Abstract
OBJECTIVE
Our laboratory has previously established in vitro that a caspase-generated RasGAP NH2-terminal moiety, called fragment N, potently protects cells, including insulinomas, from apoptotic stress. We aimed to determine whether fragment N can increase the resistance of pancreatic β-cells in a physiological setting.
RESEARCH DESIGN AND METHODS
A mouse line, called rat insulin promoter (RIP)-N, was generated that bears a transgene containing the rat insulin promoter followed by the cDNA-encoding fragment N. The histology, functionality, and resistance to stress of RIP-N islets were then assessed.
RESULTS
Pancreatic β-cells of RIP-N mice express fragment N, activate Akt, and block nuclear factor κB activity without affecting islet cell proliferation or the morphology and cellular composition of islets. Intraperitoneal glucose tolerance tests revealed that RIP-N mice control their glycemia similarly as wild-type mice throughout their lifespan. Moreover, islets isolated from RIP-N mice showed normal glucose-induced insulin secretory capacities. They, however, displayed increased resistance to apoptosis induced by a series of stresses including inflammatory cytokines, fatty acids, and hyperglycemia. RIP-N mice were also protected from multiple low-dose streptozotocin-induced diabetes, and this was associated with reduced in vivo β-cell apoptosis.
CONCLUSIONS
Fragment N efficiently increases the overall resistance of β-cells to noxious stimuli without interfering with the physiological functions of the cells. Fragment N and the pathway it regulates represent, therefore, a potential target for the development of antidiabetes tools.
Elimination of pancreatic β-cells by apoptosis is a culminating event leading to type 1 diabetes (1) and possibly type 2 diabetes (2,3). The development of tools favoring β-cell survival in patients is therefore of critical importance to delay or prevent the development of the disease.
Apoptosis is induced when a family of proteases called the caspases is activated (4,5). These enzymes cleave a subset of cellular proteins, inducing the characteristic biochemical and morphological features of apoptosis. Pancreatic islet cells undergo apoptosis in response to many stimuli (6), including anoxia (7), nutrient deprivation (8), hyperglycemia (9), and inflammatory cytokines (10). Counteracting the proapoptotic effects of caspases would therefore be advantageous to render islet cells more resistant to a series of noxious stimuli.
Many proapoptotic signaling pathways have been characterized in β-cells. These include the Fas death receptor pathway, the endoplasmic reticulum stress response, and the activation of the nuclear factor (NF)κB transcription factor (6,11). The detrimental effect of sustained NFκB activity observed in β-cells contrasts with the prosurvival effect of NFκB activation in many other cell types (7,8). An elegant in vivo support for the notion that NFκB can be deleterious in β-cells comes from the demonstration that transgenic mice expressing specifically in β-cells a degradation-resistant NFκB inhibitor are protected from diabetogenic agents (12).
On the other hand, antiapoptotic pathways can be induced in β-cells to allow for survival in stress conditions. Akt is a kinase that inhibits apoptosis in many cell types by regulating a vast variety of pro- and antiapoptotic molecules (13,14). Expression of a constitutively active form of Akt in β-cells in mice protected them from experimentally induced diabetes (15,16). In at least one of the models, this was accompanied by disturbed β-cell and islet morphology, islet hyperplasia, and, paradoxically, a very significant increase in the basal β-cell apoptotic rate (15). The increased rate of proliferation was therefore compensating for the loss of cells through apoptosis. These data indicate that expression of an active form of Akt1 in β-cells generates two opposing forces: an increase in basal apoptosis and a stimulation of proliferation/growth. The latter effect eventually promotes the development of insulinomas (17). The potential beneficial effects of Akt activity in β-cells are therefore mitigated by a predisposition toward malignancy and by an increased susceptibility to cell death that is most likely mediated by the concomitant activation of NFκB (6). Thus, unless Akt is prevented from stimulating NFκB (and hence apoptosis) and from inducing excessive cell proliferation, it remains unclear whether expression of an active form of Akt is advantageous for the long-term survival and functionality of β-cells.
RasGAP, a regulator of Ras and Rho, is a caspase-3 substrate bearing two cleavage sites. RasGAP is cleaved in a stepwise manner as caspase activity increases in cells. At low caspase-3 activity, RasGAP is cleaved only once, generating an NH2-terminal fragment, called fragment N, that induces a potent antiapoptotic response (18,19). At higher caspase activity, fragment N is further processed into two additional fragments, called fragments N1 and N2, that no longer protect cells (18,20). It is possible, however, to prevent cleavage of fragment N by replacing, in the second caspase cleavage site, the aspartate residue at position 157 with an alanine (18). Fragment N induces cell survival by activating the Ras-PI3K-Akt pathway (19). Importantly, not only does fragment N not require NFκB activity for its antiapoptotic properties, it inhibits the ability of Akt to activate NFκB (19). This indicates that different ways of activating Akt (i.e., via expression of an active mutant of Akt or via expression of fragment N) does not lead to the same cellular responses. We have recently demonstrated that expression of fragment N in β-cells in vitro leads to the stimulation of Akt-dependent protective signals while blocking the ability of Akt to activate the proapoptotic NFκB pathway (21). To determine whether fragment N would display its protective functions in an in vivo setting, a transgenic mouse was generated that expresses an uncleavable form of fragment N under the control of the rat insulin promoter to restrict its expression in pancreatic β-cells. This mouse model displayed an increased resistance to experimentally induced diabetes, and its β-cells were less susceptible to apoptosis induced by a variety of death stimuli.
RESEARCH DESIGN AND METHODS
The supplemental methods for cell culture, chemicals and antibodies, transgene detection by PCR, quantitative PCR, mouse islet isolation and dissociation, preparation of tissue sections and immunochemistry, insulin quantitation, Western blot analysis, Southern blot, nuclear protein extract preparation, and electromobility shift assay (EMSA) are found in the online appendix (available at http://diabetes.diabetesjournals.org/cgi/content/full/db09-0104/DC1).
Apoptosis assay.
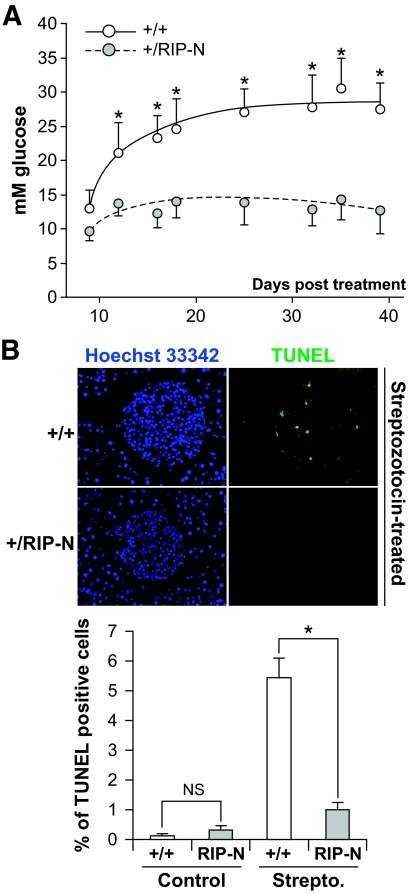
Apoptosis ex vivo was assessed by scoring the number of cells with pycnotic nuclei after Hoechst 33342 staining (20). Apoptosis in vivo was assessed by a terminal transferase dUTP nick-end labeling (TUNEL) assay (DeadEnd Fluorometric TUNEL system, catalog no. G3250; Promega, Basel, Switzerland) on islet paraffin sections as per the manufacturer's protocol.
Animal experimentation.
All procedures on mice were performed according to the Swiss legislation for animal experimentation. Unless noted otherwise, the animals were used at an age of 8–12 weeks.
Transgenic lines.
The transgenic construct (RIP-HA-N[D157A].xf3) bears fragment N of RasGAP under the control of the rat insulin promoter (RIP). It was obtained by ligation of a blunt-ended BamHI/SalI 1.4-kb fragment from plasmid HA-N(D157A).bs (22) with a blunt-ended XbaI/HindIII 4-kb fragment from RIP-vMos.xf3 plasmid. The correctness and functionality of the plasmid were controlled by sequencing and transfection into insulinoma cell lines. Finally, a BamHI 2.8-kb fragment from RIP-N.xf3 was microinjected into FVB/N oocytes at the transgenic animal facility of the University of Lausanne. Four independent RIP-N–expressing founders were obtained. Founders 1 and 2 were used in the experiments described here.
Blood glucose level measurements and intraperitoneal glucose tolerance test.
Blood glucose content of mice under feeding or fasting (16 h) conditions was determined with an Accu-Check Compact Plus glucometer (Roche Diagnostics). For the intraperitoneal glucose tolerance tests (IPGTTs), fasted (16 h) animals were injected intraperitoneally with 2 mg glucose per kilogram body weight. Blood glucose levels were determined from a blood drop taken after a short incision of the tail tip at increasing time intervals (−30, 0, 15, 30, 60, 90, 120, and 150 min) following glucose injection.
Streptozotocin-induced diabetes.
Type 1–like diabetes was induced by multiple low-dose streptozotocin injections. Briefly, 4-h–fasted female RIP-N mice were injected intraperitoneally with 50 mg streptozotocin per kg of mice. This procedure was repeated every day for a total period of 5 days. Streptozotocin was prepared and diluted in citrate buffer (pH 4.5) (sodium citrate 25 mmol/l, citric acid 23 mmol/l) just before injection. Control mice were injected with the citrate buffer alone. Blood glucose levels were assessed biweekly.
In vitro insulin secretion measurement.
Islets were isolated from mice pancreas as described in the supplemental research design and methods section in the online appendix. The islets (200 per 100-mm dish in 10 ml culture medium) were incubated overnight at 37°C, 5% CO2. The next day, the islets were hand-picked and cultured in Krebs-Ringer bicarbonate HEPES buffer (KRBH)-BSA (120 mmol/l NaCl, 4 mmol/l KH2PO4, 20 mmol/l HEPES, 1 mmol/l MgCl2, 1 mmol/l CaCl2, 5 mmol/l NAHCO3, pH 7.4, with 0.5% BSA) at 37°C and 5% CO2. The following day, well preserved and good-quality islets were again hand-picked and placed into 12-well plates (10 islets/well) in 1 ml KRBH-BSA containing 2.8 mmol/l glucose for 1 h. The islets were then transferred to new wells containing 2.8 or 20 mmol/l glucose with or without 10 nmol/l exendin-4 (catalog no. H-8370; Bachem) in 1 ml KRBH-BSA and incubated for 2 additional hours. The supernatant and islets were collected into separate tubes and placed on ice. The islets were lysed in 500 μl acid/ethanol (75% ethanol/1.5% concentrated HCl) and sonicated 15 sec (using a W-375 cell disruptor from Kontron equipped with a 3-mm tip). Insulin in the supernatant and extracted islets was measured using an radioimmunoassay kit (catalog no. RI-13K; Linco).
Statistical analysis.
Unless stated otherwise, the statistical analyses were done with Microsoft Office Excel 2003 SP1 using the two-tailed unpaired Student t test. Significance is indicated by an asterisk when P < 0.05/n, where P is the probability derived from the t test analysis and n is the number of comparisons done (Bonferroni correction). All the other statistical analyses were performed with the SAS/STAT software (version 9.1.3; SAS Institute, Cary, NC).
RESULTS
Generation of a transgenic mouse expressing fragment N in pancreatic β-cells.
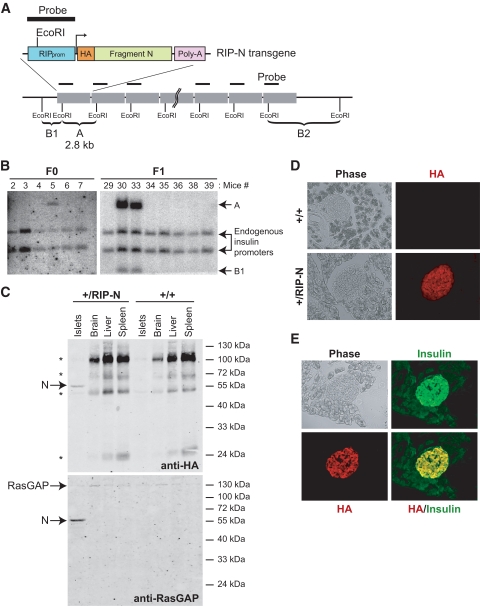
A transgenic vector was constructed (see research design and methods) so as to encode an HA-tagged form of fragment N bearing the D157A mutation (preventing it from being cleaved by caspases) under the control of the RIP and regulatory sequences of the simian virus 40 (SV40) gene (Fig. 1A). The construct was injected into FVB/N oocytes, and transgene-positive mice were identified by Southern blotting (Fig. 1B). In total, four founder mice were obtained. The results presented here all include data from founder 1 (labeled mouse 5 in Fig. 1B). When indicated, some experiments were also performed with mice derived from founder 2 (labeled mouse 28 in supplemental Fig. S1). By comparison, with the endogenous insulin promoters, it was estimated that founders 1 and 2 bore 12–15 and 1 copies of the transgene in their genome, respectively (Fig. 1B and supplemental Fig. S1).
FIG. 1.
Expression and function of fragment N in RIP-N mice. A: Schematic representation of the RIP-N transgene together with the strategy for its detection by Southern blot. An HA-tagged form of fragment N (amino acids 1–455 of RasGAP) followed by an SV40-derived poly-A sequence was placed under the control of the RIP. Band A corresponds to the transgene-specific EcoRI Southern blot fragment. B1 and B2 are examples of EcoRI Southern blot fragments derived from random insertions of the transgene into the host's genome. B: Identification of RIP-N transgenic mice. The progeny of the injected pseudo-pregnant mice were genotyped by Southern blot (see research design and methods for details). Band A (2.8 kb) is specific for the transgene. Founder 1 (mouse 5) was able to transmit the transgene to the F1 generation. C: Tissue expression of fragment N. Lysates from the indicated tissues were analyzed for the presence of fragment N by Western blot using anti-HA and anti-RasGAP antibodies. D: Expression of fragment N in the pancreas. The presence of fragment N was assessed by immunofluorescense analysis of paraformaldehyde-fixed cryosections using an antibody recognizing the HA tag borne by fragment N. E: Colocalization of insulin and fragment N. The specific location of fragment N in pancreatic β-cells was determined by immunofluorescence of paraformaldehyde-fixed cryo-sections from RIP-N mice using anti-insulin and anti-HA antibodies. (A high-quality color digital representation of this figure is available in the online issue.)
To determine the expression pattern of fragment N in the transgenic line, lysates from pancreatic islets, liver, brain, and spleen were analyzed by Western blotting using antibodies specific for the HA tag or for the NH2-terminal part of RasGAP. Figure 1C shows that fragment N was, as expected, only expressed in islet cells. Immunofluorescence analysis of both founders revealed that fragment N was restricted to the endocrine part of the pancreas (Fig. 1D and supplemental Fig. S2A and B) and that the vast majority of fragment N–expressing cells corresponded to β-cells (i.e., insulin-containing cells) (Fig. 1E).
Regulation of Akt and NFκB by fragment N in RIP-N β-cells.
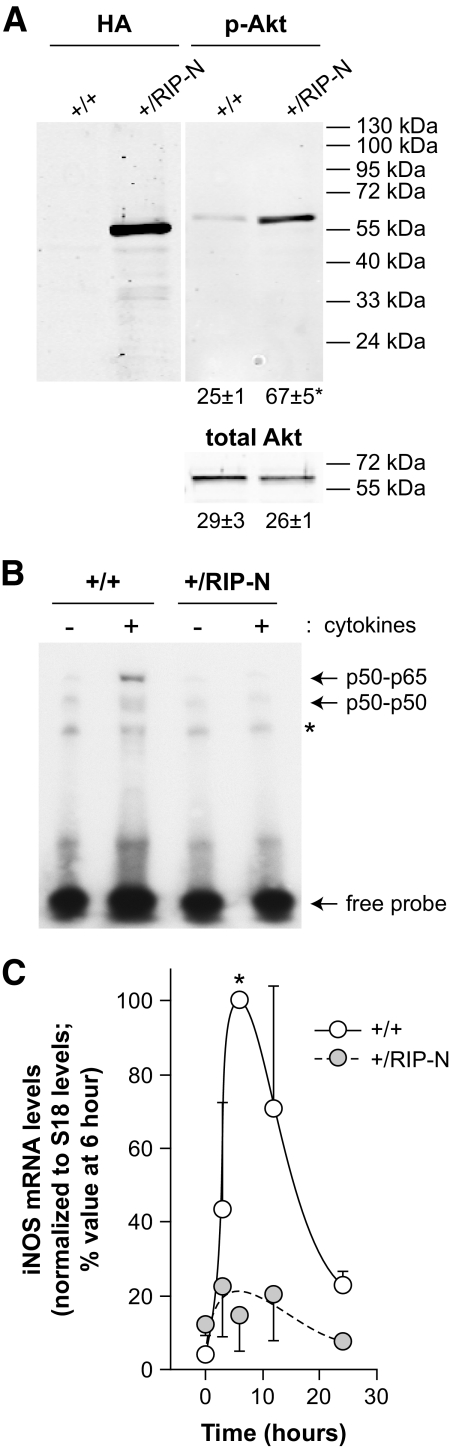
In various cell types, fragment N, when ectopically expressed or when generated in response to mild stress, activates Akt (19,21,23). As adaptive mechanisms can take place in vivo, it was important to determine whether fragment N could induce a chronic Akt activity in islet cells in mice. Islets isolated from control and RIP-N mice were therefore analyzed for the presence of activated Akt. As shown in Fig. 2A, there was a significant approximately threefold increase in Akt activity in islet cells from RIP-N compared with control islets. This indicates that fragment N can stimulate Akt on a long-term basis when expressed in vivo.
FIG. 2.
Fragment N activates Akt and inhibits NFκB in islet β cells. A: Lysates from islets isolated from the indicated mice were analyzed by Western blot for the presence of fragment N using an HA-specific antibody and for the activation of Akt using a phospho-specific anti-Akt antibody (p-Akt). An Akt-specific antibody was used to assess evenness in loading (total Akt). The numbers under the blots correspond to the quantitation (arbitrary units) of the detected bands (means ± SD of three independent determinations). The asterisk indicates a statistically significant difference as assessed by a paired t test analysis. B: Islets isolated from wild-type (+/+) and RIP-N mice (+/RIP-N) were stimulated or not for 30 min with inflammatory cytokines (1,000 units/ml tumor necrosis factor-α, 1,000 units/ml in terleukin-1β, and 50 units/ml interferon-γ). The ability of nuclear proteins to interact with an NFκB-binding element-bearing radioactive probe was then monitored by EMSA as described in research design and methods. The locations of p65-p50 and p50-p50 complexes are indicated. The asterisk denotes a nonspecific band. This experiment was repeated once with similar results. C: Islets isolated from wild-type (+/+) and RIP-N mice (+/RIP-N) were stimulated or not for the indicated periods of time with 1,000 units/ml of interleukin-1β. The expression of iNOS mRNA was then measured by quantitative real-time PCR, normalized as described in research design and methods and expressed as percent of the 6-h values. The results correspond to the means ± SE of three independent experiments performed in triplicate. The asterisk indicates a significant difference as determined by a nonparametrical Wilcoxon's signed-rank test.
A potential important property of fragment N in the context of β-cell protection is its ability to block NF-κB activation. This property, however, had so far only been evidenced in cultured immortalized cell lines (19,21). As shown in Fig. 2B, binding of nuclear factors to NFκB binding elements was markedly diminished in nuclear extracts from RIP-N mouse–isolated islet cells stimulated with cytokines compared with similarly treated islets isolated from control mice. Moreover, cytokine-induced expression of the transcript encoding inducible nitric oxide (iNOS) synthase, which participates in β-cells apoptosis (24) and the gene of which is an NFκB target (25), also appeared to be impaired in islets cells isolated from RIP mice compared with wild-type islets (Fig. 2C).
These results indicate that fragment N regulates Akt and NFκB in β-cells in vivo in a manner similar to what has been described using cultured cell lines. As cytokines can induce apoptosis of β-cells via NFκB–mediated NO production (24), these results also suggest that the ability of fragment N to protect β-cells might rely, at least in part, on its capacity to target the NFκB–iNOS axis.
No detection of fragment N in the brain of RIP-N mice.
It was reported in transgenic models done using RIP-Cre mice that the RIP promoter can also be active in the brain (more specifically in the hypothalamus) (26,27). Immunohistochemical analysis, however, did not reveal the presence of fragment N in hypothalamic sections from adult RIP-N mice (supplemental Fig. S3). This indicates that the RIP-N transgene is not expressed in adult mouse brain or, if it is expressed, at levels that are much lower than those detected in the endocrine pancreas and that are under the sensitivity limit of our assay.
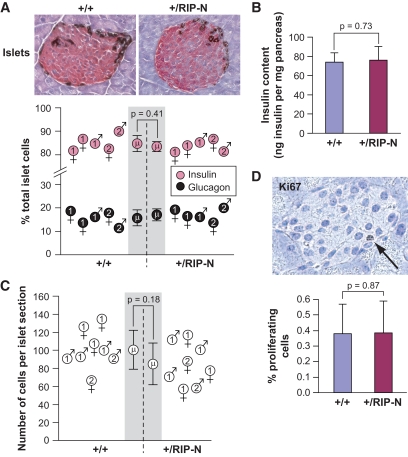
Fragment N expression does not affect islet morphology and cellularity.
Expression of fragment N in insulinomas and islet cells leads to Akt activation (Fig. 1F) (21). Since Akt signaling has the potential to stimulate cell survival and proliferation (28), and since transgenic mice expressing a constitutively active form of Akt (myr-Akt) show an increase in both β-cell size and total islet mass (15), the presence of fragment N in islets might affect the morphology and cellularity of the endocrine pancreas. However, neither the proportion of α- and β-cells (Fig. 3A), nor the insulin content of the pancreas (Fig. 3B), were affected by the presence of fragment N. Moreover, the size of the islets did not appear to be different in RIP-N transgenic mice compared with control mice (Fig. 3C). Finally, the percentage of cells positive for the nuclear protein Ki67 that is preferentially expressed in dividing cells was similar in both types of mice (Fig. 3D). These results indicate that fragment N does not favor β-cell proliferation in an in vivo setting and that it does not affect the normal development of the endocrine pancreas. Consistent with this notion is the observation that RIP-N mice did not develop insulinomas over an 18-month period (as assessed by a drop in glucose blood level and increased mortality) (Fig. 8).
FIG. 3.
Fragment N expression does not affect islet morphology and cellularity. A: The identification of α- and β-cells was determined by immunohistochemistry of paraffin-embedded pancreas sections with antibodies directed against glucagon (dark brown staining) and insulin (purple-red staining). The graph depicts the proportion of α- and β-cells in islets derived from the analysis of an average of 25 islets per 9- to 12-week-old wild-type and +/RIP-N mice. Data from individual mice are shown (the numbers in the sex symbols indicate which founder the animals are derived from) as well as the means ± SD values (indicated by the μ symbol in the gray area). B: Freshly isolated pancreata were homogenized and extracted with acid ethanol. Insulin concentration in the supernatant was determined by enzyme-linked immunosorbent assay. The results correspond to the means ± SD of nine (wild-type) and six (RIP-N) pancreata. C: The graph depicts the number of cells-per-islet section counted on hematoxylin-eosin–stained paraffin-embedded pancreas sections. The results are presented as in A and were derived from the analysis of 9- to 12-week-old wild-type and +/RIP-N animals (12 mice per genotype; an average of 90 islets per mouse were analyzed). D: The percentage of proliferating cells was determined by scoring Ki67-positive cells on paraffin-embedded pancreas sections (the arrow points to a Ki67-positive cell). The bar graph depicts the percentage of proliferating cells in islets (means ± SD) derived from the analysis of at least 20 histological slices obtained from nine mice per genotype. (A high-quality color digital representation of this figure is available in the online issue.)
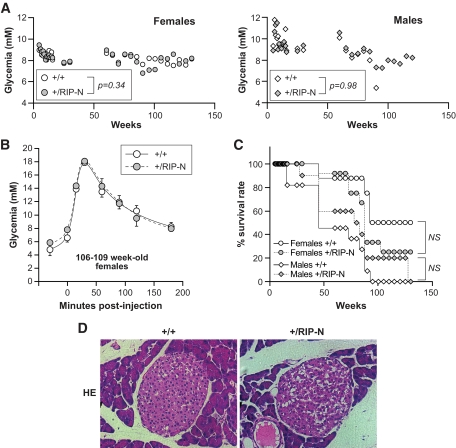
FIG. 8.
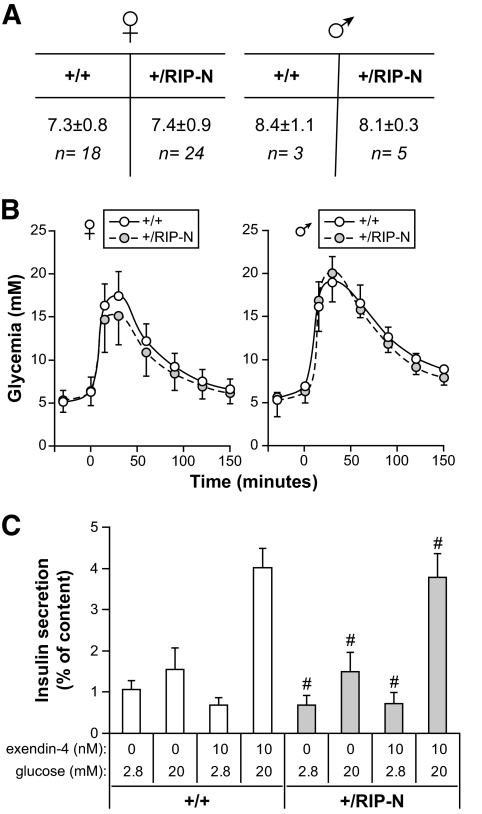
The RIP-N transgene does not affect glucose homeostasis in old mice. In the experiments presented in this figure, a cohort of 8 and 12 wild-type (+/+) and transgenic (+/RIP-N) female mice, respectively, and 11 and 10 wild-type and transgenic male mice, respectively, was used. A: Nonfasting glycemia was measured at the indicated times. The average values for the indicated groups are shown. Males had significantly higher glycemia values than females (P < 0.0001). There was also a significant decrease in glycemia as the mice aged (P < 0.0001). However, the glycemia between wild-type and RIP-N mice for a given sex was not statistically different (the P values are indicated on the figure). The statistical test used was ANOVA (repeated measures with a first-order autoregressive covariance structure). B: Four and three 106- to 109-week-old female wild-type and transgenic mice, respectively, were subjected to an IPGTT. Statistic analysis (t tests) was performed for each time point between wild-type and RIP-N mice (eight comparisons). No significant differences were recorded. This experiment was repeated once on 111- to 114-week-old females with similar results. C: The survival rate of the mice is presented. The statistical test used was a test of equality over strata (life-test procedure of the SAS/STAT software, including a log-rank test and a Wilcoxon test). Males survived significantly less than females (P = 0.0007 for the log-rank test and P = 0.0013 for the Wilcoxon test). However, the transgene did not affect survival within a given sex (P = 0.21 and 0.24 for the log-rank test and P = 0.20 and 0.33 for the Wilcoxon test, for females and males, respectively). D: Hematoxylin-eosin–stained paraffin-embedded pancreas sections from 130-week-old female control and RIP-N mice were produced. Representative images are shown. No histological differences were detected between wild-type and RIP-N islets. (A high-quality color digital representation of this figure is available in the online issue.)
Islets from RIP-N transgenic mice display increased resistance to basal- and stress-induced apoptosis.
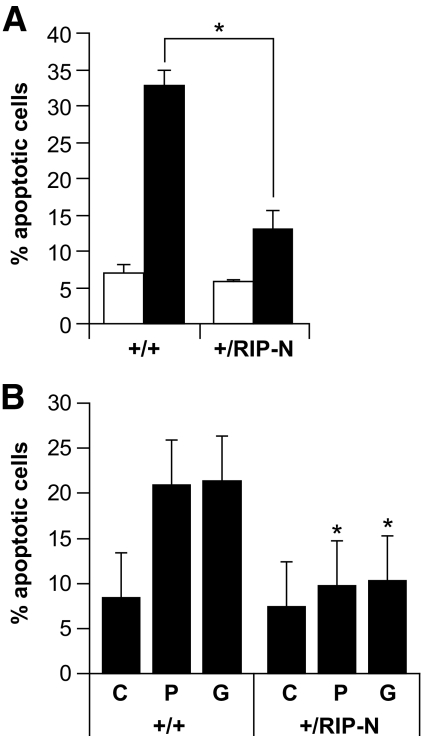
In wild-type mice, the basal apoptotic rate in islets is very low (< 0.5%; Fig. 6B) or undetectable (15). In contrast, islets from transgenic mice expressing a constitutively active form of Akt show a marked increase in β-cell apoptosis (15). Despite the ability of fragment N to activate Akt (Fig. 2A), there was no associated increase in the basal apoptotic rate in islets from RIP-N mice compared with the wild-type controls, either in vitro or in vivo (Fig. 4A and first two bars of Fig. 6B). Moreover, islets isolated from RIP-N mice were more resistant than those isolated from control mice when subjected to a variety of stress stimuli, including inflammatory cytokines, the free fatty acid palmitate, and high glucose concentrations (Fig. 4). These results demonstrate that fragment N efficiently protects pancreatic β-cells against various noxious conditions and stimuli, including some that are associated with the development of type 1 and type 2 diabetes (e.g., inflammatory cytokines and free fatty acids).
FIG. 6.
Resistance of RIP-N mice to streptozotocin-induced diabetes. Wild-type (+/+) and RIP-N (+/RIP-N) females (nine each) were subjected to multiple low-dose injections of streptozotocin (Strepto.) (see research design and methods). Glucose blood levels were then determined at the indicated times. The results are expressed as the means ± SD (statistic analysis was performed for each time point between wild-type and RIP-N mice [eight comparisons]). This experiment has been repeated two more times with similar results (A). Alternatively, the mice were killed 8 days after the first streptozotocin injection, and apoptosis on islet sections was determined by the TUNEL assay (B). The results shown in the graph correspond to the means ± SD of the quantitation performed on three and four mice for the control and streptozotocin treatment, respectively (an average of 24 islets per mice were analyzed). Statistic analysis was performed on the indicated groups. As reported by others (47), we note that the percentage of basal apoptosis in islet cells in vivo is >10-fold lower than what is detected in in vitro cultured islets (compare with Fig. 4). * indicates a statistically significant difference as described in the research design and methods section. (A high-quality color digital representation of this figure is available in the online issue.)
FIG. 4.
RIP-N islet cells are more resistant to stress-induced apoptosis. A: Freshly isolated islets were loosely dissociated (see research design and methods) and incubated or not with inflammatory cytokines (1,000 units/ml TNF-α, 1,000 units/ml interleukin-1β, and 50 units/ml interferon-γ) for an additional 24-h period. The islets were then stained with Hoechst 33342 and apoptosis scored. The results correspond to the means ± SD of three independent experiments (statistic analyses were performed for the control and stimulated conditions between wild-type and RIP-N mice [two comparisons]). □, Control; ■, cytokines. B: Freshly isolated islets were loosely dissociated and treated with vehicle (C; ethanol 1%) or incubated during 72 h with 1 mmol/l palmitate (P) or 33 mmol/l glucose (G). Apoptosis was then assessed as above. The results correspond to the means ± SD of three independent experiments (statistic analysis was performed for each condition between wild-type and RIP-N mice [three comparisons]). * indicates a statistically significant difference as described in the research design and methods section.
Fragment N does not adversely affect β-cell functions in vivo.
Transgenic mice expressing a nondegradable form of IκBα under the control of Pdx1 promoter, which drives its expression in the β-cells of the pancreas, display impaired glucose-induced insulin secretion (29). Fragment N, by blocking NFκB activity (19,21), could potentially similarly affect insulin secretion. However, fragment N expression in β-cells did not modify glycemia under nonfasted (Fig. 5A) or fasted (Fig. 5B, first points in the graphs) conditions. Moreover, the ability of the transgenic mice to metabolize glucose, assessed by IPGTTs, was not negatively affected by the presence of the transgene in β-cells (Fig. 5B). Finally, islets isolated from control and RIP-N transgenic mice had a similar ability to secrete insulin in response to glucose and the gluco-incretin exendin-4 (Fig. 5C). These results indicate that fragment N does not compromise the ability of β-cells to secrete insulin in response to augmented glucose levels.
FIG. 5.
Glycemia and glucose tolerance of RIP-N mice. A: Nonfasting glycemia of wild-type and RIP-N males and females was determined as described in research design and methods. B: Mice were subjected to an IPGTT to analyze their response to hyperglycemic conditions. Results correspond to the means ± SD of six independent experiments. Statistic analysis was performed for each time point between wild-type and RIP-N mice (eight comparisons). No significant differences were recorded. C: Islets from wild-type and RIP-N female mice were stimulated with low- or high-glucose concentration in the presence or in the absence of exendin-4 (see research design and methods). Insulin secretion was then determined. Results are expressed as the amount of insulin secreted normalized to the initial cellular insulin content (means ± SD of quadruplicate determinations). #No statistical differences between insulin secretion of wild-type and RIP-N islets for a given stimulation regimen (four comparisons).
RIP-N transgenic mice are protected against streptozotocin-induced diabetes.
Multiple low-dose streptozotocin injections in mice induce islet inflammation, ultimately leading to β-cell loss and diabetes (30,31). This model is thought to mimic the development of type 1 diabetes in humans (32). Using this protocol, it was found that RIP-N mice were resistant to diabetes induction compared with control mice (Fig. 6A). Assessment of apoptosis by the TUNEL method showed that the percentage of β-cell apoptosis induced by streptozotocin in vivo was significantly reduced in the RIP-N mice compared with the wild-type controls (Fig. 6B).
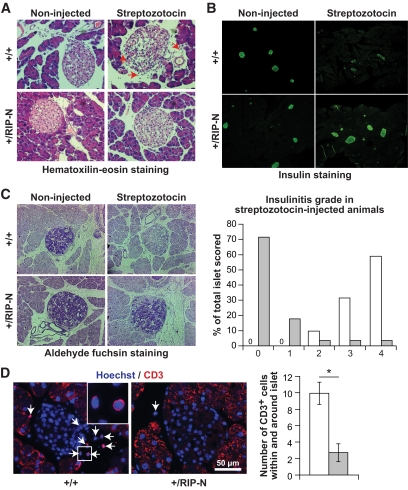
To further characterize the reduced sensitivity to streptozotocin-induced diabetes in RIP-N mice, pancreas sections from control and RIP-N mice treated or not with streptozotocin were prepared. The mice were killed 3 days after the last streptozotocin injection at a time were increased apoptosis can be detected in wild-type mice (see Fig. 6B) but before the apparition of an overt diabetes (see Fig. 6A). At this time, leukocytic infiltration can be visualized in the islets of wild-type streptozotocin-induced diabetic mice. This was accompanied by a loosening of the islet structure. This was not seen in RIP-N mice (Fig. 7A). Additionally, insulin staining was reduced in the islets of wild-type streptozotocin-induced diabetic mice (Fig. 7B). Moreover, there was almost no sign of insulinitis in the transgenic mice after the streptozotocin injections, while in similarly treated control mice, a strong insulinitis developed (Fig. 7C). Finally, there was a significant reduction in CD3-positive leukocyte infiltration in RIP-N mice compared with the control mice after the streptozotocin treatment (Fig. 7D). Taken together, these results indicate that streptozotocin induces less damage in RIP-N islets, which results in a weaker inflammatory response compared with wild-type islets.
FIG. 7.
Reduced insulinitis in streptozotocin-treated RIP-N mice. Control and RIP-N mice were subjected or not to multiple low-dose injections of streptozotocin (see research design and methods) and killed 8 days after the first streptozotocin injection. Sections were then prepared for histology and immunohistochemistry analyses. Representative images are shown. A: Hematoxylin-eosin–stained paraffin-embedded pancreas sections. The red arrows point to infiltrating leukocytes. Note also the loosened structure of islets from wild-type streptozotocin-induced diabetic mice. B: Insulin immunofluorescence staining of paraformaldehyde-fixed cryosections. C: Aldehyde fuchsin–stained sections. These were used to score insulinitis in the streptozotocin-induced diabetic animals as described by Leiter (48) (right part of the panel; the higher the grade, the stronger the insulinitis; >100 islets from two to three mice per genotype were analyzed). □, +/+;  , +/RIP-N. D: CD3 staining of paraffin-embedded pancreas sections (there was a high background staining in the exocrine pancreas, but this was not seen in the islets). Representative images are shown (arrows indicate CD3-positive cells; the inset shows an enlargement of the indicated region). The number of CD3-positive cells within and at the immediate periphery of the islets was counted (graph on the right). The results correspond to means ± SD of four mice (at least 25 islets per mouse were scored). □, +/+;
, +/RIP-N. D: CD3 staining of paraffin-embedded pancreas sections (there was a high background staining in the exocrine pancreas, but this was not seen in the islets). Representative images are shown (arrows indicate CD3-positive cells; the inset shows an enlargement of the indicated region). The number of CD3-positive cells within and at the immediate periphery of the islets was counted (graph on the right). The results correspond to means ± SD of four mice (at least 25 islets per mouse were scored). □, +/+;  , +/RIP-N. (A high-quality color digital representation of this figure is available in the online issue.)
, +/RIP-N. (A high-quality color digital representation of this figure is available in the online issue.)
The RIP-N transgene does not alter glucose homeostasis or the lifespan of mice.
In relatively young mice (8–12 weeks), glucose homeostasis and insulin secretion are unaffected by the presence of fragment N in β-cells (Fig. 5). To determine whether the transgene could nevertheless negatively affect the function of pancreatic β-cells on a longer-term basis, the glycemia of a cohort of female and male wild-type and RIP-N mice was followed for up to 130 weeks (Fig. 8A). Males displayed higher glycemia values than females. There was also a significant decrease in glycemia as the mice aged. However, the glycemia between wild-type and RIP-N mice for a given sex was not statistically different. This indicates that fragment N does not negatively affect the function of the islets of Langerhans. Consistent with this is the observation that IPGTTs performed on very old animals did not reveal differences between wild-type and RIP-N mice (Fig. 8B).
Constitutive expression of Akt in pancreatic β-cells increases the likelihood of insulinoma development, leading to a reduction of the lifespan expectancy of the mice (17). As fragment N activates Akt, it was relevant to check if fragment N would have a negative impact on the survival of the mice. Figure 8C shows that this is not the case. While females lived significantly longer than males, the presence of the transgene did not affect the percent survival rate of the mice. Finally, there was no histological difference that could be evidenced on islets from very old wild-type and RIP-N mice (Fig. 8D). Altogether, these results indicate that fragment N expressed in pancreatic β-cells displays no negative effect throughout the lifespan of mice.
DISCUSSION
Apoptosis, which is the cause of β-cell death in patients with type 1 diabetes (33), might also participate in the loss of β-cell mass observed in type 2 diabetes (34–37). The notion, however, that there is a decrease in β-cell mass in type 2 diabetes has been controversial for a number of years. Nevertheless, if one considers those studies using well-preserved pancreases obtained from autopsies, it appears that there is a 3- to 10-fold increase in the rate of β-cell apoptosis in type 2 diabetic patients compared with control subjects (38). These results indicate that failure to compensate for insulin resistance could result from decreased β-cell mass mediated by apoptosis.
Understanding the pathways leading to β-cell death and β-cell protection might therefore be of crucial importance to find new approaches to treat diabetic patients. Procedures to block β-cell death could not only potentially inhibit the development of diabetes but might also be useful in the context of islet transplantation, where apoptosis has been shown to adversely affect the number of islets that can be implanted in patients. Here, we present an in vivo model, the RIP-N transgenic mice, where the NH2-terminal fragment of RasGAP (called fragment N) effectively protects the pancreatic β-cell against apoptosis without affecting their ability to appropriately secrete insulin in physiological conditions and without favoring excessive proliferation.
Hyperlipidemia, a risk factor for the development of diabetes (39), can cause β-cell apoptosis (40,41). Islets isolated from RIP-N mice underwent less apoptosis in conditions mimicking hyperlipidemia (i.e., high concentrations of palmitate) compared with control islets. Hyperglicemia, which can lead to β-cell dysfunction and death (6), also induced less apoptosis in fragment N–expressing islets. Finally, RIP-N transgenic islets were more resistant to interleukin-1β–, tumor necrosis factor-α–, and interferon-γ–induced death. Interestingly, these inflammatory cytokines, known to be involved in the development of type 1 diabetes, have also been shown to be produced at high concentrations in diabetes-prone obese patients (42,43). These observations suggest that the protective signals elicited by fragment N can counteract prodiabetic conditions (e.g., hyperglycemia, hyperlipidemia, presence of inflammatory cytokines) that are deleterious for β-cells.
Much work on the cellular mechanisms controlling cell death and survival has been performed within the last few years. This knowledge has been used to manipulate β-cells in order to increase their survival capacities. One strategy was based on Akt because it is a potent antiapoptotic kinase in many cell types (13). Transgenic mice expressing an active form of Akt (myr-Akt) in β-cells in mice have larger β-cells and bigger islets (15) and this ultimately favors the development of insulinomas (17). These transgenic mice are resistant to experimentally induced diabetes, but, paradoxically, their β-cells have a much increased basal apoptotic rate (15). Conceivably, this higher apoptotic rate is compensated by increased β-cell renewal to maintain an adequate β-cell mass. As NFκB activation can induce β-cell death (6) and because Akt stimulates NFκB (44), the increased apoptosis response observed in myr-Akt–expressing β-cells likely results from the stimulation of the NFκB pathway. Indeed, prevention of NFκB activation using a dominant-negative IκB mutant allows mice to resist streptozotocin-induced diabetes (12,45). NFκB inhibition might, however, not always be protective against diabetes, as indicated by the increased susceptibility of nonobese diabetic (NOD) mice to develop diabetes when their β-cells express the dominant-negative IκB mutant (45). Additionally, it has been shown that expression of a dominant-negative IκB mutant in β-cells in mice can inhibit glucose-stimulated insulin secretion (29).
The RIP-N mice appear to lack some of the defects associated with the models described above. The activation of Akt by fragment N in the β-cells of RIP-N mice is not accompanied by an increased basal apoptotic rate, most likely because fragment N blocks Akt from stimulating NFκB. Moreover, in contrast to mice expressing an active form of Akt in β-cells (17), RIP-N mice do not display islet and β-cell hyperplasia, they do not develop insulinomas, and have a normal lifespan. Finally, RIP-N mice have no defect in glucose-induced insulin secretion and display normal glucose homeostasis, even in very old animals. Transgenic mice overexpressing proteins of the IAP (inhibitor of apoptosis) family specifically in the β-cells have been generated. Similarly to RIP-N mice, their β-cells are less susceptible to apoptosis, and this is not accompanied by alterations in islet morphology and function (46). It will therefore be important to define if there is a link between fragment N and IAPs that could explain the protective function of fragment N in β-cells. A detailed characterization at the molecular level of the pathways regulated by fragment N might ultimately lead to the identification of new strategies to preserve β-cell mass.
Supplementary Material
Acknowledgments
This work was supported by grants from the Juvenile Diabetes Research Foundation and from the Swiss National Science Foundation.
No potential conflicts of interest relevant to this article were reported.
We thank Jean-Christophe Stehle from the Department of Pathology, Faculty of Biology and Medicine, University of Lausanne, for his help in performing immunohistochemistry experiments. We also thank Wanda Dolci for performing the in vitro insulin secretion experiments and Dr. Onur Boyman for the gift of the anti-CD3 antibody. We thank the Transgenic Animal Facility of the Faculty of Biology and Medicine and the University Hospital (University of Lausanne) for generation of the transgenic mice.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Liadis N, Murakami K, Eweida M, Elford AR, Sheu L, Gaisano HY, Hakem R, Ohashi PS, Woo M: Caspase-3-dependent β-cell apoptosis in the initiation of autoimmune diabetes mellitus Mol Cell Biol 2005;25:3620–3629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maedler K, Donath MY: Beta-cells in type 2 diabetes: a loss of function and mass Horm Res 2004;62(Suppl. 3):67–73 [DOI] [PubMed] [Google Scholar]
- 3.Donath MY, Halban PA: Decreased beta-cell mass in diabetes: significance, mechanisms and therapeutic implications Diabetologia 2004;47:581–589 [DOI] [PubMed] [Google Scholar]
- 4.Taylor RC, Cullen SP, Martin SJ: Apoptosis: controlled demolition at the cellular level Nat Rev Mol Cell Biol 2008;9:231–241 [DOI] [PubMed] [Google Scholar]
- 5.Yan N, Shi Y: Mechanisms of apoptosis through structural biology Annu Rev Cell Dev Biol 2005;21:35–56 [DOI] [PubMed] [Google Scholar]
- 6.Donath MY, Ehses JA, Maedler K, Schumann DM, Ellingsgaard H, Eppler E, Reinecke M: Mechanisms of β-cell death in type 2 diabetes Diabetes 2005;54(Suppl. 2):S108–S113 [DOI] [PubMed] [Google Scholar]
- 7.Moritz W, Meier F, Stroka DM, Giuliani M, Kugelmeier P, Nett PC, Lehmann R, Candinas D, Gassmann M, Weber M: Apoptosis in hypoxic human pancreatic islets correlates with HIF-1α expression FASEB J 2002;16:745–747 [DOI] [PubMed] [Google Scholar]
- 8.Ilieva A, Yuan S, Wang RN, Agapitos D, Hill DJ, Rosenberg L: Pancreatic islet cell survival following islet isolation: the role of cellular interactions in the pancreas J Endocrinol 1999;161:357–364 [DOI] [PubMed] [Google Scholar]
- 9.Efanova IB, Zaitsev SV, Zhivotovsky B, Kohler M, Efendic S, Orrenius S, Berggren PO: Glucose and tolbutamide induce apoptosis in pancreatic β-cells: a process dependent on intracellular Ca2+ concentration J Biol Chem 1998;273:33501–33507 [DOI] [PubMed] [Google Scholar]
- 10.Delaney CA, Pavlovic D, Hoorens A, Pipeleers DG, Eizirik DL: Cytokines induce deoxyribonucleic acid strand breaks and apoptosis in human pancreatic islet cells Endocrinology 1997;138:2610–2614 [DOI] [PubMed] [Google Scholar]
- 11.Mandrup-Poulsen T: Apoptotic signal transduction pathways in diabetes Biochem Pharmacol 2003;66:1433–1440 [DOI] [PubMed] [Google Scholar]
- 12.Eldor R, Yeffet A, Baum K, Doviner V, Amar D, Ben Neriah Y, Christofori G, Peled A, Carel JC, Boitard C, Klein T, Serup P, Eizirik DL, Melloul D: Conditional and specific NFκB blockade protects pancreatic β cells from diabetogenic agents Proc Natl Acad Sci U S A 2006;103:5072–5077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Datta SR, Brunet A, Greenberg ME: Cellular survival: a play in three Akts Genes Dev 1999;13:2905–2927 [DOI] [PubMed] [Google Scholar]
- 14.Manning BD, Cantley LC: AKT/PKB signaling: navigating downstream Cell 2007;129:1261–1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tuttle RL, Gill NS, Pugh W, Lee JP, Koeberlein B, Furth EE, Polonsky KS, Naji A, Birnbaum MJ: Regulation of pancreatic β-cell growth and survival by the serine/threonine protein kinase Akt1/PKBα Nat Med 2001;7:1133–1137 [DOI] [PubMed] [Google Scholar]
- 16.Bernal-Mizrachi E, Wen W, Stahlhut S, Welling CM, Permutt MA: Islet beta cell expression of constitutively active Akt1/PKB alpha induces striking hypertrophy, hyperplasia, and hyperinsulinemia J Clin Invest 2001;108:1631–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alliouachene S, Tuttle RL, Boumard S, Lapointe T, Berissi S, Germain S, Jaubert F, Tosh D, Birnbaum MJ, Pende M: Constitutively active Akt1 expression in mouse pancreas requires S6 kinase 1 for insulinoma formation J Clin Invest 2008;118:3629–3638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang J-Y, Widmann C: Antiapoptotic signaling generated by caspase-induced cleavage of RasGAP Mol Cell Biol 2001;21:5346–5358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang J-Y, Widmann C: The RasGAP N-terminal fragment generated by caspase cleavage protects cells in a Ras/PI3K/Akt-dependent manner that does not rely on NFκB activation J Biol Chem 2002;277:14641–14646 [DOI] [PubMed] [Google Scholar]
- 20.Yang J-Y, Walicki J, Michod D, Dubuis G, Widmann C: Impaired Akt activity down-modulation, caspase-3 activation, and apoptosis in cells expressing a caspase-resistant mutant of RasGAP at position 157 Mol Biol Cell 2005;16:3511–3520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang J-Y, Walicki J, Abderrahmani A, Cornu M, Waeber G, Thorens B, Widmann C: Expression of an uncleavable N-terminal RasGAP fragment in insulin-secreting cells increases their resistance toward apoptotic stimuli without affecting their glucose-induced insulin secretion J Biol Chem 2005;280:32835–32842 [DOI] [PubMed] [Google Scholar]
- 22.Bulat N, Widmann C: Generation of a tightly regulated all-cis beta cell-specific tetracycline-inducible vector BioTechniques 2008;45:411–420 [DOI] [PubMed] [Google Scholar]
- 23.Yang J-Y, Michod D, Walicki J, Murphy BM, Kasibhatla S, Martin S, Widmann C: Partial cleavage of RasGAP by caspases is required for cell survival in mild stress conditions Mol Cell Biol 2004;24:10425–10436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mauricio D, Mandrup-Poulsen T: Apoptosis and the pathogenesis of IDDM: a question of life and death Diabetes 1998;47:1537–1543 [DOI] [PubMed] [Google Scholar]
- 25.Kwon G, Corbett JA, Rodi CP, Sullivan P, McDaniel ML: Interleukin-1 beta-induced nitric oxide synthase expression by rat pancreatic beta-cells: evidence for the involvement of nuclear factor kappa B in the signaling mechanism Endocrinology 1995;136:4790–4795 [DOI] [PubMed] [Google Scholar]
- 26.Gannon M, Shiota C, Postic C, Wright CV, Magnuson M: Analysis of the Cre-mediated recombination driven by rat insulin promoter in embryonic and adult mouse pancreas Genesis 2000;26:139–142 [DOI] [PubMed] [Google Scholar]
- 27.Gorogawa S, Fujitani Y, Kaneto H, Hazama Y, Watada H, Miyamoto Y, Takeda K, Akira S, Magnuson MA, Yamasaki Y, Kajimoto Y, Hori M: Insulin secretory defects and impaired islet architecture in pancreatic beta-cell-specific STAT3 knockout mice Biochem Biophys Res Commun 2004;319:1159–1170 [DOI] [PubMed] [Google Scholar]
- 28.Wendel HG, De Stanchina E, Fridman JS, Malina A, Ray S, Kogan S, Cordon-Cardo C, Pelletier J, Lowe SW: Survival signalling by Akt and eIF4E in oncogenesis and cancer therapy Nature 2004;428:332–337 [DOI] [PubMed] [Google Scholar]
- 29.Norlin S, Ahlgren U, Edlund H: Nuclear factor-κB activity in β-cells is required for glucose-stimulated insulin secretion Diabetes 2005;54:125–132 [DOI] [PubMed] [Google Scholar]
- 30.Lukic ML, Stosic-Grujicic S, Shahin A: Effector mechanisms in low-dose streptozotocin-induced diabetes Dev Immunol 1998;6:119–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zheng SJ, Lamhamedi-Cherradi SE, Wang P, Xu L, Chen YH: Tumor suppressor p53 inhibits autoimmune inflammation and macrophage function Diabetes 2005;54:1423–1428 [DOI] [PubMed] [Google Scholar]
- 32.Lenzen S: The mechanisms of alloxan- and streptozotocin-induced diabetes Diabetologia 2008;51:216–226 [DOI] [PubMed] [Google Scholar]
- 33.Mathis D, Vence L, Benoist C: β-Cell death during progression to diabetes Nature 2001;414:792–798 [DOI] [PubMed] [Google Scholar]
- 34.Rhodes CJ: Type 2 diabetes: a matter of beta-cell life and death? Science 2005;307:380–384 [DOI] [PubMed] [Google Scholar]
- 35.Wilkin TJ: The accelerator hypothesis: weight gain as the missing link between type I and type II diabetes Diabetologia 2001;44:914–922 [DOI] [PubMed] [Google Scholar]
- 36.Maedler K, Sergeev P, Ris F, Oberholzer J, Joller-Jemelka HI, Spinas GA, Kaiser N, Halban PA, Donath MY: Glucose-induced β cell production of IL-1β contributes to glucotoxicity in human pancreatic islets J Clin Invest 2002;110:851–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maedler K, Spinas GA, Lehmann R, Sergeev P, Weber M, Fontana A, Kaiser N, Donath MY: Glucose induces β-cell apoptosis via upregulation of the Fas receptor in human islets Diabetes 2001;50:1683–1690 [DOI] [PubMed] [Google Scholar]
- 38.Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC: β-Cell deficit and increased β-cell apoptosis in humans with type 2 diabetes Diabetes 2003;52:102–110 [DOI] [PubMed] [Google Scholar]
- 39.Carr MC, Brunzell JD: Abdominal obesity and dyslipidemia in the metabolic syndrome: importance of type 2 diabetes and familial combined hyperlipidemia in coronary artery disease risk J Clin Endocrinol Metab 2004;89:2601–2607 [DOI] [PubMed] [Google Scholar]
- 40.Haber EP, Procopio J, Carvalho CR, Carpinelli AR, Newsholme P, Curi R: New insights into fatty acid modulation of pancreatic β-cell function Int Rev Cytol 2006;248:1–41 [DOI] [PubMed] [Google Scholar]
- 41.Biarnes M, Montolio M, Nacher V, Raurell M, Soler J, Montanya E: β-Cell death and mass in syngeneically transplanted islets exposed to short- and long-term hyperglycemia Diabetes 2002;51:66–72 [DOI] [PubMed] [Google Scholar]
- 42.Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM: Increased adipose tissue expression of tumor necrosis factor-α in human obesity and insulin resistance J Clin Invest 1995;95:2409–2415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hotamisligil GS, Shargill NS, Spiegelman BM: Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance Science 1993;259:87–91 [DOI] [PubMed] [Google Scholar]
- 44.Ozes ON, Mayo LD, Gustin JA, Pfeffer SR, Pfeffer LM, Donner DB: NFκB activation by tumour necrosis factor requires the Akt serine-threonine kinase Nature 1999;401:82–85 [DOI] [PubMed] [Google Scholar]
- 45.Kim S, Millet I, Kim HS, Kim JY, Han MS, Lee MK, Kim KW, Sherwin RS, Karin M, Lee MS: NFκB prevents β cell death and autoimmune diabetes in NOD mice Proc Natl Acad Sci U S A 2007;104:1913–1918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dohi T, Salz W, Costa M, Ariyan C, Basadonna GP, Altieri DC: Inhibition of apoptosis by survivin improves transplantation of pancreatic islets for treatment of diabetes in mice EMBO Rep 2006;7:438–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Johnson JD, Ahmed NT, Luciani DS, Han Z, Tran H, Fujita J, Misler S, Edlund H, Polonsky KS: Increased islet apoptosis in Pdx1+/− mice J Clin Invest 2003;111:1147–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leiter EH: The NOD mouse: a model for insulidependent diabetes mellitis In Current Protocols in Immunology Coligan JE, Bierer BE, Margulies DH, Shevach EM, Strober W. Eds. John Wiley & Sons, 1997, p. 15.9.1–15.9.23 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.