Abstract
OBJECTIVE
In a genome-wide association scan, the rs738409 C>G single nucleotide polymorphism (SNP) in the patatin-like phospholipase 3 gene (PNPLA3) was strongly associated with increased liver fat but not with insulin resistance estimated from fasting values. We investigated whether the SNP determines liver fat independently of visceral adiposity and whether it may even play a role in protecting from insulin resistance.
RESEARCH DESIGN AND METHODS
Liver fat was measured by 1H magnetic resonance spectroscopy and total and visceral fat by magnetic resonance tomography in 330 subjects. Insulin sensitivity was estimated during an oral glucose tolerance test and the euglycemic-hyperinsulinemic clamp (n = 222). PNPLA3 and tumor necrosis factor-α mRNA and triglyceride content were measured in liver biopsies from 16 subjects.
RESULTS
Liver fat correlated strongly with insulin sensitivity (P < 0.0001) independently of age, sex, total fat, and visceral fat. G allele carriers of the SNP rs738409 had higher liver fat (P < 0.0001) and an odds ratio of 2.38 (95% CI 1.37–4.20) for having fatty liver compared to C allele homozygotes. Interestingly, insulin sensitivity (oral glucose tolerance test: P = 0.99; clamp: P = 0.32), serum C-reactive protein levels, lipids, or liver enzymes (all P > 0.14) were not different among the genotypes. Additional adjustment for liver fat actually revealed increased insulin sensitivity in more obese carriers of the G allele (P = 0.01). In liver biopsies triglyceride content correlated positively with expression of the proinflammatory gene tumor necrosis factor-α in C allele homozygotes (n = 6, P = 0.027) but not in G allele carriers (n = 10, P = 0.149).
CONCLUSIONS
PNPLA3 may be an important key to understand the mechanisms discriminating fatty liver with and without metabolic consequences.
Nonalcoholic fatty liver disease (NAFLD) is the most common chronic hepatic disease in Western countries affecting more than 25% of adults and 38% of obese children. It constitutes a major risk factor for progression to liver failure, cirrhosis, and hepatocellular carcinoma (1,2). In addition, NAFLD is strongly associated with insulin resistance and atherosclerosis, independently of other risk factors such as total and visceral adiposity (3–14). Moreover, NAFLD is becoming increasingly recognized as a condition strongly involved in the pathogenesis of the epidemically spreading metabolic diseases type 2 diabetes and cardiovascular disease (10,11).
Metabolic consequences of fatty liver are hyperglycemia, dyslipidemia, and subclinical inflammation (12–14). As an underlying hepatic molecular mechanism, fatty acids in the liver promote a hepatic inflammatory response, which is referred to as lipotoxicity or nonalcoholic steatohepatitis (NASH). This condition induces hepatic insulin resistance and is often, but not always, accompanied by elevation of serum liver enzymes and increased serum levels of C-reactive protein (CRP) (6,14). Furthermore, fat accumulation in the liver induces the production of the glycoprotein fetuin-A that impairs insulin signaling in insulin-sensitive tissues (15,16), promotes cytokine expression in human monocytes and in animals in vivo (17), and is a determinant of incident diabetes and cardiovascular disease (18–20).
Most recently, a genome-wide association (GWA) scan identified an unrecognized gene that is putatively strongly involved in the pathogenesis of hepatic steatosis (21,22). One nonsynonymous variant clearly stood out from the tested single nucleotide polymorphisms (SNPs), encoding an isoleucine to methionine substitution at amino acid 148 in patatin-like phospholipase 3 (PNPLA3), also known as adiponutrin. This SNP (rs738409) was strongly associated with increased hepatic fat content, measured by 1HMR (magnetic resonance) spectroscopy, in more than 2,000 individuals, supporting that PNPLA3 is an important candidate gene for fatty liver (21). However, this genetic variant was only moderately associated with elevated serum liver enzymes and did not correlate with serum lipids, relationships that are commonly found in humans with fatty liver (3–6,12). More importantly, and unexpectedly, the SNP was not associated with insulin resistance estimated from fasting glucose and insulin values (21).
It has not been solved whether the relationship of the SNP with liver fat is mediated by total and/or visceral adiposity, strong determinants of hepatic steatosis. First, we investigated whether this SNP is associated with liver fat, measured by 1HMR spectroscopy, independently of total and visceral fat, precisely measured by magnetic resonance tomography. Second, because of the unexpected finding of no relationship of the SNP with insulin resistance, we tested whether the SNP determines insulin sensitivity, precisely measured during a 2-h 75-g oral glucose tolerance test (OGTT) and during the euglycemic-hyperinsulinemic clamp, and subclinical inflammation. Third, because hepatic insulin resistance is often associated with hepatic inflammation, we investigated the associations of mRNA expression of PNPLA3 and the proinflammatory gene tumor necrosis factor (TNF)-α with triglyceride content and the effect of the SNP on these relationships in human liver tissue.
RESEARCH DESIGN AND METHODS
Metabolic data from 330 whites from the southern part of Germany were included in the analyses. They participated in an ongoing study on the pathophysiology of type 2 diabetes (23). Individuals were included into the study when they fulfilled at least one of the following criteria: a family history of type 2 diabetes, a BMI >27 kg/m2, previous diagnosis of impaired glucose tolerance, or gestational diabetes. All subjects had measurements of body fat distribution determined by magnetic resonance imaging. They were considered healthy according to a physical examination and routine laboratory tests. As assessed by means of a standard questionnaire, the participants had no history of liver disease such as hepatitis and did not consume more than two alcoholic drinks per day.
Data from 16 individuals (6 women, 10 men, age 66 ± 9 years, BMI 24 ± 4 kg/m2) undergoing liver surgery because of hepatic carcinoma or liver metastasis were also included in the present study. They tested negative for viral hepatitis and had no liver cirrhosis. Informed written consent was obtained from all participants, and the local medical ethics committee had approved the protocol.
Body fat distribution and liver fat.
Waist circumference was measured at the midpoint between the lateral iliac crest and lowest rib. Furthermore, we measured total and visceral fat with an axial T1-weighed fast spin echo technique with a 1.5 T whole-body imager (Magnetom Sonata, Siemens Healthcare) (24). Volunteers were in prone position and images were recorded from fingers to toes with a slice thickness of 10 mm and a gap between slices of 10 mm. Segmentation of images was performed by semiautomatic thresholding based on a Matlab routine (Mathworks). Visceral fat was quantified in 16 to 21 slices (depending on the size of the volunteer) between femoral heads and diaphragm, not differentiating between intra- and retroperitoneal fat, by manual delineation of the visceral compartment. Fat volume from images was calculated by multiplying the in-plane pixel dimensions with the slice thickness and the number of pixels classified as fat. Volumes between contiguous slices are calculated by simply doubling the volume of the adjacent slice. Liver fat was measured as previously described (23) by localized 1HMR spectroscopy, applying a single-voxel STEAM technique with a short echo time (TE = 10 ms) and a long repetition time (TR = 4 s) avoiding T2-related signal losses. Volunteers were in supine position, and a single element of the spine array coil was used for acquisition of spectroscopic data. Spectra were recorded in posterior part of segment seven from a volume of interest of 3 × 3 × 2 cm3 in an acquisition time of 2:08 min (32 acquisitions). Volunteers were asked to be in respiration during data acquisition to minimize movement artifacts and resulting line broadening. Postprocessing of spectra was performed by manual integration of water signal (H2O at 4.7 ppm) and sum of methylene (CH2 at 1.3 ppm) and methyl signals (CH3 at 0.95 ppm). Liver fat is given by Si((CH2)n + CH3)/Si(H2O) × 100, where Si is the corresponding signal integral in arbitrary units.
OGTT.
All individuals underwent a 75-g OGTT. We obtained venous plasma samples at 0, 30, 60, 90, and 120 min for determination of plasma glucose and insulin. Glucose tolerance was determined according to the 1997 World Health Organization diagnostic criteria (25).
Euglycemic-hyperinsulinemic clamp.
Insulin sensitivity was determined with a primed insulin infusion at a rate of 40 mU/m2 per min for 2 h as previously described (23).
Analytical procedures.
Blood glucose was determined using a bedside glucose analyzer (glucose-oxidase method; YSI, Yellow Springs Instruments, Yellow Springs, CO). Plasma insulin was determined by microparticle enzyme immunoassay (ADVIA Siemens Healthcare Diagnostics, Eschborn, Germany). Plasma fetuin-A was determined by an immunoturbidimetric method as previously described (18,20).
Calculations.
The insulin sensitivity index (in micromole per kilogram per minute per picomole per liter) for systemic glucose uptake was calculated as the mean infusion rate of glucose (in micromole per kilogram per minute) necessary to maintain euglycemia during the last 40 min of the euglycemic-hyperinsulinemic clamp divided by the steady-state plasma insulin concentration. The latter was the mean insulin concentration at minute 100, 110, and 120 of the clamp (mean for all control subjects: 532 ± 15 pmol/l). Insulin sensitivity from the OGTT was estimated as proposed by Matsuda and DeFronzo (26).
Liver samples.
Liver samples were taken from normal, nondiseased tissue during surgery, immediately frozen in liquid nitrogen, and stored at −80°C. Tissue samples were homogenized in PBS containing 1% Triton X-100 using a TissueLyser (Qiagen, Hilden, Germany) and triglyceride content was quantified using the ADVIA 1650 clinical chemistry analyzer (Siemens Healthcare Diagnostics, Eschborn, Germany).
RNA isolation, RT-PCR, and real-time quantitative PCR analysis.
Frozen tissue was homogenized in a TissueLyser (Qiagen), and RNA was extracted with the RNeasy Tissue Kit (Qiagen) according to the manufacturer's instructions. Reverse transcription of total RNA quantitative PCR of TNF-α and β-actin was performed on the Light Cycler system (Roche, Mannheim, Germany) using SYBR green. PNPLA3 mRNA expression was quantified using the QuantiTect SYBR Green PCR Kit and Quantitect Primer Assay QT00055419 (Qiagen) on a Roche LightCycler 480 (Mannheim, Germany).
Genotyping.
For genotyping, DNA was isolated from whole blood using a commercial DNA isolation kit (NucleoSpin, Macherey & Nagel, Düren, Germany). The SNP rs738409 in PNPLA3 was genotyped using TaqMan assays (Applied Biosystems, Foster City, CA). We also genotyped the SNP rs6006460, although it has a very low allelic frequency in European Americans and was found to be associated with liver fat only in blacks (21). The TaqMan genotyping reaction was amplified on a GeneAmp PCR system 7000 (50°C for 2 min, 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min), and fluorescence was detected on an ABI Prism sequence detector (Applied Biosystems). The TaqMan assay was validated by direct sequencing of the SNPs in 50 control subjects, and both methods gave identical results. None of the control subjects was polymorphic for the SNP rs6006460. The genotyping success rate for the SNP rs738409 was 99.9%, and rescreening of 3.3% of the control subjects with the TaqMan assay gave 100% identical results.
Statistical analyses.
Data are given as means ± SE. Data that were not normally distributed (e.g., liver fat, insulin sensitivity, body fat distribution; Shapiro-Wilk W test) were logarithmically transformed. To adjust the effects of covariates and identify independent relationships, we first performed forward stepwise regression analyses to identify the variables to be included into a multiple regression model. Next, we performed multivariate linear regression analyses with these variables as independent parameters. To test the effect of the genotype on the metabolically relevant parameters, the parameter to be considered was set as the dependent variable. The genotype was included in both analyses as an independent nominal variable. For each dependent variable, two models were applied. In the additive model, the effects of all possible genotypes on the dependent variable were compared; in the dominant model homozygotes for the major C allele were compared with heterozygotes and homozygotes for the minor G allele. The relationship of the SNPs with insulin sensitivity was also investigated in more lean and more obese control subjects. Men and women were separately divided by the median total body fat content into two groups. The lean men and lean women, as well as the obese men and women, were combined thereafter. Logistic regression with adjustment for covariates was applied to determine the odds ratio of the genotypes for being diagnosed with fatty liver. The statistical software package JMP 4.0 (SAS Institute, Cary, NC) was used.
RESULTS
Subject characteristics and relationships between parameters of interest.
The 330 subjects (130 men and 200 women) had a mean age of 45 (range 18–69) years. Anthropometrics and metabolic characteristics of the subjects covered a wide range that was particularly large for total body fat (4−70 kg), visceral fat (0.2–10.1 kg), liver fat (0.2–30.0%), and insulin sensitivity (OGTT: 1.6–33.4 arbitrary units; clamp: 0.008–0.347 μmol · kg−1 · min−1 · pM−1). A total of 105 control subjects had fatty liver (liver fat >5.56%) (27).
Liver fat was higher in males than females (7.3 ± 0.5 vs. 4.8 ± 0.4%, P < 0.0001) and correlated positively with age (r = 0.18, P = 0.001), total body fat (r = 0.30, P < 0.0001), visceral fat (r = 0.59, P < 0.0001), and inversely with insulin sensitivity (OGTT: r = −0.53, P < 0.0001; clamp: r = −0.56, P < 0.0001).
In forward stepwise linear regression analyses including age, sex, liver fat, total fat, and visceral fat, liver fat was the strongest determinant of insulin sensitivity estimated from the OGTT (F ratio: 56) followed by age (F ratio: 9), total fat (F ratio: 9), and visceral fat (F ratio: 5). Similar results were found for insulin sensitivity measured by the clamp (F ratios; liver fat: 39; visceral fat: 12; total fat: 10; and age: 9).
Relationships between the SNP rs738409 and subject characteristics.
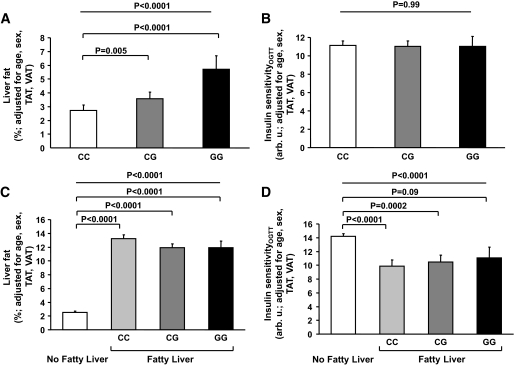
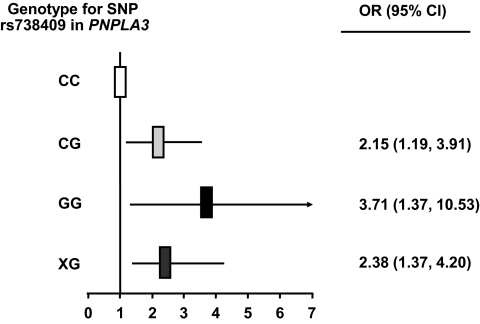
In our population, the frequency of the minor G allele of the SNP rs738409 in PNPLA3 was 0.26, similar to findings in European Americans (0.23) (21), and the SNP was in Hardy-Weinberg equilibrium (χ2 test; P > 0.05). The SNP rs738409 was strongly associated with liver fat content depicting an allele-dose effect. Carriers of the G allele had significantly higher liver fat compared to C allele homozygotes, independently of age, sex, and adiposity (Table 1) and even of visceral adiposity (P < 0.0001, Fig. 1A). Furthermore, G allele carriers had higher odds ratios (CG: 2.15 [95% CI 1.19–3.91]; GG: 3.71 [95% CI 1.37–10.53]) for being diagnosed with fatty liver compared to C allele homozygotes (Table 1, Fig. 2). However, the predictive effect of the SNP for diagnosing control subjects with fatty liver was only moderate (dominant model: positive predictive value of the SNP: 0.51; negative predictive value of the SNP: 0.61).
TABLE 1.
Associations of the SNP rs738409 in PNPLA3 with demographic and metabolic characteristics
| Characteristics | CC (Ile/Ile) | CG (Ile/Met) | GG (Met/Met) | P (additive model) | P (dominant model) |
|---|---|---|---|---|---|
| n | 188 | 111 | 31 | ||
| Sex (female/male) | 115/73 | 65/46 | 20/11 | 0.81* | 0.81* |
| Age (years) | 45 ± 1 | 46 ± 1 | 45 ± 2 | 0.76 | 0.56 |
| Body weight (kg) | 87.6 ± 1.3 | 85.1 ± 1.6 | 83.7 ± 2.8 | 0.22 | 0.08 |
| BMI (kg/m2) | 29.9 ± 0.4 | 29.1 ± 0.4 | 28.7 ± 0.8 | 0.27 | 0.11 |
| Waist circumference (cm) | 97.2 ± 0.96 | 96.3 ± 1.2 | 93.1 ± 2.4 | 0.21 | 0.15 |
| Total body fat (kg) | 26.8 ± 0.8 | 25.4 ± 1.0 | 24.9 ± 1.7 | 0.45 | 0.21 |
| Visceral fat (kg) | 3.03 ± 0.13 | 2.90 ± 0.17 | 2.66 ± 0.29 | 0.34 | 0.14 |
| Liver fat (%) | 5.40 ± 0.48 | 5.95 ± 0.56 | 7.18 ± 1.21 | 0.001 | 0.005 |
| Fatty liver (liver fat >5.56%) | 27% (n = 51) | 37% (n = 41) | 42% (n = 13) | 0.027 | 0.011 |
| Fasting glucose (mmol/l) | 5.29 ± 0.04 | 5.26 ± 0.05 | 5.12 ± 0.09 | 0.21 | 0.18 |
| 2-h glucose (mmol/l) | 7.07 ± 0.14 | 7.09 ± 0.19 | 7.01 ± 0.31 | 0.98 | 0.83 |
| Fasting insulin (pmol/l) | 65 ± 4 | 60 ± 3 | 55 ± 6 | 0.81 | 0.97 |
| 2-h insulin (pmol/l) | 513 ± 30 | 467 ± 33 | 527 ± 73 | 0.74 | 0.67 |
| Insulin sensitivity OGTT (arbitrary unit) | 12.60 ± 0.51 | 12.91 ± 0.66 | 12.88 ± 1.17 | 0.96 | 0.78 |
| ISI clamp (μmol · kg−1 · min−1 · pM−1)† | 0.065 ± 0.003 | 0.065 ± 0.005 | 0.061 ± 0.006 | 0.47 | 0.34 |
| ALT (U/l) | 29.3 ± 1.7 | 27.8 ± 1.2 | 29.1 ± 2.2 | 0.44 | 0.27 |
| AST (U/l) | 25.0 ± 0.9 | 25.0 ± 0.8 | 23.4 ± 0.9 | 0.89 | 0.78 |
| hs-CRP | 0.25 ± 0.03 | 0.25 ± 0.03 | 0.13 ± 0.02 | 0.18 | 0.91 |
| Fetuin-A (μg/ml) | 272 ± 4 | 266 ± 5 | 266 ± 9 | 0.71 | 0.41 |
Values represent means ± SE. For statistical analyses, nonnormally distributed parameters were log transformed. The genotype effect was tested using an additive and a dominant model. Body weight, BMI, waist circumference, and total body fat were adjusted for age and sex. The other parameters were additionally adjusted for total body fat.
*χ2 test. ISI, insulin sensitivity index,
†available in 222 control subjects (C/C n = 124; C/G n = 72; T/T n = 26).
FIG. 1.
Relationships of the SNP rs738409 C>G (Ile148Met) in PNPLA3 with liver fat (A) and insulin sensitivity (B) in all 330 subjects (TAT, total adipose tissue; VAT, visceral adipose tissue). Liver fat content in subjects without and with fatty liver and the relationship of the SNP rs738409 with liver fat content in subjects having fatty liver (C). Insulin sensitivity in subjects without and with fatty liver and the relationship of the SNP rs738409 with insulin sensitivity in subjects having fatty liver (D).
FIG. 2.
Odds ratios of the genotypes of the SNP rs738409 C>G (Ile148Met) in PNPLA3 for predicting fatty liver.
Interestingly, the SNP was not associated with insulin sensitivity estimated from the OGTT (Table 1, Fig. 1B) or measured by the clamp (Table 1, P = 0.32 after adjustment for age, sex, total fat, and visceral fat). Power analyses revealed that at the α-level of 0.05 and at a power of 80% the minimum detectable effect sizes among all three genotypes (genotypes CC, CG, and GG) were 8.5% for insulin sensitivity estimated from the OGTT and 9% for insulin sensitivity measured by the clamp. In a dominant model (genotype CC vs. CG + GG) the effect sizes were 8% for insulin sensitivity estimated from the OGTT and 8.3% for insulin sensitivity measured by the clamp. Furthermore, the SNP was not associated with total fat and visceral fat, liver enzymes, high-sensitivity (hs)-CRP, or fetuin-A levels (Table 1). For the liver enzymes, at the α-level of 0.05 and at a power of 80% the minimum detectable effect sizes among all three genotypes were 7.5% for alanine aminotransferase (ALT) and 5.5% for aspartate aminotransferase (AST). In a dominant model, the effect sizes were 7% for ALT and 5% for AST. For hs-CRP levels, the effect sizes were much larger (all three genotypes: 21% and dominant model: 19%).
To address in more detail the relationships of the SNP rs738409 in PNPLA3 with liver fat and insulin sensitivity, we first divided subjects in those with and without fatty liver. As expected upon definition liver fat was much higher in control subjects with fatty liver (Fig. 1C). The first noteworthy finding was that the SNP was no longer associated with liver fat content, adjusted for age, sex, total fat, and visceral fat, once subjects already displayed fatty liver, possibly because of a ceiling effect. The second interesting finding was that in control subjects with fatty liver, for a very similar liver fat content, carriers of the G allele appeared to be not just equally but actually more insulin sensitive than CC homozygotes, and insulin sensitivity in GG homozygotes was statistically not different compared to control subjects without fatty liver (Fig. 1D).
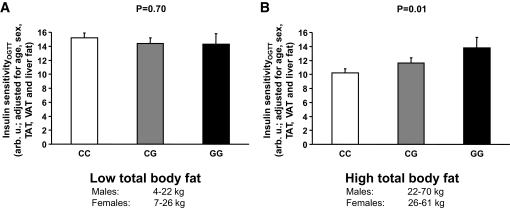
Upon this finding, based on the data in the literature showing that PNPLA3 is becoming increasingly expressed under energy excess (28–31) and because there is an interaction of the SNP rs738409 with obesity on insulin sensitivity (32), we next divided the control subjects in more lean and more obese by the median total adipose tissue mass. While in the lean group insulin sensitivity estimated by the OGTT and adjusted for age, sex, total fat, visceral fat, and liver fat was not associated with the genotype (Fig. 3A), it significantly increased with increasing copy numbers of the G allele in the obese group (Fig. 3B), indicating a protective effect of the G allele against insulin resistance.
FIG. 3.
Relationships of the SNP rs738409 C>G (Ile148Met) in PNPLA3 with insulin sensitivity in all 330 subjects separated by the median total body fat mass into more lean (A) and more obese (B) control subjects.
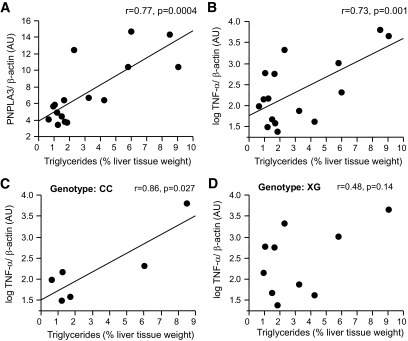
Because steatosis-induced inflammation is accompanied by insulin resistance, we further investigated in human liver biopsies the relationships of expression of the proinflammatory gene TNF-α and of PNPLA3 with the triglyceride content according to the genotype. Both PNPLA3 and TNF-α expression correlated positively with triglyceride content (Fig. 4A and B). The SNP was not associated with TNF-α expression (P = 0.64). However, liver triglyceride content correlated positively with the expression of the proinflammatory cytokine TNF-α in homozygote carriers of the C allele (n = 6) but not in carriers of the G allele (n = 10, Fig. 4C and D) of the SNP rs738409 in PNPLA3.
FIG. 4.
Relationships of hepatic triglyceride content with mRNA expression of PNPLA3 (A) and TNF-α (B) in human liver tissue. Relationships between hepatic triglyceride content and mRNA expression of TNF-α in C allele homozygotes (C) and G allele carriers (D) of the SNP rs738409 C>G (Ile148Met) in PNPLA3.
DISCUSSION
Recently, the first GWA approach searching for genetic variants associated with liver fat identified the rs738409 SNP in PNPLA3, encoding patatin-like phospholipase 3, also known as adiponutrin, as the variant that was by far most strongly associated with liver fat content in three populations (21). In the present study, using precise measurements of liver fat and body fat distribution we confirmed the strong relationship of the SNP with liver fat. Furthermore, compared to the study by Romeo et al. (21) we provide novel data that this relationship was independent of total and visceral adiposity, which are important determinants of hepatic steatosis (3–6,33). Thus, hepatic PNPLA3 activity most probably directly affects fat accumulation in the liver, a hypothesis that is supported by our finding of a strong positive correlation of hepatic PNPLA3 mRNA expression with liver triglyceride content in liver tissue samples.
Of note, in the study by Romeo et al. this variant was not associated with estimates of insulin resistance in any of the three populations or in more than 14,000 control subjects of the Atherosclerosis Risk in Communities Study (21). Considering the strong relationship of liver fat with insulin resistance (3–6,34), this finding was unexpected. Therefore, we further investigated whether the SNP rs738409 in PNPLA3 was associated with insulin sensitivity estimated from the OGTT and measured by the euglycemic-hyperinsulinemic clamp. The advantage of these techniques for estimating insulin sensitivity compared to the use of fasting values, as done in the study of Romeo et al., is that they provide a more dynamic and precise measurement of this phenotype. We found no relationships of the SNP with insulin sensitivity in our population. Furthermore, the SNP was not associated with liver enzymes or hs-CRP levels, markers of hepatic and systemic inflammation, which are commonly elevated in insulin resistance (6,14). Because fetuin-A may be involved in fatty liver–induced insulin resistance and strongly determines the incidence of diabetes and cardiovascular disease (16–20), we further investigated the relationship of the SNP with circulating fetuin-A. Although liver fat correlated with circulating fetuin-A (P = 0.01), the SNP was not associated with fetuin-A levels.
In the study by Romeo et al. (21), the SNP rs738409 was not associated with ALT or AST levels in more than 1,800 blacks or more than 1,000 European Americans. Only in 597 Hispanics, a significant relationship with ALT and AST levels was found. In a recent GWA approach aiming at identifying genes influencing liver enzymes, another SNP in PNPLA3 (rs2281135), but not the SNP rs738409, was associated with ALT levels (35). Our finding of no significant association of the SNP with liver enzymes is in agreement with the findings of Romeo et al. in blacks and European Americans. Nevertheless, we cannot exclude that we may have missed small differences because of the relatively small sample size. In the study by Romeo et al., the relationship of the SNP rs738409 with hs-CRP levels was not investigated. We did not find a significant relationship of the SNP rs738409 with hs-CRP levels. For this analysis we had a low power; therefore, we cannot exclude a relationship of the SNP with this parameter.
Because there was a clear-cut higher liver fat content, but no increase in insulin resistance in carriers of the minor G allele compared to C allele homozygotes, we hypothesized that specifically PNPLA3-mediated accumulation of liver fat appears to be metabolically benign. If this was the case, then for a similar amount of liver fat G allele carriers must be more insulin sensitive than C allele homozygotes. Indeed, in more obese control subjects, insulin sensitivity, adjusted for age, sex, total fat, visceral fat, and liver fat, was higher in carriers of the minor G allele. Furthermore, in homozygous G allele carriers with fatty liver, insulin sensitivity was no more significantly different from control subjects without fatty liver, despite the large difference in liver fat. These data support the hypothesis that PNPLA3 is involved in the generation of a metabolically benign fatty liver. Because these findings are derived from a more obese smaller subsample of the study population, the effects of PNPLA3 may be important particularly in obesity.
Two other genes are also involved in the manifestation of this interesting condition. One of them is the acyl:CoA:diacylglycerol acyltransferase 2 (DGAT2) gene that encodes the protein DGAT2 that catalyzes the final step of triacylglycerol biosynthesis (36). Liver-specific Dgat2-overexpressing mice developed hepatic steatosis with a fivefold increase in liver triglyceride content compared to control subjects, but not whole-body or hepatic insulin resistance (37). Conversely, antisense oligonucleotide treatment targeting the Dgat2 reduced liver triglycerides in mice fed a high-fat diet, without improving insulin sensitivity or glucose tolerance (38). In agreement with these data we found that a SNP in DGAT2 was associated with liver fat but not with insulin resistance in humans (39). Another candidate is the elongation of long-chain fatty acids (ELOVL) gene (Elovl6). ELOVL catalyzes the conversion of palmitate to stearate as well as palmitoleate to vaccinate, thus regulating the hepatic fatty acid composition (40). Mice deficient for Elovl6 developed obesity and hepatic steatosis, but not insulin resistance, hyperinsulinemia, or hyperglycemia under a high-fat diet (41).
What are the mechanisms leading to this dissociation between fatty liver and insulin resistance? The paradox of this finding may be because of lipotoxicity (42). According to this concept, triglycerides are probably the least toxic form in which the lipid excess can be stored in ectopic tissues, at least in the short-term. The incorporation of fatty acids into triglycerides, as well as their oxidative degradation, thus represents protection from lipotoxicity. However, when these compensatory mechanisms are overwhelmed, fatty acids induce damage to cells resulting in impaired metabolism (43,44). Several pathways are thought to be operative in this process. Among them, activation of nuclear factor-κB and c-Jun NH2-terminal kinase, which are involved in insulin resistance (14,45,46), are critical. Besides these proinflammatory pathways fatty acid and diacylglycerol (DAG) directly activate protein kinase C-ε, thus inhibiting hepatic insulin signaling (41,47,48).
What is the putative role of PNPLA3/adiponutrin in these pathways? The function of this protein has not been completely elucidated. Data in the literature strongly support that it probably has no lipase activity (49,50) but rather lipogenic functions in adipose tissue under feeding conditions, particularly under carbohydrate intake (30,31). It was further hypothesized that if this protein has similar functions in the liver, then increased hepatic expression or activity may result in increased triglyceride synthesis (31,49). PNPLA3/adiponutrin was found to have transacetylase functions and uses DAG as an acyl acceptor (31,32,49). Therefore, increased activity of the protein may also result in depletion in the hepatic DAG and fatty acid content, both of which are involved in lipotoxicity (47,48). Thus, this protective mechanism may result in a metabolically benign phenotype under increased lipid load by partitioning excess hepatic fatty acids to triglycerides that may protect against lipotoxicity. In support of this hypothesis, we found that in carriers of the minor rs738409 G allele, the mRNA expression of the proinflammatory gene TNF-α in the liver was less strongly associated with triglyceride content compared to C allele homozygotes.
What may be the clinical implication of these findings? Because fatty liver is becoming a worldwide epidemic, it is necessary not only to screen for its presence but also for control subjects at the highest risk for fatty liver–induced complications. If further studies confirm the role of PNPLA3/adiponutrin in the generation of steatosis without complications, then screening for genetic variation in PNPLA3 may help to stratify this risk.
In conclusion, the SNP rs738409 in PNPLA3 is strongly, and independently of total and visceral adiposity, associated with fatty liver but not with insulin resistance or estimates of liver injury. Mechanisms involved may be protection from hepatic fatty acid signaling. Therefore, PNPLA3/adiponutrin may be an important key to understand the mechanisms discriminating fatty liver with and without metabolic consequences and may serve as a useful tool for estimating the risk of fatty liver–induced complications.
Acknowledgments
The study was supported by grants from the Deutsche Forschungsgemeinschaft (KFO 114 and a Heisenberg-Grant to N.S., STE 1096/1-1) and the European Community's FP6 EUGENE2 (LSHM-CT-2004-512013). The supporters had no influence on the study design and on the collection, analysis, and interpretation of data.
No potential conflicts of interest relevant to this article were reported.
We thank all the participants for their cooperation and Alexander Cegan, Roman Werner, Melanie Weisser, Alke Guirguis, Mareike Walenta, and the phenotyping team for their help to collect the data.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Angulo P: Nonalcoholic fatty liver disease N Engl J Med 2002; 346: 1221– 1231 [DOI] [PubMed] [Google Scholar]
- 2.Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, Grundy SM, Hobbs HH: Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity Hepatology 2004; 40: 1387– 1395 [DOI] [PubMed] [Google Scholar]
- 3.Roden M: Mechanisms of disease: hepatic steatosis in type 2 diabetes–pathogenesis and clinical relevance Nat Clin Pract Endocrinol Metab 2006; 2: 335– 348 [DOI] [PubMed] [Google Scholar]
- 4.Utzschneider KM, Kahn SE: Review: the role of insulin resistance in nonalcoholic fatty liver disease J Clin Endocrinol Metab 2006; 91: 4753– 4761 [DOI] [PubMed] [Google Scholar]
- 5.Stefan N, Kantartzis K, Haring HU: Causes and metabolic consequences of Fatty liver Endocr Rev 2008; 29: 939– 960 [DOI] [PubMed] [Google Scholar]
- 6.Kotronen A, Yki-Jarvinen H: Fatty liver: a novel component of the metabolic syndrome Arterioscler Thromb Vasc Biol 2008; 28: 27– 38 [DOI] [PubMed] [Google Scholar]
- 7.McKimmie RL, Daniel KR, Carr JJ, Bowden DW, Freedman BI, Register TC, Hsu FC, Lohman KK, Weinberg RB, Wagenknecht LE: Hepatic steatosis and subclinical cardiovascular disease in a cohort enriched for type 2 diabetes: the Diabetes Heart Study Am J Gastroenterol 2008; 103: 3029– 3035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Targher G, Bertolini L, Poli F, Rodella S, Scala L, Tessari R, Zenari L, Falezza G: Nonalcoholic fatty liver disease and risk of future cardiovascular events among type 2 diabetic patients Diabetes 2005; 54: 3541– 3546 [DOI] [PubMed] [Google Scholar]
- 9.Villanova N, Moscatiello S, Ramilli S, Bugianesi E, Magalotti D, Vanni E, Zoli M, Marchesini G: Endothelial dysfunction and cardiovascular risk profile in nonalcoholic fatty liver disease Hepatology 2005; 42: 473– 480 [DOI] [PubMed] [Google Scholar]
- 10.Shibata M, Kihara Y, Taguchi M, Tashiro M, Otsuki M: Nonalcoholic fatty liver disease is a risk factor for type 2 diabetes in middle-aged Japanese men Diabetes Care 2007; 30: 2940– 2944 [DOI] [PubMed] [Google Scholar]
- 11.Targher G, Bertolini L, Padovani R, Rodella S, Tessari R, Zenari L, Day C, Arcaro G: Prevalence of nonalcoholic fatty liver disease and its association with cardiovascular disease among type 2 diabetic patients Diabetes Care 2007; 30: 1212– 1218 [DOI] [PubMed] [Google Scholar]
- 12.Toledo FG, Sniderman AD, Kelley DE: Influence of hepatic steatosis (fatty liver) on severity and composition of dyslipidemia in type 2 diabetes Diabetes Care 2006; 29: 1845– 1850 [DOI] [PubMed] [Google Scholar]
- 13.Kantartzis K, Rittig K, Cegan A, Machann J, Schick F, Balletshofer B, Fritsche A, Schleicher E, Haring HU, Stefan N: Fatty liver is independently associated with alterations in circulating HDL2 and HDL3 subfractions Diabetes Care 2008; 31: 366– 368 [DOI] [PubMed] [Google Scholar]
- 14.Tilg H, Hotamisligil GS: Nonalcoholic fatty liver disease: cytokine-adipokine interplay and regulation of insulin resistance Gastroenterology 2006; 131: 934– 945 [DOI] [PubMed] [Google Scholar]
- 15.Auberger P, Falquerho L, Contreres JO, Pages G, Le Cam G, Rossi B, Le Cam A: Characterization of a natural inhibitor of the insulin receptor tyrosine kinase: cDNA cloning, purification, and anti-mitogenic activity Cell 1989; 58: 631– 640 [DOI] [PubMed] [Google Scholar]
- 16.Stefan N, Hennige AM, Staiger H, Machann J, Schick F, Krober SM, Machicao F, Fritsche A, Haring HU: α2-Heremans-Schmid glycoprotein/fetuin-A is associated with insulin resistance and fat accumulation in the liver in humans Diabetes Care 2006; 29: 853– 857 [DOI] [PubMed] [Google Scholar]
- 17.Hennige AM, Staiger H, Wicke C, Machicao F, Fritsche A, Haring HU, Stefan N: Fetuin-A induces cytokine expression and suppresses adiponectin production PLoS.ONE 2008; 3: e1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stefan N, Fritsche A, Weikert C, Boeing H, Joost HG, Haring HU, Schulze MB: Plasma fetuin-A levels and the risk of type 2 diabetes Diabetes 2008; 57: 2762– 2767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ix JH, Wassel CL, Kanaya AM, Vittinghoff E, Johnson KC, Koster A, Cauley JA, Harris TB, Cummings SR, Shlipak MG: Fetuin-A and incident diabetes mellitus in older persons JAMA 2008; 300: 182– 188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weikert C, Stefan N, Schulze MB, Pischon T, Berger K, Joost HG, Haring HU, Boeing H, Fritsche A: Plasma fetuin-a levels and the risk of myocardial infarction and ischemic stroke Circulation 2008; 118: 2555– 2562 [DOI] [PubMed] [Google Scholar]
- 21.Romeo S, Kozlitina J, Xing C, Pertsemlidis A, Cox D, Pennacchio LA, Boerwinkle E, Cohen JC, Hobbs HH: Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease Nat Genet 2008; 40: 1461– 1465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mohlke KL: Nonsynonymous variants and fatty liver disease Nat Genet 2008; 40: 1394– 1395 [DOI] [PubMed] [Google Scholar]
- 23.Stefan N, Machicao F, Staiger H, Machann J, Schick F, Tschritter O, Spieth C, Weigert C, Fritsche A, Stumvoll M, Haring HU: Polymorphisms in the gene encoding adiponectin receptor 1 are associated with insulin resistance and high liver fat Diabetologia 2005; 48: 2282– 2291 [DOI] [PubMed] [Google Scholar]
- 24.Machann J, Thamer C, Schnoedt B, Haap M, Haring HU, Claussen CD, Stumvoll M, Fritsche A, Schick F: Standardized assessment of whole body adipose tissue topography by MRI J Magn Reson Imaging 2005; 21: 455– 462 [DOI] [PubMed] [Google Scholar]
- 25.Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus Diabetes Care 1997; 20: 1183– 1197 [DOI] [PubMed] [Google Scholar]
- 26.Matsuda M, DeFronzo RA: Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp Diabetes Care 1999; 22: 1462– 1470 [DOI] [PubMed] [Google Scholar]
- 27.Szczepaniak LS, Nurenberg P, Leonard D, Browning JD, Reingold JS, Grundy S, Hobbs HH, Dobbins RL: Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population Am J Physiol Endocrinol Metab 2005; 288: E462– E468 [DOI] [PubMed] [Google Scholar]
- 28.Steinberg GR, Kemp BE, Watt MJ: Adipocyte triglyceride lipase expression in human obesity Am J Physiol Endocrinol Metab 2007; 293: E958– E964 [DOI] [PubMed] [Google Scholar]
- 29.Johansson LE, Hoffstedt J, Parikh H, Carlsson E, Wabitsch M, Bondeson AG, Hedenbro J, Tornqvist H, Groop L, Ridderstrale M: Variation in the adiponutrin gene influences its expression and associates with obesity Diabetes 2006; 55: 826– 833 [DOI] [PubMed] [Google Scholar]
- 30.Wilson PA, Gardner SD, Lambie NM, Commans SA, Crowther DJ: Characterization of the human patatin-like phospholipase family J Lipid Res 2006; 47: 1940– 1949 [DOI] [PubMed] [Google Scholar]
- 31.Jenkins CM, Mancuso DJ, Yan W, Sims HF, Gibson B, Gross RW: Identification, cloning, expression, and purification of three novel human calcium-independent phospholipase A2 family members possessing triacylglycerol lipase and acylglycerol transacylase activities J Biol Chem 2004; 279: 48968– 48975 [DOI] [PubMed] [Google Scholar]
- 32.Johansson LE, Lindblad U, Larsson CA, Rastam L, Ridderstrale M: Polymorphisms in the adiponutrin gene are associated with increased insulin secretion and obesity Eur J Endocrinol 2008; 159: 577– 583 [DOI] [PubMed] [Google Scholar]
- 33.Thamer C, Machann J, Haap M, Stefan N, Heller E, Schnodt B, Stumvoll M, Claussen C, Fritsche A, Schick F, Haring H: Intrahepatic lipids are predicted by visceral adipose tissue mass in healthy subjects Diabetes Care 2004; 27: 2726– 2729 [DOI] [PubMed] [Google Scholar]
- 34.Stefan N, Kantartzis K, Machann J, Schick F, Thamer C, Rittig K, Balletshofer B, Machicao F, Fritsche A, Haring HU: Identification and characterization of metabolically benign obesity in humans Arch Intern Med 2008; 168: 1609– 1616 [DOI] [PubMed] [Google Scholar]
- 35.Yuan X, Waterworth D, Perry JR, Lim N, Song K, Chambers JC, Zhang W, Vollenweider P, Stirnadel H, Johnson T, Bergmann S, Beckmann ND, Li Y, Ferrucci L, Melzer D, Hernandez D, Singleton A, Scott J, Elliott P, Waeber G, Cardon L, Frayling TM, Kooner JS, Mooser V: Population-based genome-wide association studies reveal six loci influencing plasma levels of liver enzymes Am J Hum Genet 2008; 83: 520– 528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buhman KK, Chen HC, Farese RV, Jr: The enzymes of neutral lipid synthesis J Biol Chem 2001; 276: 40369– 40372 [DOI] [PubMed] [Google Scholar]
- 37.Monetti M, Levin MC, Watt MJ, Sajan MP, Marmor S, Hubbard BK, Stevens RD, Bain JR, Newgard CB, Farese RV, Sr, Hevener AL, Farese RV, Jr: Dissociation of hepatic steatosis and insulin resistance in mice overexpressing DGAT in the liver Cell Metab 2007; 6: 69– 78 [DOI] [PubMed] [Google Scholar]
- 38.Yu XX, Murray SF, Pandey SK, Booten SL, Bao D, Song XZ, Kelly S, Chen S, McKay R, Monia BP, Bhanot S: Antisense oligonucleotide reduction of DGAT2 expression improves hepatic steatosis and hyperlipidemia in obese mice Hepatology 2005; 42: 362– 371 [DOI] [PubMed] [Google Scholar]
- 39.Kantartzis K, Machicao F, Machann J, Schick F, Fritsche A, Haring HU, Stefan N: The DGAT2 gene is a candidate for the dissociation between fatty liver and insulin resistance in humans Clin Sci (Lond) 2009; 116: 531– 537 [DOI] [PubMed] [Google Scholar]
- 40.Moon YA, Shah NA, Mohapatra S, Warrington JA, Horton JD: Identification of a mammalian long chain fatty acyl elongase regulated by sterol regulatory element-binding proteins J Biol Chem 2001; 276: 45358– 45366 [DOI] [PubMed] [Google Scholar]
- 41.Matsuzaka T, Shimano H, Yahagi N, Kato T, Atsumi A, Yamamoto T, Inoue N, Ishikawa M, Okada S, Ishigaki N, Iwasaki H, Iwasaki Y, Karasawa T, Kumadaki S, Matsui T, Sekiya M, Ohashi K, Hasty AH, Nakagawa Y, Takahashi A, Suzuki H, Yatoh S, Sone H, Toyoshima H, Osuga J, Yamada N: Crucial role of a long-chain fatty acid elongase, Elovl6, in obesity-induced insulin resistance Nat Med 2007; 13: 1193– 1202 [DOI] [PubMed] [Google Scholar]
- 42.Unger RH: Minireview: weapons of lean body mass destruction: the role of ectopic lipids in the metabolic syndrome Endocrinology 2003; 144: 5159– 5165 [DOI] [PubMed] [Google Scholar]
- 43.Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Tuncman G, Gorgun C, Glimcher LH, Hotamisligil GS: Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes Science 2004; 306: 457– 461 [DOI] [PubMed] [Google Scholar]
- 44.Unger RH: Lipotoxicity in the pathogenesis of obesity-dependent NIDDM. Genetic and clinical implications Diabetes 1995; 44: 863– 870 [DOI] [PubMed] [Google Scholar]
- 45.Hotamisligil GS: Inflammation and metabolic disorders Nature 2006; 444: 860– 867 [DOI] [PubMed] [Google Scholar]
- 46.Shoelson SE, Lee J, Goldfine AB: Inflammation and insulin resistance J Clin Invest 2006; 116: 1793– 1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morino K, Petersen KF, Shulman GI: Molecular mechanisms of insulin resistance in humans and their potential links with mitochondrial dysfunction Diabetes 2006; 55( Suppl. 2): S9– S15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Samuel VT, Liu ZX, Wang A, Beddow SA, Geisler JG, Kahn M, Zhang XM, Monia BP, Bhanot S, Shulman GI: Inhibition of protein kinase Cepsilon prevents hepatic insulin resistance in nonalcoholic fatty liver disease J Clin Invest 2007; 117: 739– 745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lake AC, Sun Y, Li JL, Kim JE, Johnson JW, Li D, Revett T, Shih HH, Liu W, Paulsen JE, Gimeno RE: Expression, regulation, and triglyceride hydrolase activity of Adiponutrin family members J Lipid Res 2005; 46: 2477– 2487 [DOI] [PubMed] [Google Scholar]
- 50.Kershaw EE, Hamm JK, Verhagen LA, Peroni O, Katic M, Flier JS: Adipose triglyceride lipase: function, regulation by insulin, and comparison with adiponutrin Diabetes 2006; 55: 148– 157 [PMC free article] [PubMed] [Google Scholar]