Abstract
OBJECTIVE
Establishing Caenorhabditis elegans as a model for glucose toxicity–mediated life span reduction.
RESEARCH DESIGN AND METHODS
C. elegans were maintained to achieve glucose concentrations resembling the hyperglycemic conditions in diabetic patients. The effects of high glucose on life span, glyoxalase-1 activity, advanced glycation end products (AGEs), and reactive oxygen species (ROS) formation and on mitochondrial function were studied.
RESULTS
High glucose conditions reduced mean life span from 18.5 ± 0.4 to 16.5 ± 0.6 days and maximum life span from 25.9 ± 0.4 to 23.2 ± 0.4 days, independent of glucose effects on cuticle or bacterial metabolization of glucose. The formation of methylglyoxal-modified mitochondrial proteins and ROS was significantly increased by high glucose conditions and reduced by mitochondrial uncoupling and complex IIIQo inhibition. Overexpression of the methylglyoxal–detoxifying enzyme glyoxalase-1 attenuated the life-shortening effect of glucose by reducing AGE accumulation (by 65%) and ROS formation (by 50%) and restored mean (16.5 ± 0.6 to 20.6 ± 0.4 days) and maximum life span (23.2 ± 0.4 to 27.7 ± 2.3 days). In contrast, inhibition of glyoxalase-1 by RNAi further reduced mean (16.5 ± 0.6 to 13.9 ± 0.7 days) and maximum life span (23.2 ± 0.4 to 20.3 ± 1.1 days). The life span reduction by glyoxalase-1 inhibition was independent from the insulin signaling pathway because high glucose conditions also affected daf-2 knockdown animals in a similar manner.
CONCLUSIONS
C. elegans is a suitable model organism to study glucose toxicity, in which high glucose conditions limit the life span by increasing ROS formation and AGE modification of mitochondrial proteins in a daf-2 independent manner. Most importantly, glucose toxicity can be prevented by improving glyoxalase-1–dependent methylglyoxal detoxification or preventing mitochondrial dysfunction.
Because of its short life span, the relative ease in modifying its genome, and its simple insulin receptor system (daf-2) (1), Caenorhabditis elegans can be used not only to study molecular targets affected by normal glucose concentration but also by pathological glucose concentrations. It is likely a good model to study mechanisms by which high glucose might reduce life span.
Increased glycolytic flux leads to formation of mitochondrial reactive oxygen species (ROS). In turn, ROS formation decreases the activity of glyoxalase-1, an enzyme detoxifying methylglyoxal. Accumulation of methylglyoxal, a highly reactive dicarbonyl derived from triosephosphates (2), leads to rapid protein modification. Methylglyoxal is an arginine-directed glycating agent and precursor of advanced glycation end products (AGEs). It modifies proteins mainly but not exclusively on arginine residues forming methylglyoxal-derived hydroimidazolone (MG-H1) with formation of other minor AGE residues, arginine-derived argpyrimidine and lysine-derived Nε-(1-carboxyethyl)lysine and methylglyoxal-derived lysine dimer (3). Methylglyoxal-derived modification of mitochondrial proteins increases mitochondrial ROS formation, thus reducing life span in C. elegans (4). The formation of methylglyoxal-modified proteins in cells is suppressed by the activity of the enzyme glyoxalase-1 that catalyzes conversion of methylglyoxal, with the cofactor glutathione, to S-d-lactoylglutathione. This is further converted to d-lactate by glyoxalase-2 regenerating the glutathione (5). We recently showed that with increasing age, glyoxalase-1 activity decreased in C. elegans, leading to an increase of methylglyoxal accumulation and oxidative stress (4). In these settings, overexpression of a glyoxalase-1 homologue in C. elegans decreased mitochondrial AGE accumulation and increased life span, whereas knockdown of the glyoxalase-1 gene increased AGE accumulation and decreased life span, pointing to a dependence of life span on AGE production (4).
Glucose restriction extends C. elegans life span. Recent studies by Schultz et al. (6) using a model of impaired glucose metabolism indicate that an increase in ROS results in a secondary hormetic increase in stress defense, eventually resulting in reduced net stress levels. In contrast, high glucose concentrations reduce life span of C. elegans. Because an increased glycolytic flux is likely to cause a long-lasting accumulation of methylglyoxal-derived AGEs and a steady increase in ROS formation, we hypothesized that C. elegans cannot only be used for understanding the role of glucose metabolism in normal aging, but most importantly for understanding basic mechanisms of glucose toxicity. Therefore, we studied whether elevation of glucose concentrations in C. elegans extracts from 6 to 14 mmol/l (resembling the hyperglycemic conditions in diabetic patients) could limit life span in C. elegans by accumulation of methylglyoxal-derived AGEs and impairment of mitochondrial function and whether detoxifying methylglyoxal might prevent glucose toxicity and mitochondrial damage.
RESEARCH DESIGN AND METHODS
C. elegans maintenance and life span assays.
All nematodes were cultivated on nematode growth medium (NGM) agar as described previously (4) and maintained at 20°C. Strains used in this study include wild-type (N2) and eat-2 (-) mutant (eat-2[ad465]II) provided by the Caenorhabditis Genetic Center and glyoxalase-1 transgenic (4). Animals were maintained on living Escherichia coli (OP50) from a standardized overnight culture with an OD of 1.5, which was added to the surface of the NGM plates. One hundred fifty microliters of a 400 mmol/l glucose solution was used to achieve a concentration of 14 mmol/l in the worms. In experiments using dead bacteria, the bacteria were killed by sonication. In experiments using a mitochondrial uncoupler or a complex III inhibitor, C. elegans were cultivated in the presence of 50 μmol/l carbonylcyanide-p-trifluorormethoxyphenylhydrazone (FCCP, Sigma-Aldrich) or 10 μmol/l myxothiazol (Sigma-Aldrich), respectively.
Establishment of high glucose conditions.
It was our aim to reach a glucose concentration in a C. elegans whole-body extract of 10–15 mmol/l, resembling the glucose concentrations in diabetic patients under poor glucose control. Therefore, 150 μl of a glucose solution of various concentrations was added to the agar of NGM plates in the absence of C. elegans as described above for 1, 3, 5, 10, and 15 days to define the time for reaching a steady state of glucose in the agar. A piece of agar was then cut out of the middle of the NGM plate and fluidified using β-agarase (New England Biolabs) as described by the manufacturer. Glucose was measured using a Modular ISE 900 P 800 analyzer (Hitachi) according to the manufacturer's recommendations.
Wild-type C. elegans were kept for 5 days under various glucose concentrations in the agar prepared as described above, harvested, and washed. An extract of C. elegans was prepared by sonication and analyzed for the glucose concentration using a Modular ISE 900 P 800 analyzer (Hitachi). For all further experiments, a glucose concentration of 40 mmol/l in the agar was used because it resulted in a glucose concentration of 14 mmol/l in the C. elegans whole-body extract.
Scanning electron microscopy.
Wild-type C. elegans were kept under high glucose conditions for 16 days as described above. Worms were then harvested, washed with PBS buffer, and incubated with 2.5% glutaraldehyde overnight. After three times washing with PBS buffer, worms were transferred into microporous specimen capsules and dehydrated in graded ethanol solutions. Specimen were finally dried in a critical point dryer (030 Critical point dryer, Bal-Tec) and placed onto conductive sticky tape. Subsequently, samples were sputter coated with a 15-nm gold layer (MED020, Bal-Tec) and imaged in a field emission scanning electron microscope (Leo1530 Gemini).
Glyoxalase-1 activity.
Glyoxalase-1 activity was assayed as described (4,7,8). For the conversion of hemithioacetal to S-d-lactoylglutathione, the change in molar extinction coefficient, Δε240 = 2.86 mmol · l−1 · cm−1, was measured after the addition of the C. elegans protein extract to hemithioacetal, prepared by preincubating methylglyoxal and glutathione. The initial rate of change of absorbance (ΔA240, o [arbitrary unit, AU] min−1) was deduced, and the ΔA240, o for the blank value was corrected. The activity of glyoxalase-1 aGI is given in units where 1 unit of glyoxalase-1 activity catalyzes the formation of 1 μmol S-d-lactoylglutathione per minute under assay conditions. The standard assay mixture contained 2 mmol/l methylglyoxal and 2 mmol/l glutathione in a sodium phosphate buffer (100 mmol/l, pH 6.6, 37°C).
Staining of mitochondria and MG-H1.
Staining of mitochondria for MG-H1 and confocal microscopy were performed as described (4). Briefly, mitochondria were stained by incubation with 50 μmol/l MitoTracker Deep Red FM (Molecular Probes) according to the manufacturer's protocol. MG-H1 was detected using a mouse antibody and visualized by a Texas Red-labeled rabbit anti-mouse second antibody (Biozol) as described previously (4). Immunhistology was performed according to the protocol of Ruvkun (for details see http://www.wormatlas.org/anatmeth/finneyruvkun.pdf). Formation and colocalization of MG-H1 with mitochondria was quantified by using the image processing and analysis software ImageJ (10) with the Intensity Correlation Analysis plug-in (11) as described previously (4).
Staining of ROS.
Staining of ROS, confocal microscopy, and analysis with ImageJ were performed as described previously (4). Briefly, C. elegans were washed, incubated with 3 μmol/l of dihydroethidium for 30 min, and analyzed for fluorescence intensity by confocal microscopy. Raw data from confocal microscopy were then analyzed using ImageJ.
RNAi.
For RNAi experiments, the feeding technique was used as described previously (4,12,13). Individual colonies of E. coli HT115 containing plasmids of interest (Y55D5A.5 [daf-2] and C16C10.10 [glyoxalase-1]) were inoculated in LB broth containing ampicillin (100 mg/ml) (Sigma-Aldrich, München, Germany) and grown overnight at 37°C. Bacteria (150 μl) were then seeded onto NGM plates containing carbenicillin (0.025 g/l) and isopropyl-β-d-thiogalactopyranoside (IPTG) (0.25 g/l) and allowed to dry overnight. E. coli containing only the control vector were grown in parallel and seeded on control plates.
Twenty L4 stage hermaphrodites (one generation) were placed on each plate and incubated overnight at 20°C. The worms were then transferred on a fresh RNAi plate, and left to lay eggs for 24 h. The old worms were removed from the plates, and the eggs were incubated at 20°C until worms were in L4 stage. These worms were used for life span observation. Furthermore, to use active RNAi E. coli and to prevent mixture of generations, worms were transferred by picking on a new NGM-IPTG/carbenicillin plate seeded with the appropriate E. coli clone every day.
Determination of life span.
Age-synchronized worms were obtained as described before (4). Life span studies without RNAi were performed as described (4,9) on NGM plates containing 300 μg/ml 5-fluorodesoxyuridine (Sigma-Aldrich) to prevent progeny production. Life span studies using RNAi were performed on NGM plates containing carbenicillin (0.025 g/l) and IPTG (0.25 g/l), and animals were transferred to new plates daily. In all experiments, the prefertile period of adulthood was used as t = 0 for life span analysis. Animals that did not move after repeated stimulus were regarded as dead. Animals that crawled away from the plate or contained internally hatched worms were excluded. One hundred worms were used for each experiment; all experiments were performed at least three times.
Statistical analysis.
Statistical analysis was performed using StatView 5.0 software (SAS Institute, Cary, NC). In experiments in which only two groups were compared (Figs. 1–5), unpaired t tests were used to determine significance. If multiple groups were run (Table 1 and Fig. 6), the data were compared across the groups using ANOVA; additional between-group comparisons were made using Fisher protected least significant difference (PLSD) post hoc tests.
FIG. 1.
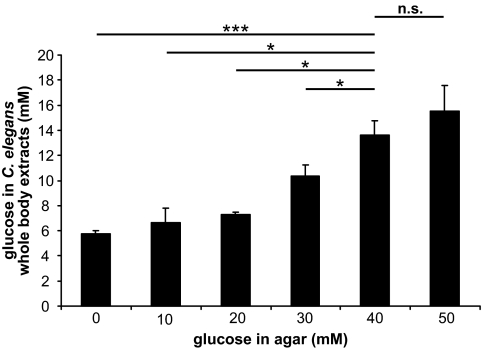
Glucose concentration in C. elegans. C. elegans were cultured on agar having the glucose concentrations of 0, 10, 20, 30, 40, and 50 mmol/l. After 5 days, 100 C. elegans were harvested and the glucose concentrations in the C. elegans whole-body extracts were determined. Results are the means ± SE of three independent experiments; n.s. (not significant) describes a t test value (P > 0.05, *P < 0.05, and ***P < 0.001) comparing two groups as indicated.
FIG. 2.
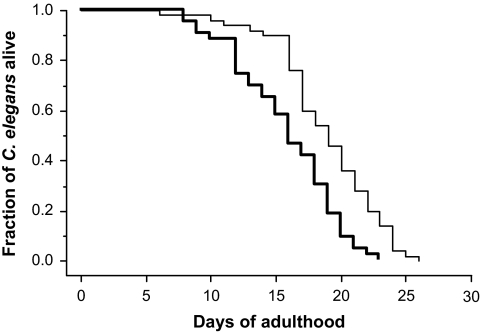
Effect of high glucose conditions on C. elegans life span. Kaplan-Meier graphs of the fraction of C. elegans alive. Shown are wild-type C. elegans cultured under standard and high glucose conditions. Life span assays were performed as described in Research Designs and Methods. The results are from a representative experiment out of three independent experiments, each including 100 nematodes. Thin line = standard conditions; bold line = high glucose conditions.
FIG. 3.
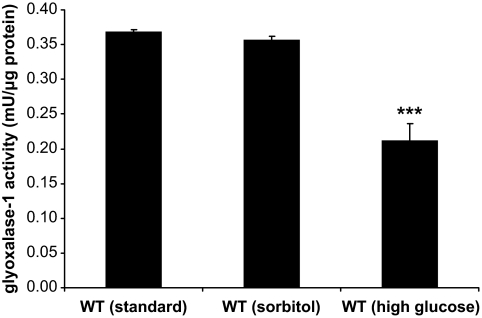
Effect of high glucose conditions on glyoxalase-1 activity. Quantification of glyoxalase-1 activity in whole-body extracts of 5-day-old wild-type (WT) animals, cultured under standard and high glucose conditions, or sorbitol as control. Results are the means ± SE of three independent experiments; value from a t test (***P < 0.001) comparing wild type under standard conditions vs. wild type under high glucose conditions.
FIG. 4.
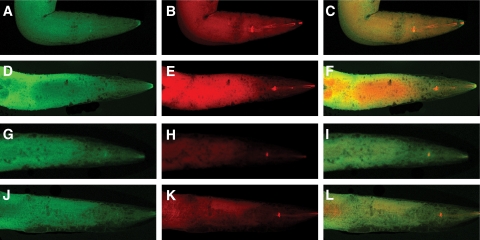
Mitochondrial MG-H1 immunoreactivity in C. elegans. Mitochondria were stained with MitoTracker Deep Red FM (A, D, G, and J), and MG-H1 was visualized by immunostaining with a Texas Red-labeled antibody directed against MG-H1 antibody (B, E, H, and K). Merged staining is shown in C, F, I, and L. Orange color indicates colocalization of MG-H1 with mitochondria. Images are shown for 15-day-old wild-type animals cultured under standard (A–C) and high glucose conditions (D–F) and 15-day-old transgenic glyoxalase-1–overexpressing animals cultured under standard (G–I) and high glucose conditions (J–L). Shown are animals from a representative experiment out of three independent experiments, each including 100 nematodes.
FIG. 5.
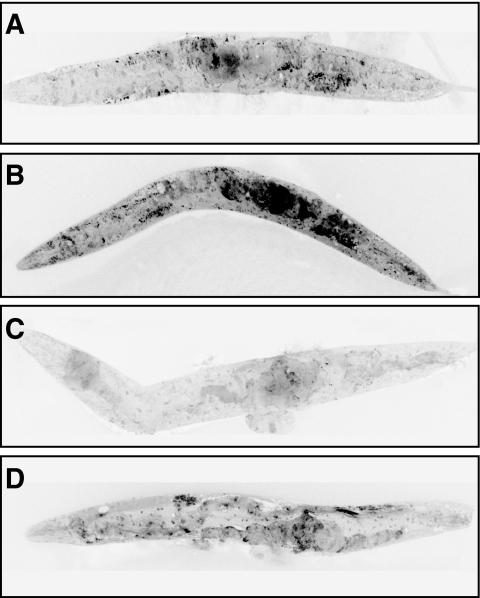
Effect of high glucose conditions on formation of ROS. Shown are ethidium-labeled wild-type animals cultured for 15 days under standard (A) and high glucose conditions (B), transgenic glyoxalase-1– overexpressing animals cultured for 15 days under standard (C) and high glucose conditions (D). Shown are animals from a representative experiment out of three independent experiments, each including 100 nematodes.
TABLE 1.
Summary of individual life span experiments
| Mean life span in days ± SE | Max life span in days ± SE |
P value vs. specific group |
||
|---|---|---|---|---|
| Mean life span | Maxlife span | |||
| WT (standard) | 18.5 ± 0.4 | 25.9 ± 0.4 | ||
| WT (high glucose) | 16.5 ± 0.6 | 23.2 ± 0.4 | <0.05a | <0.05a |
| WT + sorbitol | 17.9 ± 0.2 | 24.5 ± 0.5 | n.s. (0.6438)a | n.s. (0.4676)a |
| Glyoxalase-1 transgenic | 20.3 ± 0.2 | 29.2 ± 1.5 | <0.05a | <0.05a |
| Glyoxalase-1 transgenic (high glucose) | 20.6 ± 0.4 | 27.7 ± 2.3 | <0.001b | <0.01b |
| WT + glyoxalase-1 RNAi | 13.5 ± 1.2 | 21.0 ± 1.2 | <0.001a | <0.01a |
| WT + glyoxalase-1 RNAi (high glucose) | 13.9 ± 0.7 | 20.3 ± 1.1 | <0.01b | <0.05b |
| WT + dead bacteria | 29.3 ± 0.1 | 40.5 ± 2.5 | ||
| WT + dead bacteria (high glucose) | 25.1 ± 0.1 | 35.5 ± 0.5 | <0.01c | <0.05c |
| eat-2 (-) mutant | 24.3 ± 0.3 | 33.3 ± 0.3 | ||
| eat-2 (-) mutant (high glucose) | 21.5 ± 1.4 | 29.0 ± 1.5 | <0.05c | <0.05c |
| WT + daf-2 RNAi | 24.2 ± 2.0 | 35.0 ± 3.0 | ||
| WT + daf-2 RNAi (high glucose) | 20.6 ± 1.7 | 30.0 ± 2.0 | <0.05c | <0.05c |
| WT + FCCP | 21.7 ± 1.7 | 31.5 ± 0.5 | <0.05a | <0.01a |
| WT + FCCP (high glucose) | 19.5 ± 0.9 | 28.5 ± 1.5 | <0.05b | <0.01b |
| WT + myxothiazol | 21.1 ± 1.1 | 31.0 ± 1.7 | <0.01a | <0.01a |
| WT + myxothiazol (high glucose) | 20.4 ± 0.1 | 27.7 ± 1.9 | <0.001b | <0.01b |
Summary of mean and maximum (max) life span and statistical analysis for life span experiments. The data were compared across the groups using ANOVA; additional between-group comparisons were made using Fisher PLSD post hoc tests. P value from a Fisher PLSD post hoc test comparing a group vs.
(a) wild type (WT) under standard conditions, (b) WT under high glucose conditions, or (c) the corresponding group under standard conditions.
FIG. 6.
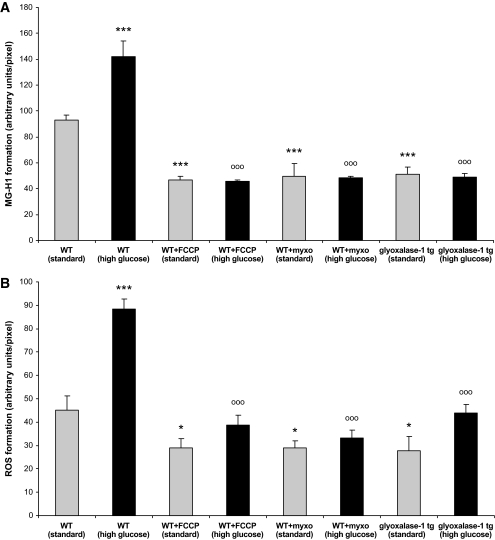
Quantification of MG-H1 and ROS in C. elegans. Formation of MG-H1 (A) and ROS (B) was quantified in 15-day-old wild-type (WT) animals, without and with 10 μmol/l myxothiazol (myxo) or 50 μmol/l FCCP, and in 15-day-old transgenic glyoxalase-1–overexpressing animals. Each group was cultured under standard and high glucose conditions. The data were compared across the groups using ANOVA; additional between-group comparisons were made using Fisher PLSD post hoc tests. Results are the means ± SE of three independent experiments with 100 nematodes each; value from a Fisher PLSD post hoc test (*P < 0.05 and ***P < 0.001) comparing the indicated group vs. wild type under standard conditions; value from a Fisher PLSD post hoc test (oooP < 0.001) comparing the indicated group vs. wild type under high glucose conditions.
RESULTS
To establish the potential rate of diffusion of glucose from the medium to the agar, a solution of glucose (400 mmol/l) was added to an agar plate and incubated for a period of 15 days. At consecutive time points, glucose concentration was determined. No significant change in glucose concentration was observed in the agar (data not shown). In all subsequent experiments, agar plates were incubated for at least 1 day with an appropriate concentration of glucose before inoculation with C. elegans.
C. elegans (ca. 100) were cultured on agar plates containing various concentrations of glucose (0 to 50 mmol/l) for 5 days. After which time, intracellular glucose concentrations were determined from whole-body extracts. It was found that culturing of C. elegans on agar plates containing 40 mmol/l glucose resulted in an intracellular concentration of 14 mmol/l (Fig. 1), which is within the range observed in poorly controlled diabetic patients. In all subsequent experiments, C. elegans cultured under high glucose conditions refer to a concentration of 40 mmol/l, whereas standard conditions refer to no additional glucose being added to the agar.
Under high glucose conditions, mean life span was reduced from 18.5 ± 0.4 to 16.5 ± 0.6 days (P < 0.05), and maximum life span was reduced from 25.9 ± 0.4 to 23.2 ± 0.4 days (P < 0.05) (Fig. 2 and Table 1). Scanning electron microscopy studies excluded nonspecific high glucose effects on the cuticle of the worms because there was no morphological difference in the appearance of C. elegans held under high glucose conditions for 16 days and the control group (data not shown). To exclude nonspecific osmotic effects, experiments were performed by adding sorbitol as control. Sorbitol affected neither mean (P > 0.05) nor maximum (P > 0.05) life span (Table 1). To exclude nonspecific effects because of bacterial metabolization of glucose, control experiments were performed using dead bacteria. In these experiments, the addition of glucose reduced mean life span from 29.3 ± 0.1 to 25.1 ± 0.1 days (P < 0.01) and maximum life span from 40.5 ± 2.5 to 35.5 ± 0.5 days (P < 0.05) (Table 1). The increased life span compared to animals fed with living bacteria could be caused by caloric restriction because of differential food preferences. These results point to a specific life-shortening effect of glucose. Thus, increasing the total body glucose concentration to 14 mmol/l resulted in a reduction of life span not explained by nonspecific effects.
Glyoxalase-1 is central in controlling the extent of methylglyoxal and methylglyoxal-derived AGE formation because of glucose metabolism. After high glucose exposure for 5 days, glyoxalase-1 activity determined in whole- body extracts of C. elegans was reduced from 0.368 ± 0.004 to 0.211 ± 0.025 mU/μg protein, whereas sorbitol had no significant effect (Fig. 3).
To test whether glucose-dependent glyoxalase-1 downregulation leads to an increase in AGE modification of mitochondria in C. elegans (4,14), the level of MG-H1 was determined using immunostaining. The staining of MG-H1 in standard (Fig. 4B) and high-glucose animals (Fig. 4E) did not differ with respect to localization, only with respect to the intensity. When mitochondria were stained with MitoTracker and counterstained for MG-H1, colocalization of MG-H1 with mitochondria could be detected (Fig. 4C and F). Because of the higher content of MG-H1, staining for colocalization was stronger under high glucose than standard glucose conditions.
Previously, we reported a C. elegans strain with transgenic stable overexpression of glyoxalase-1 (4). This resulted in a ∼200-fold increase of glyoxalase-1 activity (4). These animals with genetically controlled stable glyoxalase-1 expression were also exposed to standard and high glucose conditions. Overexpression of C. elegans glyoxalase-1 (4) resulted in a decrease in total and mitochondrial MG-H1 in standard (Fig. 4H and I) as well as in high glucose (Fig. 4K and L) conditions.
The increase of mitochondrial immunoreactive MG-H1 was paralleled by an increase in ROS formation (Fig. 5A–D). When dihydroethidium staining was used to visualize ROS generation, a strong increase was seen in C. elegans cultured for 15 days in high glucose (Fig. 5B) compared to standard conditions (Fig. 5A). Using transgenic C. elegans overexpressing glyoxalase-1, a dramatic reduction in dihydroethidium staining was observed. Under standard conditions, transgenic C. elegans had a staining significantly below control (Fig. 5A and C) and even under high glucose conditions, overexpression of glyoxalase-1 reduced staining below wild-type controls (Fig. 5B and D).
To test whether high glucose-induced mitochondrial dysfunction is responsible for increased MG-H1 and ROS formation, experiments were repeated with C. elegans cultured in the presence of the electron transport chain uncoupler FCCP (50 μmol/l) or the complex IIIQo inhibitor myxothiazol (10 μmol/l) for 15 days (Fig. 6A and B). When AUs/pixel were used to quantify MG-H1 generation, a significant increase from 93 ± 4 to 142 ± 12 (P < 0.001) was observed in worms treated with high glucose for 15 days (Fig. 6A). MG-H1 formation in high glucose wild-type C. elegans was significantly reduced by FCCP and myxothiazol. FCCP and myxothiazol reduced MG-H1 formation in standard and high glucose–treated animals to the same level, which was by ∼49% of staining observed in wild type under standard conditions and by ∼66% compared to high glucose (Fig. 6A).
A similar result was observed with ROS formation. When AUs/pixel were used to quantify ROS generation, a significant increase from 45 ± 6 to 88 ± 4 (P < 0.001) was observed in worms treated with high glucose for 15 days (Fig. 6B). FCCP and myxothiazol reduced ROS formation by 36% in standard and up to 62% in high glucose cultured C. elegans (Fig. 6B). Thus, overcoming high glucose–induced mitochondrial dysfunction reduces MG-H1 and ROS formation.
Treatment with either FCCP or myxothiazol enhanced life span under both conditions (Table 1). FCCP increased mean life span from 18.5 ± 0.4 to 21.7 ± 1.7 days (P < 0.05) and maximum life span from 25.9 ± 0.4 to 31.5 ± 0.5 days (P < 0.01), whereas myxothiazol increased mean life span from 18.5 ± 0.4 to 21.1 ± 1.1 days (P < 0.01) and maximum life span from 25.9 ± 0.4 to 31.0 ± 1.7 days (P < 0.01) under standard glucose conditions (Table 1). Under high glucose conditions, FCCP increased mean life span from 16.5 ± 0.6 to 19.5 ± 0.9 days (P < 0.05) and maximum life span from 23.2 ± 0.4 to 28.5 ± 1.5 days (P < 0.01), whereas myxothiazol increased mean life span from 16.5 ± 0.6 to 20.4 ± 0.1 days (P < 0.001) and maximum life span from 23.2 ± 0.4 to 27.7 ± 1.9 days (P < 0.01).
To determine whether overexpression of glyoxalase-1 cannot only reduce MG-H1 (Figs. 4 and 6A) and ROS (Figs. 5 and 6B) formation, but also counteract the life shortening effect of glucose, glyoxalase-1–overexpressing worms were exposed to glucose (Table 1). Overexpression of glyoxalase-1 prolonged mean life span in standard culture conditions from 18.5 ± 0.4 in wild type to 20.3 ± 0.2 in transgenic (P < 0.05) and maximum life span from 25.9 ± 0.4 to 29.2 ± 1.5 days (P < 0.05). Under high glucose conditions, mean life span of 16.5 ± 0.6 days in wild type was increased to 20.6 ± 0.4 days in transgenic animals (P < 0.001) and maximum life span of 23.2 ± 0.4 to 27.7 ± 2.3 days (P < 0.01), thus restoring life span under high glucose conditions to the life span seen in wild type under standard glucose concentration (Table 1).
In contrast, a decrease of life span under standard glucose conditions was seen when RNAi was used to knockdown glyoxalase-1 activity by 75% (4). Under normal glucose conditions mean life span of 18.5 ± 0.4 days in wild type was reduced by glyoxalase-1 RNAi to 13.5 ± 1.2 days (P < 0.001). Maximum life span was reduced from 25.9 ± 0.4 to 21.0 ± 1.2 days (P < 0.01). In the presence of high glucose, mean life span was reduced from 16.5 ± 0.6 in wild type to 13.9 ± 0.7 in glyoxalase-1 RNAi treated C. elegans (P < 0.01) and maximum life span from 23.2 ± 0.4 to 20.3 ± 1.1 days (P < 0.05) (Table 1).
The reduction of life span under high glucose conditions is independent from the pathways activated by caloric restriction because the effect of glucose was still observed in eat-2 (-) mutants with reduced pharyngeal activity and thus reduced caloric uptake (Table 1). In eat-2 mutants, mean life span was reduced from 24.3 ± 0.3 days in standard culture conditions to 21.5 ± 1.4 days in high glucose conditions (P < 0.05) and the maximum life span from 33.3 ± 0.3 to 29.0 ± 1.5 days (P < 0.05). Thus, the proportional reduction of mean and maximum life span is 12 and 13% in eat-2 mutants, respectively. Both correspond well to a reduction of mean life span of 11% and a reduction of maximum life span of 10% by high glucose in wild-type C. elegans. Thus, the glycotoxic effects described here are independent from the eat-2 controlled starvation pathway.
A decline in daf-2 insulin signaling pathway of C. elegans (1) is known to result in activation of the fork-like transcription factor daf-16 and to prolong life span (9). This effect, however, was also observed to be diminished under high glucose conditions. Administration of glucose to C. elegans knockdown for daf-2 by RNAi had similar effects as in wild-type worms, namely, a reduction of mean life span from 24.2 ± 2.0 to 20.6 ± 1.7 days (P < 0.05) and maximum life span from 35.0 ± 3.0 to 30.0 ± 2.0 days (P < 0.05) (Table 1). This points to a life span–reducing mechanism under high glucose conditions, which is independent from the eat-2 and daf-2/daf-16 pathway and dependent on glyoxalase-1–controlled mitochondrial complexes II and III (Table 1).
DISCUSSION
The major purpose of the study was to establish C. elegans as a model for diabetes research to understand basic mechanisms underlying glucose effects on cellular and mitochondrial function. Because a total body glucose concentration of 14 mmol/l was sufficient to achieve significant effects on life span, this in vivo model organism provides a reliable tool to decipher changes in cellular functions induced by glucose concentrations that are within the range observed in poorly controlled diabetic patients.
As shown here, long-lasting high glucose conditions result in a permanent accumulation of methylglyoxal-derived modifications of mitochondrial proteins, a steady increase in ROS formation and a significant reduction in life span. These effects are independent of osmotic pressure, the pathways activated by caloric restriction in eat-2 mutants, and the insulin-like receptor daf-2/daf-16 pathway, one of the central pathways involved in life span determination. Because overexpression of the C. elegans homologue of glyoxalase-1 partly reversed these specific glucose effects, while knockdown of glyoxalase-1 further shortened the life span, protective enzymes capable of reducing reactive metabolites generated by high glucose, do not only normalize the function of single cells but determine the life span of a whole organism.
In a recent study, Schulz et al. (6) analyzed life span extension in C. elegans by glucose restriction. It was concluded that impairment of glucose metabolism initially causes enhanced mitochondrial respiration and increased ROS formation resulting in a short-lasting and nonlethal stress, leading to a secondary and long-lasting increase in antioxidant defenses. Therefore, glucose restriction increases C. elegans life span by hormetic sustained reduction of net stress levels. This fits well to the previously established role of ROS in activating antioxidant response mechanisms and thereby providing tools for prolonging life span. Another study shows that mitochondria of long-lived daf-2 mutants produce increased amounts of ROS compared to wild-type mitochondria (15), probably because daf-2 mutants show a higher antioxidant activity by upregulation of antioxidant enzymes (16–18). Up to now, however, it is not known whether these life-enhancing effects of such a glucose restriction would still be evident under conditions with high extracellular glucose concentrations.
Additional studies are needed to analyze whether the changes in life span observed by glucose administration or restriction are mainly depending on extracellular or intracellular effects. Our data suggest that the excess of a certain glucose level leads to an overload and subsequent inhibition of the glyoxalase-1 system. A potential explanation is that the long-lasting high glucose conditions used here result in a sustained excess of ROS, depleting glyoxalase-1, which in turn might result in a breakdown of the mitochondrial integrity by methylglyoxal-dependent protein modification and ultimatively ends in increased ROS formation and life span reduction (4).
Future studies are required to prove whether mechanisms described in C. elegans can be translated to the situation in diabetic patients. Although total life span is an emerging goal of diabetes treatment, it is evident that simply lowering glucose concentrations is not sufficient to normalize life expectancy in patients with diabetes (19). The availability of an in vivo model of life expectancy might open new opportunities to study cellular defense systems such as glyoxalase-1 in the context of high glucose and total life span. Thus, protecting glyoxalase-1 activity from glucose-dependent downregulation might provide a promising therapeutic option in increasing the life expectancy in diabetic patients.
Acknowledgments
This work was supported by research grants from the Netzwerk Altersforschung (NAR) (M.M., P.H., and A.B.), the Hopp-Stiftung für Alternsforschung (M.M., P.H., A.B., and P.N.), the Juvenile Diabetes Research Foundation (A.B. and P.N.), and the Manfred Lautenschläger Stiftung (A.B. and P.N.).
No potential conflicts of interest relevant to this article were reported.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Kimura KD, Tissenbaum HA, Liu Y, Ruvkun G: daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Science 1997;277:942–946 [DOI] [PubMed] [Google Scholar]
- 2.Phillips SA, Thornalley PJ: The formation of methylglyoxal from triose phosphates. Investigation using a specific assay for methylglyoxal. Eur J Biochem 1993;212:101–105 [DOI] [PubMed] [Google Scholar]
- 3.Lo TW, Westwood ME, McLellan AC, Selwood T, Thornalley PJ: Binding and modification of proteins by methylglyoxal under physiological conditions. A kinetic and mechanistic study with N α-acetylarginine, N α-acetylcysteine, and N α-acetyllysine, and bovine serum albumin. J Biol Chem 1994;269:32299–32305 [PubMed] [Google Scholar]
- 4.Morcos M, Du X, Pfisterer F, Hutter H, Sayed AA, Thornalley P, Ahmed N, Baynes J, Thorpe S, Kukudov G, Schlotterer A, Bozorgmehr F, El Baki RA, Stern D, Moehrlen F, Ibrahim Y, Oikonomou D, Hamann A, Becker C, Zeier M, Schwenger V, Miftari N, Humpert P, Hammes HP, Buechler M, Bierhaus A, Brownlee M, Nawroth PP: Glyoxalase-1 prevents mitochondrial protein modification and enhances lifespan in Caenorhabditis elegans. Aging Cell 2008;7:260–269 [DOI] [PubMed] [Google Scholar]
- 5.Thornalley PJ: The glyoxalase system: new developments towards functional characterization of a metabolic pathway fundamental to biological life. Biochem J 1990;269:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schulz TJ, Zarse K, Voigt A, Urban N, Birringer M, Ristow M: Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress. Cell Metab 2007;6:280–293 [DOI] [PubMed] [Google Scholar]
- 7.Racker E: The mechanism of action of glyoxalase. J Biol Chem 1951;190:685–696 [PubMed] [Google Scholar]
- 8.McLellan AC, Thornalley PJ, Benn J, Sonksen PH: Glyoxalase system in clinical diabetes mellitus and correlation with diabetic complications. Clin Sci (Lond) 1994;87:21–29 [DOI] [PubMed] [Google Scholar]
- 9.Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R: A C. elegans mutant that lives twice as long as wild type. Nature 1993;366:461–464 [DOI] [PubMed] [Google Scholar]
- 10.Rasband WS: ImageJ http://rsb.info.nih.gov/ij/ U. S. National Institutes of Health, Bethesda, Maryland, 1997 [Google Scholar]
- 11.Li Q, Lau A, Morris TJ, Guo L, Fordyce CB, Stanley EF: A syntaxin 1, G-α (o), and N-type calcium channel complex at a presynaptic nerve terminal: analysis by quantitative immunocolocalization. J Neurosci 2004;24:4070–4081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Timmons L, Fire A: Specific interference by ingested dsRNA. Nature 1998;395:854. [DOI] [PubMed] [Google Scholar]
- 13.Kamath RS, Fraser AG, Dong Y, Poulin G, Durbin R, Gotta M, Kanapin A, Le Bot N, Moreno S, Sohrmann M, Welchman DP, Zipperlen P, Ahringer J: Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature 2003;421:231–237 [DOI] [PubMed] [Google Scholar]
- 14.Brownlee M: The pathological implications of protein glycation. Clin Invest Med 1995;18:275–281 [PubMed] [Google Scholar]
- 15.Brys K, Vanfleteren JR, Braeckman BP: Testing the rate-of-living/oxidative damage theory of aging in the nematode model Caenorhabditis elegans. Exp Gerontol 2007;42:845–851 [DOI] [PubMed] [Google Scholar]
- 16.Lee SS, Kennedy S, Tolonen AC, Ruvkun G: DAF-16 target genes that control C. elegans life-span and metabolism. Science 2003;300:644–647 [DOI] [PubMed] [Google Scholar]
- 17.Murphy CT, McCarroll SA, Bargmann CI, Fraser A, Kamath RS, Ahringer J, Li H, Kenyon C: Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature 2003;424:277–283 [DOI] [PubMed] [Google Scholar]
- 18.McElwee J, Bubb K, Thomas JH: Transcriptional outputs of the Caenorhabditis elegans forkhead protein DAF-16. Aging Cell 2003;2:111–121 [DOI] [PubMed] [Google Scholar]
- 19.Gerstein HC, Miller ME, Byington RP, Goff DC, Jr, Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, Grimm RH, Jr, Probstfield JL, Simons-Morton DG, Friedewald WT: Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358:2545–2559 [DOI] [PMC free article] [PubMed] [Google Scholar]