Abstract
OBJECTIVE
This study investigated the hypothesis that baseline calcified coronary atherosclerosis may determine cardiovascular disease events in response to intensive glycemic control within the Veterans Affairs Diabetes Trial (VADT).
RESEARCH DESIGN AND METHODS
At baseline, 301 type 2 diabetic participants in the VADT, a randomized trial comparing the effects of intensive versus standard glucose lowering on cardiovascular events, had baseline coronary atherosclerosis assessed by coronary artery calcium (CAC) measured by computed tomography. Participants were followed over the 7.5-year study for development of cardiovascular end points.
RESULTS
During a median follow-up duration of 5.2 years, 89 cardiovascular events occurred. Although intensive glucose-lowering therapy did not significantly reduce cardiovascular events in the substudy cohort as a whole, there was evidence that the response was modified by baseline CAC, as indicated by significant P values for treatment by log(CAC + 1) interaction terms in unadjusted and multivariable-adjusted models (0.01 and 0.03, respectively). Multivariable-adjusted hazard ratios (HRs) for the effect of treatment indicated a progressive diminution of benefit with increasing CAC. Subgroup analyses were also conducted for clinically relevant CAC categories: those above and below an Agatston score of 100. Among those randomized to intensive treatment, for the subgroup with CAC >100, 11 of 62 individuals had events, while only 1 of 52 individuals with CAC ≤100 had an event. The multivariable HR for intensive treatment for those with CAC >100 was 0.74 (95% CI 0.46–1.20; P = 0.21), while for the subgroup with CAC ≤100, the corresponding HR was 0.08 (0.008–0.77; P = 0.03), with event rates of 39 and 4 per 1,000 person-years, respectively.
CONCLUSIONS
These data indicate that intensive glucose lowering reduces cardiovascular events in those with less extensive calcified coronary atherosclerosis.
Despite a wealth of epidemiologic data indicating that poor glycemic control is associated with increased risk for subsequent cardiovascular disease (CVD) events (1–4), trials of glucose-lowering therapy have not reduced the incidence of CVD (5–12). The Veterans Affairs Diabetes Trial (VADT) was one of several recent large prospective randomized studies initiated to help clarify whether intensive glycemic control would reduce development of macrovascular disease events (13). After a median duration of follow-up of >5 years, the group receiving intensive glycemic control in the VADT experienced no significant reduction in CVD events despite an average 1.5% lower median A1C throughout the trial (14).
To more fully understand the relationships between glycemic control, calcified atherosclerosis, and CVD, a substudy of subclinical atherosclerosis was conducted in coordination with the VADT. This study, the Risk Factors, Atherosclerosis, and Clinical Events in Diabetes (RACED) study (15,16), examined coronary artery calcium (CAC) in a subset of the VADT participants. A major goal of this substudy was to determine whether the effect of intensive glycemic control on CVD depended on the initial extent of underlying atherosclerosis (as estimated by vascular calcium) in participants.
RESEARCH DESIGN AND METHODS
Data for this study derive primarily from baseline examinations of participants in the RACED study (15,16) and include incident CVD events during the VADT. (Study staff and investigators in the RACED study are listed in the online appendix, available at http://diabetes.diabetesjournals.org/cgi/content/full/db09-0618/DC1). Sites that had access to appropriate computed tomography scanning centers and were geographically representative of the national distribution of VADT sites were selected for this substudy. The RACED study included 324 patients with type 2 diabetes who were participating at seven study sites (distributed across the southwest, midwest, and the southeast regions of the U.S.) of the VADT and was approved by the institutional review boards of these sites. All participants gave written informed consent. All subjects enrolling into the VADT at the seven selected sites were asked to participate in this substudy; of these, ∼95% agreed to receive CAC scans. Detailed descriptions of the VADT, including exclusion and inclusion criteria, study activities, and main results, have previously been reported (13,14).
Incident CVD events.
The primary VADT end point was time to first occurrence of any of a composite of CVD events including documented myocardial infarction; cerebrovascular accident; cardiovascular death; new or worsening congestive heart failure; surgical intervention for cardiac, cerebrovascular, or peripheral vascular disease; inoperable coronary artery disease; and amputation for ischemic gangrene. All reported end points were adjudicated by an end points committee (13,14).
Coronary artery and abdominal aortic calcium scores.
Coronary artery calcium was measured using electron beam computed tomography cardiac scanners (GE Imatron, South San Francisco, CA) as previously described (15,16). Readers scoring calcium at the centralized reading center were blinded to demographic and clinical information. A threshold of four contiguous pixels and 130 Hounsfield units was used to identify calcified lesions that were scored using the algorithm developed by Agatston et al. (17). Total CAC was determined by summing individual lesion scores from each of four anatomic sites (left main, left anterior descending, circumflex, and right coronary arteries). A calibration phantom was scanned under the chest of each participant at each scanning center to calibrate the images to identical standards, as previously described (15,16).
Statistical analyses.
Statistical analyses were performed with SAS (version 9.2; SAS Institute, Cary, NC). Measures that were normally distributed are reported as means ± SD. Variables with a skewed distribution are reported as median (interquartile range) or proportions. Significant differences between groups were assessed using t tests, Wilcoxon's two-sample rank-sum test, or χ2 tests, as appropriate. Prespecified goals of the RACED study included determining whether baseline CAC [parameterized as log10(CAC + 1) and by clinical categories of CAC] influenced the relationship between treatment assignment and CVD.
Kaplan-Meier curves were used to illustrate the relationship between categories of calcium and CVD events by treatment groups. Patients without an event were censored at their date of withdrawal from the study or of the final follow-up visit. To directly test effect modification by CAC, the effects for treatment, CAC [parameterized as log(CAC + 1) or by CAC categories], and a treatment-CAC interaction term were tested in unadjusted and adjusted Cox models. Hazard ratios (HRs) for effects of intensive versus standard treatment were then separately assessed within lower- and higher-CAC subgroups. To limit the number of degrees of freedom for predictor variables to permit adjustment for appropriate covariates, and because there were no events in individuals assigned to intensive treatment who were within the lowest CAC category, a post hoc decision was made to collapse the CAC categories into clinically relevant categories (no/mild vs. moderate/severe disease) by dichotomizing at an Agatston score of 100 (18,19). To avoid overfitting of final regression models, prespecified baseline covariates were limited to age, ethnicity, and variables (diabetes duration, smoking, prior CVD, hypertension, glycemic control, and lipid levels) for which the literature strongly supports a relationship with either CVD events or CAC. Adding other variables (BMI, serum creatinine, urinary albumin/creatinine, blood pressure, and medication use) to those models did not meaningfully alter results concerning the CAC-treatment interaction. All reported P values are two sided and not adjusted for multiple testing.
RESULTS
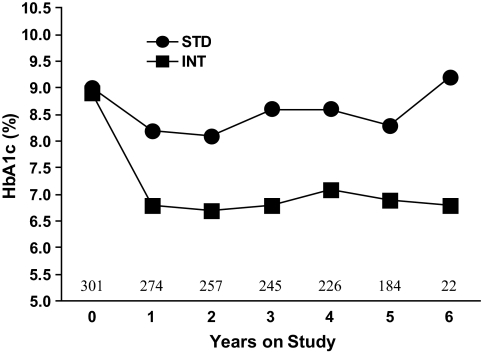
For the 301 participants with available baseline CAC scans, the mean duration of diabetes was 12.1 ± 8.0 years and mean age 61.0 ± 9.0 years, most subjects were male (95%) and non-Hispanic white (65%), and prevalence of hypertension (79%) and prior CVD (38%) was high. Clinical characteristics for the 301 RACED participants were generally similar to those for the other VADT participants (Table 1). The minimum CAC Agatston score was 0 (present in 16%), while 25th, 50th (median), and 75th percentiles for CAC were 23, 276, and 872, respectively. Clinical characteristics by treatment group are shown in Table 2. The intensive group had lower triglycerides and higher HDL cholesterol (P < 0.05 for both) but had similar levels of other risk factors. As in the VADT (14), A1C levels in intensive and standard groups in the RACED substudy were well separated after 1 year (P < 0.01), and the difference averaged ∼1.5% units throughout the study (Fig. 1).
TABLE 1.
Comparison of clinical characteristics for RACED participants and for VADT participants who did not participate in RACED
| RACED | VADT | P | |
|---|---|---|---|
| n | 301 | 1,490 | |
| Age (years) | 61 ± 9 | 60 ± 9 | 0.19 |
| Male | 94.7 | 97.6 | 0.01 |
| NHW | 65 | 61 | 0.18 |
| Duration of diabetes (years) | 12.1 ± 8.0 | 11.4 ± 7.4 | 0.15 |
| BMI (kg/m2) | 31.5 ± 4.3 | 31.2 ± 4.4 | 0.24 |
| History of CVD | 38 | 41 | 0.33 |
| Current smoker | 15 | 17 | 0.37 |
| History of hypertension | 79 | 71 | 0.01 |
| SBP (mmHg) | 131 ± 17 | 132 ± 17 | 0.77 |
| DBP (mmHg) | 75 ± 10 | 76 ± 10 | 0.04 |
| A1C | 9.3 ± 1.4 | 9.5 ± 1.6 | 0.04 |
| Total cholesterol (mmol/l) | 4.65 ± 0.98 | 4.76 ± 1.27 | 0.17 |
| HDL cholesterol (mmol/l) | 0.96 ± 0.26 | 0.93 ± 0.26 | 0.18 |
| LDL cholesterol (mmol/l) | 2.69 ± 0.80 | 2.79 ± 0.83 | 0.09 |
| Triglycerides (mmol/l) | 2.27 ± 1.48 | 2.42 ± 3.33 | 0.46 |
Data are means ± SD or percent unless otherwise indicated. P values are from independent-samples t tests or Wilcoxon or χ2 tests, as appropriate. DBP, diastolic blood pressure; NHW, non-Hispanic white; SBP, systolic blood pressure.
TABLE 2.
Comparison of clinical characteristics of RACED participants according to VADT treatment assignment
| Standard | Intensive | P | |
|---|---|---|---|
| n | 159 | 142 | |
| Age (years) | 61 ± 9 | 61 ± 9 | 0.89 |
| NHW | 69 | 62 | 0.23 |
| Duration of diabetes (years) | 12.0 ± 7.7 | 12.2 ± 8.3 | 0.77 |
| BMI (kg/m2) | 31.7 ± 4.3 | 31.3 ± 4.4 | 0.47 |
| History of CVD | 39 | 37 | 0.67 |
| Current smoker | 15 | 15 | 0.94 |
| History of hypertension | 78 | 81 | 0.49 |
| SBP (mmHg) | 132 ± 18 | 130 ± 16 | 0.32 |
| DBP (mmHg) | 75 ± 10 | 75 ± 11 | 0.72 |
| A1C | 9.3 ± 1.4 | 9.3 ± 1.5 | 0.94 |
| Total cholesterol (mmol/l) | 4.64 ± 0.96 | 4.67 ± 1.01 | 0.73 |
| HDL cholesterol (mmol/l) | 0.92 ± 0.27 | 0.98 ± 0.26 | 0.03 |
| LDL cholesterol (mmol/l) | 2.65 ± 0.79 | 2.77 ± 0.79 | 0.23 |
| Triglycerides (mmol/l) | 2.48 ± 1.64 | 2.04 ± 1.24 | 0.01 |
| Statin use | 57 | 64 | 0.23 |
| TZD use | 12 | 10 | 0.56 |
| ASA use | 86 | 86 | 0.95 |
| BP medication | 91 | 89 | 0.61 |
Data are means ± SD or percent unless otherwise indicated. ASA, aspirin; BP, blood pressure; DBP, diastolic blood pressure; NHW, non-Hispanic white; SBP, systolic blood pressure; TZD, thiazolidinedione. P values are from independent-samples t tests or Wilcoxon or χ2 tests, as appropriate.
FIG. 1.
Time course of A1C levels for intensive (INT) and standard (STD) treatment groups. Median levels of A1C are shown by study year (0 = baseline); the P value for the overall difference between groups is <0.01. Shown above the x-axis are the total number of participants at baseline and the beginning of each follow-up year through year 6.
In the RACED cohort, a total of 89 participants experienced primary events over a median follow-up duration of 5.2 years. These events included 30 coronary, carotid, or peripheral revascularizations, 19 episodes of congestive heart failure, 16 myocardial infarctions, 10 strokes, 9 CVD deaths, 3 ischemic amputations, and 2 cases of severe but inoperable coronary artery disease. RACED participants who suffered a CVD event during follow-up were older, more likely to be non-Hispanic white, and had a longer history of diabetes, a greater prevalence of prior CVD, and lower HDL cholesterol (all P < 0.05). The median CAC was significantly higher in those who had an event (P < 0.01). Other characteristics did not differ significantly between those who did or did not develop clinical events. In this cohort, the reduction in CVD observed in the intensive treatment group was not significant (unadjusted HR 0.72 [0.47 – 1.10]; P = 0.13), and was comparable to that found for the entire VADT (14).
To test whether baseline CAC modified the influence of intensive glucose management (as assigned within the VADT) on future CVD events, we considered models including interactions between treatment and calcium [parameterized as log(CAC + 1) or dichotomized at 100]. In models including log(CAC + 1) or calcium categories (0–100 and >100), treatment, and calcium-by-treatment interaction but no adjustment for other covariates, the P values for interaction were 0.01 and 0.05, respectively, indicating that effects of intensive versus standard glucose management were modified by baseline CAC. After adjustment for relevant covariates, P values for interaction terms in corresponding multivariable models were 0.03 and 0.07, respectively, providing further support for differential effects of treatment according to level of CAC. A bootstrap analysis (using 2,000 samples) was also performed and identified the treatment, baseline CAC, interaction of baseline CAC with treatment, and prior CVD history as the four most important predictors of CVD events, each occurring in >73% of the repeated samplings (data not shown).
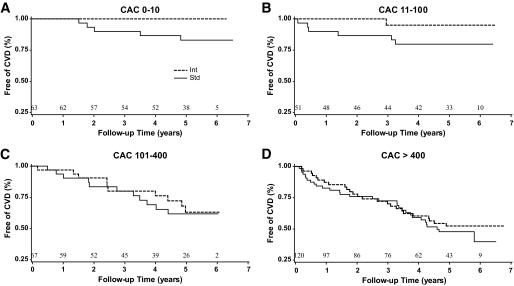
Kaplan-Meier curves for time to first CVD primary event (Fig. 2) by treatment group for each of the four categories of CAC illustrate the modification of treatment effect with changes in baseline CAC. In the lowest two categories of calcium (CAC 0–10 and 11–100), participants randomized to receive intensive treatment had a CVD risk that was substantially lower than those randomized to standard therapy (P = 0.03 for those with CAC 0–10 and P = 0.12 for those with CAC 11–100). In fact, with these two low-CAC groups combined, a very low proportion of those receiving intensive therapy developed events (only 1 of 52 participants during follow-up; median time to event 5.2 years and maximum >6 years). In comparison, with the same two groups combined, a much larger proportion (11 of 62 participants) in the standard arm had an event. This corresponded to event rates of 4 and 39 per 1,000 person-years for those receiving intensive and standard therapy, respectively. The event types by treatment group are shown in the supplemental table in the online appendix. A comparison of risk factors and CAC scores between intensive and standard treatment arms in the combined lower CAC categories revealed no significant differences (Table 3), and any potentially relevant differences in these variables were accounted for in subsequent multivariable models. Similar results were present when individuals without previous CVD were excluded. In contrast, Kaplan-Meier curves for participants in the two higher CAC categories (101–400 and >400) demonstrated similar levels of CVD risk in both treatment groups. This occurred despite the fact that at baseline, triglyceride levels were lower and HDL cholesterol levels higher in the intensive treatment group (Table 3). The distribution of CVD events (supplemental Table 1) was similar between treatment groups in the higher two CAC categories.
FIG. 2.
Kaplan-Meier curves for time to primary macrovascular end point by clinical categories of CAC (0–10 [A], 11–100 [B], 101–400 [C], and >400[D]) in those randomized to the standard (Std) or intensive (Int) therapy arm. Differences between treatment groups were significant in A (P = 0.03). Shown above the x-axes are the total numbers of participants at risk at baseline and the beginning of each follow-up year through year 6.
TABLE 3.
Comparison of clinical characteristics for RACED participants with lower (≤100) or higher (>100) CAC according to VADT treatment assignment
| Lower CAC |
P | Higher CAC |
P | |||
|---|---|---|---|---|---|---|
| Standard | Intensive | Standard | Intensive | |||
| n | 62 | 52 | 97 | 90 | ||
| Age (years) | 57 ± 9 | 57 ± 9 | 0.74 | 64 ± 9 | 64 ± 9 | 0.90 |
| NHW | 58 | 48 | 0.29 | 75 | 70 | 0.42 |
| Duration of diabetes (years) | 10.2 ± 7.2 | 9.4 ± 7.8 | 0.57 | 13.12 ± 7.2 | 13.9 ± 7.8 | 0.49 |
| BMI (kg/m2) | 31.8 ± 4.0 | 30.9 ± 4.6 | 0.28 | 31.6 ± 4.5 | 31.5 ± 4.3 | 0.92 |
| History of CVD | 16 | 6 | 0.08 | 54 | 54 | 0.91 |
| Current smoker | 18 | 15 | 0.74 | 13 | 14 | 0.84 |
| History of hypertension | 65 | 75 | 0.23 | 86 | 84 | 0.72 |
| SBP (mmHg) | 133 ± 18 | 131 ± 16 | 0.62 | 132 ± 18 | 130 ± 17 | 0.39 |
| DBP (mmHg) | 78 ± 10 | 79 ± 11 | 0.56 | 73 ± 9 | 73 ± 10 | 0.88 |
| A1C | 9.6 ± 1.4 | 9.5 ± 1.5 | 0.88 | 9.1 ± 1.3 | 9.1 ± 1.4 | 0.90 |
| Total cholesterol (mmol/l) | 4.84 ± 1.16 | 4.94 ± 1.11 | 0.70 | 4.50 ± 0.78 | 4.53 ± 0.92 | 0.83 |
| HDL cholesterol (mmol/l) | 0.96 ± 0.28 | 1.06 ± 0.31 | 0.12 | 0.89 ± 0.25 | 1.06 ± 0.21 | 0.11 |
| LDL cholesterol (mmol/l) | 2.79 ± 0.93 | 2.90 ± 0.88 | 0.52 | 2.57 ± 0.67 | 2.68 ± 0.72 | 0.28 |
| Triglycerides (mmol/l) | 2.40 ± 1.70 | 2.05 ± 1.00 | 0.21 | 2.53 ± 1.61 | 2.03 ± 1.36 | 0.02 |
| Statin use | 52 | 54 | 0.81 | 61 | 70 | 0.19 |
| TZD use | 13 | 8 | 0.37 | 11 | 11 | 0.96 |
| ASA use | 87 | 85 | 0.71 | 86 | 87 | 0.83 |
| BP medication | 89 | 85 | 0.52 | 93 | 92 | 0.88 |
| CAC (Agatston units) | 20 ± 26 | 18 ± 26 | 0.64 | 1,106 ± 1,384 | 1,106 ± 1,146 | 0.99 |
Data are means ± SD or percent unless otherwise indicated. ASA, aspirin; BP, blood pressure; DBP, diastolic blood pressure; NHW, non-Hispanic white; SBP, systolic blood pressure; TZD, thiazolidinedione. P values are from independent-samples t tests or Wilcoxon or χ2 tests, as appropriate.
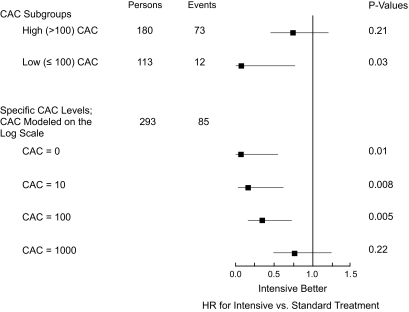
Figure 3 graphically displays HRs and CIs for the effect of intensive treatment for multivariable models fitted separately for subgroups with higher (>100) and lower (≤100) CAC. For the subgroup with higher CAC, the multivariable HR for the effect of treatment was 0.74 (95% CI 0.46–1.18; P = 0.21), while for those with lower CAC, the multivariable HR for treatment was 0.08 (0.008–0.77; P = 0.03). The magnitude of benefit of treatment across the range of CAC scores was further examined by estimating HRs for treatment from a multivariable model including age, ethnicity, diabetes duration, history of hypertension, history of smoking, prior CVD history, total and HDL cholesterol, and A1C as covariates and, additionally, log(CAC + 1), treatment, and calcium-by-treatment interaction. This analysis with CAC parameterized on the log scale was performed with data for all participants. This approach revealed that the benefit of intensive treatment diminished in a progressive manner across selected CAC scores of 0, 10, and 100, with HRs of 0.07 (0.01–0.55; P = 0.01), 0.16 (0.04–0.61; P < 0.01), and 0.34 (0.16–0.73; P < 0.01), and was completely absent at very high CAC scores (e.g., an HR of 0.75 [95% CI 0.47–1.19; P = 0.22] for a CAC score of 1,000). Additional sensitivity analyses, using CAC coded as tertiles (0–57, 58–641, and >641) or as three clinical categories (0–100, 101–400, and >400), yielded consistent results and demonstrated that the benefit of intensive glycemic control for CVD outcomes was apparent only for individuals in the lowest CAC category.
FIG. 3.
HRs (95% CI) for effect of treatment (intensive vs. standard) in multivariable-adjusted models. Boxes represent HRs, and lines indicate 95% CI. P values indicate the significance of treatment effect in the indicated models. The treatment effect was estimated for high- and low-CAC subgroups separately (upper portion of figure) or for specific CAC scores (lower portion of figure) within a multivariable model including log(CAC + 1) as a continuous variable, treatment, and the calcium-treatment interaction effect. Multivariable models included age, ethnicity, diabetes duration, history of hypertension, history of smoking, prior CVD history, total and HDL cholesterol, and A1C as covariates.
DISCUSSION
The major novel finding of this study was that in a subset of VADT participants, the benefit of intensive glycemic control on CVD outcomes was greater for those with lower CAC. Models with CAC parameterized either as a continuous variable [log(CAC + 1)] or by categories of CAC, whether or not adjustment was made for multiple covariates, provided consistent evidence for the presence of a significant treatment-calcium interaction. These results were also observed when the analysis was limited to RACED subjects without a baseline history of CVD. Both the continuous model and the model based on the categories of CAC indicated that intensive glucose therapy reduced future CVD events predominantly in participants with less extensive atherosclerotic disease. This translated to a nearly 90% reduction in incidence of CVD events in those with very low CAC (subgroup with CAC ≤100 where the median CAC was <5 or with CAC ≤10 in the log model). In this low-CAC group, the estimated number needed to treat with intensive glucose lowering to prevent one CVD event was 29. Less benefit was observed at higher levels of CAC.
These RACED substudy results suggest that intensive glycemic control may not be effective in those with more advanced vascular disease. Based on the CAC distribution in this VADT subset, nearly 40% of the full VADT cohort presumably would have had extensive atherosclerosis (with CAC >400) at baseline and >60% would have CAC >100 at study entry. Thus, intensive glycemic control would not be expected to be beneficial in that large portion of the VADT cohort which had more extensive atherosclerosis. Consistent with this, the reduction in CVD observed in the intensive treatment group in the overall VADT (unadjusted HR 0.88 [95% CI 0.74–1.05]; P = 0.14) was not significant and was comparable with that found for all subjects in this substudy (0.72 [0.47–1.10]; P = 0.13). The modestly better outcome in the RACED cohort (compared with that in the overall VADT study) may reflect a different composition of participants or more intensive glucose lowering, but this is not apparent from the baseline clinical characteristics (Table 1) or A1C values observed during the study (Fig. 1). Moreover, despite the slight trend for a more favorable outcome in response to intensive glucose reduction in the RACED cohort, those with higher levels of CAC received substantially less benefit from intensive therapy than did those with lower CAC. This conclusion seems to provide a plausible interpretation of the results from the ACCORD and ADVANCE trials (11,12). Both of those studied populations were older and included participants with established diabetes and one or more CVD risk factors, suggesting that most of the participants in these studies may also have had relatively advanced atherosclerosis. This is supported by the fact that nearly one-third of both cohorts had a history of CVD at study entry. Therefore, inferring from the current results, both the ACCORD and ADVANCE cohorts may also have had such extensive atherosclerosis that no benefit from intensive glucose lowering was observed.
The current RACED study does, however, indicate that intensive glycemic therapy may be effective in those with less extensive coronary atherosclerosis. Consistent with these results, two subgroups of subjects in the ACCORD study that could reasonably be expected to have reduced atherosclerosis—those with lower baseline A1C values (<8%) and those with no prior history of CVD—demonstrated significant reductions in the primary composite CVD end point in response to intensive glycemic control (11). This may also explain the more favorable results in the UK Prospective Diabetes Study, which evaluated improved glucose control in patients with newly diagnosed diabetes (6,20). An important implication of these findings is that it may be possible to identify patients who may obtain greater CVD benefit from intensive glucose-lowering therapy while preserving quality of life and avoiding unnecessary expenditure of resources and risk of adverse side effects of intensive treatment in those less likely to benefit.
An important, but unanswered, question is why glucose lowering is less effective in reducing CVD events in individuals with more advanced atherosclerosis. Although numerous mechanisms have been implicated in the initiation and development of atherosclerosis in diabetes, there is less information about which processes may be responsive to lowered glucose. One may speculate that when sufficient plaque development has occurred, the presence of modified lipoproteins, activated vascular cells, and altered immune cell signaling may generate a self-propagating process that maintains atherogenesis even in the face of improved glucose control. Alternatively, chronic hyperglycemia leads to advanced glycation end product formation and extensive protein cross-linking—a process that is not readily reversible, increases with age, and is enhanced by oxidative stress (21). For older individuals with a long history of diabetes, the past burden of hyperglycemia and advanced glycation end product formation is presumably extensive and may have long-term negative “legacy” effects (21,22). Although glucose lowering may prevent new advanced glycation end product formation and limit new vascular injury, 3–5 years of intensive glycemic control as occurs during clinical trials is presumably insufficient to reverse the accumulated vascular damage from decades of hyperglycemia or other metabolic abnormalities.
Several limitations of this study deserve mention. The number of incident CVD events in this substudy of the VADT is relatively modest. Although we accounted for the available cardiovascular risk factors that appeared to be relevant determinants of CVD events in this cohort, it is possible that other useful predictors may exist and were not examined. However, comparison of risk factors and relevant medication use between standard and intensive groups in RACED participants with lower CAC at baseline revealed similar levels of risk factors (Table 3), and treatment effects persisted in multivariable (i.e., adjusted) models. Although examination of baseline CAC (using either log CAC or clinical CAC categories) as an effect modifier of glycemic control was a prespecified aim of this study, the decision to dichotomize CAC at 100 was determined post hoc to facilitate analysis in the context of the very low number of events in those with low CAC. The cut point of 100 Agatston units for CAC is clinically used to stratify CVD risk and reflected expected differences in incidence of CVD between the low and high CAC categories within the RACED cohort (18,19). Moreover, when CAC was considered as a continuous variable, which fit the data somewhat better than the CAC dichotomization approach, there was a progressive increase in benefit of intensive treatment with decreasing values of CAC, and again, no treatment benefit was seen in those at the highest CAC scores. Additional sensitivity analyses, using CAC expressed either as tertiles or as three clinical categories (0–100, 101–400, and >400) also demonstrated that the benefit of intensive glycemic control for CVD was present only for individuals within the lowest CAC category. Despite these consistent results, it is important that these novel findings be confirmed in larger diabetes cohorts and in studies with a greater proportion of female participants. In summary, these data provide support for the concept that intensive glucose-lowering therapy may be most effective in reducing CVD in those with less extensive coronary atherosclerosis.
Supplementary Material
Acknowledgments
Financial support for this work was provided by the Cooperative Studies Program of the Department of Veterans Affairs Office of Research and Development, National Institutes of Health Grant RO1067690 (to P.D.R.), the Kronos Research Institute, and clinical research awards from the American Diabetes Association (to P.D.R.).
J.H.S. received grant support from Roche Pharmaceuticals. C.A. received grants from GlaxoSmithKline, sanofi-aventis, Amylin, Novo Nordisk, Roche Diagnostics, Kos Pharmaceuticals, the National Institutes of Health National Eye Institute and the American Diabetes Association, and speaker fees from sanofi-aventis and Takeda. No other potential conflicts of interest relevant to this article were reported.
Parts of this study were presented in abstract form at the 68th Scientific Sessions of the American Diabetes Association, San Francisco, California, 6–10 June 2008.
Footnotes
Clinical trial reg. no. NCT00032487, clinicaltrials.gov.
The contents of this article do not represent the views of the Department of Veterans Affairs or the U.S. government.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
See accompanying commentary, p. 2448..
REFERENCES
- 1.Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Holman RR: Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study BMJ 2000; 321: 405– 412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khaw KT, Wareham N, Bingham S, Luben R, Welch A, Day N: Association of hemoglobin A1c with cardiovascular disease and mortality in adults: the European prospective investigation into cancer in Norfolk Ann Intern Med 2004; 141: 413– 420 [DOI] [PubMed] [Google Scholar]
- 3.Selvin E, Marinopoulos S, Berkenblit G, Rami T, Brancati FL, Powe NR, Golden SH: Meta-analysis: glycosylated hemoglobin and cardiovascular disease in diabetes mellitus Ann Intern Med 2004; 141: 421– 431 [DOI] [PubMed] [Google Scholar]
- 4.Laakso M: Hyperglycemia and cardiovascular disease in type 2 diabetes Diabetes 1999; 48: 937– 942 [DOI] [PubMed] [Google Scholar]
- 5.UK Prospective Diabetes Study (UKPDS) Group UKPDS: intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet 1998; 352: 837– 853 [PubMed] [Google Scholar]
- 6.UKPDS Group Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34) Lancet 1998; 352: 854– 865 [PubMed] [Google Scholar]
- 7.Duckworth WC, McCarren M, Abraira C: Glucose control and cardiovascular complications: the VA Diabetes Trial Diabetes Care 2001; 24: 942– 945 [DOI] [PubMed] [Google Scholar]
- 8.Stettler C, Allemann S, Juni P, Cull CA, Holman RR, Egger M, Krahenbuhl S, Diem P: Glycemic control and macrovascular disease in types 1 and 2 diabetes mellitus: meta-analysis of randomized trials Am Heart J 2006; 152: 27– 38 [DOI] [PubMed] [Google Scholar]
- 9.Leibel B: An analysis of the University Group Diabetes Study Program: data results and conslusions Can Med Assoc J 1971; 105: 292– 294 [PMC free article] [PubMed] [Google Scholar]
- 10.Abraira C, Colwell JA, Nuttall FQ, Sawin CT, Nagel NJ, Comstock JP, Emanuele NV, Levin SR, Henderson W, Lee HS: Veterans Affairs Cooperative Study on glycemic control and complications in type II diabetes (VA CSDM): results of the feasibility trial: Veterans Affairs Cooperative Study in Type II Diabetes Diabetes Care 1995; 18: 1113– 1123 [DOI] [PubMed] [Google Scholar]
- 11.Gerstein HC, Miller ME, Byington RP, Goff DC, Jr, Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, Grimm RH, Jr, Probstfield JL, Simons-Morton DG, Friedewald WT: Effects of intensive glucose lowering in type 2 diabetes N Engl J Med 2008; 358: 2545– 2559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D, Hamet P, Harrap S, Heller S, Liu L, Mancia G, Mogensen CE, Pan C, Poulter N, Rodgers A, Williams B, Bompoint S, de Galan BE, Joshi R, Travert F: Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes N Engl J Med 2008; 358: 2560– 2572 [DOI] [PubMed] [Google Scholar]
- 13.Abraira C, Duckworth W, McCarren M, Emanuele N, Arca D, Reda D, Henderson W: Design of the cooperative study on glycemic control and complications in diabetes mellitus type 2: Veterans Affairs Diabetes Trial J Diabetes Complications 2003; 17: 314– 322 [DOI] [PubMed] [Google Scholar]
- 14.Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R, Warren SR, Goldman S, McCarren M, Vitek ME, Henderson WG, Huang GD: Glucose control and vascular complications in veterans with type 2 diabetes N Engl J Med 2009; 360: 129– 139 [DOI] [PubMed] [Google Scholar]
- 15.Reaven PD, Sacks J: Coronary artery and abdominal aortic calcification are associated with cardiovascular disease in type 2 diabetes Diabetologia 2005; 48: 379– 385 [DOI] [PubMed] [Google Scholar]
- 16.Reaven PD, Sacks J: Reduced coronary artery and abdominal aortic calcification in Hispanics with type 2 diabetes Diabetes Care 2004; 27: 1115– 1120 [DOI] [PubMed] [Google Scholar]
- 17.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R: Quantification of coronary artery calcium using ultrafast computed tomography J Am Coll Cardiol 1990; 15: 827– 832 [DOI] [PubMed] [Google Scholar]
- 18.Greenland P, Bonow RO, Brundage BH, Budoff MJ, Eisenberg MJ, Grundy SM, Lauer MS, Post WS, Raggi P, Redberg RF, Rodgers GP, Shaw LJ, Taylor AJ, Weintraub WS: ACCF/AHA 2007 clinical expert consensus document on coronary artery calcium scoring by computed tomography in global cardiovascular risk assessment and in evaluation of patients with chest pain: a report of the American College of Cardiology Foundation Clinical Expert Consensus Task Force (ACCF/AHA Writing Committee to Update the 2000 Expert Consensus Document on Electron Beam Computed Tomography) developed in collaboration with the Society of Atherosclerosis Imaging and Prevention and the Society of Cardiovascular Computed Tomography J Am Coll Cardiol 2007; 49: 378– 402 [DOI] [PubMed] [Google Scholar]
- 19.Rumberger JA: Coronary artery calcium scanning using computed tomography: clinical recommendations for cardiac risk assessment and treatment Semin Ultrasound CT MR 2008; 29: 223– 229 [DOI] [PubMed] [Google Scholar]
- 20.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA: 10-year follow-up of intensive glucose control in type 2 diabetes N Engl J Med 2008; 359: 1577– 1589 [DOI] [PubMed] [Google Scholar]
- 21.Yan SF, Ramasamy R, Naka Y, Schmidt AM: Glycation, inflammation, and RAGE: a scaffold for the macrovascular complications of diabetes and beyond Circ Res 2003; 93: 1159– 1169 [DOI] [PubMed] [Google Scholar]
- 22.Del Prato S: Megatrials in type 2 diabetes: from excitement to frustration? Diabetologia 2009; 52: 1219– 1226 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.