Type 2 diabetes is caused by a complex set of interactions between genetic and environmental factors. Recent work has shown that human type 2 diabetes is a constellation of disorders associated with polymorphisms in a wide array of genes, with each individual gene accounting for <1% of disease risk (1). Moreover, type 2 diabetes involves dysfunction of multiple organ systems, including impaired insulin action in muscle and adipose, defective control of hepatic glucose production, and insulin deficiency caused by loss of β-cell mass and function (2). This complexity presents challenges for a full understanding of the molecular pathways that contribute to the development of this major disease. Progress in this area may be aided by the recent advent of technologies for comprehensive metabolic analysis, sometimes termed “metabolomics.” Herein, we summarize key metabolomics methodologies, including nuclear magnetic resonance (NMR) and mass spectrometry (MS)-based metabolic profiling technologies, and discuss “nontargeted” versus “targeted” approaches. Examples of the application of these tools to diabetes and metabolic disease research at the cellular, animal model, and human disease levels are summarized, with a particular focus on insights gained from the more quantitative targeted methodologies. We also provide early examples of integrated analysis of genomic, transcriptomic, and metabolomic datasets for gaining knowledge about metabolic regulatory networks and diabetes mechanisms and conclude by discussing prospects for future insights.
In principal, metabolomics can provide certain advantages relative to other “omics” technologies (genomics, transcriptomics, proteomics) in diabetes research: 1) Estimates vary, but one current source, the Human Metabolome Database (HMDB)-Canada (3), currently lists ∼6,500 discrete small molecule metabolites, significantly less than the estimate of 25,000 genes, 100,000 transcripts, and 1,000,000 proteins. 2) Metabolomics measures chemical phenotypes that are the net result of genomic, transcriptomic, and proteomic variability, therefore providing the most integrated profile of biological status. 3) Metabolomics is in theory a precise tool for discerning mechanisms of action and possible toxicological effects of drug therapies. However, metabolomics is still a field in its infancy, with significant limitations and potential for misuse of technologies and overinterpretation of data. Here we seek to provide a critical evaluation of progress to date in application of metabolomics technologies for the understanding of diabetes and obesity mechanisms, for subclassification of different forms of diabetes to assist in tailoring of therapeutic strategies, and for more detailed evaluation of the safety and efficacy of drugs used to treat the disease.
Overview of current metabolomics technologies.
Genome-wide association studies and mRNA profiling by microarray analysis are relatively mature technologies that have developed to a point where core laboratories that provide these services are common in both the academic and private sectors. This is not yet the case for metabolomics. One reason for this is the complexity inherent in measuring large numbers of intermediary metabolites with diverse chemical properties in a quantitatively rigorous and reproducible fashion. Underlying issues include the wide-ranging concentrations of metabolites in tissues and bodily fluids (ranging from subnanomolar to millimolar), problems encountered in efficient extraction of metabolites from different biological matrices (e.g., tissues, blood, urine), and the chemical diversity of the analytes. Given these variables, it is perhaps not surprising that no single technology exists for measurement of all of the metabolites in the “metabolome.” Instead, groups that practice comprehensive metabolic profiling do so with quite diverse sets of instruments and methods for data analysis, the choice of which is influenced by factors such as cost, personal experience, and specific research goals. This places a large burden on the current core labs and their clients to ensure reproducibility of their findings in the absence of very little standardization of methods across groups. The U.S. National Institutes of Health has recognized the problem and recently convened a workshop on development of standardized methods for metabolomics, leading to a summary of recommendations (4).
The two major instrument platforms for measuring metabolite levels in biological samples are NMR and MS (5–13). Raman and infrared spectroscopy (14) and liquid chromatography coupled to ultraviolet or coulometric electrode array detection (15) have also been used by several labs. In general, research groups in the field tend to fall into two camps that adopt either “nontargeted/top-down” approaches or “targeted/bottom-up” methods as their core technologies, although a few groups, including our own, have both kinds of platforms in their repertoire.
Targeted methods.
For scientists with core interests in biological mechanisms rather than biomarkers, knowledge about the identity of metabolites being surveyed and their exact concentrations is essential. This encourages some metabolic profiling laboratories to emphasize targeted and quantitative MS-based analyses. These methods focus on quantification of discrete clusters of chemically related metabolites within a “module” using various combinations of chromatographic separations technologies and MS instruments that are most compatible with the analyte class.
Most targeted metabolic profiling methods use stable-isotope dilution for accurate quantification of analytes, involving addition of several stable isotope–labeled standards to the biological sample prior to the extraction and derivatization steps that may be necessary for the particular MS approach (Fig. 1A). Ideally, each unknown analyte will be paired with its labeled cognate (often an M+3 heavy isotope, e.g., methionine with methyl-D3 methionine, pyruvate with 13C3-pyruvate, etc.) to control for differences in analyte loss during sample processing and to compensate for ionization-suppression effects (example in Fig. 2). One limitation of this approach is the relatively narrow range of stable isotope–labeled standards that are available from commercial suppliers and their significant cost. Several groups are now engaged in expanding standard libraries through custom synthesis, but with the reagents available today, most labs are limited to measurement of ≤300 discrete metabolites by targeted methods.
FIG. 1.
Schematic summary of targeted and nontargeted metabolomics methods. A: When using targeted methods, quantification of specific analytes is facilitated by addition of stable isotope-labeled standards to the sample prior to the sample extraction and derivatization steps. This allows reporting of targeted analytes in true units of measure (e.g., μmol/l). B: In contrast, when performing nontargeted analysis, the goal is usually to obtain a global comparison of a large number of analytes across several classes. This is achieved by assaying replicate samples from contrasting conditions (e.g., drug-treated vs. control cells). Samples are processed and analyzed by MS to generate two independent datasets that are subjected to univariate and multivariate statistical analysis to identify features that are different between the two conditions, although in the absence of added standards, the concentration of the analytes is not reported in true units of measure. In addition, for many of the peaks, the chemical identity of the metabolite cannot be immediately discerned due to limitations in current spectral and chemical standard libraries. PCA, principal components analysis.
FIG. 2.
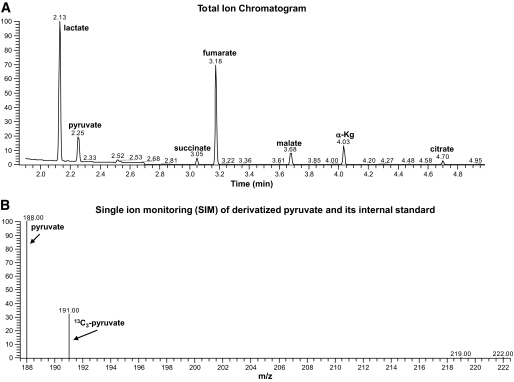
Targeted isotope-dilution analysis of organic acids by GC-MS. When performing targeted isotope-dilution analysis, a group of heavy isotope standards specific to the assay module are added immediately following sample homogenization and prior to chromatographic resolution and MS. A: Total ion chromatogram following GC of a rat liver homogenate in which multiple organic acids are resolved. B: Single ion monitoring for pyruvate and its corresponding internal standard. Each organic acid peak in the chromatogram (A) contains the target analyte and a heavy stable isotope as an internal standard, which are resolved and quantified by mass spectrometry (B) as shown for pyruvate, for example.
Although targeted metabolomics platforms tend to use conventional instruments such as electrospray ionization (ESI)-tandem MS-MS and gas chromatography (GC)-MS, significant challenges must still be overcome in building rigorous and fully vetted analysis modules for groups of metabolites. For example, when using GC-MS for measurement of fatty acids or organic acids, chromatography is performed in the gas phase at a high temperature, and analytes must be volatile and have sufficient thermal stability to survive the analysis. To help stabilize the analytes under study, reactive carboxyl, carbonyl, sulfhydryl, amine, or hydroxyl groups are derivatized by akylation, oximation, acylation, or silylation (5,11,16,17). These methods, while effective, add complexity beyond that already introduced by sample extraction to the analytical procedures and can result in batch-to-batch variation if not properly controlled. Similarly, analysis of acylcarnitines or amino acids/urea cycle intermediates by ESI-MS-MS requires module-specific derivatization, specifically treatment with acidic methanol for acylcarnitines and n-butanol for amino acids/urea cycle intermediates (17–20). Nevertheless, experience has proven that these methods can be deployed for reliable and quantitative analysis of metabolite modules, with coefficients of variation in replicate assays of major analytes of ∼15% or less.
Targeted and nontargeted MS-based metabolic analysis has been applied for years in the clinical diagnosis of metabolic disease and newborn screening, and today it is used to detect >40 different genetic diseases of lipid and amino acid metabolism (21,22). In these applications, less emphasis is placed on absolute quantification of multiple analytes in a module, since the screen only requires detection of differences in a single or discrete cluster of analytes with respect to established laboratory norms. For example, a defect in HMG-CoA lyase results in large and specific increases in 3-hydroxy-isovaleryl-carnitine and 3-methylglutaryl-carnitine species detected by MS-MS, whereas defects in long-chain 3-hydroxyacyl CoA dehydrogenase (LCHAD) or mitochondrial trifunctional protein (MTP) are associated with increases in 3-hydroxy-palmitoyl and 3-hydroxy stearoyl-carnitine metabolites (22).
In more recent years, many of the MS-based targeted metabolic profiling techniques developed for diagnosing inborn errors of metabolism have been adopted, refined, and supplemented for studies of mechanisms of disease pathogenesis. The approach taken in our laboratory has been to assemble multiple targeted MS-based assay modules that in aggregate report on several critical metabolic pathways (17–20). Using a combination of GC-MS and MS-MS, we are currently able to perform quantitative analysis of ∼180 metabolites in seven groups, as summarized in Table 1.
TABLE 1.
Analyte modules in the Stedman Center laboratory and methods of analysis
|
Although the total number of analytes measured with these tools is small relative to estimates of 6,500 total metabolites in the metabolome, they are nevertheless highly useful for understanding changes in metabolic function under different physiological and pathophysiological circumstances. Moreover, expansion of the platform to include a broader range of analytes of interest in disease pathogenesis is possible in the near term. A particular recent focus of the metabolomics community has been in the area of “lipidomics,” and methodologic advances are beginning to emerge for profiling of phospholipids, prostaglandins, and their metabolites (eicosanoids) and sphingolipids (9–13). “Shotgun lipidomics,” or the broad survey of neutral lipids such as triacylglyerols and diacylglyerols, including the profiling of the acyl side chains of these molecules, is another emergent technology. Early methods focused on separation of neutral lipid species by thin-layer chromatography followed by capillary GC and detection of lipid species with a flame-ionization detector (23). More recent studies use a four-step procedure that includes organic phase extraction (Bligh and Dyer method), intrasource separation of lipid species based on propensity for ionization, separation of ionized species by tandem-MS, and processing of data to assign molecular species and determine relative abundances (24). It should be emphasized, however, that development of new targeted modules is not a trivial or inexpensive undertaking, since it requires acquisition or synthesis of stable isotope–labeled internal standards, development of extraction procedures that are efficient for multiple analytes in a class, tailoring of protocols specific to the various biological matrices, and demonstration of quantitative reproducibility of the methods. The advantage gained is that such tools can be applied to the understanding of metabolic regulatory mechanisms in isolated cells, animal models of disease, and human disease states.
Nontargeted metabolite profiling.
Nontargeted profiling involves use of NMR, MS, or complementary technologies for measurement of as many metabolites as possible in a biological specimen simultaneously, regardless of the chemical class of the metabolites. In contrast to targeted profiling, in which added internal standards allow quantification of specific metabolites in molar units, nontargeted metabolomics generally adopts a strategy of comparison of two biological states and reporting of those analytes that qualitatively differ in the two states based on statistical analysis (Fig. 1B). When applied to nontargeted profiling, both NMR and MS have advantages and limitations, and neither technology can currently be used for surveying all of the metabolites in a sample in a quantitative fashion.
NMR spectroscopy is theoretically an excellent tool for nontargeted metabolic profiling of all small molecule metabolites, since the method detects spectral features emanating from any molecules that contain carbon or hydrogen (5,7). Moreover, analyses can be conducted directly in bodily fluids, cells, and even in intact tissues without the need for chemical extraction or derivatization of the analytes. These advantages are offset by significant technical challenges, including poor sensitivity, effects of pH and ionic strength, and the difficulty of deconvolution and normalization of spectra of complex metabolite mixtures in biological matrices like plasma, urine, or tissue extracts. Thus, although NMR spectra are information rich, lack of sensitivity and data complexity limit quantitative profiling to ≤100 metabolites in most biological samples by current methods. In some applications, NMR datasets are analyzed by statistical tools such as principal components analysis to identify spectral features (often not identified as specific metabolites) that characterize different biological or disease states (7,25).
MS has the immediate advantage of much higher sensitivity compared with NMR, and the most advanced MS platforms such as Fourier transform ion cyclotron (FT-ICR)-MS have the ability to detect metabolites in the femtomole range (11). Moreover, modern MS platforms such as those that incorporate time of flight (TOF), orbitrap, and FT-ICR mass analyzers offer very high mass resolution and mass accuracy. FT-ICR-MS, for example, is capable of achieving a mass resolution of >100,000 while providing mass accurate measurements of <1 ppm. By coupling such MS instrumentation with high-resolution chromatographic technologies (e.g., ultra high–pressure liquid chromatography [UHPLC] and sub-2 μm particle stationary phases), it has become possible to resolve literally thousands of individual small molecules. Seemingly, these platforms could circumvent problems encountered when using NMR methods for nontargeted metabolic profiling of complex biological matrices.
In practical reality, the variable lability, solubility, recovery, ionization, and detection of the different analyte species, coupled with the lack of comprehensive spectral and stable isotope–standard libraries, make these nontargeted MS methods semi-informed (with regard to analyte identities) and semiquantitative at best. Concerns about the semiquantitative nature of the methods can be overcome to some extent by focusing on tightly controlled biological conditions (e.g., cell lines in tissue culture studied in response to individual drug, nutrient, or gene manipulations) and performance of multiple replicate experiments. Under these conditions, statistical filtering can be applied to peak areas for well-resolved spectral features, thereby identifying analytes that change in a consistent fashion. However, ultimate proof of a significant change in an analyte still requires targeted analysis against an internal standard. The next challenge is identification of the chemical entities represented by the individual peaks. Help comes from high-accuracy mass measurements afforded by current high-end MS instruments. These mass measurements can be used to query databases such as METLIN, HMDB, KEGG, Madison Metabolomics Consortium Database (MMCD), and ChemSpider for chemical formulae whose theoretical masses match the experimentally determined mass, thereby usually providing a strong starting point for identifying the analyte. In most laboratories, current capabilities allow provisional or outright identification of ≤30% of analytes in complex spectra obtained through nontargeted MS methods.
Overall, it seems clear that further development of technologies for nontargeted metabolic profiling will be required before these tools can be maximally informative in the whole animal or human settings. In contrast, several examples of application of targeted profiling for gaining new insights into diabetes, obesity, and other chronic metabolic diseases have emerged recently, as will be discussed.
Use of targeted profiling and NMR-based metabolic flux analysis for studies of insulin secretion.
NMR- and MS-based tools have been used to investigate the process of glucose-stimulated insulin secretion (GSIS) in pancreatic islet β-cells (26–35). Stimulation of islet β-cells with glucose causes increases in insulin secretion within seconds to minutes, and this response is mediated by signals that are generated by β-cell glucose metabolism. A commonly accepted idea is that increases in the rate of glucose metabolism in β-cells leads to increases in ATP-to-ADP ratio, which causes inhibition of ATP-sensitive K+ channels (KATP channels), membrane depolarization, activation of voltage-gated calcium channels, and Ca2+-mediated activation of insulin granule exocytosis (36,37). However, this model clearly does not provide a complete description of signals that regulate GSIS, since pharmacologic or molecular inhibition of KATP channel activity still allows robust regulation of insulin secretion by glucose (38,39). This has led to recent investigation of alternate metabolic pathways and their byproducts in control of GSIS (rev. in 35).
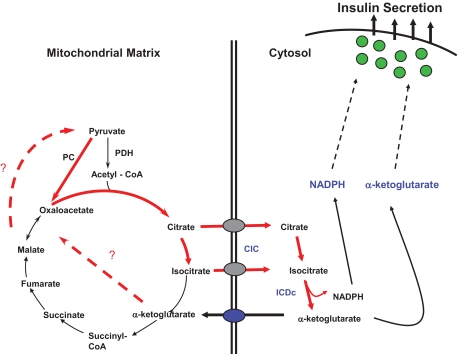
Application of 13C NMR-based isotopomer analysis and MS-based profiling of intermediary metabolites led to the discovery of a critical link between pyruvate carboxylase (PC)-mediated pyruvate exchange with tricarboxylic acid (TCA) cycle intermediates (“pyruvate cycling”) and GSIS and demonstration that these pathways are dysregulated in lipid-cultured and dysfunctional β-cells (26–35). More recent studies have focused on identification of the specific pyruvate cycling pathways that may be involved in generation of signals for insulin secretion. One important pathway appears to involve export of citrate and/or isocitrate from the mitochondria via the citrate/isocitrate carrier (CIC) and subsequent conversion of isocitrate to α-ketoglutarate (α-KG) by the cytosolic NAPD-dependent isoform of isocitrate dehydrogenase (ICDc) (30,31) (Fig. 3).
FIG. 3.
Schematic diagram of a pyruvate/isocitrate cycle implicated in control of GSIS. The cycle is initiated by anaplerotic conversion of pyruvate to oxaloacetate by PC. This leads to accumulation of the TCA cycle intermediates citrate and isocitrate and their export from the mitochondria to the cytosol by the CIC. Citrate is then converted to isocitrate by cytosolic aconitase, and isocitrate is converted to α-ketoglutarate by the cytosolic NADP-dependent ICDc. α-Ketoglutarate can then serve either as a direct signal for insulin secretion, for example, by serving as a substrate for α-ketoglutarate hydroxylases, or be recycled to pyruvate by one of several mitochondrial or cytosolic pathways that remain to be defined (dashed lines). Another byproduct of the pyruvate/isocitrate cycle with potential as an insulin secretagogue is cytosolic NAPDH, possibly acting through Kv channels or the glutathione/glutaredoxin system. This pathway was elucidated by integration of flux analysis by 13C NMR, targeted metabolic profiling by GC-MS and MS-MS, and evaluation of the effects of knockdown of key genes in the pathway, including CIC and ICDc.
In the foregoing studies, measurement of pyruvate cycling flux was accomplished by incubation of β-cells in low (3 mmol/l) or high (12 mmol/l) concentrations of U-13C glucose for several hours, followed by extraction of cells and analysis of glutamate spectra by NMR. Specific resonances for each of the carbons of glutamate are affected by the population of mass isotopomers (glutamate with varying mixtures of 13C and 12C at each of the five carbons of the molecule), and this information can be used to calculate flux through the oxidative (PDH) and anaplerotic (PC) entry points of the TCA cycle (26,27,40). These methods reveal that the capacity for GSIS in variously glucose-responsive INS-1–derived cell lines is tightly correlated with PC-catalyzed pyruvate cycling activity but not PDH-catalyzed glucose oxidation (26–28).
Metabolic flux analysis by NMR can be integrated with static metabolite profiling by MS to gain a more complete picture of fuel-sensing pathways in the β-cell. For example, GC-MS was used to demonstrate a fall in cytosolic citrate levels in response to siRNA-mediated suppression of CIC, as would be expected if a major pathway for export of citrate from the mitochondria to the cytosol is blocked (31). Other recent experiments show that glucose stimulation of β-cells results in increases in several TCA cycle intermediates in whole-cell extracts (30,41), but a selective release of only the early TCA cycle intermediates citrate and α-KG into the medium, with no change in medium levels of the later TCA cycle intermediates malate, fumarate, or succinate (M. Jensen, H.E. Hohmeier, O.I., and C.B.N., unpublished observations). These results serve to confirm that glucose causes a significant release of citrate from the mitochondria and is consistent with the conversion of this pool to α-KG via ICDc. These examples illustrate two important applications of MS-based metabolic profiling in cellular research: 1) use in validation of the expected metabolic impact of a specific genetic engineering or pharmacologic manipulation of cellular systems and 2) integration with flux analysis to provide a complete picture of changes in metabolic pathways under varying experimental conditions. Overall, these studies show that metabolic byproducts of pyruvate/isocitrate cycling may be an important amplifying signal for control of GSIS. Possible mediators now under investigation include NADPH (35,42), α-KG or its metabolites (43,44), or GTP generated by the succinyl CoA dehydrogenase reaction (45), all of which are direct or downstream products of the ICDc reaction.
Targeted MS-based metabolic profiling applied to mechanisms of insulin resistance.
Lipid infusion or the ingestion of a high-fat diet results in insulin resistance and eventual development of diabetes. A prevailing model for development of diet-induced insulin resistance holds that mitochondrial fatty acid oxidation is inadequate to deal with the large load of dietary fat, thus leading to accumulation of lipid-derived metabolites such as diacylglycerols (DAGs) and ceramides that can activate stress kinases to interfere with insulin action (46,47) (Fig. 4A). The evidence in support of such a mechanism in induction of hepatic insulin resistance is considerable and may translate to humans. For example, a short period of caloric restriction in obese humans improves hepatic insulin sensitivity in concert with a reduction in liver fat (48). A possible link between fatty liver and metabolic changes in peripheral tissues has been uncovered by liquid chromatography-MS analysis of lipids in nondiabetic women with and without increased liver fat; women with increased liver fat had elevated levels of esterifed long-chain fatty acids and ceramides in adipose tissue (49).
FIG. 4.
Mechanistic models of lipid-induced impairment of muscle insulin action and supporting metabolomics data. Feeding of diets high in fat results in muscle insulin resistance, and recent studies suggest the operation of two possible mechanisms for this effect (A). A prevailing theory is that increased delivery of fat to muscle saturates the capacity for mitochondrial β-oxidation, leading to accumulation of bioactive lipid-derived metabolites such as diacylglycerols and ceramides in the extramitochondrial space and activation of stress/serine kinases that interfere with insulin action. More recent studies have shown that fatty acid oxidation is actually increased in muscle in response to high-fat feeding but with no coordinate increase in TCA cycle activity. This results in accumulation of incompletely oxidized lipids in the mitochondria and depletion of TCA cycle intermediates, possibly resulting in mitochondrial stress and interference with insulin actions. The metabolic changes that underpin this new mechanism were identified by targeted GC-MS of organic acids and MS-MS analysis of acylcarnitines in muscle extracts from lean and obese animals, as summarized in B (data reprinted from ref. 51 with permission). Note that these mechanisms are not mutually exclusive and could work in concert to impair muscle insulin action. CPT1, carnitine palmitoyltransferase 1; ETS, electron transport system; NEFA, nonesterified fatty acid; TCAI, TCA cycle intermediates.
Also supporting an important role of hepatic steatosis in development of insulin-resistant states, whole-animal, muscle, and liver insulin resistance in rats fed a high-fat diet are all ameliorated in response to hepatic overexpression of malonyl CoA decarboxylase (MCD), a gene that reduces hepatic steatosis by partitioning lipids toward β-oxidation (18). Interestingly, the improvement in muscle insulin resistance was not correlated with changes in muscle triglyceride or fatty acyl CoA levels. Instead, metabolic profiling of 37 acylcarnitine species by MS-MS revealed a decrease in the concentration of lipid-derived metabolite β-OH-butyrylcarnitine (βHB) in muscle of MCD-overexpressing animals that was likely due to a change in intramuscular ketone metabolism.
The association of improved insulin action with a decline in a mitochondrial lipid-derived metabolite (βHB) encouraged further investigation of the mechanism of lipid-induced muscle insulin resistance with targeted MS-based metabolic profiling tools (2,50,51). These studies found that chronic exposure of muscle to elevated lipids in vitro, or in vivo as a consequence of overnutrition, resulted in an increase rather than a decrease in expression of genes of fatty acid β-oxidation (50,51). Importantly, this lipid-induced upregulation of the enzymatic machinery for β-oxidation of fatty acids in muscle was not coordinated with upregulation of downstream metabolic pathways such as the TCA cycle and electron transport chain. This resulted in incomplete metabolism of fatty acids in the β-oxidation pathway, as reflected by broad-scale accumulation of mitochondrial lipid metabolites (acylcarnitines) and a simultaneous decrease in the levels of TCA cycle intermediates, as revealed by quantitative MS-MS and GC-MS analysis (50,51) (Fig. 4B).
That these abnormalities may contribute to mitochondrial stress and development of insulin resistance is supported by the finding that exercising of obese mice normalizes the elevated acylcarnitines in muscle and restores insulin sensitivity and glucose tolerance (50). Also supporting the model, mice with global MCD knockout fed a high-fat diet have suppressed fatty acid oxidation and reduced acylcarnitine levels, coupled with improvement of glucose tolerance and insulin resistance (51). Similar improvements in insulin sensitivity and glucose uptake have been reported in human myocytes in response to siRNA-mediated suppression of MCD (52). Also consistent with these findings, transgenic mice with muscle-specific overexpression of peroxisome proliferator–activated receptor (PPAR)-α, a nuclear receptor that activates β-oxidative genes, developed both local and systemic glucose intolerance (53). In contrast, one recent study reported improved rather than impaired insulin sensitivity in response to overexpression of CPT1 (carnitine palmitoyltransferase 1) in muscle (54). Nevertheless, the weight of recent evidence is consistent with a model in which lipid-induced insulin resistance in muscle is explained at least in part by “overload” of mitochondrial lipid oxidation, accumulation of incompletely oxidized fats, and depletion of TCA intermediates, leading to a condition of mitochondrial stress that activates signaling pathways (still to be defined) that interfere with insulin action. These findings do not preclude an important role for accumulation of DAGs, ceramides, or other lipid-derived metabolites in muscle of animals with diet-induced obesity, and the two mechanisms could in fact work together to cause harmful effects.
Other metabolic profiling studies have led to the identification of a specific lipid metabolite that might serve to enhance insulin action (55). Thus, liquid and GC-based methods were used for quantitative profiling of ∼400 lipid species in mice lacking expression of fatty acid binding proteins (FABPs) in adipose tissue (aP2-mal1−/−). FABP-deficient mice fed a high-fat diet fail to accumulate lipids in adipose tissue and remain insulin sensitive. Adipose tissue from these animals was enriched in C16:1n7-palmitoleate, both as the free fatty acid and in multiple esterified species. Infusion of triglycerides containing exclusively palmitoleate (C16:1) or palmitate (C16:0) into mice for 6 h resulted in suppression of the entire insulin-signaling pathway in the case of triglyceride-palmitate, versus a clear enhancement in insulin action in the case of triglyceride-palmitoleate. Although interesting, this experiment would have been more convincing if it had included an additional control of another monounsaturated fatty acid such as oleate (C18:1). Also, the role of palmitoleate in control of insulin action in physiological or pathophysiological states remains to be defined, given that palmitoleate levels rise in concert with levels of other fatty acids in obesity (17) and fasting, even though these are states of clear insulin resistance.
Another study used LC-MS-MS to profile lipids secreted from the small intestine in response to ingestion of fat (56). This approach showed an increase in N-acylphosphatidylethanolamines (NAPEs) in the circulation. Systemic infusion of the most abundant NAPE decreased food intake in rats, an effect that could not be ascribed to taste aversion. The authors also demonstrated that systemically administered NAPE enters the brain and accumulates in the hypothalamus. Chronic administration of NAPEs reduces food intake and decreases body weight, suggesting a possible medicinal application in treatment of obesity.
The studies summarized above are examples of use of targeted metabolic profiling technologies for development of new and testable models of disease pathogenesis and novel therapeutic strategies. We chose to highlight these examples because, in each case, the investigators were not satisfied with simple reporting of a metabolic signature of nutritional manipulation (information) and moved beyond this point to explore the significance of their findings in regulation of metabolic fuel homeostasis. Unfortunately, taking such extra steps to investigate mechanism has been the exception rather than the rule in application of metabolomics to diabetes research to this point, and this must change if the full potential of the technology is to be realized.
Nuclear receptors, including the family of PPAR-α, -δ, and -γ, have emerged as important mediators of insulin sensitivity. 1H-NMR–, LC-MS–, and GC-MS–based methods were used to compare PPAR-α null and wild-type mice. The authors reported decreases in glucose, glutamine, and alanine levels and an increase in lactate, suggesting an increase in utilization of glucose and amino acids, as might be predicted from the known effects of PPAR-α to promote the opposing pathways of β-oxidation, ketogenesis, and gluconeogenesis (57). More recently, targeted GC-MS and LC-MS-MS analysis has provided deeper insights by showing that PPAR-α knockout results in fasting hypoglycemia accompanied by depletion of TCA cycle intermediates and free carnitine and short-chain acylcarnitines, as well as accumulation of long-chain acyl CoAs in skeletal muscle (58). Several of these metabolic abnormalities could be partially ameliorated by carnitine supplementation. Another study surveyed changes in lipid metabolites in obese and diabetic mice in response to treatment with rosiglitazone. These experiments revealed that rosiglitazone induced circulating hypolipidemia, caused substantial alterations in multiple lipid species in the heart, and caused accumulation of polyunsaturated lipid species in adipose tissue (23). These findings are of particular interest given the propensity of thiazolidinedione drugs to cause weight gain and accumulation of adipose tissue and the recent report of a link between these drugs and increased risk of cardiovascular disease (59), although the exact relationship between the changes in lipid metabolism and these drug side effects, if any, remain to be defined. Finally, a very recent study has investigated the effects of rosiglitazone in normal and diabetic mice with targeted quantitative measurement of a remarkable 800 metabolites by tandem MS. The authors report that methylglutarylcarnitine levels are oppositely affected in healthy and diabetic mice by rosiglitazone and that an enrichment in phosphatidylcholine relative to lysophosphatidylcholine levels occurs in diabetic versus normal animals (60). However, no mechanistic follow-up studies were attempted, and the functional significance of either of these changes therefore remains to be elucidated.
Integration of metabolomics with other “omics” technologies for studies of metabolic disease mechanisms.
Recent studies in plants (61), animal models of disease (20), and human families with early-onset cardiovascular disease (62) have demonstrated that metabolite profiles are heritable and can be integrated with whole-genome association and microarray profiling datasets to define gene/metabolite networks. In one such study, diabetes-resistant C57BL/6-ob/ob mice were bred with diabetes-susceptible BTBR-ob/ob mice to create an F2 cohort in which blood glucose and insulin levels were distributed across a wide range. Liver samples from F2 mice were subjected to targeted metabolomics and microarray analyses, and integration of these datasets with whole-genome single nucleotide polymorphism (SNP) analysis showed clusters of liver metabolites (e.g., a group of amino acids) mapped to distinct chromosomal regions, suggesting the presence of genes at those loci that exert metabolic control on a whole class of metabolites (20) (Fig. 5). Using refined statistical techniques (63), correlations between genetic loci, transcripts, and metabolites were used to develop a model that predicts that the amino acid glutamine acts through alanine:glyoxylate aminotransferase (Agxt) and arginase 1 (Arg1) to affect phosphoenolpyruvate carboxykinase (Pck1) expression (Fig. 6). Consistent with this predicted network, glutamine addition to primary hepatocytes causes strong upregulation of Agxt, Arg1, and PEPCK (20). Moreover, glutamine and PEPCK mRNA levels are reduced with obesity in diabetes-resistant B6-ob/ob mice but increased in liver of diabetes-susceptible BTBR-ob/ob animals (C. Ferrara, C.B.N., A.D. Attie, unpublished observations).
FIG. 5.
Metabolite levels are heritable and can be mapped to specific chromosomes in mice. In this particular study, diabetes-resistant B6-ob/ob mice were bred with diabetes-susceptible BTBR-ob/ob mice to create a cohort of F2 mice in which individual mice had a wide variation in blood glucose levels. Whole-genome SNP analysis was integrated with microarray and targeted GC-MS– and MS-MS–based analysis of metabolites of liver extracts from the F2 animals. This analysis revealed that metabolites can be mapped to specific chromosomes. Each row represents a SNP marker, and each column represents a metabolite. The LOD color scale is indicated, showing blue when the B6 allele at that marker results in an elevated level of the metabolite and red/yellow when the BTBR allele is dominant. Note the clusters of amino acids that map in common to regions on chromosomes 8 and 9, suggesting the presence of a gene that controls their levels. Figure reproduced with permission from ref. 20. LOD, logarithm of odds.
FIG. 6.
A novel metabolic regulatory network predicted by integration of genomic, transcriptomic, and metabolomic profiling. Whole-genome mapping was integrated with transcriptomic and metabolomic analysis of liver samples in F2 mice from a cross of diabetes-resistant B6-ob/ob mice and diabetes-susceptible BTBR-ob/ob mice. Networks of transcripts (grey ovals) and metabolites (rectangle) were identified by computational analysis (63). The network shown here predicts that the amino acid glutamate/glutamine (Glx) regulates expression of the key gluconeogenic enzyme phosphoenolpyruvate carboxykinase (Pck1) via regulation of alanine/glyoxylate aminotransferase (Agxt) and arginase1 (Arg1). Consistent with this prediction, glutamine addition to mouse hepatocytes strongly induces expression of Agxt, Arg1, and Pck1 (20). Figure reproduced with permission from ref. 19.
In another study with some similarities in design, F2 rats from a cross of diabetic Goto-Kakizaki (GK) and normoglycemic Brown Norway (BN) rats were surveyed by nontargeted NMR-based metabolic profiling, and this information was integrated with information about physiologic quantitative trait loci (QTL) in the same cross (64). As with the MS-based study described above, the authors were able to map spectral features to specific chromosomal regions. Candidate metabolites for some of the most significant QTLs were identified, including glucose and benzoate. Subsequent transcriptomic analysis of the parent strains revealed the absence of transcripts for uridine diphosphate (UDP)-glucuronosyl-transerase-2b (Ugt2b), an enzyme that metabolizes benzoate and other xenobiotics in mammals. The absence of Ugt2b expression was subsequently found to be the result of a chromosomal deletion in the GK strain, demonstrating the ability of metabolomics to uncover otherwise undetected chromosomal abnormalities.
Targeted metabolomics has also recently been integrated with genotyping for understanding the biochemical impact of common genetic polymorphisms (65). These authors performed ESI-MS-MS to measure 363 metabolites in serum of 284 male participants in the KORA study (a general population study from Germany). Significant associations were observed between frequent SNPs and changes in specific metabolites. Moreover, polymorphisms in four specific genes (FADS1, LIPC, SCAD, MCAD) encoding metabolic enzymes were linked to perturbations in the metabolic pathways in which the enzymes are known to reside. The authors suggest that the combination of genotyping and metabolic phenotyping may provide new roadmaps for application of personalized medicine.
These studies represent very early efforts to integrate metabolomics with other forms of “omics” sciences but nevertheless serve to illustrate the potential power of integrated approaches for identification of novel metabolic regulatory networks that contribute to chronic disease such as type 2 diabetes. As targeted metabolic profiling methods evolve to encompass a larger set of metabolites, computational methods for integrating this information with other broad-scale profiling datasets must continue to evolve.
Targeted MS-based metabolic profiling for understanding of human metabolic disease pathogenesis
Human diabetes and insulin resistance.
Targeted MS-based metabolic profiling has been increasingly applied to studies of human diseases and conditions (17,19,62,66–73). For example, profiling of obese (median BMI 37 kg/m2) versus lean (median BMI 23 kg/m2) humans revealed a branched-chain amino acid (BCAA)-related metabolite signature that differentiates the two groups, is suggestive of increased catabolism of BCAA, and correlates with insulin resistance (17) (Fig. 7). The signature includes several metabolites that are byproducts of BCAA catabolism, such as glutamate, α-ketoglutarate, C3 acylcarnitine (propionylcarnitine), and C5 acylcarnitines (α-methylbutyryl and isovalerylcarnitines). A subsequent cross-sectional study in sedentary hyperlipidemic subjects of varying BMI (range 25–35 kg/m2) identified several metabolite clusters (principal components) that explained most of the data variance and found that the principal component most related to insulin sensitivity (Si) in subjects tested by the frequently sampled oral glucose tolerance test was again one comprised of BCAA and related metabolites (67). To test the possible relevance of this finding for development of obesity-related insulin resistance, rats were fed one of several diets—high fat (HF), HF with supplemented BCAA (HF/BCAA), or standard diet. Despite having reduced food intake and weight gain equivalent to the standard diet group, HF/BCAA rats were equally as insulin resistant as HF rats. Insulin resistance induced by HF/BCAA feeding was accompanied by chronic activation of mTOR and JNK and serine phosphorylation of IRS1(ser307). Moreover, HF/BCAA-induced insulin resistance was reversed by the mTOR inhibitor rapamycin. These findings show that in the context of a poor dietary pattern of increased consumption of fat, BCAAs make an independent contribution to development of obesity-associated insulin resistance.
FIG. 7.
A branched-chain amino acid–related metabolite cluster that correlates with insulin resistance in humans. Targeted metabolic profiling by MS-MS and GC-MS was performed on obese, insulin-resistant, but nondiabetic humans and a group of lean controls. Principal components analysis (PCA) revealed that the cluster of statistically related metabolites (principal component) with the strongest differences between obese and lean subjects was one comprised of the branched-chain amino acids leucine/isoleucine and valine, glutamate/glutamine (glx), C3 and C5 acylcarnitines, and the aromatic amino acids phenylalanine and tyrosine. A plot of each individual's principal component score against their homeostasis model assesment index score (a measure of insulin sensitivity) is shown, indicating significant correlation (r = 0.58; P < 0.0001). Data reprinted with permission from ref. 17.
Targeted profiling has also been applied during an oral glucose tolerance test in human subjects (68,69). In two small independent groups of normal non–insulin-resistant individuals (n = 22 and 25), glucose ingestion caused significant changes in 18 metabolites (other than glucose) among 191 measured, including some that are involved in pathways not known to be affected by a glucose load (bile acids and purine degradation products) (68). The metabolites that changed significantly were also reflective of the known effects of insulin on proteolysis, lipolysis, ketogenesis, and glycolysis. A third group of mildly glucose intolerant and hyperinsulinemic subjects were also studied and found to have blunted glucose-induced changes in the metabolic markers of key anabolic pathways relative to those reported for the normal subjects. Moreover, 6 of the 18 metabolites identified as “glucose responsive” in the studies of normal subjects were found to correlate significantly with fasting insulin levels, used as a surrogate of insulin sensitivity. Among these were all three branched-chain amino acids, lactate, and β-hydroxybutyrate. Another group applied nontargeted UPLC-qTOF-MS analysis during oral glucose tolerance testing of 16 normal individuals and found by multivariate statistical analysis that free fatty acids, acylcarnitines, bile acids, and lysophosphatidylcholines were the most discriminating biomarkers of the glucose bolus (69). These studies demonstrate how metabolomics can provide a more detailed picture of metabolic status of normal and pre-diabetic subjects, which with further development could contribute to more exact subclassification of different forms of diabetes, leading to more judicious and effective use of drug therapies. However, in order for this to be fully realized, more effort must be focused on the testing of mechanistic hypotheses that emanate from initial associations of metabolic signatures and disease states.
Human cardiovascular disease.
MS-based metabolic profiling has been applied to cardiovascular disease. In one study, LC-MS analysis was applied to subjects with exercise-inducible ischemia compared with normal control subjects (70). Blood samples were taken immediately before and immediately after exercise, and levels of 173 known and several more minor intermediary metabolites were measured. A number of metabolites were found to be discordant between the two groups, including lactate, byproducts of AMP metabolism, and metabolites of the citric acid cycle. Using the six most discordantly regulated metabolites, a metabolic ischemia risk score was derived. As the number of subjects was quite small in this study, follow-up studies will be required to confirm or refute these interesting initial findings.
MS–MS– and GC-MS–based metabolomics have also been applied to subjects from multiple generations within eight multiplex families with familial early-onset cardiovascular disease (CVD) (62). Even after adjusting for variables such as diabetes, hypertension, dyslipidemia, BMI, age, and sex, multiple individual metabolites and metabolite clusters identified by principal components analysis were found to be highly heritable within families, including groups of amino acids (arginine, glutamate, alanine, ornithine, valine, leucine/isoleucine), free fatty acids (arachidonic, linoleic), and acylcarnitines. Interestingly, families in this study showed two distinct metabolite profiles that tracked with their clinical characteristics, suggesting different genetic backgrounds and consequent variation in control of key metabolic pathways that converge on CVD. Current studies are focused on applying the same metabolic profiling tools to nonfamilial populations of patients at risk for cardiovascular events. Based on our understanding thus far, targeted metabolomic profiles show promise for predicting CVD and subsequent events in high-risk families and even in the general population.
Other studies have recently emerged in the realm of application of metabolomics for the understanding of metabolic lesions in heart failure and myocardial infarction. In one study, LC-MS–based profiling of ∼200 metabolites was performed on subjects undergoing planned myocardial infarction via alcohol-mediated septal ablation or on subjects with spontaneous myocardial infarction or undergoing elective coronary angiography, as positive and negative control subjects, respectively (71). Five metabolites were altered in both spontaneous and planned myocardial infarction, and this metabolic signature may become useful for early detection of myocardial injury with further validation. Similarly, GC-MS was used for targeted analysis of serum samples of 52 patients with systolic heart failure (ejection fraction of <40% and symptoms of failure) and 57 control subjects, resulting in identification of pseudouridine and 2-oxoglutarate (α-ketoglutarate) as two potential biomarkers of the failing heart (72). One limitation of this study was the extensive differences between groups with regard to use of medications, including β-blockers, ACE inhibitors, and diuretics, all of which could have influenced the metabolic profiles. Finally, targeted ESI-MS was used to measure 63 metabolites in arterial and coronary sinus blood obtained during cardiac surgery, before and after ischemia/reperfusion (73). This work demonstrates that the preexisting ventricular state (left ventricular dysfunction [LVD], coronary artery disease, or neither condition) is associated with clear differences in myocardial fuel uptake, both at baseline and following ischemia/reperfusion. In particular, LVD was associated with global suppression of metabolic fuel intake (glucose, fatty acid, and amino acids) and limited myocardial metabolic reserve and flexibility following ischemia/reperfusion. Moreover, altered metabolic profiles following ischemia/reperfusion were associated with a postoperative hemodynamic course. The growing number of metabolomics studies in the area of heart failure may ultimately facilitate optimal design of perioperative treatment regimens based on the particular form of CVD and the metabolic status of the heart.
Nontargeted metabolic profiling applied to metabolic disease research.
Given its inherently nonquantitative nature and lack of ability to identify the majority of analytes in a given profile, is nontargeted metabolic profiling with MS platforms suitable for studies of disease mechanisms and etiology? Our perspective is that these tools must be used with caution in whole-animal or human studies and are currently best suited to in vitro applications where the biology can be more tightly controlled. Support for this position comes from a recent and careful study of application of nontargeted UPLC-MS to human serum samples, in which it was concluded that significant analytical drift can be introduced when using new chromatographic columns or when attempting to analyze more than 100 samples in a block (74).
An interesting recent example from the cancer research field further illustrates both the limitations and the promise of nontargeted approaches (75). LC and GC coupled with MS was used to perform nontargeted profiling on >1,100 individual metabolites in prostate tumor explants, blood, and urine from biopsy-positive cancer patients and biopsy-negative control subjects. Analytes were not measured in physical units (nmol or μmol) but rather in relative units in the cancer patients versus the control subjects. No meaningful differences were found in metabolite profiles in urine or blood of cancer subjects compared with control subjects. In contrast, statistically meaningful increments were found in a small subset of metabolites in tumor explants, particularly in metastatic tumors relative to benign prostate. Six metabolites were found to increase with progression from benign prostate to localized cancer to metastatic cancer, including sarcosine, a glycine metabolite. Importantly, the authors then developed a targeted stable isotope–dilution method for quantitative measurement of sarcosine and found it to be elevated by 10- to 20-fold in metastatic tumors compared with benign prostrate. They also showed that manipulation of enzymes of sarcosine metabolism influenced prostate cancer invasion. These results show that nontargeted MS methods are able to detect changes in metabolites within the tissue of origin of the metabolic variability. However, the changes in sarcosine may only have been by the semiquantitative nontargeted approach because the changes were very large in magnitude. Nevertheless, this study is one of the rare but welcome examples of translation of a metabolic profile associated with disease to actual mechanistic investigation.
There has been limited application of nontargeted MS-based metabolomics to diabetes research to date. In one study comparing pre-diabetic insulin-resistant to healthy and insulin-sensitive individuals, a complex set of technologies including LC-MS and Fourier-transform ion cyclotron resonance (FTICR)-MS coupled with multivariate statistical analysis was used to identify a single metabolite, 3-hydoxyhippuric acid, as a biomarker of the insulin-resistant state (76). In another study, GC-MS coupled with multivariate statistical analysis was used to evaluate the metabolic impact of three diabetes drugs, rosiglitazone, metformin, and repaglinide, in newly diagnosed type 2 diabetic subjects (77). Abnormalities in several amino acids and fatty acids were reported in diabetic compared with healthy subjects, and rosiglitazone was shown to correct more of these abnormalities than the other two drugs. The significance of these profiles in terms of molecular mechanisms or disease progression remains to be addressed.
Similar issues and other problems have emerged when using nontargeted 1H NMR for human studies. A recent study reported that NMR-based metabolic profiles can predict the presence and severity of coronary artery disease (78). Partial least-squares discriminant analysis was used to identify peaks in the major lipid regions of the spectra that appeared to provide separation between the groups. The specific lipid species involved were not identified by this analysis, although it was suggested that choline-containing metabolites were particularly diagnostic. However, a subsequent study using very similar techniques demonstrated that the predictive value of the NMR-based metabolic profiles was weak when other factors such as sex and use of medical interventions such as statins were taken into account (79). This second group of authors demonstrated that the 1H NMR technique could identify male versus female subjects with 100% accuracy but was much less able to identify statin users or subjects with CVD, despite expectations of substantial changes in lipid profile in the former group. Based on these findings, it seems clear that 1H NMR is currently not a substitute for the more invasive procedure of angiography in the diagnosis and staging of CVD.
An intriguing and more promising recent application of 1H NMR–based metabolic analysis has been to study the influence of intestinal bacteria (microbiota) on development of obesity and metabolic diseases (80). Indeed, inoculation of germ-free mice with microbiota from the cecum of normal mice causes an increase in body fat content and appearance of insulin resistance within 14 days of transfer (81). 1H NMR–based metabolic profiling of plasma and urine samples from a mouse strain known to be susceptible to hepatic steatosis and insulin resistance (129S6) versus a strain with relative resistance (BALBc) revealed low circulating levels of plasma phosphatidylcholine and high levels of methylamines in urine in the 129S6 strain (80). The authors propose that the increased propensity of the 129S6 strain for metabolic disease could be due to increased metabolism of phosphatidylcholine to methylamines by intestinal bacteria, resulting in a reduced pool for the assembly of VLDL particles, leading to deposition of triglycerides in liver. This hypothesis remains to be tested.
1H NMR has also been applied to research on type 1 diabetes (82,83). One study involved 613 patients with type 1 diabetes and used a novel set of statistical methods to identify a set of metabolites that stratified subgroups in the population according to micro- and macrovascular complications and mortality (83). Another study used LC-MS for lipid profiling, and two-dimensional GC-MS for profiling of organic acids, amino acids, and other small molecule metabolites to implicate gut microbiota in development of type 1 diabetes (84). These findings were made in a prospective study of Finnish children who progressed to type 1 diabetes versus control subjects who remained nondiabetic and autoantibody negative. Children progressing to diabetes had reduced serum levels of phosphatidylcholine and succinic acid at birth, possibly suggestive of increased metabolism of choline by intestinal microbes in the mother or the child. Type 1 diabetic children also had very high levels of glutamate and branched-chain amino acids in blood appearing prior to emergence of autoantibodies, for example, against GAD and insulin. The source of these very interesting surges in amino acid levels and their potential mechanistic significance remain to be established.
Conclusions and future directions.
In the postgenomic era, biologists and translational investigators alike have gained a new appreciation for metabolic analysis as a critical tool for assessing the physiological and pathophysiological impact of genetic variation. The current surge in methods development in the field of metabolomics is built on the foundation of decades of analytical biochemistry and its use in detecting inborn errors of metabolism. The major difference between then and now is that the current emphasis is on methods that allow simultaneous measurement of multiple analytes in a biological sample, whereas earlier work was often focused on one or a small number of metabolites per assay. Despite significant advances, no single profiling method currently allows simultaneous analysis of all of the metabolites in the metabolome. Ultimate achievement of this goal will require continued intensive development of deeper libraries of chemical standards, instrument platforms with broad sensitivity range and high mass accuracy, and likely integration of MS and NMR methods to gain full analyte coverage. These advances must be coupled with continued development of computational methods for analysis of complex metabolomic datasets and their integration with equally complex genomic, transcriptomic, and proteomic profiles. Meanwhile, considerable progress can be made with the currently available “targeted” technologies that allow profiling of key intermediates of lipid, carbohydrate, purine, pyrimidine, and protein metabolism. The examples provided herein about scientific insights gained by application of current tools suggest a broad horizon and provide strong encouragement for further technology development in this area. However, it may be apparent to the reader that, to date, only a subset of the studies cited in this article have gone beyond the description of metabolic “signatures” that characterize different physiological, pathophysiological, or drug-treated states (information) to actual use of the signatures to pose and then test new hypotheses (knowledge). The paramount challenge of the next phase of metabolomics investigation is to better harvest the information from large datasets to create knowledge about metabolic regulatory mechanisms, perhaps leading to better understanding of perturbations in chronic diseases and conditions such as type 2 diabetes, obesity, CVD, and cancer.
Acknowledgments
Work from the authors' laboratories cited in this article was supported by National Institutes of Health Grants PO1DK58398 and R37DK46492 to C.B.N. and P30-AG028716 to D.M.M.
No potential conflicts of interest relevant to this article were reported.
We are thankful to numerous colleagues and collaborators who have worked with the Stedman Center Metabolomics core laboratory on projects cited in this article, including senior colleagues Dr. Laura Svetkey, Dr. Alan Attie, Dr. David Millington, Dr. William Kraus, and Dr. Mark Butler.
REFERENCES
- 1.Ridderstråle M, Groop L: Genetic dissection of type 2 diabetes. Mol Cell Endocrinol 2009;297:10–17 [DOI] [PubMed] [Google Scholar]
- 2.Muoio DM, Newgard CB: Molecular and metabolic mechanisms of insulin resistance and β-cell failure in type 2 diabetes. Nat Rev Mol Cell Biol 2008;9:193–205 [DOI] [PubMed] [Google Scholar]
- 3.Human Metabolome Project: Human Metabolome Database-Canada [website]. Available at http://www.hmdb.ca/ Accessed 19 September 2009
- 4.Castle AL, Fiehn O, Kaddurah-Daouk R, Lindon JC: Metabolomics Standards Workshop and the development of international standards for reporting metabolomics experimental results. Brief Bioinform 2006;7:159–165 [DOI] [PubMed] [Google Scholar]
- 5.German JB, Hammock BD, Watkins SM: Metabolomics: building on a century of biochemistry to guide human health. Metabolomics 2005;1:3–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hollywood K, Brison DR, Goodacre R: Metabolomics: current technologies and future trends. Proteomics 2006;6:4716–4723 [DOI] [PubMed] [Google Scholar]
- 7.Lindon JC, Holmes E, Nicholson JK: Metabonomics in pharmaceutical R&D. FEBS J 2007;274:1140–1151 [DOI] [PubMed] [Google Scholar]
- 8.Han X, Gross RW: Global analyses of cellular lipidomes directly from crude extracts of biological samples by ESI mass spectrometry: a bridge to lipidomics. J Lipid Res 2003;44:1071–1079 [DOI] [PubMed] [Google Scholar]
- 9.Watson AD: Thematic review series: systems biology approaches to metabolic and cardiovascular disorders: lipidomics: a global approach to lipid analysis in biological systems. J Lipid Res 2006;47:2101–2111 [DOI] [PubMed] [Google Scholar]
- 10.Han X, Gross RW: Shotgun lipidomics: electrospray ionization mass spectrometric analysis and quantitation of cellular lipodomes directly from crude extracts of biological samples. Mass Spectrom Rev 2005;24:367–412 [DOI] [PubMed] [Google Scholar]
- 11.Dettmer K, Aronov PA, Hammock BD: Mass spectrometry-based metabolomics. Mass Spectrom Rev 2007;26:51–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu C, van der Heijden R, Wang M, van der Greef J, Hankemeier T, Xu G: Analytical strategies in lipidomics and applications in disease biomarker discovery. J Chromatogr B Analyt Technol Biomed Life Sci 2009;877:2836–2846 [DOI] [PubMed] [Google Scholar]
- 13.Oresic M, Hänninen VA, Vidal-Puig A: Lipidomics: a new window to biomedical frontiers. Trends Biotechnol 2008;26:647–652 [DOI] [PubMed] [Google Scholar]
- 14.Ellis DI, Goodacre R: Metabolic fingerprinting in disease diagnosis: biomedical applications of infrared and Raman spectroscopy. Analyst 2006;131:875–885 [DOI] [PubMed] [Google Scholar]
- 15.Shurubor YI, Matson WR, Willett WC, Hankinson SE, Kristal BS: Biological variability dominates and influences analytical variance in HPLC-ECD studies of the human plasma metabolome. BMC Clin Pathol 2007;7:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patterson BW, Zhao G, Elias N, Hachey DL, Klein S: Validation of a new procedure to determine plasma fatty acid concentration and isotopic enrichment. J Lipid Res 1999;40:2118–2124 [PubMed] [Google Scholar]
- 17.Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, Lien LF, Haqq AM, Shah SH, Arlotto M, Slentz CA, Rochon J, Gallup D, Ilkayeva O, Wenner BR, Yancy WS, Jr, Eisenson H, Musante G, Surwit RS, Millington DS, Butler MD, Svetkey LP: A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab 2009;9:311–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.An J, Muoio DM, Shiota M, Fujimoto Y, Cline GW, Shulman GI, Koves TR, Stevens R, Millington D, Newgard CB: Hepatic expression of malonyl-CoA decarboxylase reverses muscle, liver and whole-animal insulin resistance. Nat Med 2004;10:268–274 [DOI] [PubMed] [Google Scholar]
- 19.Haqq AM, Lien LF, Boan J, Arlotto M, Slentz CA, Muehlbauer MJ, Rochon J, Gallup D, McMahon RL, Bain JR, Stevens R, Millington D, Butler MD, Newgard CB, Svetkey LP: The Study of the Effects of Diet on Metabolism and Nutrition (STEDMAN) weight loss project: rationale and design. Contemporary Clinical Trials 2005;26:616–625 [DOI] [PubMed] [Google Scholar]
- 20.Ferrara CT, Wang P, Neto EC, Stevens RD, Bain JR, Wenner BR, Ilkayeva OR, Keller MP, Basiole DA, Kendziorski CM, Yandell BS, Newgard CB, Attie AD: Genetic networks of liver metabolism revealed by integration of metabolic and transcriptomic profiling. PLoS Genet 2008;4:e1000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shekhawat PS, Matern D, Strauss AW: Fetal fatty acid oxidation disorders, their effect on maternal health and neonatal outcome: impact of expanded newborn screening on their diagnosis and management. Ped Res 2005;57:78R–86R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frazier DM, Millington DS, McCandless SE, Koeberl DD, Weavil SD, Chaing SH, Muenzer J: The tandem mass spectrometry newborn screening experience in North Carolina: 1997–2005. J Inherit Metab Dis 2006;29:76–85 [DOI] [PubMed] [Google Scholar]
- 23.Watkins SM, Reifsnyder PR, Pan HJ, German JB, Leiter EH: Lipid metabolome-wide effects of the PPARgamma agonist rosiglitazone. J Lipid Res 2002;43:1809–1817 [DOI] [PubMed] [Google Scholar]
- 24.Gross RW, Han X: Shotgun lipidomics of neutral lipids as an enabling technology for elucidation of lipid-related diseases. Am J Physiol Endocrinol Metab 2009;297:E297–E303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nicholson JK, Connelly J, Lindon JC, Holmes E: Metabonomics: a platform for studying drug toxicity and gene function. Nat Rev Drug Discov 2002;1:153–161 [DOI] [PubMed] [Google Scholar]
- 26.Lu D, Mulder H, Zhao P, Burgess SC, Jensen MV, Kamzolova S, Newgard CB, Sherry AD: 13C NMR isotopomer analysis reveals a connection between pyruvate cycling and glucose-stimulated insulin secretion (GSIS). Proc Natl Acad Sci U S A 2002;99:2708–2713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boucher A, Lu D, Burgess SC, Telemaque-Potts S, Jensen MV, Mulder H, Wang MY, Unger RH, Sherry AD, Newgard CB: Biochemical mechanism of lipid-induced impairment of glucose-stimulated insulin secretion and reversal with a malate analogue. J Biol Chem 2004;279:27263–27271 [DOI] [PubMed] [Google Scholar]
- 28.Cline GW, Lepine RL, Papas KK, Kibbey RG, Shulman GI: 13C NMR isotopomer analysis of anaplerotic pathways in INS-1 cells. J Biol Chem 2004;279:44370–44375 [DOI] [PubMed] [Google Scholar]
- 29.Jensen MV, Joseph JW, Ilkayeva O, Burgess S, Lu D, Ronnebaum SM, Odegaard M, Becker TC, Sherry AD, Newgard CB: Compensatory responses to pyruvate carboxylase suppression in islet beta-cells: preservation of glucose-stimulated insulin secretion. J Biol Chem 2006;281:22342–22351 [DOI] [PubMed] [Google Scholar]
- 30.Ronnebaum S, Joseph JW, Burgess SC, Lu D, Ilkayeva O, Stevens R, Becker TC, Muehlbauer J, Sherry AD, Newgard CB, Jensen MV: A pyruvate cycling pathway involving cytosolic NAPD-dependent isocitrate dehydrogenase regulates glucose-stimulated insulin secretion. J Biol Chem 2006;281:31041–31049 [DOI] [PubMed] [Google Scholar]
- 31.Joseph JW, Jensen MV, Ilkayeva O, Palmieri F, Alárcon C, Rhodes CJ, Newgard CB: The mitochondrial citrate/isocitrate carrier plays a regulatory role in glucose-stimulated insulin secretion. J Biol Chem 2006;281:35624–35632 [DOI] [PubMed] [Google Scholar]
- 32.Joseph JW, Odegaard ML, Ronnebaum SM, Burgess SC, Muehlbauer J, Sherry AD, Newgard CB: Normal flux through ATP-citrate lyase or fatty acid synthase is not required for glucose-stimulated insulin secretion. J Biol Chem 2007;282:31592–31600 [DOI] [PubMed] [Google Scholar]
- 33.Ronnebaum SM, Joseph JW, Ilkayeva O, Burgess SC, Lu D, Becker TC, Sherry AD, Newgard CB: Chronic suppression of acetyl-CoA carboxylase 1 in beta-cells impairs insulin secretion via inhibition of glucose rather than lipid metabolism. J Biol Chem 2008;283:14248–14256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ronnebaum SM, Jensen MV, Hohmeier HE, Burgess SC, Zhou YP, Qian S, MacNeil D, Howard A, Thornberry N, Ilkayeva O, Lu D, Sherry AD, Newgard CB: Silencing of cytosolic or mitochondrial isoforms of malic enzyme has no effect on glucose-stimulated insulin secretion from rodent islets. J Biol Chem 2008;283:28909–28917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jensen MV, Joseph JW, Ronnebaum SM, Burgess SC, Sherry AD, Newgard CB: Metabolic cycling in control of glucose-stimulated insulin secretion. Am J Physiol 2008;295:E1287–E1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Newgard CB, McGarry JD: Metabolic coupling factors in pancreatic beta-cell signal transduction. Annu Rev Biochem 1995;64:689–719 [DOI] [PubMed] [Google Scholar]
- 37.Newgard CB, Matschinsky FM: Substrate control of insulin release. In Handbook of Physiology Vol. II.Jefferson J, Cherrington A. Eds. Oxford University Press, 2001, p. 125–152 [Google Scholar]
- 38.Henquin JC, Ravier MA, Nenquin M, Jonas JC, Gilon P: Hierarchy of the beta-cell signals controlling insulin secretion. Eur J Clin Invest 2003;33:742–750 [DOI] [PubMed] [Google Scholar]
- 39.Nenquin M, Szollosi A, Aguilar-Bryan L, Bryan J, Henquin JC: Both triggering and amplifying pathways contribute to fuel-induced insulin secretion in the absence of sulfonylurea receptor-1 in pancreatic beta-cells. J Biol Chem 2004;279:32316–32324 [DOI] [PubMed] [Google Scholar]
- 40.Sherry AD, Jeffrey FM, Malloy CR: Analytical solutions for (13)C isotopomer analysis of complex metabolic conditions: substrate oxidation, multiple pyruvate cycles, and gluconeogenesis. Metab Eng 2004;6:12–24 [DOI] [PubMed] [Google Scholar]
- 41.Fernandez C, Fransson U, Hallgard E, Spégel P, Holm C, Krogh M, Wårell K, James P, Mulder H: Metabolomic and proteomic analysis of a clonal insulin-producing beta-cell line (INS-1 832/13). J Proteome Res 2008;7:400–411 [DOI] [PubMed] [Google Scholar]
- 42.MacDonald MJ, Fahien LA, Brown LJ, Hasan NM, Buss JD, Kendrick MA: Perspective: emerging evidence for signaling roles of mitochondrial anaplerotic products in insulin secretion. Am J Physiol Endocrinol Metab 2005;288:E1–E15 [DOI] [PubMed] [Google Scholar]
- 43.Rabaglia ME, Gray-Keller MP, Frey BL, Shortreed MR, Smith LM, Attie AD: Alpha-ketoisocaproate-induced hypersecretion of insulin by islets from diabetes-susceptible mice. Am J Physiol Endocrinol Metab 2005;289:E218–E224 [DOI] [PubMed] [Google Scholar]
- 44.Li C, Buettger C, Kwagh J, Matter A, Daikhin Y, Nissim IB, Collins HW, Yudkoff M, Stanley CA, Matschinsky FM: A signaling role of glutamine in insulin secretion. J Biol Chem 2004;279:13393–13401 [DOI] [PubMed] [Google Scholar]
- 45.Kibbey RG, Pongratz RL, Romanelli AJ, Wollheim CB, Cline GW, Shulman GI: Mitochondrial GTP regulates glucose-stimulated insulin secretion. Cell Metab 2007;5:253–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Savage DB, Petersen KF, Shulman GI: Disordered lipid metabolism and the pathogenesis of insulin resistance. Physiol Rev 2007;87:507–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Holland WL, Summers SA: Sphingolipids, insulin resistance, and metabolic disease: new insights from in vivo manipulation of sphingolipid metabolism. Endocr Rev 2008;29:381–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Petersen KF, Dufour S, Befroy D, Lehrke M, Hendler RE, Shulman GI: Reversal of nonalcoholic hepatic steatosis, hepatic insulin resistance, and hyperglycemia by moderate weight reduction in patients with type 2 diabetes. Diabetes 2005;54:603–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kolak M, Westerbacka J, Velagapudi VR, Wågsäter D, Yetukuri L, Makkonen J, Rissanen A, Häkkinen AM, Lindell M, Bergholm R, Hamsten A, Eriksson P, Fisher RM, Oresic M, Yki-Järvinen H: Adipose tissue inflammation and increased ceramide content characterize subjects with high liver fat content independent of obesity. Diabetes 2007;56:1960–1968 [DOI] [PubMed] [Google Scholar]
- 50.Koves TR, Li P, An J, Akimoto T, Slentz D, Ilkayeva O, Dohm GL, Yan Z, Newgard CB, Muoio DM: Peroxisome proliferator-activated receptor-gamma co-activator 1alpha-mediated metabolic remodeling of skeletal myocytes mimics exercise training and reverses lipid-induced mitochondrial inefficiency. J Biol Chem 2005;280:33588–33598 [DOI] [PubMed] [Google Scholar]
- 51.Koves TR, Ussher JR, Noland RC, Slentz D, Mosedale M, Ilkayeva O, Bain J, Stevens R, Dyck JR, Newgard CB, Lopaschuk GD, Muoio DM: Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab 2008;7:45–56 [DOI] [PubMed] [Google Scholar]
- 52.Bouzakri K, Austin R, Rune A, Lassman ME, Garcia-Roves PM, Berger JP, Krook A, Chibalin AV, Zhang BB, Zierath JR: Malonyl coenzymeA decarboxylase regulates lipid and glucose metabolism in human skeletal muscle. Diabetes 2008;57:1508–1516 [DOI] [PubMed] [Google Scholar]
- 53.Finck BN, Bernal-Mizrachi C, Han DH, Coleman T, Sambandam N, LaRiviere LL, Holloszy JO, Semenkovich CF, Kelly DP: A potential link between muscle peroxisome proliferator-activated receptor-alpha signaling and obesity-related diabetes. Cell Metab 2005;1:133–144 [DOI] [PubMed] [Google Scholar]
- 54.Bruce CR, Hoy AJ, Turner N, Watt MJ, Allen TL, Carpenter K, Cooney GJ, Febbraio MA, Kraegen EW: Overexpression of carnitine palmitoyltransferase-1 in skeletal muscle is sufficient to enhance fatty acid oxidation and improve high-fat diet-induced insulin resistance. Diabetes 2009;58:550–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cao H, Gerhold K, Mayers JR, Wiest MM, Watkins SM, Hotamisligil GS: Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell 2008;134:933–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gillum MP, Zhang D, Zhang XM, Erion DM, Jamison RA, Choi C, Dong J, Shanabrough M, Duenas HR, Frederick DW, Hsiao JJ, Horvath TL, Lo CM, Tso P, Cline GW, Shulman GI: N-acylphosphtidylethanolamine, a gut-derived circulating factor induced by fat ingestion, inhibits food intake. Cell 2008;135:813–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Atherton HJ, Bailey NJ, Zhang W, Taylor J, Major H, Shockcor J, Clarke K, Griffin JL: A combined 1H-NMR spectroscopy- and mass spectrometry-based metabolomic study of the PPAR-alpha null mutant mouse defines profound systemic changes in metabolism linked to the metabolic syndrome. Physiol Genomics 2006;27:178–186 [DOI] [PubMed] [Google Scholar]
- 58.Makowski L, Noland RC, Koves TR, Xing W, Ilkayeva OR, Muehlbauer MJ, Stevens RD, Muoio DM: Metabolic profiling of PPARα−/− mice reveals defects in carnitine and amino acid homeostasis that are partially reversed by oral carnitine supplementation. FASEB J 2009;23:586–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nissen SE, Wolski K: Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 2007;356:2457–2471 [DOI] [PubMed] [Google Scholar]
- 60.Altmaier E, Ramsay SL, Graber A, Mewes HW, Weinberger KM, Suhre K: Bioinformatics analysis of targeted metabolomics: uncovering old and new tales of diabetic mice under medication. Endocrinology 2008;149:3478–3489 [DOI] [PubMed] [Google Scholar]
- 61.Wentzell AM, Rowe HC, Hansen BG, Ticconi C, Halkier BA, Kliebenstein DJ: Linking metabolic QTLs with network and cis-eQTLs controlling biosynthetic pathways. PLoS Genetics 2007;3:1687–1701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shah SH, Hauser ER, Bain JR, Muehlbauer MJ, Stevens RD, Wenner BR, Dowdy E, Granger CB, Ginsburg GS, Newgard CB, Kraus WE: High heritability of metabolomic profiles in families burdened with early-onset cardiovascular disease. Mol Syst Biol 2009;5:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chaibub , Neto E, Ferrara CT, Attie AD, Yandell BS: Inferring causal phenotype networks from segregating populations. Genetics 2008;179:1089–1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dumas ME, Wilder SP, Bihoreau MT, Barton RH, Fearnside JF, Argoud K, D'Amato L, Wallis RH, Blancher C, Keun HC, Baunsgaard D, Scott J, Sidelmann UG, Nicholson JK, Gauguier D: Direct quantitative trait locus mapping of mammalian metabolic phenotypes in diabetic and normoglycemic rat models. Nat Genet 2007;39:666–672 [DOI] [PubMed] [Google Scholar]
- 65.Gieger C, Geistlinger L, Altmaier E, Hrabe de Angelis M, Kronenberg F, Meitinger T, Mewes H-W, Wichmann H-E, Weinberger KM, Adamski J, Illig T, Suhre K: Genetics meets metabolomics: a genome-wide association study of metabolite profiles in human serum. PLoS Genet 2008;4:e1000282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lien LF, Haqq AM, Arlotto M, Slentz CA, Muehlbauer MJ, McMahon RL, Rochon J, Gallup D, Bain JR, Ilkayeva O, Wenner BR, Stevens RD, Millington DS, Muoio DM, Butler MD, Newgard CB, Svetkey LP: The STEDMAN Project: biophysical, biochemical and metabolic effects of a behavioral weight loss intervention during weight loss, maintenance, and regain. Omics 2009;13:21–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Huffman KM, Shah SH, Stevens RD, Bain JR, Muehlbauer M, Slentz CA, Tanner CJ, Kuchibhatla M, Houmard JA, Newgard CB, Kraus WE: Relationships between circulating metabolic intermediates and insulin action in overweight to obese, inactive men and women. Diabetes Care 2009;32:1678–1683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shaham O, Wei R, Wang TJ, Ricciardi C, Lewis GD, Vasan RS, Carr SA, Thadhani R, Gerszten RE, Mootha VK: Metabolic profiling of the human response to a glucose challenge reveals distinct axes of insulin sensitivity. Mol Syst Biol 2008;4:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhao X, Peter A, Fritsche J, Elenerova M, Fritsche A, Haring H-U, Schleicher ED, Xu G, Lehmann R: Changes of the plasma metabolome during an oral glucose tolerance test: is there more than glucose to look at? Am J Physiol Endocrin Metab 2009;296:E384–E393 [DOI] [PubMed] [Google Scholar]
- 70.Sabatine MS, Liu E, Morrow DA, Heller E, McCarroll R, Wiegand R, Berriz GF, Roth FP, Gerszten RE: Metabolomic identification of novel biomarkers of myocardial ischemia. Circulation 2005;112:3868–3875 [DOI] [PubMed] [Google Scholar]
- 71.Lewis GD, Wei R, Liu E, Yang E, Shi X, Martinovic M, Farrell L, Asnani A, Cyrille M, Ramanathan A, Shaham O, Berriz G, Lowry PA, Palacios IF, Taşan M, Roth FP, Min J, Baumgartner C, Keshishian H, Addona T, Mootha VK, Rosenzweig A, Carr SA, Fifer MA, Sabatine MS, Gerszten RE: Metabolite profiling of blood from individuals undergoing planned myocardial infarction reveals early markers of myocardial injury. J Clin Invest 2008;118:3503–3512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dunn WB, Broadhurst DI, Deepak SM, Buch MH, McDowell G, Spasic I, Ellis DI, Brooks N, Kell DB, Neyses L: Serum metabolomics reveals many novel metabolic markers of heart failure, including pseudouridine and 2-oxoglutarate. Metabolomics 2007;3:413–426 [Google Scholar]
- 73.Turer AT, Stevens RD, Bain JR, Muehlbauer MJ, van der Westhuizen J, Mathew JP, Schwinn DA, Glower DD, Newgard CB, Podgoreanu MV: Metabolomic profiling reveals distinct patterns of metabolic substrate use in humans with coronary artery disease or left ventricular dysfunction during surgical ischemia/reperfusion. Circulation 2009;119:1736–1746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zelena E, Dunn WB, Broadhurst D, Francis-McIntyre S, Carroll KM, Begley P, O'Hagan S, Knowles JD, Halsall A, HUSERMET Consortium. Wilson ID, Kell DB: Development of a robust and repeatable UPLC-MS method for the long-term metabolomic study of human serum. Anal Chem 2009;81:1357–1364 [DOI] [PubMed] [Google Scholar]
- 75.Sreekumar A, Poisson LM, Rajendiran TM, Khan AP, Cao Q, Yu J, Laxman B, Mehra R, Lonigro RJ, Li Y, Nyati MK, Ahsan A, Kalyana-Sundaram S, Han B, Cao X, Byun J, Omenn GS, Ghosh D, Pennathur S, Alexander DC, Berger A, Shuster JR, Wei JT, Varambally S, Beecher C, Chinnaiyan AM: Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature 2009;457:910–914 [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 76.Chen J, Zhao X, Fritsche J, Yin P, Schmitt-Kopplin P, Wang W, Lu X, Häring HU, Schleicher ED, Lehmann R, Xu G: Practical approach for the identification and isomer elucidation of biomarkers detected in a metabonomic study for the discovery of individuals at risk for diabetes by integrating the chromatographic and mass spectrometric information. Anal Chem 2008;80:1280–1289 [DOI] [PubMed] [Google Scholar]
- 77.Bao Y, Zhao T, Wang X, Qiu Y, Su M, Jia W, Jia W: Metabonomic variations in the drug-treated type 2 diabetes mellitus patients and healthy volunteers. J Proteome Res 2009;8:1623–1630 [DOI] [PubMed] [Google Scholar]
- 78.Brindle JT, Antti H, Holmes E, Tranter G, Nicholson JK, Bethell HW, Clarke S, Schofield PM, McKilligin E, Mosedale DE, Grainger DJ: Rapid and noninvasive diagnosis of the presence and severity of coronary heart disease using 1H-NMR-based metabonomics. Nat Med 2002;8:1439–1444 [DOI] [PubMed] [Google Scholar]
- 79.Kirschenlohr HL, Griffin JL, Clarke SC, Rhydwen R, Grace AA, Schofield PM, Brindle KM, Metcalfe JC: Proton NMR analysis of plasma is a weak predictor of coronary artery disease. Nat Med 2006;12:705–710 [DOI] [PubMed] [Google Scholar]
- 80.Dumas ME, Barton RH, Toye A, Cloarec O, Blancher C, Rothwell A, Fearnside J, Tatoud R, Blanc V, Lindon JC, Mitchell SC, Holmes E, McCarthy MI, Scott J, Gauguier D, Nicholson JK: Metabolic profiling reveals a contribution of gut microbiota to fatty liver phenotype in insulin-resistant mice. Proc Natl Acad Sci U S A 2006;103:12511–12516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bäckhed F, Manchester JK, Semenkovich CF, Gordon JI: Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc Natl Acad Sci U S A 2007;104:979–984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mäkinen VP, Soininen P, Forsblom C, Parkkonen M, Ingman P, Kaski K, Groop PH, Ala-Korpela MFinnDiane Study Group Diagnosing diabetic nephropathy by 1H NMR metabonomics of serum. MAGMA 2006;19:281–296 [DOI] [PubMed] [Google Scholar]
- 83.Mäkinen VP, Soininen P, Forsblom C, Parkkonen M, Ingman P, Kaski K, Groop PH, Ala-Korpela MFinnDiane Study Group 1H NMR metabonomics approach to the disease continuum of diabetic complications and premature death. Mol Syst Biol 2008;4:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Oresic M, Simell S, Sysi-Aho M, Nanto-Salonen , Seppanen-Laakso T, Parikka V, Katajamaa M, Hekkala A, Mattila I, Keskinen P, Yetukuri L, Reinikainen A, Lahde J, Suortti T, Hakalax J, Simell T, Hyoty H, Veijola R, Ilonen J, Lahesmaa R, Knip M, Simell O: Dysregulation of lipid and amino acid metabolism precedes islet autoimmunity in children who later progress to type 1 diabetes. J Expt Med 2008;205:2975–2984 [DOI] [PMC free article] [PubMed] [Google Scholar]