Abstract
OBJECTIVE
This randomized, four-arm, placebo-controlled, dose-ranging phase 2 trial was conducted to determine whether repeated subcutaneous injections of the altered peptide ligand, NBI-6024, designed to inhibit autoreactive T-cells, improves β-cell function in patients with recently diagnosed type 1 diabetes.
RESEARCH DESIGN AND METHODS
A total of 188 patients, aged 10–35 years, with recently diagnosed type 1 diabetes were randomly assigned for a treatment consisting of the subcutaneous administration of placebo or 1, 0.5, or 0.1 mg NBI-6024 at baseline, weeks 2 and 4, and then monthly until month 24. Fasting, peak, and area under the curve (AUC) C-peptide concentrations during a 2-h mixed-meal tolerance test were measured at 3-month intervals during treatment. Immune function parameters (islet antibodies and CD4 and CD8 T-cells) were also studied.
RESULTS
The mean peak C-peptide concentration at 24 months after study entry showed no significant difference between the groups treated with 0.1 mg (0.59 pmol/ml), 0.5 mg (0.57 pmol/ml), and 1.0 mg NBI-6024 (0.48 pmol/ml) and the placebo group (0.54 pmol/ml). Fasting, stimulated peak, and AUC C-peptide concentrations declined linearly in all groups by ∼60% over the 24-month treatment period. The average daily insulin needs at month 24 were also comparable between the four groups. No treatment-related changes in islet antibodies and T cell numbers were observed.
CONCLUSIONS
Treatment with altered peptide ligand NBI-6024 at repeated doses of 0.1, 0.5, or 1.0 mg did not improve or maintain β-cell function.
Type 1 diabetes results from a T-cell–mediated autoimmune attack against the insulin-producing cells of the pancreatic islets (1–3). There is no curative treatment available to control these autoreactive T-cells, rendering the patients dependent on insulin injections for normoglycemia. A treatment that could stop or reduce autoimmune destruction of pancreatic β-cells would be a major advance in diabetes treatment and could potentially prevent diabetes in individuals genetically predisposed to developing the disease (4).
There is potential to target specific populations of autoreactive T-cells by identifying the dominant antigens responsible for their activation and producing a soluble altered peptide ligand (APL) to block or change this response. The insulin B (9-23) peptide has been shown to be an important antigen of T-cells in autoimmune diabetes in animals and humans (5). NBI-6024 is an APL and contains two natural l-amino acid substitutions in the (9-23) sequence of the B-chain of insulin. Alanine is substituted for tyrosine at position 16, which is a key contact site at the T-cell receptor and at position 19 for cysteine. The resulting APL (Ala16,19), known as NBI-6024, does not activate insulin B (9-23)–reactive murine or human T-cells (6). Nonobese diabetic mice treated with NBI-6024 are protected from developing diabetes, even though other T-cells with different antigenic specificities were present, suggesting that the immune response induced by the APL may regulate pathogenic T-cells through the production of regulatory cytokines such as interleukin-4 (6).
Preliminary results of three studies in adult male patients with type 1 diabetes had indicated that NBI-6024 administration is safe and well tolerated (7,8). To investigate the pharmacological potential of NBI-6024 to improve β-cell function, a multicenter, randomized, four-arm, placebo-controlled phase 2 trial was performed. The primary objective of the trial was to assess the effect of repeated administrations of NBI-6024 on endogenous insulin production as measured by C-peptide concentration in adult and adolescent patients with recent-onset type 1 diabetes. Insulin usage, glycemic control, and immune function were also assessed.
RESEARCH DESIGN AND METHODS
Patients with recent-onset type 1 diabetes were selected according to the following criteria: age 10–17 years (adolescent group) or 18–35 years (adult group), symptom duration for no longer than 6 months, treatment with insulin for <3 months, positive result on testing for islet autoantibodies (anti-GAD antibodies or anti-islet cell [ICA512] antibodies or anti-insulin antibodies provided that the patient had not been receiving insulin therapy for >2 weeks), stimulated C-peptide peak concentration between 0.4 and 3.0 pmol/ml, BMI <28 kg/m2, laboratory and electrocardiogram results within normal ranges, and compliance with insulin treatment. Pregnant or lactating women were excluded, and female patients with childbearing potential had to practice an acceptable contraceptive technique from 30 days before enrollment until 30 days after the last dose of study drug. Written informed consent was obtained from each patient. The trial was approved by the ethics committee at each center.
Study centers
A total of 22 centers participated in the study including six centers in South Africa (103 patients randomized), one in the U.K. (three patients), two in the Czech Republic (23 patients), four in Spain (10 patients), one in Finland (5 patients), two in Germany (33 patients), four in Canada (9 patients), and two in France (2 patients).
Study design
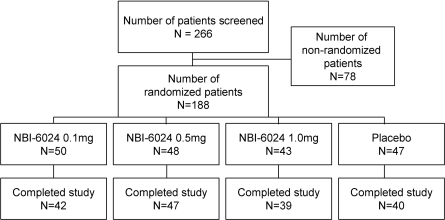
The trial was performed as a phase 2, multicenter, randomized, double-blind, placebo-controlled, parallel, dose-ranging study between 2001 and 2006. Of 266 patients screened, a total of 188 patients were randomly assigned, including 111 adolescents (Fig. 1). Eligible patients were randomly assigned in a 1:1:1:1 ratio to one of the following treatments: 0.1 mg NBI-6024 (n = 50), 0.5 mg NBI-6024 (n = 48), 1 mg NBI-6024 (n = 43), or placebo (n = 47). On day 1, patients received their first dose of study drug and were discharged ∼2 h postdose at the discretion of the investigator. The study drug was administered subcutaneously for a total of 26 times over a 24-month period. The first three doses were administered every 2 weeks (induction phase); all subsequent dosing occurred monthly (maintenance phase). Dose and dosing frequency were selected after considering immune efficacy and tolerability in animal studies. Patients returned to the study center to receive the study drug and have efficacy and safety assessments performed. Patients continued with their normal insulin regimen throughout the study, unless changes were clinically indicated. To avoid possible confounding through differences in glycemic control among the groups, diabetes management and glycemic targets were standardized as much as possible in all patients. Patients with A1C levels >8% had additional contacts with the investigator to improve their metabolic control. All patients were treated with intensive insulin therapy.
Figure 1.
Study participation and randomization.
End point assessments
The primary efficacy variable was the 2-h peak C-peptide level at 24 months. A mixed-meal tolerance test (Boost High Protein from Novartis in an amount of 6 ml/kg body weight, up to 360 ml) was administered to collect stimulated C-peptide samples (0, 30, 60, 90, and 120 min) every 3 months and at the follow-up visit. Secondary efficacy variables were the area under the curve (AUC0–120 min), C-peptide at 24 months, the prescribed insulin usage and glycemic control, assessed by A1C levels, and the hypo- and hyperglycemic events documented in the patient's diary. C-peptide concentrations and A1C were measured at a central laboratory (ICON Central Laboratories, Farmingdale, NY) with a radioimmunoassay (Diagnostic Systems Laboratories) and high-pressure liquid chromatography, respectively. The reported interassay coefficient of variation of the C-peptide assay is 5.3% at a concentration of 0.55 nmol/l and the lower limit of detection is 0.03 nmol/l.
Safety parameters and hypoglycemic events
Safety parameters included vital signs (body temperature, supine heart rate, and blood pressure) before and 1 h after study drug administration, laboratory tests (hematology, clinical chemistry, and urinalysis with microscopy), and quarterly physical examinations as well as an electrocardiogram and documentation of adverse events and concomitant medication. Hypoglycemic events were defined as any blood glucose level <50 mg/dl (3 mmol/l) with or without typical symptoms or typical symptoms if the blood glucose level was ≥50 mg/dl. If the patient required intervention by another person, the event was defined as a “major hypoglycemic event.”
Immune markers
CD4 and CD8 T-cell numbers were measured by flow cytometry on whole blood using a standard protocol at a central laboratory (ICON Central Laboratories, Dublin, Ireland). Antibodies to insulin, GAD65, and IA-2 were measured by ICON Central Laboratories (Farmingdale, NY) using radiobinding assays (Kronus, Boise, ID) according to the manufacturer's instructions.
Statistical methods
All data are summarized by treatment group. All randomly assigned patients were included in the modified intent-to treat (ITT) population. All randomly assigned patients who had taken at least one dose of study drug and had some postbaseline data for the primary efficacy parameter (2-h peak C-peptide) were included in this population. The efficacy evaluable population included patients in the ITT population who received at least 13 doses and did not have any major protocol deviations. The safety population was defined as all randomly assigned patients who received at least one dose of study drug. All hypotheses testing was two-sided and performed at the 5% significance level.
RESULTS
Of 188 patients who were randomly assigned, 168 completed the study (Fig. 1). The 20 patients who dropped out of the study included 12 who withdrew consent, 3 who had an adverse event, 2 who became pregnant, and 3 who discontinued for other reasons. The four treatment groups were similar in terms of age, duration of disease, ethnicity, and baseline C-peptide concentrations (Table 1).
Table 1.
Patient demographics and history of diabetes by treatment group
| Treatment group |
Placebo | |||
|---|---|---|---|---|
| 0.1 mg NBI-6024 | 0.5 mg NBI-6024 | 1.0 mg NBI-6024 | ||
| n | 50 | 48 | 43 | 47 |
| Age (years) | 17.9 ± 6.3 | 17.4 ± 6.4 | 17.9 ± 6.4 | 18.7 ± 7.1 |
| Sex | ||||
| Male | 31 | 26 | 30 | 31 |
| Female | 19 | 22 | 13 | 16 |
| Peak C-peptide baseline (pmol/ml) | 1.36 ± 0.63 | 1.35 ± 0.63 | 1.41 ± 0.63 | 1.42 ± 0.61 |
| A1C baseline (%) | 7.9 ± 1.6 | 7.8 ± 1.6 | 8.1 ± 2.0 | 8.1 ± 2.0 |
| Daily insulin dose (IU) | 31.3 ± 19.4 | 33.1 ± 15.8 | 30.5 ± 11.9 | 32.1 ± 14.9 |
| Time since symptoms (months) | 2.1 ± 1.1 | 2.1 ± 1.4 | 2.1 ± 1.1 | 2.3 ± 1.9 |
| Time since diagnosis (months) | 1.2 ± 0.5 | 1.2 ± 0.7 | 1.2 ± 0.7 | 1.2 ± 0.8 |
Data are means ± SD or n.
Safety parameters
Adverse events reported by ≥10% of patients were upper respiratory tract infection, nasopharyngitis, headache, pharyngitis, influenza, nausea, rhinitis, and vomiting. There was no clear pattern of relationship between dose, occurrence, and frequency of adverse events. The frequencies of adverse events in the NBI-6024 treatment groups and the placebo group were comparable. There was no clinically significant change in laboratory or vital sign parameters. Overall, 39 (20.7%) patients were reported as experiencing at least one serious adverse event; none of the serious adverse events was considered to be drug-related.
Efficacy parameters
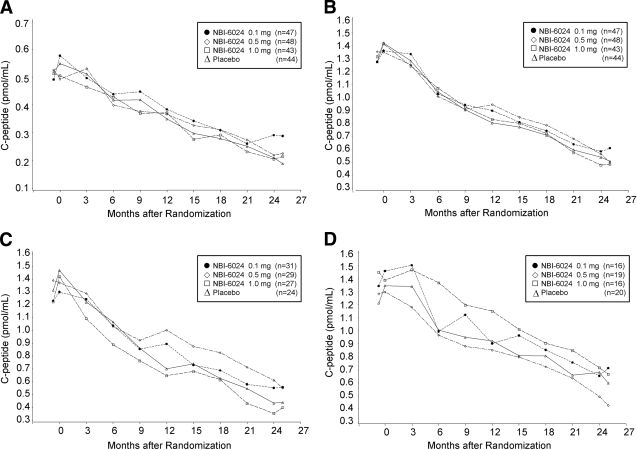
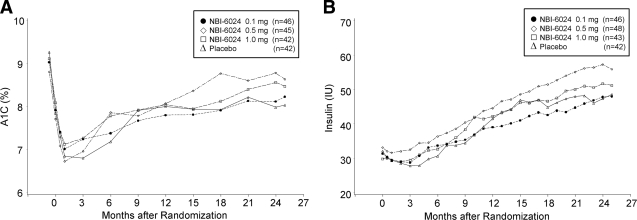
The mean peak C-peptide value at month 24 and the change in the mean values from baseline to month 24 were comparable in the NBI-6024 treatment groups and the placebo group in the ITT as well as the efficacy evaluable population (ITT population mean peak C-peptide at 24 months: 0.1 mg, 0.39 pmol/ml; 0.5 mg, 0.39 pmol/ml; 1 mg, 0.33 pmol/ml; and placebo, 0.39 pmol/ml; P = 0.4, 0.3, and 0.6) (Fig. 2). Similarly, the mean AUCs of C-peptide at baseline and at month 24 in the NBI-6024 treatment groups and in the placebo group were comparable (Table 2). The average daily insulin doses needed at 24 months were 47.8 ± 20.4, 57.2 ± 36.5, and 51.6 ± 21.8 IU in the 0.1, 0.5, and 1 mg NBI-6024 treatment groups, respectively, and 47.3 ± 17.6 IU in the placebo group (P = 0.9, 0.05, and 0.2) (Fig. 3). The A1C values at month 24 were slightly higher in the NBI-6024 treatment groups compared with that the placebo group (0.1 mg, 8.1 ± 2.1; 0.5 mg, 8.8 ± 3.0; 1 mg, 8.6 ± 2.6; and placebo, 8.0 ± 2.1; P = 0.7, 0.01, and 0.1) (Fig. 3). The percentages of patients who reached an A1C <7% at 24 months were 34, 32, 35, and 29% in the NBI-6024 treatment groups and the placebo group, respectively (P = 0.6, 0.9, and 0.6).
Figure 2.
Mean fasting C-peptide levels (for all subjects [A]) and mean peak C-peptide levels (all subjects [B], adolescents [C], and adults [D])after mixed-meal stimulation in the four treatment groups. No significant difference in C-peptide levels was observed among treatment groups. The decline in C-peptide secretion from baseline to 24 months was similar between adolescent patients and adults.
Table 2.
AUC0–120 min C-peptide by treatment group
| Treatment group |
Placebo | |||
|---|---|---|---|---|
| 0.1 mg NBI-6024 | 0.5 mg NBI-6024 | 1.0 mg NBI-6024 | ||
| Baseline | 47 | 48 | 43 | 44 |
| 134 ± 63 | 131 ± 62 | 135 ± 57 | 134 ± 59 | |
| Month 24 | 42 | 46 | 37 | 40 |
| 57 ± 71 | 53 ± 56 | 44 ± 55 | 50 ± 45 | |
| P | 0.5 | 0.6 | 0.9 | |
Data are n or means ± SD (pmol × min/ml).
Figure 3.
Mean A1C values (A) and mean daily insulin requirement (B) in the four treatment groups.
When the analysis was stratified by age, no significant difference was found for peak C-peptide at month 24 or for the decline in peak C-peptide from baseline to 24 months between adolescent patients and adults (Fig. 2). Furthermore, there was no effect of sex or ethnicity.
Hypoglycemic events
Most hypoglycemic events were deemed to be minor events. A pairwise treatment comparison showed no statistically significant difference between NBI-6024 treatment groups compared with placebo in the monthly rate of hypoglycemic events (monthly rate: 0.1 mg, 1.9 ± 2.0; 0.5 mg, 1.7 ± 1.5; 1 mg, 1.9 ± 1.6; and placebo 1.8 ± 2.4; P = 0.6, 0.3, and 0.4). Approximately 9% of patients experienced a major hypoglycemic event during this study; these patients were evenly distributed across treatment groups.
Immune function parameters
Insulin antibodies increased in concentration from the baseline visit to month 1 and remained stable thereafter in all groups. No differences in antibodies to insulin, GAD65, and IA-2 were observed between treatment and placebo groups at all study visits (supplementary Figure S1, available in an online appendix at http://care.diabetesjournals.org/cgi/content/full/dc09-0449/DC1). No differences were observed for CD4 and CD8 peripheral blood T-cell numbers between treatment and placebo groups (supplementary Figure S2).
CONCLUSIONS
For the first time, an APL was used for treatment of autoimmune diabetes in a trial that included 188 patients with recent-onset disease. The APL NBI-6024, at the doses studied, was well tolerated. There were no significant safety issues, and most adverse events were considered mild or moderate and unrelated or not likely to be related to the study drug. The frequency of injection site reactions, a surrogate marker of immunoreactivity, was comparable among placebo and active treatment groups. Treatment with NBI-6024 at the doses studied, however, provided no protection against the decline of β-cell function after diabetes onset, as measured by fasting or stimulated C-peptide. Moreover, NBI-6024 did not cause significant changes in insulin requirement, metabolic control, hypoglycemic and hyperglycemic events, autoantibody concentrations, or CD4 and CD8 T-cell numbers.
The NBI-6024 clinical trial was an intensively monitored intervention study that collected data on C-peptide, glucose, and A1C every 3 months for 24 months with central measurement of these variables. It provided a detailed natural history of β-cell function after diabetes onset in unprecedented numbers of well characterized and selected adolescents and adults with recent-onset type 1 diabetes. One limitation of the current trial is that no T-cell response data were obtained to verify an immunological effect at the doses given. However, the study included three doses over a 10-fold range, which had previously been shown to cause T-cell unresponsiveness (7,8). The lack of response may reflect a fundamental defect in the proposed mechanism of action or inadequacy of exposure (dose), frequency, or timing of the investigational drug.
With respect to the natural history of β-cell function, the study suggests a linear continuous decline of β-cell function over a period of 2 years after disease onset. The rate of decline was similar between adolescents and adults with slope estimates of −0.034 and −0.031, respectively, for peak C-peptide values. All three parameters of β-cell function examined (fasting C-peptide, peak C-peptide, and AUC of C-peptide response) in the mixed-meal test showed similar declines with an overall loss of ∼30% by 12 months of follow-up and 60% by 24 months of follow-up. These data are likely to be useful as reference for other trials and for future trial planning. For comparison, there was a 50–60% decline in fasting and stimulated C-peptide concentrations within 21 months in the recently reported GAD-alum vaccination trial in patients aged 10–18 years (9).
In summary, this study failed to demonstrate efficacy of APL treatment in patients at the stage of overt disease. The continuous decline of β-cell function after onset, however, provides important data for design and validation of future clinical trials in patients with type 1 diabetes and confirms the need for novel treatment strategies (10) to reduce this progressive β-cell loss.
Supplementary Material
Acknowledgments
R.J. is an employee of Neurocrine Biosciences, Inc. As an employee of the company, he receives a monthly salary and has been granted stock options. A.P. and F.B. received financial support in the form of institutional research grants from Neurocrine Biosciences, Inc., during the performance of this study. No other potential conflicts of interest relevant to this article were reported.
We thank the Neurocrine Biosciences, Inc., Development Team for their efforts, i3 Research for study management, all technicians and study nurses who contributed to this project, and all patients who participated in the study.
APPENDIX
Members of the NBI-6024 Study Group are the following: South Africa: L. Distiller, A. Philotheou, R. Moore, L.I. Robertson, J. Wing, G. Ellis, B.D. Kramer, F.A. Mahomed, B.I. Joffe, F. Bonnici, P.J. Wormald, S. Brown, A. Murphy, N. Gurtunca, L. Hofmann, M.-T. van der Merwe, S. Goldburg, and H. Wellmann; U.K.: S. Greene and V. Franklin; Czech Republic: J. Vavrinec, J. Venhacova, Z. Sumnik, S. Kolouskova, O. Cinek, K. Stechova, and P. Venhacova; Spain: R. Gomis, P. Martul, J.P. López-Siguero, D. Acosta-Delgado, I. Conget, E. Aguilera, J.A. Vazquez Garcia, L. Castaño, F. Vazquez San Miguel, I. Rica, V. Martín, MaS. Ruiz de Adana Navas, A. del Pino de la Fuente, M.J. Garcia Arias, M.A. Mangas Cruz, and R. Guerrero; Finland: I. Sipilä, T. Saukkonen, P.J. Miettinen, and T. Otonkoski; Germany: A.-G. Ziegler, T. Danne, M. Füchtenbusch, M. Walter, T. Kaupper, C. Bittner, W. von Schutz, A. Krautzig, A. Hackenberg, and N. Datz; Canada: A. Belanger, P. Perron, D. Pacaud, M. Lawson, J. Palardy, N. Kandalaft, R. Duma, M.F. Langlois, K. Khoury, J.P. Baillargeon, S. Lawrence, and A. Hadjiyannakis; and France: P.-F. Bougnères, M. Nicolino, M.C. Andre, J.C. Carel, P.P. Boileau, M. François, N. Bendelac, F. Texier, P. Bretones, and C.-L. Gay.
Footnotes
Clinical trial reg. no. NCT00873561, clinicaltrials.gov.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Eisenbarth GS: Type 1 diabetes mellitus: a chronic autoimmune disease. N Engl J Med 1986;314:1360–1368 [DOI] [PubMed] [Google Scholar]
- 2. Roep BO, Atkinson MA, van Endert PM, Gottlieb PA, Wilson SB, Sachs JA: Autoreactive T cell responses in insulin-dependent (type 1) diabetes mellitus. J Autoimmun 1999;13:267–282 [DOI] [PubMed] [Google Scholar]
- 3. Lejon K, Fathman CG: Isolation of self antigen-reactive cells from inflamed islets of nonobese diabetic mice using CD4 high expression as a marker. J Immunol 1999;163:5708–5714 [PubMed] [Google Scholar]
- 4. Stiller CR, Dupre J, Gent M, Jenner MR, Keown PA, Laupacis A, Martell R, Rodger NW, von Graffenried B, Wolfe BM: Effects of cyclosporine immunosuppression in insulin-dependent diabetes mellitus of recent onset. Science 1984;223:1362–1367 [DOI] [PubMed] [Google Scholar]
- 5. Wegmann DR, Gill RG, Norbury-Glaser M, Schloot N, Daniel D: Analysis of the spontaneous T cell response to insulin in NOD mice. J Autoimmun 1994;7:833–843 [DOI] [PubMed] [Google Scholar]
- 6. Alleva DG, Gaur A, Jin L, Wegmann D, Gottlieb PA, Pahuja A, Johnson EB, Motheral T, Putnam A, Crowe PD, Ling N, Boehme SA, Conlon PJ: Immunological characterization and therapeutic activity of an altered-peptide ligand, NBI-6024, based on the immunodominant type 1 diabetes autoantigen insulin B-chain (9-23) peptide. Diabetes 2002;51:2126–2134 [DOI] [PubMed] [Google Scholar]
- 7. Giannoukakis N: NBI-6024 (Neurocrine Biosciences). IDrugs 2002;5:1162–1167 [PubMed] [Google Scholar]
- 8. Alleva DG, Maki RA, Putnam AL, Robinson JM, Kipnes MS, Dandona P, Marks JB, Simmons DL, Greenbaum CJ, Jimenez RG, Conlon PJ, Gottlieb PA: Immunomodulation in type 1 diabetes by NBI-6024, an altered peptide ligand of the insulin B epitope. Scand J Immunol 2006;63:59–69 [DOI] [PubMed] [Google Scholar]
- 9. Ludvigsson J, Faresjö M, Hjorth M, Axelsson S, Chéramy M, Pihl M, Vaarala O, Forsander G, Ivarsson S, Johansson C, Lindh A, Nilsson NO, Aman J, Ortqvist E, Zerhouni P, Casas R: GAD treatment and insulin secretion in recent-onset type 1 diabetes. N Engl J Med 2008;359:1909–1920 [DOI] [PubMed] [Google Scholar]
- 10. Keymeulen B, Vandemeulebroucke E, Ziegler AG, Mathieu C, Kaufman L, Hale G, Gorus F, Goldman M, Walter M, Candon S, Schandene L, Crenier L, De Block C, Seigneurin JM, De Pauw P, Pierard D, Weets I, Rebello P, Bird P, Berrie E, Frewin M, Waldmann H, Bach JF, Pipeleers D, Chatenoud L: Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N Engl J Med 2005;352:2598–2608 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.