 1991;40:280–289 [DOI] [PubMed] [Google Scholar]
- 5.Park SY, Cho YR, Kim HJ, Higashimori T, Danton C, Lee MK, Dey A, Rothermel B, Kim YB, Kalinowski A, Russell KS, Kim JK: Unraveling the temporal pattern of diet-induced insulin resistance in individual organs and cardiac dysfunction in C57BL/6 mice. Diabetes 2005;54:3530–3540 [DOI] [PubMed] [Google Scholar]
- 6.Oakes ND, Cooney GJ, Camilleri S, Chisholm DJ, Kraegen EW: Mechanisms of liver and muscle insulin resistance induced by chronic high-fat feeding. Diabetes 1997;46:1768–1774 [DOI] [PubMed] [Google Scholar]
- 7.Dobbins RL, Szczepaniak LS, Bentley B, Esser V, Myhill J, McGarry JD: Prolonged inhibition of muscle carnitine palmitoyltransferase-1 promotes intramyocellular lipid accumulation and insulin resistance in rats. Diabetes 2001;50:123–130 [DOI] [PubMed] [Google Scholar]
- 8.Turner N, Bruce CR, Beale SM, Hoehn KL, So T, Rolph MS, Cooney GJ: Excess lipid availability increases mitochondrial fatty acid oxidative capacity in muscle: evidence against a role for reduced fatty acid oxidation in lipid-induced insulin resistance in rodents. Diabetes 2007;56:2085–2092 [DOI] [PubMed] [Google Scholar]
- 9.Hancock CR, Han DH, Chen M, Terada S, Yasuda T, Wright DC, Holloszy JO: High-fat diets cause insulin resistance despite an increase in muscle mitochondria. Proc Natl Acad Sci U S A 2008;105:7815–7820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bruce CR, Hoy AJ, Turner N, Watt MJ, Allen TL, Carpenter K, Cooney GJ, Febbraio MA, Kraegen EW: Overexpression of carnitine palmitoyltransferase-1 in skeletal muscle is sufficient to enhance fatty acid oxidation and improve high-fat diet–induced insulin resistance. Diabetes 2009;58:550–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buettner R, Parhofer KG, Woenckhaus M, Wrede CE, Kunz-Schughart LA, Scholmerich J, Bollheimer LC: Defining high-fat-diet rat models: metabolic and molecular effects of different fat types. J Mol Endocrinol 2006;36:485–501 [DOI] [PubMed] [Google Scholar]
- 12.St-Onge MP: Dietary fats, teas, dairy, and nuts: potential functional foods for weight control? Am J Clin Nutr 2005;81:7–15 [DOI] [PubMed] [Google Scholar]
- 13.Papamandjaris AA, MacDougall DE, Jones PJ: Medium chain fatty acid metabolism and energy expenditure: obesity treatment implications. Life Sci 1998;62:1203–1215 [DOI] [PubMed] [Google Scholar]
- 14.St-Onge MP, Bourque C, Jones PJ, Ross R, Parsons WE: Medium- versus long-chain triglycerides for 27 days increases fat oxidation and energy expenditure without resulting in changes in body composition in overweight women. Int J Obes Relat Metab Disord 2003;27:95–102 [DOI] [PubMed] [Google Scholar]
- 15.St-Onge MP, Ross R, Parsons WD, Jones PJ: Medium-chain triglycerides increase energy expenditure and decrease adiposity in overweight men. Obes Res 2003;11:395–402 [DOI] [PubMed] [Google Scholar]
- 16.Baba N, Bracco EF, Hashim SA: Enhanced thermogenesis and diminished deposition of fat in response to overfeeding with diet containing medium chain triglyceride. Am J Clin Nutr 1982;35:678–682 [DOI] [PubMed] [Google Scholar]
- 17.Shinohara H, Ogawa A, Kasai M, Aoyama T: Effect of randomly interesterified triacylglycerols containing medium- and long-chain fatty acids on energy expenditure and hepatic fatty acid metabolism in rats. Biosci Biotechnol Biochem 2005;69:1811–1818 [DOI] [PubMed] [Google Scholar]
- 18.Han J, Hamilton JA, Kirkland JL, Corkey BE, Guo W: Medium-chain oil reduces fat mass and down-regulates expression of adipogenic genes in rats. Obes Res 2003;11:734–744 [DOI] [PubMed] [Google Scholar]
- 19.Takeuchi H, Noguchi O, Sekine S, Kobayashi A, Aoyama T: Lower weight gain and higher expression and blood levels of adiponectin in rats fed medium-chain TAG compared with long-chain TAG. Lipids 2006;41:207–212 [DOI] [PubMed] [Google Scholar]
- 20.Eckel RH, Hanson AS, Chen AY, Berman JN, Yost TJ, Brass EP: Dietary substitution of medium-chain triglycerides improves insulin-mediated glucose metabolism in NIDDM subjects. Diabetes 1992;41:641–647 [PubMed] [Google Scholar]
- 21.Han JR, Deng B, Sun J, Chen CG, Corkey BE, Kirkland JL, Ma J, Guo W: Effects of dietary medium-chain triglyceride on weight loss and insulin sensitivity in a group of moderately overweight free-living type 2 diabetic Chinese subjects. Metabolism 2007;56:985–991 [DOI] [PubMed] [Google Scholar]
- 22.Ye JM, Iglesias MA, Watson DG, Ellis B, Wood L, Jensen PB, Sorensen RV, Larsen PJ, Cooney GJ, Wassermann K, Kraegen EW: PPARalpha/gamma ragaglitazar eliminates fatty liver and enhances insulin action in fat-fed rats in the absence of hepatomegaly. Am J Physiol Endocrinol Metab 2003;284:E531–E540 [DOI] [PubMed] [Google Scholar]
- 23.Folch J, Lees M, Sloane Stanley GH: A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 1957;226:497–509 [PubMed] [Google Scholar]
- 24.Lepage G, Roy CC: Direct transesterification of all classes of lipids in a one-step reaction. J Lipid Res 1986;27:114–120 [PubMed] [Google Scholar]
- 25.DeFronzo RA, Jacot E, Jequier E, Maeder E, Wahren J, Felber JP: The effect of insulin on the disposal of intravenous glucose: results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes 1981;30:1000–1007 [DOI] [PubMed] [Google Scholar]
- 26.Levak-Frank S, Radner H, Walsh A, Stollberger R, Knipping G, Hoefler G, Sattler W, Weinstock PH, Breslow JL, Zechner R: Muscle-specific overexpression of lipoprotein lipase causes a severe myopathy characterized by proliferation of mitochondria and peroxisomes in transgenic mice. J Clin Invest 1995;96:976–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garcia-Roves P, Huss JM, Han DH, Hancock CR, Iglesias-Gutierrez E, Chen M, Holloszy JO: Raising plasma fatty acid concentration induces increased biogenesis of mitochondria in skeletal muscle. Proc Natl Acad Sci U S A 2007;104:10709–10713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hegarty BD, Cooney GJ, Kraegen EW, Furler SM: Increased efficiency of fatty acid uptake contributes to lipid accumulation in skeletal muscle of high-fat–fed insulin-resistant rats. Diabetes 2002;51:1477–1484 [DOI] [PubMed] [Google Scholar]
- 29.Evans RM, Barish GD, Wang YX: PPARs and the complex journey to obesity. Nat Med 2004;10:355–361 [DOI] [PubMed] [Google Scholar]
- 30.Forman BM, Chen J, Evans RM: Hypolipidemic drugs, polyunsaturated fatty acids, and eicosanoids are ligands for peroxisome proliferator-activated receptors alpha and delta. Proc Natl Acad Sci U S A 1997;94:4312–4317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu HE, Lambert MH, Montana VG, Parks DJ, Blanchard SG, Brown PJ, Sternbach DD, Lehmann JM, Wisely GB, Willson TM, Kliewer SA, Milburn MV: Molecular recognition of fatty acids by peroxisome proliferator-activated receptors. Mol Cell 1999;3:397–403 [DOI] [PubMed] [Google Scholar]
- 32.Gerhart-Hines Z, Rodgers JT, Bare O, Lerin C, Kim SH, Mostoslavsky R, Alt FW, Wu Z, Puigserver P: Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC-1alpha. EMBO J 2007;26:1913–1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guillot E, Vaugelade P, Lemarchal P, Rerat A: Intestinal absorption and liver uptake of medium-chain fatty acids in non-anaesthetized pigs. Br J Nutr 1993;69:431–442 [DOI] [PubMed] [Google Scholar]
- 34.Friedman MI, Ramirez I, Bowden CR, Tordoff MG: Fuel partitioning and food intake: role for mitochondrial fatty acid transport. Am J Physiol 1990;258:R216–R221 [DOI] [PubMed] [Google Scholar]
- 35.Franck N, Stenkula KG, Ost A, Lindstrom T, Stralfors P, Nystrom FH: Insulin-induced GLUT4 translocation to the plasma membrane is blunted in large compared with small primary fat cells isolated from the same individual. Diabetologia 2007;50:1716–1722 [DOI] [PubMed] [Google Scholar]
- 36.Salans LB, Dougherty JW: The effect of insulin upon glucose metabolism by adipose cells of different size: influence of cell lipid and protein content, age, and nutritional state. J Clin Invest 1971;50:1399–1410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Geliebter A, Torbay N, Bracco EF, Hashim SA, Van Itallie TB: Overfeeding with medium-chain triglyceride diet results in diminished deposition of fat. Am J Clin Nutr 1983;37:1–4 [DOI] [PubMed] [Google Scholar]
- 38.Dias VC, Fung E, Snyder FF, Carter RJ, Parsons HG: Effects of medium-chain triglyceride feeding on energy balance in adult humans. Metabolism 1990;39:887–891 [DOI] [PubMed] [Google Scholar]
- 39.Chanez M, Bois-Joyeux B, Arnaud MJ, Peret J: Metabolic effects in rats of a diet with a moderate level of medium-chain triglycerides. J Nutr 1991;121:585–594 [DOI] [PubMed] [Google Scholar]
- 40.Geelen MJ, Schoots WJ, Bijleveld C, Beynen AC: Dietary medium-chain fatty acids raise and (n-3) polyunsaturated fatty acids lower hepatic triacylglycerol synthesis in rats. J Nutr 1995;125:2449–2456 [DOI] [PubMed] [Google Scholar]
- 41.Lieber CS, DeCarli LM, Leo MA, Mak KM, Ponomarenko A, Ren C, Wang X: Beneficial effects versus toxicity of medium-chain triacylglycerols in rats with NASH. J Hepatol 2008;48:318–326 [DOI] [PubMed] [Google Scholar]
- 42.Baldermann H, Wicklmayr M, Rett K, Banholzer P, Dietze G, Mehnert H: Changes of hepatic morphology during parenteral nutrition with lipid emulsions containing LCT or MCT/LCT quantified by ultrasound. JPEN J Parenter Enteral Nutr 1991;15:601–603 [DOI] [PubMed] [Google Scholar]
- 43.Nosaka N, Kasai M, Nakamura M, Takahashi I, Itakura M, Takeuchi H, Aoyama T, Tsuji H, Okazaki M, Kondo K: Effects of dietary medium-chain triacylglycerols on serum lipoproteins and biochemical parameters in healthy men. Biosci Biotechnol Biochem 2002;66:1713–1718 [DOI] [PubMed] [Google Scholar]
- 44.Hill JO, Peters JC, Swift LL, Yang D, Sharp T, Abumrad N, Greene HL: Changes in blood lipids during six days of overfeeding with medium or long chain triglycerides. J Lipid Res 1990;31:407–416 [PubMed] [Google Scholar]
- 45.Swift LL, Hill JO, Peters JC, Greene HL: Plasma lipids and lipoproteins during 6 d of maintenance feeding with long-chain, medium-chain, and mixed-chain triglycerides. Am J Clin Nutr 1992;56:881–886 [DOI] [PubMed] [Google Scholar]
- 46.Tsuji H, Kasai M, Takeuchi H, Nakamura M, Okazaki M, Kondo K: Dietary medium-chain triacylglycerols suppress accumulation of body fat in a double-blind, controlled trial in healthy men and women. J Nutr 2001;131:2853–2859 [DOI] [PubMed] [Google Scholar]
- 47.St-Onge MP, Bosarge A: Weight-loss diet that includes consumption of medium-chain triacylglycerol oil leads to a greater rate of weight and fat mass loss than does olive oil. Am J Clin Nutr 2008;87:621–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Natali A, Ferrannini E: Effects of metformin and thiazolidinediones on suppression of hepatic glucose production and stimulation of glucose uptake in type 2 diabetes: a systematic review. Diabetologia 2006;49:434–441 [DOI] [PubMed] [Google Scholar]