Abstract
OBJECTIVE
Previous studies, largely in northern Europe, have suggested an association between type 1 diabetes and reduced serum 25-hydroxy(OH) vitamin D levels, a concept we tested in individuals residing in a solar-rich region (Florida).
RESEARCH DESIGN AND METHODS
Serum samples from 415 individuals residing in Florida were cross-sectionally analyzed: 153 control subjects, 46 new-onset type 1 diabetic patients, 110 established type 1 diabetic patients (samples ≥5 months from diagnosis), and 106 first-degree relatives of the diabetic patients.
RESULTS
In this study, 25-OH vitamin D levels (median, range, interquartile range [IQR]) were similar among control subjects (20.1, below detection [bd]–163.5, 13.0–37.4 ng/ml), new-onset type 1 diabetic patients (21.2, bd–48.6, 12.2–30.2 ng/ml), established type 1 diabetic patients (23.2, bd–263.8, 13.8–33.9 ng/ml), and first-degree relatives (22.2, bd–59.9, 12.7–33.1 ng/ml) (P = 0.87). Mean 25-OH vitamin D levels were less than the optimal World Health Organization level of 30 ng/ml in all study groups.
CONCLUSIONS
Reduced serum 25-OH vitamin D levels were not specifically associated with type 1 diabetes. The uniform suboptimal 225-OH vitamin D levels, despite residence in a zone with abundant sunshine, support additional dietary vitamin D fortification practices.
The role for environment in the development of type 1 diabetes has remained elusive, with multiple factors purported to modulate risk for this disease (e.g., viruses, breast-feeding, age for cereal introduction, and childhood immunizations) (1,2). Further to this list is vitamin D levels (3), with previous studies suggesting type 1 diabetic patients had lower serum concentrations of this metabolite than healthy control subjects (4–6) as well as disease-associated polymorphisms in a vitamin D metabolism gene (7). Although certainly intriguing, we note the aforementioned studies were largely undertaken in northern European countries (4,5), whereas the one study performed in the U.S. failed to provide values among healthy control subjects and, hence, did not identify disease specificity (6). Therefore, we measured serum 25-hydroxy (OH) vitamin D levels from type 1 diabetic patients, their first-degree relatives, and healthy control subjects all residing in a solar-rich region (Florida).
RESEARCH DESIGN AND METHODS
Serum from 415 individuals was obtained from healthy control subjects (type 1 diabetes autoantibody negative, no family history of type 1 diabetes, median age 22.0 years, age range 5.0–65.1 years, 84 female, and 153 total), new-onset type 1 diabetic patients with diabetes ≤5 months duration (12.2 years, 5.9–35.0 years, 23 female, and 46 total), established type 1 diabetic patients with diabetes >5 months duration (16.0 years, 5.1–62.6 years, 50 female, and 110 total), and relatives of those with type 1 diabetes (21.0 years, 1.0–62.6 years, 54 female, and 106 total). All samples were collected with informed consent with University of Florida Institutional Review Board approval. As a retrospective study of de-identified samples, no information regarding sun avoidance routines or dietary practices (including vitamin D fortification) were available, nor were methods for case matching permissible.
25-OH vitamin D levels were quantified in duplicate with a commercial enzyme immunosorbent assay kit (ALPCO, Salem, NH), an analyte shown previously as stable in storage (8). This assay measures both D2 and D3 forms of 25-OH vitamin D. The intra- and interassay coefficients of variation for this assay were 10.7 and 13.2%, respectively. The lower limit of detection was 2.56 ng/ml. 25-OH vitamin D deficiency was defined as ≤20 ng/ml, insufficiency as 21–30 ng/ml, and sufficiency as >30 ng/ml (9,10).
Analysis of multiple unpaired group comparisons were achieved using the nonparametric Kruskal-Wallis with Dunn posttest to correct for multiple comparisons (11). Age and 25-OH vitamin D relationships were analyzed by linear regression, with two-tailed Fisher exact test used to compare proportions of subjects deemed insufficient. Power calculations were performed post hoc with GraphPad StatMate version 2.00 (GraphPad Software, San Diego, CA; www.graphpad.com), revealing an 80% power to detect a 10.5 ng/ml difference in 25-OH vitamin D levels.
RESULTS
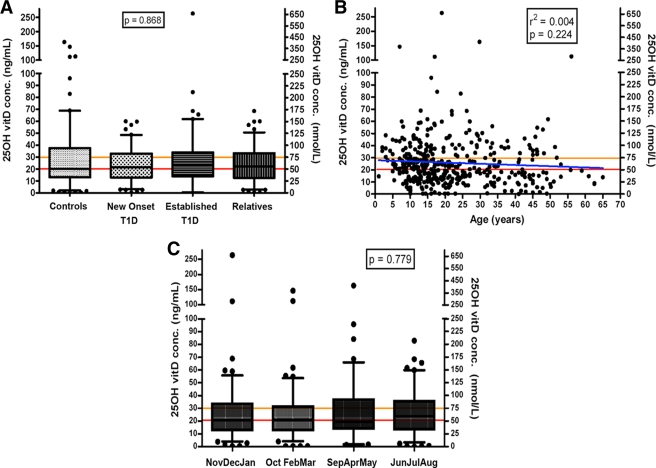
25-OH vitamin D levels (median, range, interquartile range [IQR]) were as follows: healthy control subjects (20.1, below detection [bd]–163.5, 13.0–37.4 ng/ml), new-onset type 1 diabetic patients (21.2, bd–48.6, 12.2–30.2 ng/ml), established type 1 diabetic patients (23.2, bd–263.8, 13.8–33.9 ng/ml), and first-degree relatives (22.2, bd–59.9, 12.7–33.1 ng/ml) (Fig. 1A). The medians were not different among individuals in these cohorts (P = 0.87). Suboptimal 25-OH vitamin D levels (≤30 ng/ml) were observed in 70.1% of control subjects, 76.1% of new-onset type 1 diabetic patients, 68.5% of established type 1 diabetic patients, and 68.8% of first-degree relatives; values, although low, were not significantly different from each other (P = 0.46).
Figure 1.
25-OH vitamin D levels in cohorts based on parameters of disease, age, or estimated solar exposure. For disease status (A), values are presented as a function of study group with definitions of insufficiency (orange line) and deficiency (red line) provided. With respect to age (B), values for all study participants independent of cohort are shown with the definitions of insufficiency and deficiency as defined in A along with age correlation (blue line). C: Estimated average UVB exposure for the entire study population is presented. UVI climatological data were obtained from the National Weather Service (NWS) and U.S. Environmental Protection Agency (EPA) Web sites (http://www.cpc.ncep.noaa.gov and http://www.epa.gov) to determine relative UV exposure. Based on data for the previous 5 years, for the proximate city of Jacksonville, Florida, we established the mean UV exposure for each month: January, 3.215; February, 4.08; March, 5.96; April, 7.68; May, 8.238; June, 8.578; July, 8.976; August, 8.254; September, 6.902; October, 5.11; November, 3.694; and December, 2.79. The numbers correspond to the UVI scale (1–11+) developed by the NWS and EPA and implemented by the World Health Organization. The samples were grouped according to month drawn and placed into one of four possible 3-month blocks, each block formed on the basis of similar UVB indexes. The 25-OH vitamin D levels (reported as median, range, IQR) for the November/December/January group of 112 samples (20.7, bd–263.8, 12.7–33.6 ng/ml) with an average estimated UV exposure of 3.23. The October/February/March group of 113 samples (20.8, bd–146.8, 12.7–31.5 ng/ml) with an average estimated UV exposure of 5.05. The September/April/May group of 84 samples (19.3, bd–163.5, 14.0–36.9 ng/ml) with an average estimated UV exposure of 7.61. The June/July/August group of 106 samples (23.9, bd–82.9, 13.4–35.6 ng/ml) with an average estimated UV exposure of 8.60.
Comparison of age with serum 25-OH vitamin D levels indicated that for all groups combined, r2 = 0.004 and P = 0.22. Comparison of age with serum 25-OH vitamin D levels for individual groups were for healthy control subjects, r2 = 0.010 and P = 0.21; new-onset type 1 diabetic patients, r2 = 0.0001 and P = 0.96; established type 1 diabetic patients, r2 = 0.013 and P = 0.24; and first-degree relatives, r2 = 0.075 and P = 0.005. Hence, regression analysis revealed no trend in 25-OH vitamin D levels as it pertained to overall age.
Because sunlight plays a major role in vitamin D synthesis, we then examined 25-OH vitamin D levels as a function of the month the sample was drawn as a surrogate marker of ultraviolet (UV) B exposure (Fig. 1C). Comparison of the 25-OH vitamin D levels among each 3-month block showed no significant difference (P = 0.78). Further analysis revealed no significant differences on a monthly exposure basis when examining control subjects versus new-onset type 1 diabetic patients, established type 1 diabetic patients, or first-degree relatives (P = 0.71).
CONCLUSIONS
Our study did not find significant differences in 25-OH vitamin D levels among healthy control subjects, type 1 diabetic patients, and first-degree relatives of diabetic patients in samples obtained in a solar-rich region of the U.S. However, more surprisingly, we identified that within each group, there exists a high frequency of vitamin D insufficiency, even in the sun-rich environment of Florida.
For analysis of UV exposure, we elected to group samples by similarity in UV index (UVI) (to increase statistical power). In addition, we performed analysis in a month-by-month fashion. By either method, no differences among the study groups were observed. Although we may have introduced an ecological fallacy bias in assigning UVI aggregate data to individual subjects, other factors such as sun avoidance practices to inadequate supplementation may also account for the low 25-OH vitamin D levels observed in this cross-sectional study. These biases may also be present in studies examining geographical distribution (12). Another possibility is that at Florida's latitude, the duration of sunlight hours per day does not vary as dramatically as that which occurs closer to the geographical poles, thereby providing a mechanism to explain our lack in seasonal variation for 25-OH vitamin D levels.
With respect to race/ethnicity, a variable that has previously been noted to influence 25-OH vitamin D levels (13), our study was reflective of the prevalence of type 1 diabetes among all races, and given the predominance for this disease in Caucasians, it was not of sufficient power to analyze such a variable. However, the frequency of samples from non-Caucasians did not differ significantly among the study groups (data not shown). Indeed, the issue of sample size is one worth noting. As mentioned previously, the study herein was of a size larger than those reporting a negative association between serum 25-OH vitamin D levels and type 1 diabetes, with our current study (based on its size) having a 20% chance of reporting a statistical type 2 error (false negative). Hence, future efforts that are larger, prospective, and take into account analysis of additional factors (e.g., skin type, vitamin D fortification practices, photoprotection behaviors, time spent outdoors, etc.) that may influence serum 25-OH vitamin D would be beneficial. This, as well as the question addressing 25-OH vitamin D levels in a northern U.S. state, should be addressed in the future.
Given the amount of UV available to those residing in Florida and the fortification of milk products with vitamin D, the low serum levels of 25-OH vitamin D that were found add credence to the recent recommendation by the American Academy of Pediatrics to double the amount of vitamin D supplementation provided to children (14). Given that our results contrast with those of several other efforts (4–6), additional studies using a prospective cohort design to further define the role of vitamin D in the pathogenesis of type 1 diabetes are urgently needed because trials using active forms of this metabolite for type 1 diabetes prevention are actively being considered.
Acknowledgments
This study was supported, in part, by funds obtained from the National Institutes of Health, the Keene Professorship, and the Brehm Coalition for Type 1 Diabetes.
No potential conflicts of interest relevant to this article were reported.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Atkinson MA, Eisenbarth GS: Type 1 diabetes: new perspectives on disease pathogenesis and treatment. Lancet 2001; 358: 221– 229 [DOI] [PubMed] [Google Scholar]
- 2. Gale EA: Spring harvest? Reflections on the rise of type 1 diabetes. Diabetologia 2005; 48: 2445– 2450 [DOI] [PubMed] [Google Scholar]
- 3. Peechakara SV, Pittas AG: Vitamin D as a potential modifier of diabetes risk. Nat Clin Pract Endocrinol Metab 2008; 4: 182– 183 [DOI] [PubMed] [Google Scholar]
- 4. Pozzilli P, Manfrini S, Crinò A, Picardi A, Leomanni C, Cherubini V, Valente L, Khazrai M, Visalli N, IMDIAB group4. Pozzilli P, Manfrini S, Crino A, Picardi A, Leomanni C, Cherubini V, Valente L, Khazrai M, Visalli N; IMDIAB group. Low levels of 25-hydroxyvitamin D3 and 1,25-dihydroxyvitamin D3 in patients with newly diagnosed type 1 diabetes. Horm Metab Res 2005; 37: 680– 683 [DOI] [PubMed] [Google Scholar]
- 5. Littorin B, Blom P, Schölin A, Arnqvist HJ, Blohmé G, Bolinder J, Ekbom-Schnell A, Eriksson JW, Gudbjörnsdottir S, Nyström L, Ostman J, Sundkvist G: Lower levels of plasma 25-hydroxyvitamin D among young adults at diagnosis of autoimmune type 1 diabetes compared with control subjects: results from the nationwide Diabetes Incidence Study in Sweden (DISS). Diabetologia 2006; 49: 2847– 2852 [DOI] [PubMed] [Google Scholar]
- 6. Svoren BM, Volkening LK, Wood JR, Laffel LM: Significant vitamin D deficiency in youth with type 1 diabetes mellitus. J Pediatr 2009; 154: 132– 134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bailey R, Cooper JD, Zeitels L, Smyth DJ, Yang JH, Walker NM, Hyppönen E, Dunger DB, Ramos-Lopez E, Badenhoop K, Nejentsev S, Todd JA: Association of the vitamin D metabolism gene CYP27B1 with type 1 diabetes. Diabetes 2007; 56: 2616– 2621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bodnar LM, Catov JM, Wisner KL, Klebanoff MA: Racial and seasonal differences in 25-hydroxyvitamin D detected in maternal sera frozen for over 40 years. Br J Nutr 2009; 101: 278– 284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Holick MF: Vitamin D deficiency. N Engl J Med 2007; 357: 266– 281 [DOI] [PubMed] [Google Scholar]
- 10. Holick MF: Diabetes and the vitamin D connection. Curr Diab Rep 2008; 8: 393– 398 [DOI] [PubMed] [Google Scholar]
- 11. Motulsky HJ: Intuitive biostatistics. Oxford University Press; 1995 [Google Scholar]
- 12. Holick MF: The influence of vitamin D on bone health across the life cycle. J Nutr 2005; 135: 2726– 2727 [DOI] [PubMed] [Google Scholar]
- 13. Hagenau T, Vest R, Gissel TN, Poulsen CS, Erlandsen M, Mosekilde L, Vestergaard P: Global vitamin D levels in relation to age, gender, skin pigmentation and latitude: an ecologic meta-regression analysis. Osteoporos Int 2009; 20: 133– 140 [DOI] [PubMed] [Google Scholar]
- 14. Wagner CL, Greer FR; American Academy of Pediatrics Section on Breastfeeding; American Academy of Pediatrics Committee on Nutrition; American Academy of Pediatrics Section on Breastfeeding; American Academy of Pediatrics Committee on Nutrition. Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics 2008; 122: 1142– 1152 [DOI] [PubMed] [Google Scholar]