Abstract
OBJECTIVE
The G-protein–coupled receptor GPR40 mediates fatty acid potentiation of glucose-stimulated insulin secretion, but its contribution to insulin secretion in vivo and mechanisms of action remain uncertain. This study was aimed to ascertain whether GPR40 controls insulin secretion in vivo and modulates intracellular fuel metabolism in islets.
RESEARCH DESIGN AND METHODS
Insulin secretion and sensitivity were assessed in GPR40 knockout mice and their wild-type littermates by hyperglycemic clamps and hyperinsulinemic euglycemic clamps, respectively. Transcriptomic analysis, metabolic studies, and lipid profiling were used to ascertain whether GPR40 modulates intracellular fuel metabolism in islets.
RESULTS
Both glucose- and arginine-stimulated insulin secretion in vivo were decreased by ∼60% in GPR40 knockout fasted and fed mice, without changes in insulin sensitivity. Neither gene expression profiles nor intracellular metabolism of glucose and palmitate in isolated islets were affected by GPR40 deletion. Lipid profiling of isolated islets revealed that the increase in triglyceride and decrease in lyso-phosphatidylethanolamine species in response to palmitate in vitro was similar in wild-type and knockout islets. In contrast, the increase in intracellular inositol phosphate levels observed in wild-type islets in response to fatty acids in vitro was absent in knockout islets.
CONCLUSIONS
These results indicate that deletion of GPR40 impairs insulin secretion in vivo not only in response to fatty acids but also to glucose and arginine, without altering intracellular fuel metabolism in islets, via a mechanism that may involve the generation of inositol phosphates downstream of GPR40 activation.
Since its deorphanization in 2003 (1,2), the G-protein–coupled fatty acid receptor GPR40, highly expressed in pancreatic β-cells, has drawn considerable attention as a potential therapeutic target for type 2 diabetes. Fatty acids amplify insulin secretion from the β-cell only in the presence of glucose. This incretin-like effect could be exploited to develop novel therapeutic agents to enhance glucose-stimulated insulin secretion (GSIS) in type 2 diabetes (3). Previous studies in our laboratory have shown that GPR40 contributes to ∼50% of fatty acid potentiation of insulin secretion in vitro and in vivo (4). In addition, recent reports (4–7) provide evidence that activation of GPR40 may represent a beneficial approach to enhance insulin secretion in type 2 diabetes. However, several key questions remain to be answered regarding the role of GPR40 in insulin secretion. Whereas it is clear that GPR40 mediates, at least in part, fatty acid potentiation of insulin secretion, its contribution to the secretory action of other fuel and nonfuel stimuli in vivo is not known. This issue is important because 1) fatty acids are always present in the circulation and therefore may influence the response to various secretagogues and thereby the overall regulation of insulin secretion in vivo, 2) lipid signaling is an integral part of the regulation of insulin secretion in response to glucose and other secretagogues (8), and 3) our previous results suggest impaired insulin secretion in response to glucose in high-fat–fed GPR40 knockout mice (5), and a recent study reported increased GSIS in vitro in islets from GPR40 transgenic mice (9). In addition, the mechanisms of action of GPR40 are incompletely understood. It was shown to couple to the G-protein subunit Gαq/11 (2) and in turn activate phospholipase C (PLC)-mediated hydrolysis of phosphatidylinositol 4,5-biphosphate into diacylglycerol (DAG) and inositol phosphates (InsPs), which activate protein kinase C and mobilize Ca2+ from the endoplasmic reticulum, respectively (rev. in 10). A rise in cytoplasmic Ca2+ has been linked to the activation of dehydrogenases involved in pyruvate mitochondrial metabolism, such as pyruvate-, α-ketoglutarate–, and isocitrate-dehydrogenase (11), raising the possibility that GPR40 activation modulates intracellular fuel metabolism. The present study was therefore aimed to determine whether deletion of GPR40 1) alters insulin secretion in response to fuel and nonfuel secretagogues and insulin sensitivity in vivo and 2) affects intracellular fuel metabolism in islets.
RESEARCH DESIGN AND METHODS
Reagents.
Twenty percent dextrose solution was from Baxter (Mississauga, ON, Canada), RPMI 1640, and FBS were from Invitrogen (Burlington, ON, Canada). Fatty acid–free BSA was from Equitech-Bio (Kerrville, TX). Radioactive tracers were from GE Healthcare (Baie d'Urfé, QC, Canada), and all other reagents were from Sigma (St. Louis, MO), unless otherwise noted. The fatty acid enzymatic kit was from Wako Chemicals (Neuss, Germany).
Animals.
GPR40 knockout mice were generated as described (4) and backcrossed to the C57BL/6 strain for more than seven generations at Amgen (San Francisco, CA). All procedures were approved by the institutional committee for the protection of animals at the Centre Hospitalier de l'Université de Montréal.
β-Cell mass.
Freshly excised whole pancreata (from fed 12- to 14-week-old mice) were trimmed of fat, weighed, fixed in 4% buffered paraformadehyde, and embedded in paraffin with 5-μm sections mounted on glass slides for immunohistochemical and β-cell mass analyses, as previously described (4).
Whole-body fat content.
The percentage of whole-body fat content in fed GPR40 wild-type and knockout (12–14 weeks old) mice was assessed using an EchoMRI-700 (Houston, TX).
Assessment of insulin secretion and sensitivity by hyperglycemic and euglycemic-hyperinsulinemic clamp.
One-step hyperglycemic clamps were performed on conscious animals. A 20% dextrose solution was infused through the jugular vein to clamp plasma glucose at ∼350 mg/dl for 60 min and was adjusted based on glucose measurements (Roche Accu-Check; Roche, Indianapolis, IN). At 60 min, an arginine bolus injection was performed (1 mmol/kg; Sandoz Canada) to assess the maximal insulin response. Plasma samples were collected from the tail at several time points during the clamp for insulin measurements using a mouse insulin enzyme-linked immunosorbent assay (ELISA) kit (Alpco Diagnostics, Salem, NH). Plasma samples for C-peptide measurements were collected at 45 min and analyzed using a mouse C-peptide ELISA kit (Alpco Diagnostics). Two-hour hyperinsulinemic-euglycemic clamps were performed in 5-h food-restricted GPR40 wild-type and knockout mice. Following a 1-min bolus insulin infusion (85 mU/kg; Humulin R), insulin was infused at 5 mU · kg−1 · min−1. Twenty percent dextrose was infused starting 5 min after the insulin infusion to clamp glycemia at ∼140 mg/dl. The insulin sensitivity index (M/I) was calculated as the glucose infusion rate (M) divided by the average insulinemia during the last 30 min of the clamp (I).
Transcriptomic study.
Islets were isolated from GPR40 wild-type and knockout fed mice (13–15 weeks old, n = 5 for each genotype) as previously described (4). Total RNA was extracted from pancreatic islets using an RNeasy Micro kit (Qiagen) in accordance with the manufacturer's protocol as previously described (5). Total RNA was stored at −80°C for microarray hybridization (see supplemental research design and methods in the online appendix [available at http://diabetes.diabetesjournals.org/cgi/content/full/db09-0362/DC1]).
Metabolic studies in islets.
Islets were isolated from 24-h–fasted or ad libitum–fed mice (12–14 weeks old) as previously described (4). Freshly isolated islets were recovered at 37°C for 30 min in Krebs-Ringer bicarbonate HEPES buffer (KRBH)/0.25% BSA supplemented by 2.8 mmol/l glucose. Palmitate and control solutions were prepared as previously described (12). For measurements of glucose oxidation and utilization, batches of 20 islets were incubated in KRBH/0.25% BSA containing 1μCi d-[U-14C]-glucose (250 mCi/mmol) and 0.5 μCi d-[5-3H]-glucose (16 Ci/mmol) at 2.8 or 16.7 mmol/l glucose for 2 h. Glucose oxidation was measured by the generation of KOH-trapped 14CO2, and glucose utilization was determined by measuring the amount of 3H2O generated as described previously (13). To measure fatty acid oxidation and incorporation in total lipids, batches of 50 islets were incubated in KRBH/0.25% BSA, 0.1 mmol/l palmitate, 1 mmol/l carnitine, 2μCi/ml [9,10(n)-3H] palmitate (51 Ci/mmol), and 2.8 or 16.7 mmol/l glucose for 2 h. Supernatants were transferred to Eppendorf tubes for separation of 3H2O from labeled fatty acids as previously described (14). The remaining islets were washed and sonicated in water. Total lipids were extracted using the Folch method. Radioactivity incorporation was counted on the lipid soluble fractions.
Fatty acid esterification.
Islets isolated from fed mice were cultured overnight in RPMI supplemented with 10% FCS (complete RPMI) at 11 mmol/l glucose. Fatty acid esterification was determined in batches of 70 islets incubated for 4 h in the same conditions as described above. After the incubation, total lipids were extracted and subjected to separation by thin-layer chromatography as described (15).
Lipid profiling.
Islets isolated from fed mice were cultured overnight in complete RPMI medium at 11 mmol/l glucose. Batches of 250 islets each were incubated for 1 h at 2.8 mmol/l glucose in RPMI and then exposed to 16.7 mmol/l glucose with or without 0.5 mmol/l palmitate for 1 h. Total lipids were extracted using the Folch method, dried, and stored at −80°C for liquid chromatography–mass spectrometry analysis (see supplemental research design and methods).
Arachidonic acid release.
Islets isolated from fed mice were cultured overnight in complete RPMI medium at 5.6 mmol/l glucose and 4 μCi/ml [5, 6, 8, 9, 11, 12, 14, 15(n)-3H] arachidonic acid (AA) (250 Ci/mmol 3H-AA). After prelabeling, 3H-AA release was measured in islets incubated at 16.7 mmol/l glucose, with or without 0.5 mmol/l palmitate or 100 μmol/l carbachol in KRBH/0.6% BSA for 30 min as described (16). After incubation, islets were lysed to measure incorporation of the total radioactivity.
Intracellular inositol phosphate accumulation.
Islets isolated from fed mice were cultured overnight in complete RPMI medium at 11.1 mmol/l glucose. Batches of 40 islets were prelabeled with 7.5 μCi of [3H]myo-inositol (95 Ci/mmol) in KRBH/0.01% BSA for 3 h. After prelabeling, InsPs accumulation was measured in islets incubated at 16.7 mmol/l glucose, with or without 30 μmol/l oleate or 1 mmol/l carbachol in KRBH/0.01% BSA/10 mmol/l LiCl for 20 min. After incubation, islets were lysed in 20 mmol/l formic acid (150 μl), and 3H-InsPs content was measured using the Yttrium silicate scintillation proximity assay (YSi SPA) beads (GE Healthcare) (17). Briefly, YSi SPA beads were diluted eightfold with water, 100 μl of diluted beads were added to each well of a Wallac Isoplate-96 white plate (Perkin Elmer, Woodbridge, ON, Canada) followed by 50 μl of islets extracts, and radioactivity was measured using a MicroBeta TriLux scintillation counter (Perkin Elmer) after a 2-h incubation.
Expression of data and statistics.
Data are expressed as means ± SE. Intergroup comparisons were performed by ANOVA, with post hoc adjustments for two-by-two comparisons or Student's t test, as appropriate. P < 0.05 was considered significant.
RESULTS
Insulin secretion is impaired in GPR40 knockout mice during hyperglycemic clamps.
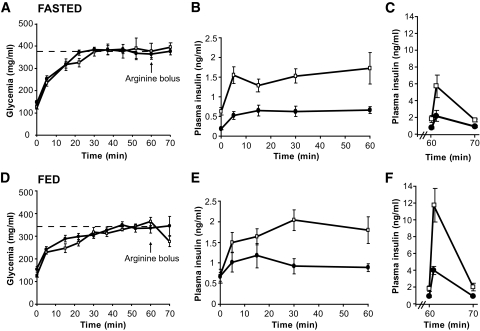
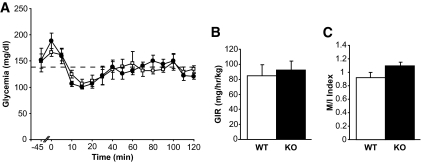
Basal metabolic parameters were first compared between 24-h–fasted and ad libitum–fed male GPR40 knockout mice (back-crossed onto the C57Bl/6J for seven generations) and their wild-type littermates (Table 1). As expected, plasma fatty acid levels were significantly increased, while both glucose and insulin levels were significantly decreased, in fasted versus fed mice. Blood glucose, fatty acids, insulin, and C-peptide levels were similar in GPR40 knockout and wild-type mice in both the fed and fasted state, consistent with our previous report (4). Insulin secretion in response to glucose and arginine was assessed in vivo using 1-h hyperglycemic clamps (Fig. 1A and D). As expected, the glucose infusion rate was markedly lower in fasted than in fed animals, although it was not affected by the genotype when taking into account the prevailing insulin levels (Table 2). Insulin secretion during the clamp was markedly decreased in both fasted and fed GPR40 knockout mice (Fig. 1B and E). The areas under the curve for insulin over the first 60 min of the clamp were reduced in fasted and fed GPR40 knockout mice by approximately twofold and approximately threefold, respectively (Table 2). Accordingly, C-peptide concentrations 45 min into the clamp were significantly lower in GPR40 knockout fed animals versus wild type (1.45 ± 0.13 ng/ml [n = 7] vs. 2.04 ± 0.17 ng/ml [n = 9]; P < 0.05). Arginine potentiation of GSIS was also decreased by approximately threefold in fasted and fed GPR40 knockout mice (Table 2) (Fig. 1C and F). Similar observations were made in female GPR40 knockout mice (not shown). β-Cell mass (Table 1) and islet size distribution (not shown) were not different between GPR40 wild-type and knockout mice. Insulin sensitivity was then examined in hyperinsulinemic-euglycemic clamps. Both the glucose infusion rate (Fig. 2B) required to maintain glycemia at ∼140 mg/dl (Fig. 2A) during the clamp and the insulin sensitivity index (Fig. 2C) were similar in GPR40 wild-type and knockout mice. Thus, GPR40 deletion does not affect whole-body insulin sensitivity, consistent with previous reports using insulin tolerance tests (4,18). Altogether, our data demonstrate that GPR40 deletion impairs insulin secretion in vivo in response to glucose and arginine without altering insulin sensitivity.
TABLE 1.
Metabolic parameters of GPR40 wild-type and knockout mice and β-cell mass
| Wild type | Knockout | |
|---|---|---|
| Basal 24-h–fasted mice | ||
| Body weight (g) | 24.1 ± 0.2 | 25.1 ± 0.5 |
| Basal glycemia (mg/dl) | 120 ± 5 | 119 ± 9 |
| Free fatty acids (mmol/l) | 0.50 ± 0.03 | 0.55 ± 0.03 |
| Basal insulinemia (ng/ml) | 0.06 ± 0.01 | 0.08 ± 0.01 |
| C-peptide (ng/ml) | 0.51 ± 0.04 | 0.49 ± 0.01 |
| Basal fed mice | ||
| Body weight (g) | 28.2 ± 0.9* | 27.8 ± 0.5* |
| Basal glycemia (mg/dl) | 151 ± 3† | 154 ± 5† |
| Free fatty acids (mmol/l) | 0.09 ± 0.01† | 0.11 ± 0.02† |
| Basal insulinemia (ng/ml) | 0.48 ± 0.01† | 0.51 ± 0.03† |
| C-peptide (ng/ml) | 0.64 ± 0.03* | 0.71 ± 0.02* |
| β-Cell mass (mg) | 1.55 ± 0.09 | 2 ± 0.22 |
| Fat (% of body weight) | 12 ± 1 | 10 ± 1 |
Data are means ± SE. n = 7–9 mice/group.
*P < 0.05;
†P < 0.01 vs. fasted.
FIG. 1.
Hyperglycemic clamps in 24-h–fasted and ad libitum–fed GPR40 wild-type and knockout mice. Glucose (A and D) and insulin (B and E) levels during the course of the hyperglycemic clamp in fasted and fed GPR40 wild-type and knockout mice. Insulin levels (C and F) in response to an arginine bolus (1 mmol/kg). Values are expressed as means ± SE of seven to nine mice per group. □, Wild type; ●, knockout.
TABLE 2.
Insulin secretion in GPR40 wild-type and knockout during hyperglycemic clamps
| Wild type | Knockout | |
|---|---|---|
| Hyperglycemic clamp in 24-h–fasted mice | ||
| AUCInsulin T0–60 min | 51.6 ± 9.6 | 24.9 ± 6.9* |
| AIRMax (ng/ml) T61 min | 5.7 ± 1.3 | 2.1 ± 0.6* |
| GIR (mg · kg−1 · min−1) | 16 ± 5 | 10.5 ± 1 |
| Hyperglycemic clamp in fed mice | ||
| AUCInsulin T0–60 min | 65.4 ± 17.2 | 19.3 ± 6.9* |
| AIRMax (ng/ml) T61 min | 11.8 ± 1.9 | 3.9 ± 0.5† |
| GIR (mg · kg−1 · min−1) | 71 ± 9§ | 39 ± 4†§ |
Data are means ± SE. n = 7–9 mice/group.
*P < 0.05;
†P < 0.01 vs. wild type.
§P < 0.01 vs. fasted. AIR Max, maximal arginine-induced insulin response; AUCInsulin, area under the curve of insulin; GIR, glucose infusion rate.
FIG. 2.
Hyperinsulinemic-euglycemic clamps in GPR40 wild-type and knockout mice. Glucose levels (A) and glucose infusion rate (GIR) (B) during the course of the hyperinsulinemic clamp in 5-h-food–restricted GPR40 wild-type and knockout mice. The insulin sentivity index (M/I) (C) was calculated as the glucose infusion rate (M) divided by the average insulinemia during the last 30 min of the clamp (I). Values are expressed as means ± SE of five to six mice per group. □, Wild type; ●, knockout.
Intracellular metabolism of glucose and fatty acids in islets is not affected by GPR40 deletion.
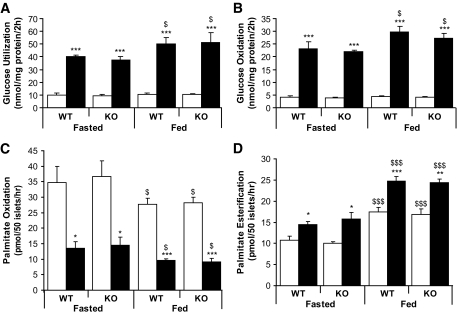
Transcriptomic analysis, metabolic studies, and lipid profiling were used to address whether GPR40 modulates intracellular fuel metabolism in islets. We first assessed the impact of GPR40 deletion on the islet transcriptome using expression microarrays. Approximately 1,770 genes were significantly modulated (1,109 downregulated and 661 upregulated) in knockout versus wild type (P < 0.05). However, expression of none of these genes was changed more than twofold (supplementary Fig. S1). Thus, GPR40 deletion does not have a major impact on the islet transcriptome. To explore the functional impact of GPR40 deletion on fuel partitioning, glucose and palmitate metabolism were assessed in islets isolated from 24-h–fasted and fed mice. Raising glucose from 2.8 to 16.7 mmol/l significantly increased glucose utilization and oxidation, decreased palmitate oxidation by ∼3-fold, and increased palmitate esterification by ∼1.5-fold in both fasted and fed wild-type islets (Fig. 3A–D). No differences were observed between wild-type and knockout islets for any of these measurements (Fig. 3A–D). Consistent with previous studies (19,20), fasting decreased glucose oxidation and palmitate esterification and increased palmitate oxidation (Fig. 3A–D).
FIG. 3.
Glucose and palmitate metabolism in GPR40 wild-type and knockout islets. Glucose utilization (A), glucose oxidation (B), palmitate oxidation (C), and palmitate incorporation into total lipids (D) in islets isolated from fasted or fed mice incubated at 2.8 (□) or 16.7 (■) mmol/l glucose for 2 h. Data are expressed as means ± SE of three to five independent experiments. *P < 0.05, **P < 0.01, or ***P < 0.001 vs. 2.8 mmol/l glucose. $P < 0.05, $$$P < 0.001 vs. fasted.
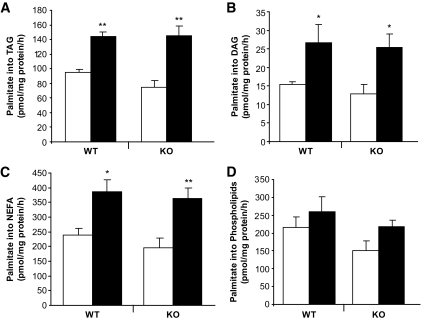
Palmitate esterification into phospholipids (PLs), triacylglycerol (TAG), DAG, and fatty acids was assessed in islets isolated from fed wild-type and knockout mice. Raising glucose from 2.8 to 16.7 mmol/l increased palmitate esterification into DAG and TAG and increased fatty acid content similarly in wild-type and knockout islets (Fig. 4A and B). The effect of elevated glucose on palmitate esterification in PLs, DAG, TAG, and on intracellular fatty acid levels was not different between wild-type and knockout islets. These data indicate that GPR40 deletion does not affect glucose or palmitate metabolism in islets, nor does it contribute to changes in intracellular fuel metabolism in response to fasting.
FIG. 4.
Palmitate esterification in GPR40 wild-type and knockout islets. Palmitate esterification into triacylglycerol (A), diacylglycerol (B), nonesterified fatty acid (C), and phospholipids (D) in islets incubated at 2.8 (□) or 16.7 (■) mmol/l glucose for 4 h. Data are expressed as means ± SE of five independent experiments. *P < 0.05 or **P < 0.01 vs. 2.8 mmol/l glucose.
The islet lipid profile is modulated acutely by palmitate independently of GPR40.
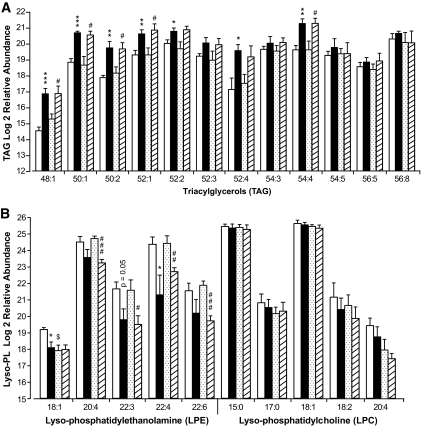
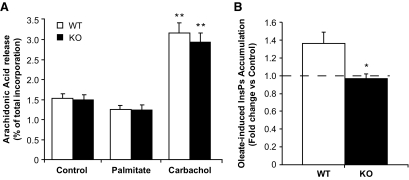
Data presented in Figs. 3 and 4 indicate that GPR40 deletion does not alter fluxes through major glucose and palmitate metabolic pathways. This does not rule out the possibility that GPR40 might modulate the abundance of individual lipid species in islets in response to palmitate. This possibility was tested using liquid chromatography–mass spectrometry–based lipid profiling. Islets isolated from wild-type and knockout mice were incubated for 1 h at 16.7 mmol/l glucose ± 0.5 mmol/l palmitate. Lipid-soluble fractions were separated by liquid chromatography followed by tandem mass spectrometry analysis as described in the supplemental research design and methods. The data are presented as relative abundance (i.e., comparative mass measurements across samples) after log2 transformation (Fig. 5). Palmitate treatment significantly modulated the relative abundance of 59 lipid species in wild-type islets and 62 in knockout islets (P < 0.05) (see supplementray Fig. S2), among which 13 were identified based on their match with the accurate mass and time tag database (see supplemental research design and methods). Several TAG species were markedly increased by palmitate treatment both in wild-type and knockout islets (Fig. 5A). For example, the abundance of TAG 48:1 was increased fivefold (2.3 log2 units) in wild-type islets in response to palmitate. Although the effect of palmitate was more pronounced for some TAG in wild-type versus knockout islets, when calculated as fold increase over control, no significant differences were observed between wild-type and knockout islets. These results indicate that acute palmitate treatment induces a robust increase in several TAG species in islets independently of GPR40. Palmitate treatment decreased the abundance of all identified lyso-phosphatidylethanolamine (LPE) species (Fig. 5B) but did not have a major effect on lyso-phosphatidylcholines (LPC) (Fig. 5B), phosphatidylethanolamines (PEs) (supplementary Fig. S3A), phosphatidylcholines (PCs) (supplementary Fig. S3A), or sphingomyelins (supplementary Fig. S3B). Although the effect of palmitate was more pronounced for some LPE in knockout versus wild-type islets (Fig. 5B), when calculated as fold decrease over control, no difference was observed between the two genotypes. These data indicate that palmitate modulates LPE abundance independently of GPR40. Several lines of evidence suggest that hydrolysis of membrane PLs by phospholipase A2 (PLA2) into lyso-PLs and AA participates in the control of insulin secretion (21–24). We therefore tested whether palmitate lowering of LPE content results from PLA2-mediated PL hydrolysis. PLA2 activity was indirectly assessed in islets by measuring the release of AA as previously described (16). The muscarinic agonist carbachol stimulated AA release to a similar extent in wild-type and knockout islets, while palmitate had no effect (Fig. 6A). We conclude that 1) palmitate potentiation of GSIS is independent of AA release and 2) activation of the PLA2 pathway is not affected by GPR40 deletion.
FIG. 5.
Lipid profiles in GPR40 wild-type and knockout islets. Log2 relative abundances of triacylglycerols (A) and lyso-phospholipids (B) in islets incubated at 16.7 mmol/l glucose with or without 0.5 mmol/l palmitate for 1 h. Lipid extracts were subjected to liquid chromatography–mass spectrometry analysis (see supplementary research design and methods). Data are expressed as means ± SE of five independent experiments as relative abundance (i.e., comparative mass measurements across samples) after log2 transformation. *P < 0.05, **P < 0.01, or ***P < 0.001 vs. wild-type control. #P < 0.05, ##P < 0.01, or ###P < 0.001 vs. knockout control. □, Wild-type control; ■, wild-type palmitate;  , knockout control;
, knockout control;  , knockout palmitate.
, knockout palmitate.
FIG. 6.
Arachidonic acid release and InsPs accumulation in GPR40 wild-type and knockout islets. A: AA release in islets incubated during 30 min at 16.7 mmol/l glucose with or without 0.5 mmol/l palmitate and 100 μmol/l carbachol. AA efflux is expressed as the percentage release of total incorporated radioactivity. Data are expressed as means ± SE of three to four independent experiments. **P < 0.01 vs. control. B: InsPs accumulation in islets incubated during 20 min at 16.7 mmol/l glucose with or without 30 μmol/l oleate. InsPs accumulation is expressed as fold changes over control conditions (16.7 mmol/l glucose). Data are expressed as means ± SE of three independent experiments. *P < 0.05 vs. control. □, Wild type; ■, knockout.
Deletion of GPR40 abolishes intracellular accumulation of inositol phosphates in response to oleate.
Previous studies suggest that GPR40 couples to the G-protein subunit Gαq/11 (2) and in turn activates PLC-mediated hydrolysis of phosphatidylinositol into DAG and InsPs (25). Furthermore, pharmacological inhibition of Gαq (4,26) or PLC (26,27) in β-cells prevents fatty acid potentiation of GSIS. However, whether GPR40 mediates fatty acid–induced InsPs generation in islets is unknown. To address this question, we compared InsPs generation from tritiated phosphoinositides in response to oleate in islets from wild-type and GPR40 knockout mice (Fig. 6B). Remarkably, the increase in InsPs accumulation observed in wild-type islets in response to oleate was absent in knockout islets. In contrast, InsPs accumulation in response to the muscarinic agonist carbachol was similar in both groups (1.9 ± 0.3–fold increase in knockout islets vs. 2.0 ± 0.7–fold increase in wild-type islets; n = 3; NS). These data indicate that fatty acid–induced InsPs accumulation is dependent upon GPR40 in mouse islets.
DISCUSSION
The objectives of this study were to examine whether deletion of GPR40 impairs insulin secretion in vivo and modulates intracellular fuel metabolism in islets. We found that, in GPR40 knockout mice, insulin secretion in response to both glucose and arginine is impaired in fed and fasted animals during hyperglycemic clamps, without changes in insulin sensitivity, as measured by hyperinsulinemic-euglycemic clamps. This was associated with a complete absence of fatty acid–induced intracellular InsPs accumulation in islets, without detectable changes in intracellular glucose or fatty acid metabolism. We conclude that GPR40 signaling is implicated in the regulation of insulin secretion in vivo, independently from changes in fuel metabolism.
Our results demonstrate that deletion of GPR40 impairs insulin secretion in response to glucose and arginine in vivo under fasted and fed conditions. Since neither β-cell mass nor insulin content (not shown) were affected by GPR40 deletion, we infer that the decrease in insulin levels in GPR40 knockout mice results from a bona fide secretory defect. This conclusion is further supported by the observed decrease in circulating C-peptide levels during the hyperglycemic clamp. Since fatty acids are always present in the circulation in vivo, we suggest that GPR40 mediates their potentiating action on glucose- and arginine-stimulated insulin secretion. Consistent with this view, GSIS ex vivo was similar in wild-type and knockout islets isolated from fasted mice (not shown). In addition, we have previously shown that the islet response to glucose alone in the absence of exogenous fatty acids is not affected by GPR40 deletion, whereas the response to fatty acids is markedly decreased (4). The observed impairment of insulin secretion in this study using hyperglycemic clamps is in apparent contradiction with our previous findings that insulin secretion was similar in wild-type and knockout mice when assessed by intravenous glucose tolerance tests (IVGTTs) (4,5). One possible explanation for this discrepancy is that the single bolus of glucose in the IVGTT mostly stimulates first-phase insulin release, which relies less on the amplification pathway of insulin secretion than the full first- and second-phase secretory profile induced under clamp conditions. Also, sustained glucose stimulation during the clamp evokes a greater integrated insulin response than the IVGTT bolus and is therefore more likely to detect secretory defects.
Using transcriptomic, metabolic, and lipid-profiling approaches, we tested the hypothesis that impaired insulin secretion in GPR40 knockout mice involves modulation of intracellular fuel metabolism in islets. First, microarray analyses indicated that GPR40 deletion does not alter the expression of islet genes by more than twofold, suggesting that the defect in insulin secretion is not related to major changes in islet phenotype. Second, neither glucose nor palmitate metabolism (as assessed by tracer studies) was modified in fasted or fed GPR40 knockout islets, nor were there any significant differences in lipid profiles using liquid chromatography–mass spectrometry between wild-type and knockout islets in response to palmitate. Third, AA generation was unaltered by GPR40 deletion. In contrast, InsPs accumulation in response to oleate was completely absent in GPR40 knockout islets. Together with previous studies from our (4) and other (26,27) groups using pharmacological inhibitors of the Gαq/11 signaling pathway, these data therefore reinforce the notion that the mode of regulation of insulin secretion by GPR40 involves receptor-mediated signaling rather than fuel-derived metabolic signals. This conclusion has important implications for the understanding of the mechanisms of action of fatty acids on the β-cell. On the one hand, molecular and pharmacological approaches have shown that fatty acid activation to fatty acyl-CoA and intracellular metabolism are required for their potentiating effects on GSIS (8). On the other hand, several studies have reported that blockade of GPR40 signaling prevents, at least in part, fatty acid potentiation of GSIS (2,4,26,28,29). This suggested that perhaps these two mechanisms of action (intracellular metabolism and GPR40 activation) were interrelated. The results of the present study show that, to the contrary, fatty acid signaling through GPR40 is independent from their intracellular metabolism. They further suggest that in this context, the GPR40-dependent rise in intracellular Ca2+ levels does not significantly affect the activity of the tricarboxylic acid cycle, as measured by fuel oxidation. Based on our previous observation that GPR40 deletion results in a 50% decrease in insulin secretion in response to fatty acids in vivo (4), we propose that GPR40 signaling contributes approximately half of the potentiating effects of fatty acids on GSIS.
Liquid chromatography–mass spectrometry–based lipid profiling showed that acute palmitate treatment markedly increased several TAG species and decreased LPE species independently from GPR40. To our knowledge, this is the first direct demonstration that acute exposure of islets to palmitate rapidly results in a dramatic (fivefold for some species) increase in different TAG species. It suggests that intermediates generated along the TAG synthesis pathway might be implicated in GPR40-independent amplification of GSIS by fatty acids. Whether this is also associated with increased rates of lipolysis and an increase in TAG–fatty acid cycling (30) remains to be determined. A potential mechanism for the palmitate-induced decrease in LPE involves LPE reesterification by lyso-PL-acyltransferase (Lands cycle) (31). Such a mechanism is consistent with the observed increase in PE 36:4 and PE 18:0p/20:4 (supplementary Fig. S3A). Whether this mechanism plays a role in fatty acid potentiation of GSIS remains to be investigated. LPC was recently shown to potently enhance GSIS via activation of the GPR119 receptor (32,33). Both LPE and LPC are generated by plasma membrane–associated PLA2 activities known to stimulate insulin secretion (34), and a recent report suggested that fatty acids modulate the PLA2 pathway and the generation of AA via GPR40 in primary hepatocytes (35). However, although we have not directly measured the activity of the enzyme, our finding that AA release is not increased by palmitate does not support the hypothesis that the effects of palmitate on GSIS are mediated by PLA2.
GPR40 expression has been detected in the ileum (1,2), monocytes (1), pancreatic α-cells (36), some areas of the brain (1,37), entero-endocrine cells (38), and osteoclasts (39), albeit at much lower levels than in β-cells. Consequently, we cannot exclude that deletion of GPR40 in these tissues in our global knockout model could have contributed to the insulin secretory defect. However, there was not apparent change in body weight, food intake, or insulin sensitivity in these mice, and the main phenotype appears restricted to the β-cell. Therefore, we believe that the contribution of non–β-cells to the observed phenotype, if any, is minimal, although a tissue-specific knockout model would be required to formally exclude this possibility.
In conclusion, this study identifies GPR40 as necessary for insulin secretion in vivo. The impaired insulin secretion observed in GPR40 knockout mice is independent of nutrient metabolism in islets and more likely involves the canonical GPR40 signaling pathway.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grants R21-DK070598 to V.P., R01-DK068329 to T.L.J., and R33-DK070146 to R.D.S. and T.O.M. and the Canadian Institutes of Health Research (MOP 177381 to V.P.). T.A. was supported by a postdoctoral fellowship from the Canadian Diabetes Association. V.P. holds the Canada Research Chair in Diabetes and Pancreatic β-Cell Function. M.P. holds the Canada Research Chair in Diabetes and Metabolism. The Pacific Northwest National Laboratory (PNNL) is operated by Battelle Memorial Institute for the DOE under contract no. DE-AC06-76LO-1830.
Portions of the work were performed in the Environmental Molecular Sciences Laboratory, a national scientific user facility located at the PNNL and supported by the U.S. Department of Energy Office of Biological and Environmental Research.
No potential conflicts of interest relevant to this article were reported.
We thank the staff of the Poitout, Prentki, Jetton, and Metz laboratories for technical assistance and Drs. Daniel C.-H. Lin and Hélène Baribault (Amgen) for providing GPR40 knockout breeders.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Briscoe CP, Tadayyon M, Andrews JL, Benson WG, Chambers JK, Eilert MM, Ellis C, Elshourbagy NA, Goetz AS, Minnick DT, Murdock PR, Sauls HR, Jr, Shabon U, Spinage LD, Strum JC, Szekeres PG, Tan KB, Way JM, Ignar DM, Wilson S, Muir AI: The orphan G protein-coupled receptor GPR40 is activated by medium and long chain fatty acids J Biol Chem 2003; 278: 11303– 11311 [DOI] [PubMed] [Google Scholar]
- 2.Itoh Y, Kawamata Y, Harada M, Kobayashi M, Fujii R, Fukusumi S, Ogi K, Hosoya M, Tanaka Y, Uejima H, Tanaka H, Maruyama M, Satoh R, Okubo S, Kizawa H, Komatsu H, Matsumura F, Noguchi Y, Shinohara T, Hinuma S, Fujisawa Y, Fujino M: Free fatty acids regulate insulin secretion from pancreatic beta cells through GPR40 Nature 2003; 422: 173– 176 [DOI] [PubMed] [Google Scholar]
- 3.Alquier T, Poitout V: GPR40: good cop, bad cop? Diabetes 2009; 58: 1035– 1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Latour MG, Alquier T, Oseid E, Tremblay C, Jetton TL, Luo J, Lin DC, Poitout V: GPR40 is necessary but not sufficient for fatty acid stimulation of insulin secretion in vivo Diabetes 2007; 56: 1087– 1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kebede M, Alquier T, Latour MG, Semache M, Tremblay C, Poitout V: The fatty acid receptor GPR40 plays a role in insulin secretion in vivo after high-fat feeding Diabetes 2008; 57: 2432– 2437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lan H, Hoos LM, Liu L, Tetzloff G, Hu W, Abbondanzo SJ, Vassileva G, Gustafson EL, Hedrick JA, Davis HR: Lack of FFAR1/GPR40 does not protect mice from high-fat diet-induced metabolic disease Diabetes 2008; 57: 2999– 3006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tan CP, Feng Y, Zhou YP, Eiermann GJ, Petrov A, Zhou C, Lin S, Salituro G, Meinke P, Mosley R, Akiyama TE, Einstein M, Kumar S, Berger JP, Mills SG, Thornberry NA, Yang L, Howard AD: Selective small-molecule agonists of G protein-coupled receptor 40 promote glucose-dependent insulin secretion and reduce blood glucose in mice Diabetes 2008; 57: 2211– 2219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roduit R, Nolan C, Alarcon C, Moore P, Barbeau A, Delghingaro-Augusto V, Przybykowski E, Morin J, Masse F, Massie B, Ruderman N, Rhodes C, Poitout V, Prentki M: A role for the malonyl-CoA/long-chain acyl-CoA pathway of lipid signaling in the regulation of insulin secretion in response to both fuel and nonfuel stimuli Diabetes 2004; 53: 1007– 1019 [DOI] [PubMed] [Google Scholar]
- 9.Nagasumi K, Esaki R, Iwachidow K, Yasuhara Y, Ogi K, Tanaka H, Nakata M, Yano T, Shimakawa K, Taketomi S, Takeuchi K, Odaka H, Kaisho Y: Overexpression of GPR40 in pancreatic beta-cells augments glucose-stimulated insulin secretion and improves glucose tolerance in normal and diabetic mice Diabetes 2009; 58: 1067– 1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stoddart LA, Smith NJ, Milligan G: International Union of Pharmacology. LXXI. Free fatty acid receptors FFA1, -2, and -3: pharmacology and pathophysiological functions Pharmacol Rev 2008; 60: 405– 417 [DOI] [PubMed] [Google Scholar]
- 11.McCormack JG, Halestrap AP, Denton RM: Role of calcium ions in regulation of mammalian intramitochondrial metabolism Physiol Rev 1990; 70: 391– 425 [DOI] [PubMed] [Google Scholar]
- 12.Kelpe CL, Moore PC, Parazzoli SD, Wicksteed B, Rhodes CJ, Poitout V: Palmitate inhibition of insulin gene expression is mediated at the transcriptional level via ceramide synthesis J Biol Chem 2003; 278: 30015– 30021 [DOI] [PubMed] [Google Scholar]
- 13.Massa ML, Borelli MI, Del Zotto H, Gagliardino JJ: Changes induced by sucrose administration on glucose metabolism in pancreatic islets in normal hamsters J Endocrinol 2001; 171: 551– 556 [DOI] [PubMed] [Google Scholar]
- 14.Saddik M, Lopaschuk GD: Myocardial triglyceride turnover and contribution to energy substrate utilization in isolated working rat hearts J Biol Chem 1991; 266: 8162– 8170 [PubMed] [Google Scholar]
- 15.Nolan CJ, Leahy JL, Delghingaro-Augusto V, Moibi J, Soni K, Peyot ML, Fortier M, Guay C, Lamontagne J, Barbeau A, Przybytkowski E, Joly E, Masiello P, Wang S, Mitchell GA, Prentki M: Beta cell compensation for insulin resistance in Zucker fatty rats: increased lipolysis and fatty acid signalling Diabetologia 2006; 49: 2120– 2130 [DOI] [PubMed] [Google Scholar]
- 16.Simonsson E, Karlsson S, Ahren B: Ca2+-independent phospholipase A2 contributes to the insulinotropic action of cholecystokinin-8 in rat islets: dissociation from the mechanism of carbachol Diabetes 1998; 47: 1436– 1443 [DOI] [PubMed] [Google Scholar]
- 17.Brandish PE, Hill LA, Zheng W, Scolnick EM: Scintillation proximity assay of inositol phosphates in cell extracts: high-throughput measurement of G-protein-coupled receptor activation Anal Biochem 2003; 313: 311– 318 [DOI] [PubMed] [Google Scholar]
- 18.Steneberg P, Rubins N, Bartoov-Shifman R, Walker MD, Edlund H: The FFA receptor GPR40 links hyperinsulinemia, hepatic steatosis, and impaired glucose homeostasis in mouse Cell Metab 2005; 1: 245– 258 [DOI] [PubMed] [Google Scholar]
- 19.Hedeskov CJ, Capito K: The effect of starvation on insulin secretion and glucose metabolism in mouse pancreatic islets Biochem J 1974; 140: 423– 433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tamarit-Rodriguez J, Vara E, Tamarit J: Starvation-induced changes of palmitate metabolism and insulin secretion in isolated rat islets stimulated by glucose Biochem J 1984; 221: 317– 324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramanadham S, Song H, Hsu FF, Zhang S, Crankshaw M, Grant GA, Newgard CB, Bao S, Ma Z, Turk J: Pancreatic islets and insulinoma cells express a novel isoform of group VIA phospholipase A2 (iPLA2 beta) that participates in glucose-stimulated insulin secretion and is not produced by alternate splicing of the iPLA2 beta transcript Biochemistry 2003; 42: 13929– 13940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bao S, Bohrer A, Ramanadham S, Jin W, Zhang S, Turk J: Effects of stable suppression of group VIA phospholipase A2 expression on phospholipid content and composition, insulin secretion, and proliferation of INS-1 insulinoma cells J Biol Chem 2006; 281: 187– 198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bao S, Song H, Wohltmann M, Ramanadham S, Jin W, Bohrer A, Turk J: Insulin secretory responses and phospholipid composition of pancreatic islets from mice that do not express group VIA phospholipase A2 and effects of metabolic stress on glucose homeostasis J Biol Chem 2006; 281: 20958– 20973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacobson DA, Weber CR, Bao S, Turk J, Philipson LH: Modulation of the pancreatic islet beta-cell-delayed rectifier potassium channel Kv2.1 by the polyunsaturated fatty acid arachidonate J Biol Chem 2007; 282: 7442– 7449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salehi A, Flodgren E, Nilsson NE, Jimenez-Feltstrom J, Miyazaki J, Owman C, Olde B: Free fatty acid receptor 1 (FFA(1)R/GPR40) and its involvement in fatty-acid-stimulated insulin secretion Cell Tissue Res 2005; 322: 207– 215 [DOI] [PubMed] [Google Scholar]
- 26.Shapiro H, Shachar S, Sekler I, Hershfinkel M, Walker MD: Role of GPR40 in fatty acid action on the beta cell line INS-1E Biochem Biophys Res Commun 2005; 335: 97– 104 [DOI] [PubMed] [Google Scholar]
- 27.Fujiwara K, Maekawa F, Yada T: Oleic acid interacts with GPR40 to induce Ca2+ signaling in rat islet beta-cells: mediation by PLC and L-type Ca2+ channel and link to insulin release Am J Physiol Endocrinol Metab 2005; 289: E670– E677 [DOI] [PubMed] [Google Scholar]
- 28.Briscoe CP, Peat AJ, McKeown SC, Corbett DF, Goetz AS, Littleton TR, McCoy DC, Kenakin TP, Andrews JL, Ammala C, Fornwald JA, Ignar DM, Jenkinson S: Pharmacological regulation of insulin secretion in MIN6 cells through the fatty acid receptor GPR40: identification of agonist and antagonist small molecules Br J Pharmacol 2006; 148: 619– 628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schnell S, Schaefer M, Schofl C: Free fatty acids increase cytosolic free calcium and stimulate insulin secretion from beta-cells through activation of GPR40 Mol Cell Endocrinol 2007; 263: 173– 180 [DOI] [PubMed] [Google Scholar]
- 30.Prentki M, Madiraju SR: Glycerolipid metabolism and signaling in health and disease Endocr Rev 2008; 29: 647– 676 [DOI] [PubMed] [Google Scholar]
- 31.Shindou H, Shimizu T: Acyl-CoA: lysophospholipid acyltransferases J Biol Chem 2009; 284: 1– 5 [DOI] [PubMed] [Google Scholar]
- 32.Soga T, Ohishi T, Matsui T, Saito T, Matsumoto M, Takasaki J, Matsumoto S, Kamohara M, Hiyama H, Yoshida S, Momose K, Ueda Y, Matsushime H, Kobori M, Furuichi K: Lysophosphatidylcholine enhances glucose-dependent insulin secretion via an orphan G-protein-coupled receptor Biochem Biophys Res Commun 2005; 326: 744– 751 [DOI] [PubMed] [Google Scholar]
- 33.Chu ZL, Jones RM, He H, Carroll C, Gutierrez V, Lucman A, Moloney M, Gao H, Mondala H, Bagnol D, Unett D, Liang Y, Demarest K, Semple G, Behan DP, Leonard J: A role for beta-cell-expressed G protein-coupled receptor 119 in glycemic control by enhancing glucose-dependent insulin release Endocrinology 2007; 148: 2601– 2609 [DOI] [PubMed] [Google Scholar]
- 34.Poitout V: Phospholipid hydrolysis and insulin secretion: a step toward solving the Rubik's cube Am J Physiol Endocrinol Metab 2008; 294: E214– E216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suh HN, Huong HT, Song CH, Lee JH, Han HJ: Linoleic acid stimulates gluconeogenesis via Ca2+/PLC, cPLA2, and PPAR pathways through GPR40 in primary cultured chicken hepatocytes Am J Physiol Cell Physiol 2008; 295: C1518– C1527 [DOI] [PubMed] [Google Scholar]
- 36.Flodgren E, Olde B, Meidute-Abaraviciene S, Winzell MS, Ahren B, Salehi A: GPR40 is expressed in glucagon producing cells and affects glucagon secretion Biochem Biophys Res Commun 2007; 354: 240– 245 [DOI] [PubMed] [Google Scholar]
- 37.Ma D, Lu L, Boneva NB, Warashina S, Kaplamadzhiev DB, Mori Y, Nakaya MA, Kikuchi M, Tonchev AB, Okano H, Yamashima T: Expression of free fatty acid receptor GPR40 in the neurogenic niche of adult monkey hippocampus Hippocampus 2008; 18: 326– 333 [DOI] [PubMed] [Google Scholar]
- 38.Edfalk S, Steneberg P, Edlund H: Gpr40 is expressed in enteroendocrine cells and mediates free fatty acid stimulation of incretin secretion Diabetes 2008; 57: 2280– 2287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cornish J, MacGibbon A, Lin JM, Watson M, Callon KE, Tong PC, Dunford JE, van der Does Y, Williams GA, Grey AB, Naot D, Reid IR: Modulation of osteoclastogenesis by fatty acids Endocrinology 2008; 149: 5688– 5695 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.