Abstract
OBJECTIVE
To examine the association of A1C levels and fasting plasma glucose (FPG) with diabetic retinopathy in the U.S. population and to compare the ability of the two glycemic measures to discriminate between people with and without retinopathy.
RESEARCH DESIGN AND METHODS
This study included 1,066 individuals aged ≥40 years from the 2005–2006 National Health and Nutrition Examination Survey. A1C, FPG, and 45° color digital retinal images were assessed. Retinopathy was defined as a level ≥14 on the Early Treatment Diabetic Retinopathy Study severity scale. We used joinpoint regression to identify linear inflections of prevalence of retinopathy in the association between A1C and FPG.
RESULTS
The overall prevalence of retinopathy was 11%, which is appreciably lower than the prevalence in people with diagnosed diabetes (36%). There was a sharp increase in retinopathy prevalence in those with A1C ≥5.5% or FPG ≥5.8 mmol/l. After excluding 144 people using hypoglycemic medication, the change points for the greatest increase in retinopathy prevalence were A1C 5.5% and FPG 7.0 mmol/l. The coefficients of variation were 15.6 for A1C and 28.8 for FPG. Based on the areas under the receiver operating characteristic curves, A1C was a stronger discriminator of retinopathy (0.71 [95% CI 0.66–0.76]) than FPG (0.65 [0.60 – 0.70], P for difference = 0.009).
CONCLUSIONS
The steepest increase in retinopathy prevalence occurs among individuals with A1C ≥5.5% and FPG ≥5.8 mmol/l. A1C discriminates prevalence of retinopathy better than FPG.
Tests of glycemia and their thresholds for diabetes diagnosis is an area of long-standing debate. The presence of diabetic retinopathy is arguably the best criterion from which to compare glycemic measures because it is a specific and early clinical complication usually related to diabetes, and it represents a specific and relevant clinical end point for judging an alternative test (1). For these reasons, diabetic retinopathy has served as the basis for diagnostic criteria of type 2 diabetes (2–4) and provides the rationale for the American Diabetes Association's recommendation of a threshold of a fasting plasma glucose (FPG) of 7.0 mmol/l to define the presence of diabetes (4,5). However, an analysis of three recent population-based cross-sectional studies suggested that there may be considerable variation across populations and that the association of FPG with retinopathy prevalence may be more of a continuous relationship than previously thought (5).
A1C levels are being considered as an alternative diagnostic tool for diabetes diagnosis (6). Unlike FPG, A1C does not require an overnight fast, is not affected by short-term lifestyle changes, and has less variability within individuals than FPG (7–9). Nevertheless, few studies have examined the prevalence of retinopathy across the spectrum of A1C levels, which could assist in the designation of ideal A1C diagnostic cut points (2,3).
The newly released National Health and Nutrition Examination Survey (NHANES) 2005–2006 incorporated a multiple-field retinal photograph examination, presenting an opportunity to reassess the selection of glucose and A1C cut points for diabetes diagnosis. Our objectives were to examine the relation between levels of A1C and FPG and prevalence of retinopathy in the U.S. population and to compare the ability of both measures to differentiate people with and without retinopathy.
RESEARCH DESIGN AND METHODS
We analyzed 2005–2006 data from NHANES, a cross-sectional nationally representative sample of the U.S. civilian noninstitutionalized population. The sample was obtained using a stratified multistage probability design with planned oversampling of older people and minority groups. Detailed descriptions of the design and data collection of the survey are published on the National Center for Health Statistics website (10). A total of 3,056 people aged 40 years and older were interviewed, and their sociodemographic, medical, and family information were obtained. Of those who attended the mobile examination center, 1,393 people (46%) were randomized to a morning session where blood was drawn for the measurement of FPG and A1C and retinal fundus photography examinations were performed. After excluding people who fasted <8 or ≥24 h (n = 144), pregnant women (n = 2), those with invalid FPG (n = 24) or A1C values (n = 4), and those without completed retinopathy grading (n = 153), the final analytic sample consisted of 1,066 adults. For the latter exclusion, fundus photography was not completed for individuals because of lack of time available to complete the examination (n = 64), physical limitations (n = 25), eye-specific limitations (n = 16), refusal (n = 19), communication problems (n = 5), accompanying child (n = 5), illness (n = 4), and equipment failure or unspecified problems (n = 15). The NHANES protocol was approved by a human subjects review board, and informed consent was obtained from all participants.
Two 45° nonmydriatic color digital images of the retina were taken of each eye by a technologist using a Canon CR6-45NM ophthalmic digital imaging system and Canon EOS 10D digital camera. The first image was centered on the macula, and the second was centered on the optic nerve. Retinopathy lesions were graded at the University of Wisconsin Ocular Epidemiology Reading Center according to the modified Airlie House classification system, as used in the Early Treatment Diabetic Retinopathy Study (ETDRS) (11). Participants were dichotomized based on ETDRS severity level as having retinopathy (≥14) or not having retinopathy (<14).
A1C was measured by high-performance liquid chromatography (HPLC), as used in the Diabetes Control and Complications Trial (10). FPG was measured in the morning after an 8- to 24-h fast at a central laboratory using a hexokinase enzymatic method (10).
Participants were asked if a doctor or health care professional had ever told them they have diabetes (other than gestational diabetes). Those who responded “yes” were classified as having diagnosed diabetes.
Hypertension was defined as use of antihypertensive medication or the mean of three or four readings of systolic blood pressure ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg. Time since diagnosis of diabetes, diabetes treatment, age, sex, and race/ethnicity (non-Hispanic white, non-Hispanic black, others) were self-reported.
Statistical analysis
We compared three approaches to categorizing A1C and FPG. First, we used deciles, a widely used approach, to group data. Second, we applied the cut points used in an analysis of the Pima Indians (2). Third, since both approaches may not yield a precise change point, we also used a moving average smoothing technique. Taking a 0.1-unit increment each time from the lowest to the highest levels of A1C and FPG, we created a series of subsets with a 0.5-unit range of A1C or FPG (window) and then calculated the mean A1C and FPG and the prevalence of retinopathy for each subset. SAS callable SUDAAN (version 9.0.1; SUDAAN Statistical Software Center, Research Triangle Park, NC) was used to calculate standard errors based on Taylor Series linearization.
Joinpoint regression, in which the relationship between the dependent and independent variables is modeled as piecewise linear phases, is often useful to describe changes in trend data. It is also called piecewise regression, segmented regression, broken line regression, or multiphase regression with the continuity constraint (12). We used logistic regression, accounting for the complex sampling design, to obtain predicted prevalences and standard errors; we then tested the null hypothesis of no change points of these prevalences by glucose categories using joinpoint regression software developed by the Surveillance, Epidemiology, and End Results program of the National Cancer Institute (version 3.3; Rockville, MD). We tested a constant prevalence of A1C or FPG level below the joinpoint and a linear association above. In addition, to summarize and compare the ability of A1C and FPG to identify retinopathy cases, we used Stata (version 10.1; StataCorp, College Station, TX) and applied logistic regression, accounting for the complex survey design, to calculate the predicted probability of retinopathy for each participant and the areas under receiver operating characteristic curves (AUCs). Larger values of AUC indicate a better ability to discriminate. Because there is no postestimation command of AUC calculation specifically for complex sampling of survey data, the variation of AUC in this study might be underestimated.
Our primary analyses included people with and without diabetes. Because of the potential for confounding from hypoglycemic treatment, we conducted sensitivity analyses in which we excluded participants receiving hypoglycemic medications. This exclusion eliminated 34% of those with retinopathy.
RESULTS
The overall study population, weighted to be representative of the U.S. noninstitutionalized population aged ≥40 years, had a mean age of 56 years, 47% were male, 79% were non-Hispanic white, 9% were non-Hispanic black, and 12% were of “other” race and ethnicity. Mean A1C and FPG were 5.7% and 5.9 mmol/l (106 mg/dl), respectively. Among the participants with diabetes, 88% were using hypoglycemic medication and 40% of those taking hypoglycemic medication had FPG <7.0 mmol/l. In this study population, the prevalence of any retinopathy was 11% and was appreciably higher in those with (36%) than in those without diagnosed diabetes (8%).
Table 1 shows that people with retinopathy were older, more likely to be men, had a higher prevalence of diagnosed diabetes and diabetes treatment, and were more likely to have hypertension. The coefficients of variation (CVs) (%), calculated as (100 × standard deviation)/mean, where standard deviation = [(sample size × standard error2)/design effect)0.5] are unitless and therefore can be compared between datasets having different units; the CVs were 15.6 and 28.8 for A1C and FPG (P < 0.001), respectively.
Table 1.
Characteristics of analytic population by diabetic retinopathy status
| No retinopathy | Retinopathy | P | |
|---|---|---|---|
| n | 913 | 153 | |
| Age (years) | 55.9 ± 0.61 | 60.3 ± 1.83 | 0.024 |
| Men (%) | 46.7 ± 1.61 | 56.0 ± 3.97 | 0.010 |
| Race/ethnicity (%) | 0.048 | ||
| Non-Hispanic white | 79.8 ± 2.55 | 70.1 ± 5.21 | |
| Non-Hispanic black | 8.3 ± 1.46 | 15.4 ± 4.14 | |
| Other | 11.9 ± 1.82 | 14.6 ± 4.91 | |
| FPG (mmol/l) | 5.8 ± 0.07 | 6.9 ± 0.24 | <0.001 |
| A1C (%) | 5.6 ± 0.02 | 6.4 ± 0.12 | <0.001 |
| Diabetes (%) | 7.8 ± 0.92 | 35.6 ± 3.40 | <0.001 |
| Diabetes treatment (%) | 6.4 ± 0.81 | 34.0 ± 3.16 | <0.001 |
| Hypertension (%) | 42.1 ± 2.26 | 60.1 ± 6.49 | 0.009 |
Data are means ± SE.
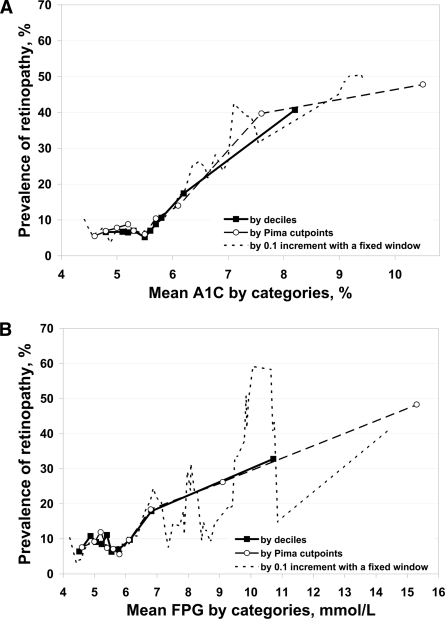
Figure 1A shows retinopathy prevalence by A1C deciles (5.0, 5.2, 5.3, 5.5, 5.6, 5.7, 5.8, 6.0, and 6.7%), cut points of the Pima Indian study (4.8, 5.0, 5.1, 5.3, 5.5, 5.7, 5.9, 6.6, and 9.4%) (2), and a 0.1% increment with a fixed 0.5% width window. Regardless of the approach used, there was a sharp increase in retinopathy prevalence above an A1C of 5.5% (95% CI 5.3–5.6 for the decile approach, 5.1–6.1 for the Pima Indian approach, and 5.3–5.8 by the moving average approach) (all P values <0.05). The linear regression coefficients of retinopathy prevalence by the 1% increment approach were 0.7 (P = 0.756) and 12.7 (P < 0.001) for before and after the change point of 5.5%, respectively. That is, above an A1C of 5.5%, the prevalence of retinopathy rose 12.7% for each 1% A1C increment.
Figure 1.
Relation between prevalence of retinopathy and A1C (A) and FPG (B).
Figure 1B describes retinopathy prevalence by FPG deciles (4.8, 5.0, 5.2, 5.4, 5.5, 5.7, 6.0, 6.4, and 7.7 mmol/l), cut points of the Pima Indian study (4.9, 5.2, 5.3, 5.5, 5.8, 6.0, 6.4, 7.5, and 12.4 mmol/l) (2), and a 0.1-mmol/l increment with a fixed 0.5-mmol/l–width window. Regardless of the approach to categorize FPG, there was a sharp increase in retinopathy prevalence after 5.8 mmol/l (95% CI 5.1–6.1 for the decile approach, 5.2–6.8 for the Pima Indian approach, and 5.3–6.3 by the moving average approach) (all P values <0.05). The linear regression coefficients of retinopathy prevalence by the FPG 1% increment approach were 0.8 (P = 0.476) and 3.9 (P < 0.001) for before and after the change point of 5.8 mmol/l, respectively.
After excluding participants taking hypoglycemic medications, by using the moving average approach, the change point for A1C (%) remained at 5.5 (95% CI 5.2–5.7); the regression coefficients were 0.8 (P = 0.409) and 10.5 (P < 0.001) for before and after the change point of 5.5, respectively. However, for FPG, the change point increased from 5.8 to 7.0 mmol/l (6.8–7.2). The regression coefficients for retinopathy prevalence were 1.1 (P = 0.079) and 4.3 (P < 0.001) for before and after the change point, respectively.
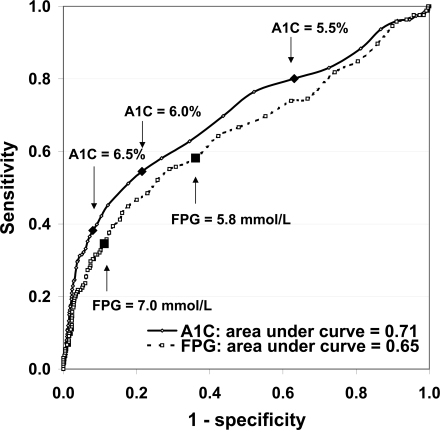
Based on the total study population, AUC indicated that A1C was more accurate than FPG in discriminating retinopathy cases from noncases: AUC 0.71 (95% CI 0.66–0.76) for A1C and 0.65 (0.60–0.70) for FPG, P for difference = 0.009 (Fig. 2). At A1C cut points of 5.5, 6.0, and 6.5%, the sensitivities and specificities were 80 and 37%, 55 and 79%, and 38 and 92%, respectively. At an FPG cut point of 5.8, 6.5, 7.0, and 7.5 mmol/l, sensitivities and specificities were 58 and 64%, 43 and 84%, 35 and 89%, and 30 and 92%, respectively.
Figure 2.
Receiver operating characteristic curves for A1C (%) and FPG (mmol/l) and prevalent retinopathy.
We reran the models using multivariate logistic regression on the total study population that included selected covariates of glycemia and retinopathy risk (age, sex, race/ethnicity, BMI, waist circumference, time since diagnoses of diabetes, diabetes treatment, and hypertension status). The AUC of A1C and prevalence of retinopathy increased from 0.71 to 0.75 (P = 0.060), and the AUC of FPG and prevalence of retinopathy increased from 0.65 to 0.74 (P < 0.001). For both A1C and FPG, only time since diagnosis of diabetes and sex were significantly related to prevalence of retinopathy, and their inclusion improved the discrimination of prevalence of retinopathy in these two full models (both P values <0.05).
CONCLUSIONS
Using nationally representative data, we examined the associations of A1C and FPG with retinopathy prevalence in the U.S. noninstitutionalized population aged ≥40 years. For both measures of glycemia, we identified points at which retinopathy prevalence began to rise sharply. Retinopathy prevalence began to rise precipitously when A1C exceeded 5.5% (corresponding to the 5th decile) and after FPG exceeded 5.8 mmol/l (corresponding to the 7th decile). This study also demonstrates that the change points are helpful in finding the lowest cut point for the diagnosis of retinopathy. However, to be used clinically, at a minimum, further analyses to determine cut points for the diagnosis of retinopathy would need to include sensitivity and specificity analyses.
Our A1C change point is similar to that observed in a recent Japanese study (5.3–5.5%) (13) but lower than that observed in some previous studies, including the Pima Indian study (6.2%), the Egyptian study (6.3%), and NHANES III (6.0%) (14). There are at least three reasons for this finding. First, in our study, retinopathy was assessed by two retinal photographs in each eye, while previous studies used either one retinal photograph in one eye or direct ophthalmoscopy to detect retinopathy. Thus, our use of a more sensitive assessment of retinopathy (5) may have identified the presence of retinopathy at lower A1C levels. Second, a higher proportion of the Egyptian and the Pima Indian populations were at high risk for or had diabetes (2,3); thus, compared with the U.S. population, the whole A1C distribution may be shifted to the left. Third, discrepancies in cutoff points may be due to differences in laboratory methods for A1C measurement (15).
In the Multi-Ethnic Study of Atherosclerosis (MESA) population, there was a continuous relation between prevalent retinopathy (defined as ETDRS level ≥20) and A1C and, based on change point analysis, no clear evidence of a threshold (5). Moreover, retinopathy in the absence of diabetes (FPG <7 mmol/l) was more frequent among individuals of minority racial groups than whites (16), suggesting a higher likelihood of retinopathy not due to hyperglycemia among minority populations. This may have influenced the capacity of identifying a clear A1C threshold. In our study, minorities represented 30% of the population, but in MESA they represented 60% of the population.
In the overall population, retinopathy prevalence increased precipitously after FPG levels of 5.8 mmol/l, but the change point was higher (7.0 mmol/l) among those not receiving hypoglycemic treatment. This suggests that treatment affects FPG level and shifts the FPG distribution among people with diabetes to the left. The FPG change points observed in our study span the range of levels observed in previous studies, including the Pima Indian (6.8 mmol/l) and the Egyptian studies (6.4 mmol/l) (2,14). However, in contrast with our findings, a recent analysis of three population-based studies (Blue Mountains Eye Study, Australian Diabetes Obesity and Lifestyle Study, and MESA) did not detect a clear FPG threshold for the prevalence of any or moderate retinopathy (5). Demographic differences (age, sex, and race/ethnicity), differences in status of hypoglycemic treatment, and criteria of diagnosis of retinopathy could explain these discrepancies.
There are several advantages to A1C as a diagnostic criterion for diabetes. A1C is less affected by short-term lifestyle changes, and its measurement has been improved and standardized during the last decade. While hemoglobinopathies and race/ethnicity may reduce the validity of A1C as a diagnostic tool (17,18), A1C level has less variability than FPG (7–9). Nevertheless, the interindividual variability of A1C is far more complicated than that of FPG. Genetic factors account for a significant part of the variation in A1C among people without diabetes (19). Glycation rate is also influenced by factors other than the level of plasma glucose (20). In our study, A1C had a smaller CV and a larger AUC than FPG. Though FPG had a similar level of AUC after adjusting for other variables, these adjustments are not practical in clinical practice. These results suggested that in some circumstances A1C may discriminate prevalent retinopathy better than FPG.
Our findings provide additional support for the current diagnostic threshold of FPG in diabetes while providing guidance for potential diagnostic thresholds based on A1C. However, we caution against the overreliance of these data for several reasons. First, even though retinopathy is more specific than other diabetes complications, the Diabetes Prevention Program recruited a population at high risk for developing diabetes and found that 8% of people with FPG below diabetic levels had retinopathy (21). Similar findings have been reported by others (5). In our study, 8% of participants with FPG <7.0 mmol/l had retinopathy. Longitudinal studies have reported that the presence of retinopathy in people with normal glucose levels at baseline predicts the development of diabetes (22,23), suggesting that some factors may play a role in the pathogenesis of both microvascular changes and diabetes. The presence of retinopathy among nondiabetic individuals may also be related to other conditions such as hypertension. In our subset analysis among people without diabetes, the prevalence of retinopathy was 10% in those with hypertension and 6% in those without hypertension (P = 0.222). The presence of these retinal lesions in nondiabetic people is likely to attenuate the detection of the change point of glucose level for retinopathy. Examining the prevalence of more advanced retinopathy by A1C levels may help in the identification of diagnostic cut points. However, because only 2 years of NHANES data are available, there is insufficient power to explore this issue further.
Second, our main analyses included people with previous hypoglycemic treatment. The inclusion of people on such treatment could artificially reduce levels of glycemia in the population and lead to an overestimation of the steepness in the prevalence of retinopathy by glycemic level. Theoretically, a preferable study design would follow people without diabetes treatment prospectively until the development of retinopathy; however, such a design is impractical due to the ethical problem of following high-risk people without starting hypoglycemic treatment. In a sensitivity analysis, we excluded participants taking diabetes-related medications. This had no effect on the A1C change point but raised the change point for FPG.
Third, variability among different assay methods of A1C is a potential source of inaccuracy whenever A1C results are interpreted relative to universal, fixed, clinical decision thresholds (15). The mean and range of percent A1C and percent of total glycated hemoglobin measured by the ion-exchange method (which this study used) and the affinity method are similar. Nevertheless, Nuttall (24,25) argued that affinity chromatography has significant advantages over the ion-exchange HPLC, especially among people with diabetes, including less interference by hemoglobinopathies, more reliability, and no need for validation by the complicated mass spectrometry method.
In summary, based on the latest nationally representative sample, our analysis examined the association of A1C with retinopathy and provides new information on defining cut points for diagnosing diabetes. While the A1C and FPG levels of 5.5% and 5.8 mmol/l provide start points at which retinopathy prevalence increases most precipitously, A1C appears to discriminate between the presence and absence of retinopathy at least as well as FPG and offers some advantages over FPG.
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
Footnotes
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention, the National Institute of Diabetes and Digestive and Kidney Diseases, or the University of Wisconsin School of Medicine and Public Health.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
See accompanying editorial, p. 2140.
References
- 1. McCance DR, Hanson RL, Pettitt DJ, Bennett PH, Hadden DR, Knowler WC: Diagnosing diabetes mellitus: do we need new criteria? Diabetologia 1997;40:247–255 [DOI] [PubMed] [Google Scholar]
- 2. McCance DR, Hanson RL, Charles MA, Jacobsson LT, Pettitt DJ, Bennett PH, Knowler WC: Comparison of tests for glycated haemoglobin and fasting and two hour plasma glucose concentrations as diagnostic methods for diabetes. BMJ 1994;308:1323–1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Engelgau MM, Thompson TJ, Herman WH, Boyle JP, Aubert RE, Kenny SJ, Badran A, Sous ES, Ali MA: Comparison of fasting and 2-hour glucose and HbA1c levels for diagnosing diabetes. Diagnostic criteria and performance revisited. Diabetes Care 1997;20:785–791 [DOI] [PubMed] [Google Scholar]
- 4. Expert Committee on the Diagnosis and Classification of Diabetes Mellitus: Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2003;26(Suppl. 1):S5–S20 [DOI] [PubMed] [Google Scholar]
- 5. Wong TY, Liew G, Tapp RJ, Schmidt MI, Wang JJ, Mitchell P, Klein R, Klein BE, Zimmet P, Shaw J: Relation between fasting glucose and retinopathy for diagnosis of diabetes: three population-based cross-sectional studies. Lancet 2008;371:736–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Buell C, Kermah D, Davidson MB: Utility of A1C for diabetes screening in the 1999–2004 NHANES population. Diabetes Care 2007;30:2233–2235 [DOI] [PubMed] [Google Scholar]
- 7. Petersen PH, Jørgensen LG, Brandslund I, De Fine Olivarius N, Stahl M: Consequences of bias and imprecision in measurements of glucose and hba1c for the diagnosis and prognosis of diabetes mellitus. Scand J Clin Lab Invest Suppl 2005;240:51–60 [DOI] [PubMed] [Google Scholar]
- 8. Ollerton RL, Playle R, Ahmed K, Dunstan FD, Luzio SD, Owens DR: Day-to-day variability of fasting plasma glucose in newly diagnosed type 2 diabetic subjects. Diabetes Care 1999;22:394–398 [DOI] [PubMed] [Google Scholar]
- 9. Sacks DB, Bruns DE, Goldstein DE, Maclaren NK, McDonald JM, Parrott M: Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Clin Chem 2002;48:436–472 [PubMed] [Google Scholar]
- 10. National Center for Health Statistics: NHANES 2005–2006 Manuals, Brochures, Consent Documents [Internet]. Centers for Disease Control and Prevention. Available at http://www.cdc.gov/nchs/about/major/nhanes/nhanes2005–2006/current_nhanes_05_06.htm. Accessed 15 May 2009
- 11. Diabetic Retinopathy Study Research Group. Diabetic retinopathy study: Report number 6: design, methods, and baseline results; Report number 7: a modification of the Airlie House classification of diabetic retinopathy. Invest Ophthalmol Vis Sci 1981;21:1–226 [PubMed] [Google Scholar]
- 12. Kim HJ, Fay MP, Feuer EJ, Midthune DN: Permutation tests for joinpoint regression with applications to cancer rates. Stat Med 2000;19:335–351 [DOI] [PubMed] [Google Scholar]
- 13. Miyazaki M, Kubo M, Kiyohara Y, Okubo K, Nakamura H, Fujisawa K, Hata Y, Tokunaga S, Iida M, Nose Y, Ishibashi T. Hisayama study. Comparison of diagnostic methods for diabetes mellitus based on prevalence of retinopathy in a Japanese population: the Hisayama Study. Diabetologia 2004;47:1411–1415 [DOI] [PubMed] [Google Scholar]
- 14. Davidson MB, Schriger DL, Peters AL, Lorber B: Relationship between fasting plasma glucose and glycosylated hemoglobin: potential for false-positive diagnoses of type 2 diabetes using new diagnostic criteria. JAMA 1999;281:1203–1210 [DOI] [PubMed] [Google Scholar]
- 15. Holmes EW, Erşahin C, Augustine GJ, Charnogursky GA, Gryzbac M, Murrell JV, McKenna KM, Nabhan F, Kahn SE: Analytic bias among certified methods for the measurement of hemoglobin A1c: a cause for concern? Am J Clin Pathol 2008;129:540–547 [DOI] [PubMed] [Google Scholar]
- 16. Wong TY, Klein R, Islam FM, Cotch MF, Folsom AR, Klein BE, Sharrett AR, Shea S: Diabetic retinopathy in a multi-ethnic cohort in the United States. Am J Ophthalmol 2006;141:446–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Saudek CD, Derr RL, Kalyani RR: Assessing glycemia in diabetes using self-monitoring blood glucose and hemoglobin A1c. JAMA 2006;295:1688–1697 [DOI] [PubMed] [Google Scholar]
- 18. Herman WH, Ma Y, Uwaifo G, Haffner S, Kahn SE, Horton ES, Lachin JM, Montez MG, Brenneman T, Barrett-Connor E. Diabetes Prevention Program Research Group. Differences in A1C by race and ethnicity among patients with impaired glucose tolerance in the Diabetes Prevention Program. Diabetes Care 2007;30:2453–2457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cohen RM, Snieder H, Lindsell CJ, Beyan H, Hawa MI, Blinko S, Edwards R, Spector TD, Leslie RD: Evidence for independent heritability of the glycation gap (glycosylation gap) fraction of HbA1c in nondiabetic twins. Diabetes Care 2006;29:1739–1743 [DOI] [PubMed] [Google Scholar]
- 20. Cohen RM, Smith EP: Frequency of HbA1c discordance in estimating blood glucose control. Curr Opin Clin Nutr Metab Care 2008;11:512–517 [DOI] [PubMed] [Google Scholar]
- 21. Diabetes Prevention Program Research Group. The prevalence of retinopathy in impaired glucose tolerance and recent-onset diabetes in the Diabetes Prevention Program. Diabet Med 2007;24:137–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tapp RJ, Tikellis G, Wong TY, Harper CA, Zimmet PZ, Shaw JE. Australian Diabetes Obesity and Lifestyle Study Group. Longitudinal association of glucose metabolism with retinopathy: results from the Australian Diabetes Obesity and Lifestyle (AusDiab) study. Diabetes Care 2008;31:1349–1354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Klein R, Klein BE, Moss SE, Wong TY: The relationship of retinopathy in persons without diabetes to the 15-year incidence of diabetes and hypertension: Beaver Dam Eye Study. Trans Am Ophthalmol Soc 2006;104:98–107 [PMC free article] [PubMed] [Google Scholar]
- 24. Nuttall FQ: Comparison of percent total GHb with percent HbA1c in people with and without known diabetes. Diabetes Care 1998;21:1475–1480 [DOI] [PubMed] [Google Scholar]
- 25. Nuttall FQ: Total glycohemoglobin or hemoglobin A1C? Endocr Pract 2007;13:317–318 [DOI] [PubMed] [Google Scholar]