Abstract
OBJECTIVE
A loss of skeletal muscle mass is frequently observed in older adults. The aim of the study was to investigate the impact of type 2 diabetes on the changes in body composition, with particular interest in the skeletal muscle mass.
RESEARCH DESIGN AND METHODS
We examined total body composition with dual-energy X-ray absorptiometry annually for 6 years in 2,675 older adults. We also measured mid-thigh muscle cross-sectional area (CSA) with computed tomography in year 1 and year 6. At baseline, 75-g oral glucose challenge tests were performed. Diagnosed diabetes (n = 402, 15.0%) was identified by self-report or use of hypoglycemic agents. Undiagnosed diabetes (n = 226, 8.4%) was defined by fasting plasma glucose (≥7 mmol/l) or 2-h postchallenge plasma glucose (≥11.1 mmol/l). Longitudinal regression models were fit to examine the effect of diabetes on the changes in body composition variables.
RESULTS
Older adults with either diagnosed or undiagnosed type 2 diabetes showed excessive loss of appendicular lean mass and trunk fat mass compared with nondiabetic subjects. Thigh muscle CSA declined two times faster in older women with diabetes than their nondiabetic counterparts. These findings remained significant after adjusting for age, sex, race, clinic site, baseline BMI, weight change intention, and actual weight changes over time.
CONCLUSIONS
Type 2 diabetes is associated with excessive loss of skeletal muscle and trunk fat mass in community-dwelling older adults. Older women with type 2 diabetes are at especially high risk for loss of skeletal muscle mass.
Age-related loss of skeletal muscle mass or sarcopenia results in decreased skeletal muscle strength, mobility limitations, physical disability, and eventually high mortality among the elderly (1–3). However, little is known about the causes or risk factors associated with loss of skeletal muscle mass in older adults. In addition, although weight gain and accumulation of abdominal fat have been known as strong risk factors for the development of type 2 diabetes (4), the changes in body composition after the onset of diabetes are not well documented. We have observed cross-sectionally that older adults with type 2 diabetes have an altered body composition and low skeletal muscle strength compared with nondiabetic older adults (5). We also reported that older adults with type 2 diabetes lost their knee extensor strength more rapidly than their nondiabetic counterparts (6).
The effects of type 1 diabetes on protein metabolism seem to be clear, as insulin deprivation causes a profound increase in catabolism, especially in skeletal muscle (7,8). However, the effect of type 2 diabetes on protein metabolism is less clear, since the results of previous studies are inconsistent (9–12). Few studies have examined the effect of type 2 diabetes on the quantity of skeletal muscle mass in humans.
In the Health, Aging, and Body Composition Study (Health ABC Study), we assessed the changes in total and regional lean and fat mass over 6 years with precise measures of body composition with dual-energy X-ray absorptiometry (DEXA) and computed tomography (CT). The aim of the study was to investigate the impact of type 2 diabetes on the changes in body composition, with particular interest on the skeletal muscle, in community-dwelling well-functioning older adults. We hypothesized that older adults with type 2 diabetes would show more loss of lean skeletal muscle mass than older adults without diabetes.
RESEARCH DESIGN AND METHODS
Study population
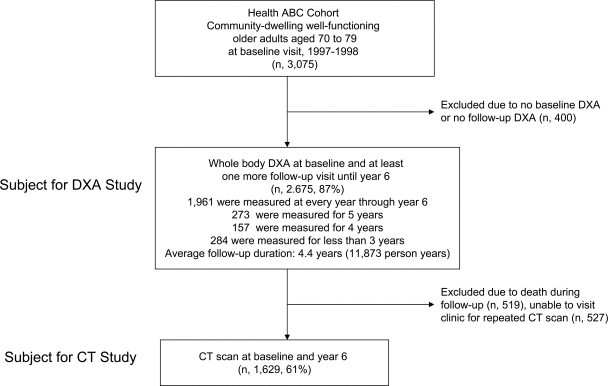
The Health ABC cohort consisted of well-functioning community-dwelling older adults age 70–79 years. (Detailed information on enrollment can be found elsewhere [5].) The flow of subjects for the DEXA study and the CT study is summarized in Fig. 1. All participants gave written informed consent before participating in the study. The consent forms and study protocols were approved by the institutional review boards at each field center.
Figure 1.
Flow of study population: the Health ABC Study.
Assessment of diabetes status
At baseline, diagnosed diabetes was defined by a report of physician-diagnosed type 2 diabetes or the current use of oral hypoglycemic agents or insulin with onset after age 25 years. We also performed 75-g oral glucose challenge tests for all participants without diagnosed diabetes. Undiagnosed diabetes was defined by a fasting plasma glucose concentration ≥7.0 mmol/l or a 2-h postchallenge plasma glucose ≥11.1 mmol/l. The average duration of diagnosed diabetes was 13.3 ± 10.9 years from the time of diagnosis. Plasma glucose was measured by an automated glucose oxidase reaction (Vitros 950 analyzer; Johnson & Johnson, Rochester, NY). A1C was measured by the enzymatic method (Bio-Rad, Hercules, CA).
Body composition by DEXA
Body weight and height were measured in patients wearing a hospital gown and without shoes on a calibrated balance beam scale and stadiometer, respectively, and BMI was calculated as weight in kilograms divided by the square of height in meters. We used fan-beam dual-energy X-ray absorptiometry (model QDR 4.500, software version 8.21; Hologic, Bedford, MA) to measure total body mass and body composition. Total body fat and fat-free mass were measured and separated into trunk and appendicular components. Then, bone mineral content was subtracted from the total and regional fat-free mass to define total and regional nonbone lean mass. Appendicular lean mass was calculated as the sum of lean soft tissue (nonfat, nonbone) mass in the arms and legs, which represents primarily skeletal muscle mass in the extremities. The validity and reproducibility of the body composition data in the Health ABC Study were previously reported (13,14).
Body composition by CT
Axial CT scans at the abdomen and mid-thigh levels were obtained at baseline (year 1) and 5 years later (year 6). CT images were acquired in either Pittsburgh, Pennsylvania (9800 Advantage; General Electric, Milwaukee, WI), or Memphis, Tennessee (Somaton Plus; Simens, Iselin, NJ, or PQ2000S; Picker, Cleveland, OH). We used the mid-thigh muscle cross-sectional area (CSA) as an indicator of skeletal muscle mass. Quality control ensured the reproducibility and quality of the repeated CT scans. Scans with any artifacts or poor quality were removed, abdominal scans obtained at or above the L3/L4 level or at or below the L5/S1 level were removed, and mid-thigh scans obtained from a different leg or a slice location on the femur >2 cm of the baseline scan were removed.
Inflammatory cytokines
Interleukin (IL)-6 and tumor necrosis factor (TNF)-α were measured in duplicate with an ultrasensitive enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN). The lower limit of detection was <0.10 pg/ml for IL-6 and 0.18 pg/ml for TNF-α, with coefficients of variation of 6.3 and 16.0%, respectively.
Statistical analyses
Baseline characteristics of the cohort are presented separately for three groups defined by baseline diabetes status (Table 1). ANOVA, χ2, and Kruskal-Wallis tests were used to examine differences in the descriptive characteristics of the study population. The longitudinal changes in body composition were analyzed with the generalized estimating equation model (using SAS Version 8.1 Proc Genmod) developed by Liang and Zeger (15). This method simultaneously examines the cross-sectional relation between each independent variable and body composition and the longitudinal relation between these variables and changes in body composition over time. Included in the models are potential confounding factors that are associated with body composition and its changes over time. The initial model included age, sex, race, clinic site, BMI, baseline body composition, weight loss intention assessed by questionnaire at each year, two dummy variables for new diabetes (new-DM) and known diabetes (known-DM), examination year (YR) as a time-dependent covariate, and cross-product terms between YR and the two dummy variables for diabetes (YR × new-DM, YR × known-DM) to assess changes in body composition over time between groups. Interactions between sex, race, and clinic site with diabetes variables (e.g., sex × new-DM × YR) were assessed. There was no significant interaction effect (P < 0.10) between sex, race, or clinic site with diabetes variables on changes in body composition. The final model included changes in body weight at each examination year as time-dependent covariates to examine the effects of diabetes on the changes of each body composition parameters while adjusting for the changes in overall body weight.
Table 1.
Characteristics of participants by baseline diabetes status in the Health ABC Study
| Without diabetes | Undiagnosed diabetes | Diagnosed diabetes | P * | |
|---|---|---|---|---|
| n | 2047 | 226 | 402 | |
| Sociodemographic | ||||
| Age (years) | 73.6 ± 2.9 | 73.7 ± 2.8 | 73.6 ± 2.7 | NS |
| Men (%) | 47.6 | 55.8 | 55.5 | <0.001 |
| Blacks (%) | 36.7 | 42.0 | 57.7 | <0.001 |
| Body composition | ||||
| BMI (kg/m2) | 26.8 ± 4.6 | 28.5 ± 4.8 | 29.1 ± 4.7 | <0.001 |
| Total body mass (kg) | 74.2 ± 14.4 | 79.9 ± 15.8 | 81.2 ± 14.0 | <0.001 |
| Total lean mass (kg) | 45.9 ± 9.8 | 49.1 ± 10.3 | 50.4 ± 9.3 | <0.001 |
| Trunk lean | 23.1 ± 4.8 | 24.8 ± 5.1 | 25.3 ± 4.7 | <0.001 |
| Appendicular lean | 19.8 ± 4.9 | 21.1 ± 5.1 | 21.9 ± 4.6 | <0.001 |
| Total fat mass (kg) | 26.0 ± 8.4 | 28.4 ± 9.0 | 28.5 ± 8.7 | <0.001 |
| Trunk fat | 12.9 ± 4.6 | 14.9 ± 5.3 | 15.0 ± 5.0 | <0.001 |
| Appendicular fat | 12.6 ± 4.5 | 13.0 ± 4.6 | 12.8 ± 4.3 | NS |
| Biochemical | ||||
| Fasting glucose (mmol/l) | 5.14 ± 0.52 | 6.98 ± 2.19 | 8.56 ± 3.26 | <0.001 |
| A1C (%) | 6.0 ± 0.5 | 6.9 ± 1.3 | 8.0 ± 1.6 | <0.001 |
| IL-6 (pg/ml)† | 1.72 (1.17–2.62) | 2.10 (1.38–3.08) | 2.16 (1.52–3.19) | <0.001 |
| TNF-α (pg/ml)† | 3.08 (2.38–3.95) | 3.28 (2.49–4.34) | 3.46 (2.58–4.42) | <0.001 |
Data are means ± SD, proportions, or median (interquartile range). NS, not significant;
*P values from ANOVA or χ2 tests;
†Kruskal-Wallis tests.
For the CT-derived body composition data, changes in abdominal subcutaneous fat, visceral fat, thigh subcutaneous fat, thigh intermuscular fat, and thigh muscle CSA were calculated in both absolute terms (year 6 value − year 1 value) and relative terms (percent change from baseline). Differences between the groups were assessed by general linear models controlling for age, sex, race, clinic site, and baseline values when using absolute changes. We found a significant interaction effect (P < 0.10) of sex and diabetes variables on the changes in thigh muscle area. Therefore, further analyses were stratified by sex for CT data. Additional adjustments were made for baseline BMI, weight change, IL-6, and TNF-α. We used Bonferroni correction methods for multiple comparisons between groups. A P value of <0.05 was accepted as statistically significant. The analyses were performed with SPSS (version 12.0.0; SPSS, Chicago, IL) and SAS (version 8.1; SAS Institute, Cary, NC).
RESULTS
The annual changes in each body composition parameter adjusting for age, sex, race, clinic site, baseline BMI, and weight loss intention are summarized in Table 2. Loss of total body mass was the most profound in older adults with undiagnosed diabetes followed by diagnosed diabetes and those without diabetes (−435 ± 79 vs. −293 ± 72 vs. −193 ± 22 g/year, respectively, P < 0.01). Most of the declines in total body mass were from lean mass, particularly in the extremities (appendicular lean mass). The annual declines in appendicular lean mass were higher in older adults with undiagnosed and diagnosed diabetes than in those without diabetes. Total and trunk fat mass also declined in older adults with undiagnosed and diagnosed diabetes in contrast to no change or a slight gain in those without diabetes (Table 2).
Table 2.
Annual changes in body composition assessed with DEXA by baseline diabetes status in the Health ABC Study
| Without diabetes | Undiagnosed diabetes | Diagnosed diabetes | |
|---|---|---|---|
| n | 2047 | 226 | 402 |
| Model 1 | |||
| Total body mass (g/year) | −193 ± 22 | −435 ± 79* | −293 ± 72 |
| Total lean mass (g/year) | −198 ± 10 | −340 ± 37* | −222 ± 29 |
| Trunk lean (g/year) | −44 ± 6 | −103 ± 22* | −27 ± 16 |
| Appendicular lean (g/year) | −150 ± 5 | −226 ± 20* | −187 ± 16† |
| Total fat mass (g/year) | 25 ± 16 | −94 ± 53† | −66 ± 53 |
| Trunk fat (g/year) | 44 ± 10 | −39 ± 35† | −34 ± 32† |
| Appendicular fat (g/year) | −17 ± 7 | −51 ± 24 | −27 ± 24 |
| Model 2 | |||
| Total lean mass (g/year) | −125 ± 7 | −186 ± 25† | −106 ± 20 |
| Trunk lean (g/year) | −10 ± 5 | −32 ± 17 | 26 ± 13† |
| Appendicular lean (g/year) | −113 ± 4 | −149 ± 14† | −130 ± 11 |
| Total fat mass (g/year) | 163 ± 7 | 203 ± 23 | 160 ± 20 |
| Trunk fat (g/year) | 125 ± 5 | 136 ± 17 | 96 ± 14† |
| Appendicular fat (g/year) | 41 ± 4 | 73 ± 14† | 64 ± 12 |
Data are adjusted means ± SE estimated by generalized estimating equations. Model 1: adjustment for age, sex, race, clinic site, baseline BMI, and weight loss intention; model 2: further adjustment for changes in body weight.
*P < 0.01,
†P < 0.05 vs. those without diabetes.
Model 2 in Table 2 shows disproportional changes in body composition. In general, lean skeletal mass decreased but fat mass increased over time in all three groups. The rates of decline in total and appendicular lean mass were greater in older adults with undiagnosed diabetes than in those without diabetes, even after adjusting for the changes in body weight over time. In older adults with diagnosed diabetes, trunk lean and fat mass were slightly increased when the changes in body weight were accounted for.
Longitudinal changes in thigh muscle mass assessed by CT scan are summarized in Table 3. Because we found a significant interaction effect of sex and diabetes status on the changes in thigh muscle area (P = 0.044), the results were shown by sex. Men showed more rapid declines in thigh muscle CSA than women in all three groups. Even in men without diabetes, the decline in thigh muscle CSA was about two- to threefold higher than in women without diabetes (−13.0 ± 0.8 vs. −5.1 ± 0.5 cm2 in 5 years). In men, the declines in thigh muscle CSA were not significantly different between groups. However, in women, the declines in thigh muscle CSA were significantly higher in those with either diagnosed or undiagnosed diabetes than in their nondiabetic counterparts (−11.1 ± 1.4 and −11.7 ± 1.8 vs. −5.1 ± 0.5 cm2, P < 0.001). Adjustments for baseline weight, weight change over 5 years, IL-6, and TNF-α attenuated the rapid declines in thigh muscle CSA. But older women with either diagnosed or undiagnosed diabetes still showed about a twofold greater loss of thigh muscle CSA than their nondiabetic counterparts when adjusted for the differences in body size, weight changes, and inflammatory cytokines (models 2–4, Table 3).
Table 3.
Longitudinal changes in thigh muscle CSA (cm2) by baseline diabetes status in the Health ABC Study, stratified by sex
| Without diabetes | Undiagnosed diabetes | Diagnosed diabetes | P | |
|---|---|---|---|---|
| n | 1,290 | 125 | 214 | |
| Men | ||||
| Model 1 | −13.0 ± 0.8 | −17.6 ± 2.2 | −14.0 ± 1.7 | 0.153 |
| Model 2 | −13.3 ± 0.7 | −17.0 ± 2.2 | −13.1 ± 1.7 | 0.282 |
| Model 3 | −13.4 ± 0.6 | −15.2 ± 1.7 | −13.4 ± 1.3 | 0.632 |
| Model 4 | −13.6 ± 0.6 | −15.9 ± 1.9 | −12.8 ± 1.4 | 0.411 |
| Women | ||||
| Model 1 | −5.1 ± 0.5 | −11.7 ± 1.8* | −11.1 ± 1.4* | <0.001 |
| Model 2 | −5.2 ± 0.5 | −11.3 ± 1.8* | −10.6 ± 1.4* | <0.001 |
| Model 3 | −5.3 ± 0.4 | −10.8 ± 1.4* | −10.0 ± 1.1* | <0.001 |
| Model 4 | −5.2 ± 0.4 | −10.6 ± 1.5* | −9.3 ± 1.2* | <0.001 |
Data are adjusted means ± SE. Model 1: adjusted for age, race, and clinic site; model 2: additionally adjusted for baseline body weight; model 3: additionally adjusted for changes in body weight; and model 4: additionally adjusted for IL-6 and TNF-α.
*P < 0.01 vs. those without diabetes after Bonferroni correction for multiple comparisons.
CONCLUSIONS
In this study, we found rapid declines in appendicular lean mass in older adults with type 2 diabetes, especially in undiagnosed cases. The declines in appendicular lean mass, which represent skeletal muscle mass, are independent of weight changes over time, confirming an excessive loss of skeletal muscle mass in older adults with type 2 diabetes. The CT data reaffirmed the rapid loss of thigh muscle mass in older adults with type 2 diabetes, although it was significant only in women.
We found a significant interaction effect of sex and diabetes on the changes in thigh muscle mass assessed by CT scan. Older women with type 2 diabetes showed about twofold rapid declines in thigh muscle mass compared with nondiabetic women. It is interesting that the amount of thigh muscle lost in women with type 2 diabetes was comparable with that of men without diabetes, suggesting that women with type 2 diabetes lost the beneficial effect of female sex on preserving lean muscle mass. The finding of our study on sex difference is very consistent with previous studies showing that declines in muscle mass were almost always greater in men than in women (16,17). Higher background declines of thigh muscle mass in older men without diabetes may make it difficult to detect subtle additional changes associated with diabetes. It is also possible that survival bias or selection bias for year 6 CT measurement may obscure the true association, particularly in men. Our previous report on the changes of muscle strength in the same population suggested that differential follow-up rate or nonrandom missing data might influence the results biased to the null (6).
The reason for an accelerated loss of muscle mass in older adults with type 2 diabetes is not clear. It can be postulated that metabolic abnormalities in type 2 diabetes may negatively affect muscle mass. Although the effect on protein metabolism is not as clear as it is in type 1 diabetes, the net balance of body protein metabolism is diminished in type 2 diabetes (9–11). Insulin resistance in type 2 diabetes may also result in the reduced synthesis of whole-body proteins (12).
Interestingly, we found that those with undiagnosed diabetes showed the greatest declines in appendicular lean mass, suggesting that the effect of type 2 diabetes on skeletal muscle mass seems to be manifested in the early stages of the disease. In diagnosed cases, the long duration of diabetes (average 13.3 years) could already affect the baseline body composition, which might make it difficult to detect further changes. It is also possible that various treatments in subjects with diagnosed diabetes might modify the association of diabetes and the changes in body composition. For example, treatment with sulfonylurea or insulin is often accompanied by improved protein metabolism (18,19). Thiazolidinediones may also result in weight gain and/or edema, which may obscure accurate assessments of body composition changes. Unfortunately, we were unable to evaluate the effects of various medications in the current study because of the small numbers in any one treatment and the substantial changes in treatment during follow-up.
There are several limitations in our study. Although we have shown the temporal relationship between baseline diabetes status and longitudinal changes in muscle mass, it does not confirm causality. We could not identify the factors associated with the rapid loss of muscle mass in older adults with type 2 diabetes other than those in the early stages of diabetes, as evidenced in cases of undiagnosed diabetes. Our study was not designed to examine the effect of glycemic control, specific treatments, comorbidities, and other hormones, etc. These would be better addressed in a study of diabetes with a detailed characterization of diabetic management over time.
Despite any limitations, the results of our study have important implications given that both sarcopenia and type 2 diabetes increase with age. Both conditions often remain unrecognized since one-third of type 2 diabetic subjects are still undiagnosed (20,21). If older adults with undiagnosed type 2 diabetes were left untreated, they would be at higher risk for developing sarcopenia. Excessive loss of muscle mass in older adults with type 2 diabetes may result in poor muscle strength, functional limitations, and physical disability. Future research should find the factors responsible for excessive loss of lean mass in older adults with type 2 diabetes and develop strategies to prevent the adverse outcomes of sarcopenia in this high-risk population (22). In conclusion, type 2 diabetes is associated with the excessive loss of skeletal muscle mass in older adults. Older adults with undiagnosed diabetes are at particularly high risk for the loss of skeletal muscle mass.
Acknowledgments
This study was supported by contracts N01-AG-6-2101, N01-AG-6-2103, and N01-AG-6-2106 and in part by the Intramural Research Program of the National Institutes of Health National Institute on Aging.
No potential conflicts of interest relevant to this article were reported.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
See accompanying editorial, p. 2136.
References
- 1. Landers KA, Hunter GR, Wetzstein CJ, Bamman MM, Weinsier RL: The interrelationship among muscle mass, strength, and the ability to perform physical tasks of daily living in younger and older women. J Gerontol A Biol Sci Med Sci 2001; 56: B443– B448 [DOI] [PubMed] [Google Scholar]
- 2. Visser M, Goodpaster BH, Kritchevsky SB, Newman AB, Nevitt M, Rubin SM, Simonsick EM, Harris TB: Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci 2005; 60: 324– 333 [DOI] [PubMed] [Google Scholar]
- 3. Newman AB, Kupelian V, Visser M, Simonsick EM, Goodpaster BH, Kritchevsky SB, Tylavsky FA, Rubin SM, Harris TB: on behalf of the Health, Aging and Body Composition Study Investigators. Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J Gerontol A Biol Sci Med Sci 2006; 61: 72– 77 [DOI] [PubMed] [Google Scholar]
- 4. Koh-Banerjee P, Wang Y, Hu FB, Spiegelman D, Willett WC, Rimm EB: Changes in body weight and body fat distribution as risk factors for clinical diabetes in US men. Am J Epidemiol 2004; 159: 1150– 1159 [DOI] [PubMed] [Google Scholar]
- 5. Park SW, Goodpaster BH, Strotmeyer ES, De Rekeneire N, Harris TB, Schwartz AV, Tylavsky FA, Newman AB: Decreased muscle strength and quality in older adults with type 2 diabetes: the Health, Aging, and Body Composition Study. Diabetes 2006; 55: 1813– 1818 [DOI] [PubMed] [Google Scholar]
- 6. Park SW, Goodpaster BH, Strotmeyer ES, Kuller LH, Broudeau R, Kammerer C, De Rekeneire N, Harris TB, Schwartz AV, Tylavsky FA, Cho Y-W, Newman AB. for the Health, Aging, and Body Composition Study. Accelerated loss of skeletal muscle strength in older adults with type 2 diabetes: the Health, Aging, and Body Composition Study. Diabetes Care 2007; 30: 1507– 1512 [DOI] [PubMed] [Google Scholar]
- 7. Charlton M, Nair KS: Protein metabolism in insulin-dependent diabetes mellitus. J Nutr 1998; 128( S2): 323S– 327S [DOI] [PubMed] [Google Scholar]
- 8. Karakelides H, Asmann YW, Bigelow ML, Short KR, Dhatariya K, Coenen-Schimke J, Kahl J, Mukhopadhyay D, Nair KS: Effect of insulin deprivation on muscle mitochondrial ATP production and gene transcript levels in type 1 diabetic subjects. Diabetes 2007; 56: 2683– 2689 [DOI] [PubMed] [Google Scholar]
- 9. Denne SC, Brechtel G, Johnson A, Liechty EA, Baron AD: Skeletal muscle proteolysis is reduced in noninsulin-dependent diabetes mellitus and is unaltered by euglycemic hyperinsulinemia or intensive insulin therapy. J Clin Endocrinol Metab 1995; 80: 2371– 2377 [DOI] [PubMed] [Google Scholar]
- 10. Halvatsiotis P, Short KR, Bigelow M, Nair KS: Synthesis rate of muscle proteins, muscle functions, and amino acid kinetics in type 2 diabetes. Diabetes 2002; 51: 2395– 2404 [DOI] [PubMed] [Google Scholar]
- 11. Gougeon R, Morais JA, Chevalier S, Pereira S, Lamarche M, Marliss EB: Determinants of whole-body protein metabolism in subjects with and without type 2 diabetes. Diabetes Care 2008; 31: 128– 133 [DOI] [PubMed] [Google Scholar]
- 12. Pereira S, Marliss EB, Morais JA, Chevalier S, Gougeon R: Insulin resistance of protein metabolism in type 2 diabetes. Diabetes 2008; 57: 56– 63 [DOI] [PubMed] [Google Scholar]
- 13. Visser M, Fuerst T, Lang T, Salamone L, Harris TB: Validity of fan beam dual-energy x-ray absorptiometry for measuring fat-free and leg muscle mass. Health, Aging, and Body Composition Study–Dual-Energy X-ray Absorptiometry and Body Composition Working Group. J Appl Physiol 1999; 87: 1513– 1520 [DOI] [PubMed] [Google Scholar]
- 14. Tylavsky FA, Lohman TG, Dockrell M, Lang T, Schoeller DA, Wan JY, Fuerst T, Cauley JA, Nevitt M, Harris TB: Comparison of the effectiveness of 2 dual-energy X-ray absorptiometers with that of total body water and computed tomography in assessing changes in body composition during weight change. Am J Clin Nutr 2003; 77: 356– 363 [DOI] [PubMed] [Google Scholar]
- 15. Liang KY, Zeger SL: Longitudinal data analysis using generalized linear models. Biometrica 1986; 73: 13– 22 [Google Scholar]
- 16. Visser M, Pahor M, Tylavsky F, Kritchevsky SB, Cauley JA, Newman AB, Blunt BA, Harris TB: One- and two-year change in body composition as measured by DXA in a population-based cohort of older men and women. J Appl Physiol 2003; 94: 2368– 2374 [DOI] [PubMed] [Google Scholar]
- 17. Hughes VA, Frontera WR, Roubenoff R, Evans WJ, Singh MA: Longitudinal changes in body composition in older men and women: role of body weight change and physical activity. Am J Clin Nutr 2002; 76: 473– 481 [DOI] [PubMed] [Google Scholar]
- 18. Gougeon R, Styhler K, Morais JA, Jones PJ, Marliss EB: Effects of oral hypoglycemic agents and diet on protein metabolism in type 2 diabetes. Diabetes Care 2000; 23: 1– 8 [DOI] [PubMed] [Google Scholar]
- 19. Gougeon R, Marliss EB, Jones PJ, Pencharz PB, Morais JA: Effect of exogenous insulin on protein metabolism with differing nonprotein energy intakes in type 2 diabetes mellitus. Int J Obes Relat Metab Disord 1998; 22: 250– 261 [DOI] [PubMed] [Google Scholar]
- 20. Wild S, Roglic G, Green A, Sicree R, King H: Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004; 27: 1047– 1053 [DOI] [PubMed] [Google Scholar]
- 21. Franse LV, Di Bari M, Shorr RI, Resnick HE, van Eijk JT, Bauer DC, Newman AB, Pahor M. for the Health, Aging, and Body Composition Study Group. Type 2 diabetes in older well-functioning people: Who is undiagnosed? Data from the Health, Aging, and Body Composition Study. Diabetes Care 2001; 24: 2065– 2070 [DOI] [PubMed] [Google Scholar]
- 22. Lanza IR, Short DK, Short KR, Raghavakaimal S, Basu R, Joyner MJ, McConnell JP, Nair KS: Endurance exercise as a countermeasure for aging. Diabetes 2008; 57: 2933– 2942 [DOI] [PMC free article] [PubMed] [Google Scholar]