Abstract
OBJECTIVE
To assess the magnitude and independence of the effects of routine blood pressure lowering and intensive glucose control on clinical outcomes in patients with long-standing type 2 diabetes.
RESEARCH DESIGN AND METHODS
This was a multicenter, factorial randomized trial of perindopril-indapamide versus placebo (double-blind comparison) and intensive glucose control with a gliclazide MR–based regimen (target A1C ≤6.5%) versus standard glucose control (open comparison) in 11,140 participants with type 2 diabetes who participated in the Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation (ADVANCE) trial. Annual event rates and risks of major macrovascular and microvascular events considered jointly and separately, renal events, and death during an average 4.3 years of follow-up were assessed, using Cox proportional hazards models.
RESULTS
There was no interaction between the effects of routine blood pressure lowering and intensive glucose control for any of the prespecified clinical outcomes (all P > 0.1): the separate effects of the two interventions for the renal outcomes and death appeared to be additive on the log scale. Compared with neither intervention, combination treatment reduced the risk of new or worsening nephropathy by 33% (95% CI 12–50%, P = 0.005), new onset of macroalbuminuria by 54% (35–68%, P < 0.0001), and new onset of microalbuminuria by 26% (17–34%). Combination treatment was associated with an 18% reduction in the risk of all-cause death (1–32%, P = 0.04).
CONCLUSIONS
The effects of routine blood pressure lowering and intensive glucose control were independent of one another. When combined, they produced additional reductions in clinically relevant outcomes.
Type 2 diabetes affects >240 million people worldwide and significantly contributes to the global burden of vascular disease, of which both blood pressure and glucose are important determinants (1). Large-scale, randomized controlled trials have clearly documented the benefits of blood pressure–lowering treatment on the risk of macrovascular and microvascular complications and on survival among patients with type 2 diabetes (2–4). Intensive glucose control has been shown to reduce the risk of microvascular complications (5–8), although it did not improve patient survival or alter the course of macrovascular complications in the first 3–10 years (7–10). Current guidelines recommend a multifactorial approach with simultaneous targeting of elevated blood pressure and glucose levels in individuals with type 2 diabetes (11–13). However, it is not known whether combining blood pressure lowering and glucose control can reduce the risk of vascular complications to a greater extent than either treatment alone.
Results from the UK Prospective Diabetes Study (UKPDS) have suggested that the effects of blood pressure– and glucose-lowering interventions might be additive (14). In post hoc analyses, the associations between vascular outcomes and systolic blood pressure or A1C levels achieved during follow-up were independent, and there was a trend toward the greatest benefit with the combination of intensive blood pressure– and glucose-lowering interventions (14). However, because only a small subset of hypertensive subjects received both interventions, the UKPDS had insufficient power to determine conclusively whether the effects of the treatments were additive in this group or in the broader population with type 2 diabetes. Moreover, the reported excess mortality in the intensive glucose control arm of the Action to Control Cardiovascular Risk in Diabetes (ACCORD) study compared with that for those randomized to standard glucose control has raised concerns about whether intensive glucose lowering could modify or even negate the benefits of blood pressure lowering in patients with type 2 diabetes (9).
Recently the Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation (ADVANCE) study, a large-scale, double-blind, randomized factorial trial among 11,140 people with type 2 diabetes, demonstrated the separate vascular benefits of routine blood pressure lowering with a fixed combination of perindopril and indapamide after 4.3 years of follow-up and of intensive glucose control, based on gliclazide MR, after 5 years of follow-up (4,8). In this report, we present new analyses that explore the extent to which blood pressure–lowering treatment and intensive glucose control provide independent and additive benefits with respect to vascular outcomes in patients with type 2 diabetes after 4.3 years of follow-up.
RESEARCH DESIGN AND METHODS
ADVANCE was a factorial randomized controlled trial evaluating the effects of blood pressure lowering and intensive blood glucose control on vascular outcomes. Detailed descriptions of the study methods have been published previously (4,8,15). In brief, a total of 11,140 individuals with type 2 diabetes aged ≥55 years, with a history of major macrovascular or microvascular disease or at least one other risk factor for vascular disease, were enrolled from 215 centers in 20 countries. There were no A1C or blood pressure inclusion criteria. Approval for the trial was obtained from each center's institutional review board, and all participants provided written informed consent. After a 6-week active run-in period with fixed-combination perindopril and indapamide (2 mg/0.625 mg), during which usual glucose control was continued, participants were randomly assigned, in a factorial design, to continued perindopril-indapamide (2 mg/0.625 mg for the first 3 months then 4 mg/1.25 mg thereafter) or matching placebo and to either an intensive glucose control strategy aiming for an A1C of ≤6.5% or a standard glucose control strategy (target A1C levels defined by local guidelines) using a central, computer-based randomization service. Participants randomly assigned to intensive glucose control were given gliclazide MR (30–120 mg daily) and other glucose-lowering drugs as required by their primary physicians (8). Participants randomly assigned to the standard control who were using gliclazide MR at study entry were required to substitute this with another sulfonylurea, if continued therapy was required (8).
Outcomes and follow-up
The primary outcomes were a composite of macrovascular (cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke) and microvascular (new or worsening nephropathy or retinopathy) events, considered jointly and separately (4,8). The secondary outcomes examined were death from all causes, cardiovascular death, major coronary events (death due to coronary heart disease [including sudden death] or nonfatal myocardial infarction), major cerebrovascular events (death due to cerebrovascular disease or nonfatal stroke), new or worsening nephropathy (requirement for renal replacement therapy, death from renal disease, development of macroalbuminuria, or a doubling of serum creatinine to a level of at least 200 μmol/l), new onset of microalbuminuria (urinary albumin-to-creatinine ratio 30–300 μg/mg) and total renal events (defined post hoc as the composite of new or worsening nephropathy or new onset of microalbuminuria), and new or worsening retinopathy. Primary outcomes and all deaths were reviewed and validated by an independent end point adjudication committee blinded to treatment allocation (4,8). For the purposes of this study, the joint effects of randomized treatments were studied after 4.3 years of follow up, that is, at completion of the blood pressure intervention, although the glucose-lowering intervention was completed after an average of 5 years.
Statistical analysis
Differences between randomized groups in blood pressure and A1C during follow-up were estimated using linear mixed models. Annual event rates were calculated with the person-years method. Combined treatment effects on study end points were estimated from unadjusted Cox proportional hazard models, using survival time to the first relevant end point in any individual patient, according to the principle of intention to treat. Only the first event of the relevant outcome type was included in each analysis. Participants were censored at their date of death, date of last visit (for those still alive at the end of follow-up), or date when last known to be alive (for those with unknown vital status). For each outcome, interactions between treatment effects were tested by adding interaction terms to the relevant Cox models and comparing the likelihood statistics for the models with and without interaction; this will thus be termed a test for an additive interaction here. However, because Cox models work on the logarithmic scale, the null hypothesis being tested is that the joint effect of the two treatments is the product of their individual effects, with all effects measured through hazard ratios (HRs) (16). All P values were calculated from two-tailed tests of statistical significance with a type I error rate of 5%. All analyses were performed using SAS (version 9.1).
RESULTS
Baseline characteristics
The four treatment groups were well balanced for a range of baseline characteristics (Table 1). In all four groups, 32% of participants had a history of major macrovascular disease and 10% had a history of major microvascular disease. Mean duration of diabetes was 8 years, and mean entry A1C and blood pressure were 7.5% and 145/81 mmHg, respectively (Table 1).
Table 1.
Characteristics of randomized participants at baseline
| Characteristic | Intensive glucose and perindopril-indapamide | Standard glucose and perindopril-indapamide | Intensive glucose and placebo | Standard glucose and placebo |
|---|---|---|---|---|
| n | 2,783 | 2,786 | 2,788 | 2,783 |
| Age (years) | 65.8 ± 6.3 | 65.8 ± 6.4 | 65.7 ± 6.5 | 65.8 ± 6.4 |
| Female sex | 1,198 (43.0) | 1,168 (41.9) | 1,181 (42.4) | 1,188 (42.7) |
| Age when diabetes first diagnosed (years) | 57.8 ± 8.5 | 57.9 ± 8.9 | 57.8 ± 8.7 | 57.8 ± 8.8 |
| Diabetes duration (years) | 8.0 ± 6.4 | 8.0 ± 6.4 | 7.8 ± 6.3 | 8.0 ± 6.3 |
| Prior vascular disease | ||||
| History of major macrovascular disease | 896 (32.2) | 902 (32.4) | 898 (32.2) | 894 (32.1) |
| History of myocardial infarction | 339 (12.2) | 339 (12.2) | 329 (11.8) | 327 (11.7) |
| History of stroke | 254 (9.1) | 248 (8.9) | 261 (9.4) | 259 (9.3) |
| History of major microvascular disease | 281 (10.1) | 287 (10.3) | 288 (10.3) | 296 (10.6) |
| History of macroalbuminuria | 94 (3.5) | 103 (3.9) | 93 (3.5) | 111 (4.2) |
| History of microvascular eye disease | 195 (7.0) | 194 (7.0) | 206 (7.4) | 198 (7.1) |
| History of microalbuminuria | 726 (27.4) | 715 (26.9) | 711 (26.7) | 710 (26.7) |
| Blood glucose control | ||||
| A1C concentration (%) | 7.5 ± 1.6 | 7.6 ± 1.6 | 7.5 ± 1.6 | 7.5 ± 1.5 |
| Fasting blood glucose (mmol/l) | 8.6 ± 2.8 | 8.5 ± 2.8 | 8.5 ± 2.7 | 8.4 ± 2.8 |
| Other major risk factors | ||||
| Systolic blood pressure (mmHg) | 145.3 ± 22.1 | 144.8 ± 21.6 | 144.6 ± 21.3 | 145.3 ± 21.2 |
| Diastolic blood pressure (mmHg) | 80.9 ± 11.1 | 80.5 ± 11.0 | 80.6 ± 11.0 | 80.5 ± 10.7 |
| Currently treated hypertension | 1,909 (68.6) | 1,893 (67.9) | 1,907 (68.4) | 1,946 (69.9) |
| Serum total cholesterol (mmol/l) | 5.2 ± 1.2 | 5.2 ± 1.2 | 5.2 ± 1.2 | 5.2 ± 1.2 |
| Serum LDL cholesterol (mmol/l) | 3.1 ± 1.0 | 3.1 ± 1.0 | 3.1 ± 1.0 | 3.1 ± 1.0 |
| Serum HDL cholesterol (mmol/l) | 1.3 ± 0.3 | 1.3 ± 0.4 | 1.3 ± 0.4 | 1.3 ± 0.3 |
| Serum triglycerides (mmol/l) | 1.6 (1.2–2.3) | 1.6 (1.2–2.3) | 1.6 (1.2–2.4) | 1.7 (1.2–2.3) |
| Urinary albumin-to-creatinine ratio (μg/mg) | 15 (7–40) | 15 (7–40) | 15 (7–39) | 14 (7–40) |
| BMI (kg/m2) | 28.4 ± 5.1 | 28.3 ± 5.3 | 28.4 ± 5.2 | 28.3 ± 5.1 |
| Current smoking | 387 (13.9) | 346 (12.4) | 406 (14.6) | 411 (14.8) |
Data are means ± SD, n (%), or median (interquartile range).
Characteristics during follow-up
At the end of 4.3 years of follow-up, use of concomitant treatment was similar among the four treatment groups (supplementary Table, available in an online appendix at http://care.diabetesjournals.org/cgi/content/full/dc09-0959/DC1). Over the duration of 4.3 years, blood pressure was reduced by an average ± SEM of 7.1 ± 0.3 mmHg systolic and 2.9 ± 0.2 mmHg diastolic blood pressure in patients assigned to joint treatment compared with those assigned to neither treatment (P < 0.001). Similarly, A1C was reduced by 0.61 ± 0.02% after 4.3 years follow-up in patients assigned to joint treatment compared with those assigned to neither treatment (P < 0.001).
Joint effects of routine blood pressure lowering and intensive glucose control
The HRs for the combination of blood pressure lowering with perindopril-indapamide and intensive glucose control with gliclazide MR and for either active treatment compared with those for placebo and standard glucose control are shown for all clinical outcomes in Table 2. There was no interaction between the effects of routine blood pressure lowering and intensive glucose control for any of the prespecified clinical end points (all P > 0.1), indicating that the effects of these two interventions were independent.
Table 2.
Joint effects of routine blood pressure lowering and intensive glucose control on all clinical outcomes
| Randomized treatments |
Pinteraction | ||||
|---|---|---|---|---|---|
| Intensive glucose and perindopril-indapamide | Standard glucose and perindopril-indapamide | Intensive glucose and placebo | Standard glucose and placebo | ||
| n | 2,783 | 2,786 | 2,788 | 2,783 | |
| Primary outcomes | |||||
| Combined major microvascular and macrovascular events | |||||
| No. events | 431 | 430 | 440 | 498 | |
| HR (95% CI) | 0.85 (0.75–0.97) | 0.85 (0.74–0.96) | 0.87 (0.77–0.99) | 1.00 (reference) | 0.13 |
| Major microvascular events | |||||
| No. events | 213 | 226 | 217 | 260 | |
| HR (95% CI) | 0.81 (0.68–0.97) | 0.85 (0.72–1.02) | 0.83 (0.69–0.99) | 1.00 (reference) | 0.32 |
| Major macrovascular events | |||||
| No. events | 246 | 234 | 255 | 265 | |
| HR (95% CI) | 0.92 (0.77–1.10) | 0.87 (0.73–1.04) | 0.96 (0.81–1.14) | 1.00 (reference) | 0.44 |
| Other outcomes | |||||
| Death from any cause | |||||
| No. events | 198 | 210 | 231 | 240 | |
| HR (95% CI) | 0.82 (0.68–0.99) | 0.87 (0.72–1.04) | 0.96 (0.80–1.15) | 1.00 (reference) | 0.90 |
| Death from cardiovascular causes | |||||
| No. events | 104 | 107 | 121 | 136 | |
| HR (95% CI) | 0.76 (0.59–0.98) | 0.78 (0.60–1.00) | 0.89 (0.70–1.14) | 1.00 (reference) | 0.62 |
| Major coronary heart events | |||||
| No. events | 133 | 132 | 139 | 155 | |
| HR (95% CI) | 0.92 (0.77–1.10) | 0.87 (0.73–1.04) | 0.90 (0.71–1.13) | 1.00 (reference) | 0.47 |
| Major cerebrovascular events | |||||
| No. events | 111 | 104 | 111 | 107 | |
| HR (95% CI) | 1.03 (0.79–1.35) | 0.96 (0.73–1.26) | 1.03 (0.79–1.35) | 1.00 (reference) | 0.85 |
| All renal events | |||||
| No. events | 590 | 630 | 686 | 777 | |
| HR (95% CI) | 0.72 (0.65–081) | 0.77 (0.69–0.85) | 0.88 (0.79–0.97) | 1.00 (reference) | 0.33 |
| New or worsening nephropathy | |||||
| No. events | 81 | 100 | 96 | 120 | |
| HR (95% CI) | 0.67 (0.50–0.88) | 0.82 (0.63–1.07) | 0.80 (0.61–1.05) | 1.00 (reference) | 0.93 |
| New or worsening retinopathy | |||||
| No. events | 147 | 142 | 133 | 153 | |
| HR (95% CI) | 0.96 (0.76–1.20) | 0.92 (0.73–1.16) | 0.86 (0.69–1.09) | 1.00 (reference) | 0.27 |
| New onset of microalbuminuria | |||||
| No. events | 525 | 542 | 605 | 673 | |
| HR (95% CI) | 0.75 (0.67–0.84) | 0.77 (0.68–0.86) | 0.90 (0.80–100) | 1.00 (reference) | 0.29 |
| New onset of macroalbuminuria | |||||
| No. events | 44 | 74 | 73 | 95 | |
| HR (95% CI) | 0.46 (0.32–0.65) | 0.77 (0.56–1.04) | 0.77 (0.57–1.04) | 1.00 (reference) | 0.30 |
A total of 1,799 participants had a major macrovascular or microvascular event during follow-up: 431 (15.5%) in the joint treatment group and 498 (17.9%) in the group that received neither active intervention, a relative risk reduction of 15% (95% CI 3–25%, P = 0.02). Compared with neither active intervention, combined treatment reduced the risk of microvascular events by 19% (3–32%, P = 0.02) but had no significant effect on the incidence of macrovascular events (8% [−10 to 23%], P = 0.35).
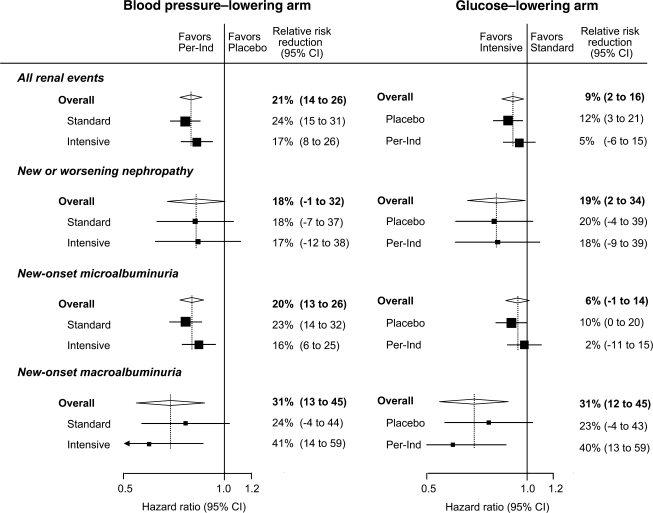
A total of 2,683 participants had a major renal event during follow-up: 590 (21.2%) in the joint treatment group and 777 (27.9%) in the group that received neither active intervention. Compared with neither active intervention, combined treatment reduced the risk of all renal events by 28% (95% CI 19–35%, P < 0.0001), which included a 33% reduction in the risk of new or worsening nephropathy (12–50%, P = 0.005), a 54% reduction in the risk of new onset of macroalbuminuria (35–68%, P < 0.0001), and a 25% reduction in the risk of new onset of microalbuminuria (16–33%, P < 0.001). The effects of each intervention on all renal events were intermediate between those of joint treatment and neither active intervention. When considered separately, the effects of the two interventions were additive on the log scale, and the effect of each intervention was not altered by the other treatment (Fig. 1).
Figure 1.
Relative effects of routine blood pressure–lowering and intensive glucose control strategy on all prespecified renal events. The effects of treatment (HRs) were estimated from unadjusted Cox proportional hazards models that used all available data at 4.3 years of follow-up. The diamonds incorporate the point estimates, represented by the vertical dashed lines, and the 95% CIs of the overall effects within categories; for subcategories, black squares represent point estimates (with the area of each square proportional to the inverse variance of each estimate), and horizontal lines represent 95% CIs. The HRs and relative risk reductions are given for intensive glucose control compared with standard glucose control in the blood pressure–lowering arm and for perindopril-indapamide (Per-Ind) compared with placebo in the glucose-lowering arm.
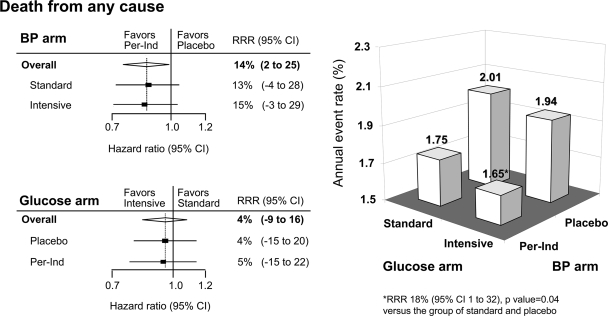
A total of 879 participants died during follow-up: 198 (7.1%) in the joint treatment group and 240 (8.6%) in the group that received neither active intervention. Compared with those in neither active intervention, those allocated to joint treatment had a significantly lower annual mortality rate (relative risk reduction 18% [95% CI 1–32%], P = 0.04) with the effects of the two treatments being independent of each other (Fig. 2).
Figure 2.
Combined effects of routine blood pressure–lowering and intensive glucose control strategy on the incidence of death from any cause. Incidence of death from any cause is presented as the annual event rate (percent) by the four randomized treatment groups: intensive glucose control and perindopril-indapamide (Per-Ind), standard glucose control and perindopril-indapamide, intensive glucose control and placebo, and standard glucose control and placebo. The effects of treatment (HRs and P values) were estimated from unadjusted Cox proportional hazards models that used all available data at 4.3 years of follow-up. The diamond incorporates the point estimate, represented by the vertical dashed line and the 95% CI of the overall effect. The HRs and relative risk reductions (RRRs) are given for intensive glucose control compared with standard glucose control in the blood pressure (BP)-lowering arm and for perindopril-indapamide compared with placebo in the glucose-lowering arm.
CONCLUSIONS
The ADVANCE trial has reported the separate beneficial effects of routine blood pressure lowering with perindopril-indapamide and intensive glucose control with a gliclazide MR–based regimen on a range of vascular complications in patients with type 2 diabetes (4,8). In the present analyses we show that the combined effect of both treatments was at least as great as the effect of either treatment alone for all clinical outcomes and appeared to be greater for some. This finding was most clearly apparent where both blood pressure lowering and intensive glucose control had separate significant beneficial effects, as was observed for all renal events and the components of new or worsening nephropathy, new onset of microalbuminuria, and new onset of macroalbuminuria (Table 2). Where a separately significant beneficial effect was observed only for blood pressure lowering (as for all-cause mortality and cardiovascular death), the addition of intensive glucose control did not negate that effect. In fact, for all-cause mortality (Table 2, Fig. 2) and cardiovascular death (Table 2), the estimates of the effect size for the joint intervention were slightly greater than they were for blood pressure lowering alone. These findings provide considerable reassurance that the widely used clinical approach of joint management of blood pressure and glucose in patients with type 2 diabetes is both appropriate and effective.
Our findings support and strengthen those of the UKPDS (14) and provide further evidence for the benefits of a multifactorial treatment approach that includes blood pressure lowering and intensive glucose control in patients with type 2 diabetes. The previously reported benefits of multifactorial risk management in the STENO 2 study were obtained through a combination of optimal blood pressure, glucose control, lipid modification, and antiplatelet therapy, although only 20% of participants achieved A1C levels of <7% (11). Our data show that intensification of glycemic control to achieve A1C levels of ≤6.5% augments the benefits obtained with blood pressure–lowering treatment, particularly with respect to renal events. Our study also shows that such an approach has benefits not only for individuals with type 2 diabetes who also have hypertension (14) or microalbuminuria (11) but for a much broader cross-section of the population with type 2 diabetes.
The substantial renal benefits observed in both arms of the ADVANCE trial and magnified in the group receiving both interventions may also translate into future cardiovascular benefits. Elevated levels of albuminuria have clearly been demonstrated to be a risk factor for cardiovascular disease in patients with type 2 diabetes (17), with current screening strategies aimed to identify affected patients so that appropriate preventive treatments may be introduced. Recent studies have suggested that treatment of albuminuria per se may reduce cardiovascular events (18). Long-term follow-up of the UKPDS study (19) has also demonstrated that the cardiovascular and mortality benefits of intensive glucose control emerge over time. The combined treatment strategy used in ADVANCE would therefore be anticipated to further reduce cardiovascular risk in the long term.
In the ACCORD study, intensive glucose control increased the risk of death (9). However, the overall mortality rate was much lower than predicted, probably as a consequence of the factorial study design that included intensive blood pressure– and lipid-lowering interventions. Our analyses provide no evidence of such an increase in mortality either in the glucose-lowering arm of the ADVANCE trial as reported previously (8) or in the present analyses. On the contrary, the combination of both active interventions compared with neither active intervention significantly reduced the risk of all-cause and cardiovascular mortality. There was also no indication that the benefits of routine blood pressure lowering were offset by the intensive glucose control intervention. If anything, the magnitude of risk reduction was somewhat greater in the group receiving both interventions than that observed in the group receiving routine blood pressure lowering alone. It is noteworthy that the intensive glucose control strategies applied in ADVANCE and in ACCORD differed substantially in the way the glucose target was achieved (8,9). Our results indicate that intensive glucose control can be implemented safely through a conventional approach, using the gliclazide MR–based regimen and stepwise increase in oral agents and, eventually, basal insulin (8).
Although we found no evidence for an interaction between the blood pressure– and glucose-lowering interventions for any of the outcomes, we are unable to fully exclude the presence of an interaction, particularly for some of the less common outcomes. Exclusion of any interaction between the two interventions of the ADVANCE trial would have required a sample size 4 times as large to be adequately powered (20). The absence of an interaction is further supported by observational data demonstrating that associations of blood pressure levels and measures of glycemic control with the risks of mortality and coronary heart disease in patients with type 2 diabetes are independent and additive (14,21,22).
When the treatments were not demonstrated to have separate significant benefits, as for macrovascular events, such effects were also not observed with combined treatment. One possible explanation is the relatively short length of study follow-up. This may particularly influence the effect of the glucose-lowering strategy for which the rate of macrovascular events only began to diverge in the last 12 months of the average 5-year period of follow-up, whereas the present analyses were limited to 4.3 years of follow up (8). The resultant loss of a large number of events that occurred with prolongation of the randomized glucose intervention therefore limited the statistical power to detect significant effects. In addition, the lower-than-anticipated event rates, possibly reflecting the comprehensive background management of cardiovascular risk in both arms (4,8), and less-than-projected separation in A1C between the glucose treatment groups may have further reduced the ability to detect an effect reliably. The recent report from the 10-year post-trial follow-up of the UKPDS cohort of significant long-term benefits observed with intensive glucose control for macrovascular events (19) suggests that the ADVANCE trial may have required much longer follow-up to demonstrate clear benefits of intensive glucose (8) or combined treatment for this outcome.
In summary, the ADVANCE trial has demonstrated that a combined approach of routine blood pressure lowering and intensive glucose control resulted in substantial reductions in major renal events and all-cause death. For the major renal outcomes, the separately significant beneficial effects of the two interventions were additive. This suggests that the multifactorial management of type 2 diabetes should incorporate routine blood pressure lowering and more intensive glucose control to reduce the burden of adverse clinical sequelae in individuals with established diabetes.
Supplementary Material
Acknowledgments
The ADVANCE trial was funded by grants from the National Health and Medical Research Council of Australia and Institut de Recherches Internationales Servier. S.Z. was supported by a National Health and Medical Research Council of Australia Health Professional Research Fellowship. T.N. was supported by a fellowship awarded by the Banyu Life Science Foundation and by an International Society of Hypertension Visiting Postdoctoral Fellowship awarded by the Foundation for High Blood Pressure Research Council of Australia. A.P. was supported by a National Heart Foundation of Australia Career Development award.
S.Z. reports being a member of an advisory board for Merck and receiving lecture fees from Servier, GlaxoSmithKline, and sanofi-aventis and grant support from Novo Nordisk and Pfizer. B.E.d.G. reports receiving lecture fees from Servier. D.G. reports receiving lecture fees from Servier and consulting and lecture fees and grant support from Pfizer, AstraZeneca, Novartis, and sanofi-aventis. P.H. reports receiving consulting or lecture fees from Servier, Neurochem, Prognomix, Medpharmgene, Novartis, Bristol-Myers Squibb, and Pfizer. S.H. reports receiving consulting and lecture fees from Novo Nordisk, Eli Lilly, Amylin, Servier, and Merck and grant support from Novo Nordisk and GW Pharmaceuticals. S.M. reports being a member of advisory boards for Servier, Pfizer, and Novartis and receiving lecture fees from Servier and Pfizer and grant support from Servier, Pfizer, and Novartis. M.M. reports receiving lecture fees from Servier and being a member of advisory boards for Servier, Novo Nordisk, and sanofi-aventis. B.N. reports receiving lecture fees from Servier and GlaxoSmithKline and grant support from Pfizer. A.P. reports receiving lecture fees from Servier, Pfizer, and Abbott and grant support from Servier, Pfizer. and sanofi-aventis. M.W. reports receiving lecture fees from Servier and Pfizer and grant support from Pfizer and sanofi-aventis. J.C. reports being a member of an advisory board for Servier and receiving lecturing fees from Servier, Pfizer, and Daiichi and grant support from Servier. No other potential conflicts of interest relevant to this article were reported.
Parts of this study were presented in abstract form at the annual meeting of the American Society of Nephrology, Philadelphia, Pennsylvania, 4–9 November 2008.
We thank the patients and all the investigators at the participating centers.
Footnotes
Clinical trial reg. no. NCT00145925, clinicaltrials.gov.
This study was designed, conducted, analyzed, and interpreted by the investigators independently of all sponsors.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.International Diabetes Federation Diabetes Atlas 3rd ed.Brussels, International Diabetes Federation, 2006 [Google Scholar]
- 2.UK Prospective Diabetes Study Group Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ 1998;317:703–713 [PMC free article] [PubMed] [Google Scholar]
- 3.Heart Outcomes Prevention Evaluation (HOPE) Study Investigators Effects of cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Lancet 2000;355: 253–259 [PubMed] [Google Scholar]
- 4.Patel A, MacMahon S, Chalmers J, MacMahon S, Chalmers J, Neal B, Woodward M, Billot L, Harrap S, Poulter N, Marre M, Cooper M, Glasziou P, Grobbee DE, Hamet P, Heller S, Liu LS, Mancia G, Mogensen CE, Pan CY, Rodgers A, Williams B: Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet 2007;370:829–840 [DOI] [PubMed] [Google Scholar]
- 5.Ohkubo Y, Kishikawa H, Araki E, Miyata T, Isami S, Motoyoshi S, Kojima Y, Furuyoshi N, Shichiri M: Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract 1995;28:103–117 [DOI] [PubMed] [Google Scholar]
- 6.Shichiri M, Kishikawa H, Ohkubo Y, Wake N: Long-term results of the Kumamoto Study on optimal diabetes control in type 2 diabetes patients. Diabetes Care 2000;23(Suppl. 2):B21–B29 [PubMed] [Google Scholar]
- 7.UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837–853 [PubMed] [Google Scholar]
- 8.The ADVANCE Collaborative Group Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008;358:2560–2572 [DOI] [PubMed] [Google Scholar]
- 9.The Action to Control Cardiovascular Risk in Diabetes Study Group Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358:2543–2553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R, Warren SR, Goldman S, McCarren M, Vitek ME, Henderson WG, Huang GDVADT Investigators Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009;360:129–139 [DOI] [PubMed] [Google Scholar]
- 11.Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Perdersen O: Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med 2003;348:383–393 [DOI] [PubMed] [Google Scholar]
- 12.American Diabetes Association Standards of medical care in diabetes—2008. Diabetes Care 2008;31(Suppl. 1):S12–S54 [DOI] [PubMed] [Google Scholar]
- 13.IDF Clinical Guidelines Task Force Global Guideline for Type 2 Diabetes Brussels, International Diabetes Federation, 2005 [Google Scholar]
- 14.Stratton IM, Matthews DR: Additive effects of glycaemia and blood pressure exposure on risk of complications in type 2 diabetes: a prospective observational study (UKPDS 75). Diabetologia 2006;49:1761–1769 [DOI] [PubMed] [Google Scholar]
- 15.ADVANCE Management Committee Study Rationale and Design of ADVANCE: Action in Diabetes and Vascular Disease: Preterax and Diamicron MR controlled evaluation. Diabetologia 2001;44:1118–1120 [DOI] [PubMed] [Google Scholar]
- 16.Woodward M: epidemiology: study design and data analysis. 2nd ed.Boca Raton, FL, Chapman and Hall/CRC Press, 2005 [Google Scholar]
- 17.Perkovic V, Verdon C, Ninomiya T, Barzi F, Cass A, Patel A, Jardine M, Gallagher M, Turnbull F, Chalmers J, Craig J, Huxley R: The relationship between proteinuria and coronary risk: a systematic review and meta-analysis. PLoS Med 2008;5:e207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Zeeuw D, Remuzzi G, Parving HH, Keane WF, Zhang Z, Shahinfar S, Snapinn S, Cooper ME, Mitch WE, Brenner BM: Albuminuria, a therapeutic target for cardiovascular protection in type 2 diabetic patients with nephropathy. Circulation 2004;110:921–927 [DOI] [PubMed] [Google Scholar]
- 19.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA: 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359:1577–1589 [DOI] [PubMed] [Google Scholar]
- 20.Piantadosi S: Clinical Trials: a Methodologic Perspective 2nd ed.Hoboken, NJ, John Wiley & Sons, 2005 [Google Scholar]
- 21.Stamler J, Vaccaro O, Neaton JD, Wentworth D: Diabetes, other risk factors, and 12-year cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care 1993;16:434–444 [DOI] [PubMed] [Google Scholar]
- 22.Hanefeld M, Fischer S, Julius U, Schulze J, Schwanebeck U, Schmechel H, Ziegelasch HJ, Lindner J.DIS Group. Risk factors for myocardial infarction and death in newly detected NIDDM: the Diabetes Intervention Study, 11-year follow up. Diabetologia 1996;39:1577–1583 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.