Abstract
OBJECTIVE
To clarify the dose-response relationship between alcohol consumption and type 2 diabetes.
RESEARCH DESIGN AND METHODS
A systematic computer-assisted and hand search was conducted to identify relevant articles with longitudinal design and quantitative measurement of alcohol consumption. Adjustment was made for the sick-quitter effect. We used fractional polynomials in a meta-regression to determine the dose-response relationships by sex and end point using lifetime abstainers as the reference group.
RESULTS
The search revealed 20 cohort studies that met our inclusion criteria. A U-shaped relationship was found for both sexes. Compared with lifetime abstainers, the relative risk (RR) for type 2 diabetes among men was most protective when consuming 22 g/day alcohol (RR 0.87 [95% CI 0.76–1.00]) and became deleterious at just over 60 g/day alcohol (1.01 [0.71–1.44]). Among women, consumption of 24 g/day alcohol was most protective (0.60 [0.52–0.69]) and became deleterious at about 50 g/day alcohol (1.02 [0.83–1.26]).
CONCLUSIONS
Our analysis confirms previous research findings that moderate alcohol consumption is protective for type 2 diabetes in men and women.
Diabetes is a major public health problem with long-term consequences including loss of vision; kidney failure; amputations; gastrointestinal, genitourinary, and cardiovascular symptoms; and sexual dysfunction (1). Several factors increase the risk of diabetes, including being overweight, lack of physical activity, and family history of diabetes (2). There is growing consensus that alcohol consumption is an influencing factor. The biological mechanism is uncertain, but there are several factors that may explain the relationship, including increases in insulin sensitivity after moderate alcohol consumption (3), changes in levels of alcohol metabolites (4), increases in HDL cholesterol concentrations (5), or via the anti-inflammatory effect of alcohol (6).
The exact nature of the dose-response relationship remains unclear (7). Several reviews have suggested a U-shaped relationship or a protective effect of moderate consumption with some question about the effect of higher levels of alcohol consumption (7–10). However, these reviews are narrative. Two quantitative reviews have been conducted. Carlsson et al. (11) categorized consumption into predetermined moderate- and high-consumption groups and used current abstainers or low consumers as the reference group. In their analysis, moderate consumption was associated with a 30% reduced risk of diabetes among men (relative risk [RR] 0.72 [95% CI 0.67–0.77]) and women (0.68 [0.61–0.75]). The risk associated with high consumption was described as being unclear. In the other meta-analysis, in which alcohol consumption was treated continuously, a U-shaped relationship was found for both men and women, with a more protective effect of moderate consumption observed for women (12). However, in both of these reviews, the reference group was composed of former drinkers and lifetime abstainers. Because former drinkers may be inspired to abstain due to health concerns, they may actually be at increased risk of developing diabetes, known as the sick-quitter effect (13). Our goal, therefore, was to examine the relationship between alcohol consumption and the risk of type 2 diabetes by conducting a meta-analysis that uses a flexible modeling approach and that, for the first time, uses lifetime abstention as the reference category.
RESEARCH DESIGN AND METHODS
Non–insulin-dependent diabetes (type 2) was the outcome. Although this outcome can be measured in various ways, the current World Health Organization (WHO) clinical diagnostic criteria were considered the gold standard for this meta-analysis. These criteria define diabetes by a fasting plasma glucose (FPG) level ≥7.0 mmol/l or a venous plasma concentration ≥11.1 mmol/l 2 h after a 75-g oral glucose challenge (14). The American Diabetes Association (ADA) also includes as sufficient criteria symptoms of hyperglycemia and a random plasma glucose concentration ≥11.1 mmol/l (1). The criteria, however, changed in 1996 (ADA) and 1999 (WHO) from an FPG ≥7.8 mmol/l.
Articles were found via a search of the following sources: Medline (via OVID and PubMed), the Cumulative Index to Nursing and Allied Health Literature (CINAHL), the Excerpta Medica Database (EMBASE), CAB Abstracts, World Health Organization Library Information System (WHOLIS), the System for Information on Grey Literature in Europe (SIGLE), the Alcohol and Alcohol Problems Science (ETOH), Web of Science, and the Alcohol In Moderation (AIM; an alcohol industry database) databases. The databases were searched for reports published from 1 January 1980 to 31 January 2008, with the following keywords: alcohol or ethanol, diabetes, case-control or cohort or prospective, and risk. Animal studies, commentaries, editorials, letters, and review articles were excluded. No language restriction was applied. A simplified search using the terms alcohol or ethanol as well as diabetes was used for WHOLIS and SIGLE, which could not support the complex strategy. AIM is not a searchable index, but a selective list of articles was reviewed.
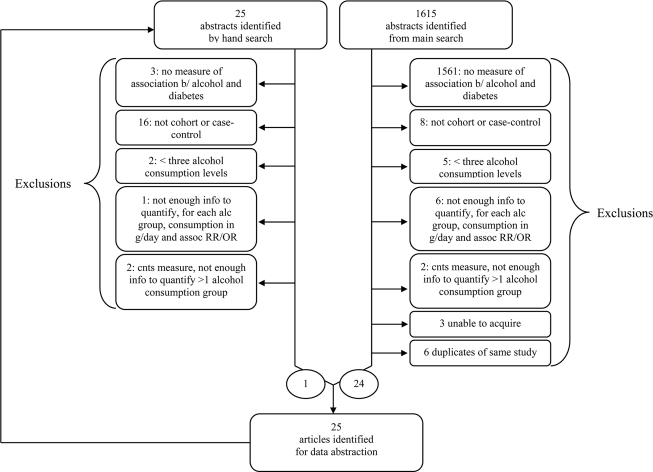
The results of the search are shown in Fig. 1. The strategy resulted in 1,615 hits after the removal of duplicates. The abstracts or complete publications were reviewed and excluded if they contained no indication of a measure of association between alcohol and either morbidity or mortality due to diabetes (n = 1,561), if the measure was cross-sectional (n = 8), or if fewer than three levels of alcohol consumption (i.e., no dose-response data) were reported (n = 5). For all non-English articles, the authors were able to ascertain eligibility. Of the 41 publications remaining, 3 (student theses) could not be obtained and 6 did not contain enough information to quantify, for each alcohol group, consumption in grams per day and/or the measure of association. Two publications reporting consumption using continuous measures could not be revised to provide a measure of association. In cases where more than one publication was generated from a given study, the most comprehensive analysis was used. After exclusion of 6 such duplicates, 24 articles remained. The references of these and relevant review articles were checked for additional publications, and 25 articles were identified. One remained after applying the same criteria described above. Combining the database and hand searches, 25 articles were identified for data abstraction. However, four were excluded because the number of cases of diabetes per alcohol exposure group, which was required for the analysis, was not reported. One was excluded because the measure was for a recent drinking occasion and not a typical day. Thus, 20 articles were included in the analysis (15–34).
Figure 1.
Flow diagram of literature search for the relationship between alcohol consumption and risk of type 2 diabetes.
Data abstracted included descriptors of study design in addition to measures of association. Alcohol consumption was converted to grams per day (if not originally reported as such). For studies that reported ranges of alcohol consumption for the categories, the midpoint was used. When the highest category was open ended, three-quarters the width of the previous interval was added to the lower limit. Where consumption was reported in drinks and not grams, the grams of pure alcohol equivalent described in the article, if stated, was used as a conversion factor; if not stated, conversion was based on typical drink sizes of the country (35). In one case, due to ambiguity over a suspected misprint, the authors were contacted via e-mail for clarification (16). 1.
The measures of effect abstracted were hazard ratios, odds ratios, and RRs but are referred to hereafter using the general term RR. Where RRs were not specifically presented but sufficient information was available, they were calculated. Where some consumption group was used as the reference, the RRs were reformulated to make abstainers the reference group. For studies for which various estimates including more or less covariates were reported, and a choice existed as to which to include, those that controlled for the most potential confounders not on the causal pathway were chosen. In five cases, crude measures were used when no other measure was available. In one study, where males and females were analyzed together, the RRs were applied to both men and women.
The studies contained two types of reference groups: lifetime and current alcohol abstainers. In order to use lifetime abstention as the reference, RRs for those studies that had current abstention as the reference were adjusted; for each sex, studies that had both current and lifetime abstainers were used to determine the overall prevalence of former drinkers among current abstainers and the RR of former drinkers relative to lifetime abstainers (weighted by precision). The RR for current abstention was then reweighted by the overall prevalence and the RR calculated above and then multiplied by the dose-specific RRs.
The literature search, review, and abstraction were carried out by D.B. To ensure accuracy in abstraction, a limited double entry was performed by B.T. and the results compared. Both authors agreed on 5/5 articles reviewed for inclusion/exclusion and 605/664 data points abstracted over 10 articles. Where disagreements existed, both authors reviewed the materials together until a consensus was reached.
To assess potential publication bias, separate funnel plots were drawn for consumption <20 g/day and ≥20 g/day. In the absence of a known cutoff for decreasing and increasing diabetes risk, these categories were chosen because they correspond to the WHO's low-risk drinking level guidelines (36). The estimates were prepooled using the inverse variance–weighted method because funnel plot methodology assumes one overall RR per article. We assessed publication bias using the tests of Egger and Begg (37,38). The Q test was used to assess the presence of heterogeneity (39). Additionally, the I2 statistic was used to measure inconsistency across studies and represents the proportion of variability in the estimates that is due to between-study variation (40).
We conducted the meta-regression using linear, first-order, and second-order fractional polynomial regression of the inverse variance–weighted data to estimate a best-fitting curve (41). Best-fit curves were assessed using decreased deviance compared with the reference model. Comparisons of curves to determine the best fit were made using a χ2 distribution (41).
Two sensitivity analyses were conducted. Because the meta-analysis by Koppes et al. (12) suggested that studies that used self-report of diabetes status reported a more protective effect than those that tested for diabetes, we assessed whether the model varied by self-reported outcome. Also, we abstracted estimates that did not adjust for potential intermediates on the causal pathway to avoid over adjustment. However, this may have resulted in using estimates that were not sufficiently adjusted. Therefore, we repeated the analysis using the most-adjusted estimates available, selecting models for men and women based on fit and comparability with the main analysis. All analyses were conducted using Stata software (version 10.1; StatCorp, College Station, TX).
RESULTS
Characteristics of the 20 studies included are presented in Table In total, data from 477,200 individuals, including 12,556 incident cases of diabetes, were included. Six studies included only men, five only women, eight both men and women (separately), and one men and women combined together. All were prospective cohorts. Adjustment for confounders varied. All but four adjusted for age at minimum; for these, only crude measures were available once those that adjusted for factors on the causal pathway were excluded. Diabetes ascertainment varied from self-report and data linkage to national registers to an oral glucose tolerance test (OGTT), the current clinical gold standard.
Table 1.
Characteristics of 20 cohort studies included in the analyses
| First author (year of publication) | Location | Sex | Age at baseline (years) | n | Follow-up |
Alcohol consumption |
Diabetes definition | Adjustments | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Duration (years) | Events (no.) | No. categories | Description | |||||||
| Ajani (2000) | U.S. | Men | 40–85 | 20,951 | 12.7 | 766 | 6 | No. of drinks: rarely/never, 1–3/month, 1/week, 2–4/week, 5–6/week, or ≥1/day | Self-report (physician participants) | Age, BMI, treatment assignment, smoking status, and physical activity |
| Beulens (2005) | The Netherlands | Women | 49–70 | 16,330 | 6.2 | 760 | 7 | g/week: teetotaler, 0–4.9, 5–29.9, 30–69.9, 70–139.9, 140–209.9, or ≥210 | Self-report and/or urinary glucose strip and/or Dutch register of hospital discharge diagnosis | Age and BMI |
| Carlsson (2003) | Finland | Men | Not reported | 10,970 | 19.4 | 277 | 4 | g/day: abstainers, <5.0, 5.0–29.9, or ≥30.0 | Linkage to Finnish national hospital discharge register and national drug register; patients who received only oral medication, dietary therapy, or insulin as an adjuvant treatment to hypoglycemic drugs were categorized as type 2 diabetic | Age and BMI |
| Women | Not reported | 11,808 | 19.8 | 297 | 4 | g/day: abstainers, <5.0, 5.0–19.9, or ≥20.0 | ||||
| Conigrave (2001) | U.S. | Men | 40–75 | 46,892 | 10.9 | 1,571 | 7 | g/day: 0, 0.1–4.9, 5.0–9.9, 10.0–14.9, 15.0–29.9, 30.0–49.9, or ≥50.0 | Self-report; criteria: any of 1) one or more classic symptoms with FPG ≥7.8 mmol/l, nonfasting glucose ≥11.1 mmol/l, or OGTT ≥11.1 mmol/l, 2) elevated plasma glucose levels on two different occasions, 3) hypoglycemia treatment (but not type 1 diabetic) | Age and BMI |
| de Vegt (2002) | The Netherlands | Men and women combined | 50–75 | 1,322 | Not reported | 241 | 3 | g/day: 0, <10, or ≥10 | FPG ≥7.0 mmol/l or OGTT ≥11.1 mmol/l; or subjects already being treated for diabetes | Age and sex |
| Djoussé (2007) | U.S. | Men | 64–95 | 1,909 | 6.3 | 109 | 4 | Drinks/week: never, former, <1, 1–7, or ≥7 | Use of insulin or oral hypoglycemic agents; or FPG ≥7.0 mmol | Age, BMI, education, and smoking status |
| Women | 63–95 | 2,746 | 6.3 | 125 | 4 | Drinks/week: never, former, <1, 1–7, or ≥7 | ||||
| Hodge (2006) | Australia | Men | 40–69 | 12,214 | 4 | 179 | 5 | g/day: never drinker, former drinker, <10, 10–20, 20–30, or >30 | Participants mailed questionnaire covering diagnosis of diabetes, with verification from primary care physician | Age, BMI, country of birth, dietary glucose intake, dietary energy intake, and waist-to-hip ratio |
| Women | 40–69 | 19,208 | 4 | 183 | 4 | g/day: never drinker, former drinker, <10, 10–20, or >20 | ||||
| Holbrook (1990) | U.S. | Men | 40–79 | 221 | 14 | 31 | 4 | g/week: nondrinker, 0.1–84.3, 84.4–176.0, or ≥176.1 | FPG ≥140 mg/dl, OGTT ≥200 mg/dl (11.1 mmol/l), or self-report of diabetes diagnosis by a physician | Age |
| Women | 40–79 | 303 | 14 | 44 | 4 | g/week: nondrinker, 0.1–41.3, 41.4–117.4, or ≥117.5 | ||||
| Hu (2006) | Finland | Men | 35–74 | 10,188 | 13.4 | 517 | 3 | g/week: none, 1–100, or >100 | Linkage of info in Finnish national hospital discharge register and national drug register | Age; BMI; study year; education; systolic blood pressure; bread, vegetable, fruit, sausage, coffee, and tea consumption; smoking status; and physical activity |
| Women | 35–74 | 11,197 | 13.4 | 447 | 3 | g/week: none, 1–100, or >100 | ||||
| Kao (2001) | U.S. | Men | 45–64 | 5,423 | 5.3 | 547 | 6 | Drinks/week: lifetime abstainer, former drinker, ≤1, 1.1–7, 7.1–14, 14.1–21, or ≥21 | Any of 1) FPG ≥7.0 mmol, 2) nonfasting glucose ≥11.1 mmol, 3) current use of diabetic medications, or 4) positive response to “Has a doctor ever told you that you had diabetes?” | None |
| Women | 45–64 | 6,838 | 5.4 | 569 | 6 | Drinks/week: lifetime abstainer, former drinker, ≤1, 1.1–7, 7.1–14, 14.1–21, or ≥21 | ||||
| Lee (2003) | Korea | Men | 25–55 | 4,055 | 4 | 83 | 5 | g/week: abstainer, 1–90, 91–180, 181–360 g, or >360 g | Serum fasting glucose concentration ≥126 mg/dl or taking diabetes medication | None |
| Lee (2004) | U.S. | Women | 55–69 | 35,698 | 11 | 1,921 | 3 | g/day: 0, 1–14, or ≥15 | Self-report | None |
| Meisinger (2002) | Germany | Men | 35–74 | 3,052 | 7.5 | 128 | 3 | g/day: 0, 0.1–39.9, or ≥40.0 | Self-report diagnosis or taking antidiabetes medications | Age, BMI, and survey |
| Women | 35–74 | 3,114 | 7.6 | 85 | 3 | g/day: 0, 0.1–19.9, or ≥20.0 | ||||
| Stampfer (1988) | U.S. | Women | 34–59 | 85,051 | 4 | 524 | 5 | g/day: 0, <1.5, 1.5–4.9, 5.0–14.9, or ≥15.0 | Self-report by questionnaire, then supplementary questionnaire regarding classic symptoms with FPG ≥140 mg/dl or random plasma glucose ≥200 mg/dl (or at least two elevated plasma glucose levels if no symptoms) | Age, BMI, and caloric intake |
| Strodl (2006) | Australia | Women | 70–74 | 8,896 | 3 | 231 | 3 | Drinks/day: none or rarely, 1–2, or ≥3 | Asked whether a doctor had told them they had a diagnosis of diabetes | None |
| Tsumura (1999) | Japan | Men | 35–61 | 6,362 | 9.7 | 456 | 5 | ml/day: abstainer, 0.1–19.0, 19.1–29.0, 29.1–50.0, or ≥50.1 | FPG ≥7.8 mmol/l, OGTT ≥11.1 mmol/l, or FPG ≥7.0 mmol/l | Age, BMI, smoking status, leisure time physical activity, parental diabetes, and FPG level |
| Waki (2004) | Japan | Men | 40–59 | 12,913 | Not reported | 703 | 4 | g/day: abstainer and infrequent drinker, ≤23.0, 23.1–46.0, or ≥46.1 | Self-report “Has a doctor ever told you that you have diabetes?” | Age, BMI, smoking status, family history of diabetes, leisure time physical activity, and hypertension |
| Women | 40–59 | 15,980 | Not reported | 480 | 4 | g/day: abstainer and infrequent drinker, ≤4.9, 5.0–11.5, or ≥11.6 | ||||
| Wannamethee (2002) | U.K. | Men | 40–59 | 5,221 | 16.8 | 198 | 5 | Units/week: none, <1, 1–15, 15–42, or >42 | Diagnosis of diabetes not accepted on basis of self-completed questionnaire unless confirmed in primary care records | Age and BMI |
| Wannamethee (2003) | U.S. | Women | 25–42 | 109,705 | 8.1 | 935 | 5 | g/day: lifelong abstainer, former drinker, <5.0, 5.0–14.9, 15.0–29.9, or ≥30.0 | Report on biennial questionnaire, then supplementary questionnaire regarding symptoms: before 1997 1) ≥1 classic symptoms and FPG ≥7.8 mmol/l or random plasma glucose ≥11.1 mmol/l, 2) ≥2 elevated plasma glucose results on different occasions (FPG ≥7.8 mmol/l, random plasma glucose ≥11.1 mmol/l, and/or OGTT ≥11.1 mmol/l in absence of symptoms), or 3) treatment with hypoglycemia medications | Age |
| Wei (2000) | U.S. | Men | 30–79 | 8,633 | 6 | 149 | 5 | g/week: abstainer, 1–61.8, 61.9–122.7, 122.8–276.6, or >276.6 | FPG ≥7.0 mmol/l or history of diabetes plus current insulin therapy | Age, parental diabetes, and years of follow-up |
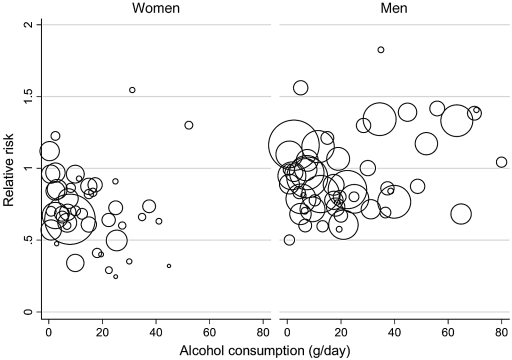
Figure 2 shows the RRs for the individual studies in the meta-analyses for women and men, respectively. Marked heterogeneity was found for both women (Q = 69.97, P = 0.004, I2 = 40%, 95% CI 13–58%) and men (Q = 79.58, P = 0.008, I2 = 35%, 95% CI 8–53%). Random-effects models were used for all subsequent analyses. No significant publication bias was detected.
Figure 2.
Scatter plot of the RR estimates of type 2 diabetes reported in the 20 studies included in the analyses. Each study provides more than one RR estimate. The area of each circle is proportional to the precision of the RR estimate.
Among women, the best-fitting model was the second-degree model with powers 0.5 and 3 (and function β1x0.5 + β2x3) (P < 0.001). Among men, the best-fitting model was the second-degree fractional polynomial with powers 1 and 1 (and function β1x + β2xlnx) (P = 0.007).
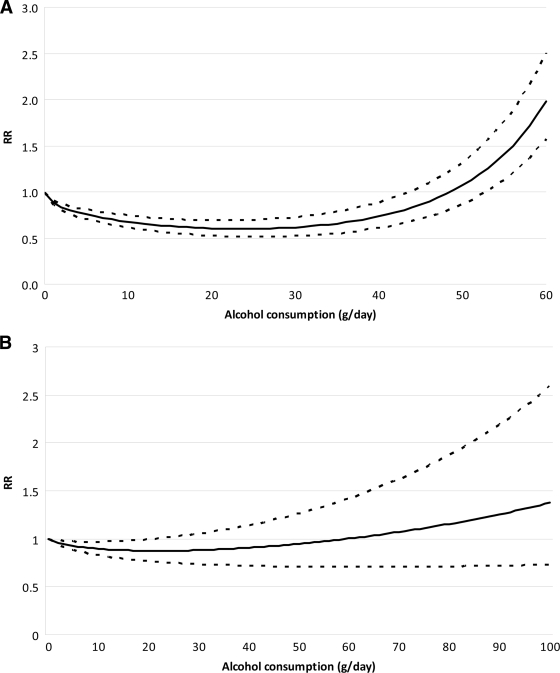
Figure 3 displays the relationship between the risk of alcohol consumption and type 2 diabetes among women and men. For both sexes, the relationship was U shaped. For women, the protective effect was greatest at the 24 g/day level, with a risk reduction of 40% compared with lifetime abstainers (95% CI 0.52–0.69). Alcohol consumption remained protective until just under 50 g/day. For men, the protective effect of alcohol consumption was greatest at 22 g/day, with the risk of diabetes being ∼0.87 times that of lifetime abstainers (95% CI 0.76–1.00), and remained protective until consumption of ∼60 g/day. Thus, for both women and men, the protective effect of alcohol consumption on incident type 2 diabetes was greatest with the consumption of about two drinks per day. Similarly, for both men and women, higher levels of consumption (above ∼50 g/day for women and 60 g/day for men) were no longer protective but actually increased the risk for diabetes.
Figure 3.
Pooled and fitted RR estimates and 95% CI band. A: The highest single alcohol consumption measure for women was 52.35 g/day, thus x-axis is scaled to 60 g/day. B: Among men, the single highest alcohol consumption measure was 80.04 g/day.
The mode of ascertainment of diabetes, self-report versus objective measurement, did impact the risk relation with volume of alcohol exposure but only for men; there was no effect for women. Accordingly, we repeated the analyses separately for self-report versus no self-report in men and found a linearly decreasing dose-response relationship in the studies with self-report (15,18,27,31),as well as a model similar to the main analysis in the rest of the studies. The result in the group based on self report was mainly influenced by two studies (15,18) that accounted for 81% of the observations.
The second sensitivity analysis, performed using the most adjusted estimates available, resulted in models remarkably similar to those in the main analysis for both sexes. For men, the U-shaped relationship was most protective at a consumption of 22 g/day and crossed back over the RR 1 at 62 g/day. For women, the U-shaped relationship, as with the main analysis, was most protective at a consumption of 25 g/day and crossed back over to deleterious effect at 51 g/day.
CONCLUSIONS
Our meta-analysis confirms the U-shaped relationships between average amount of alcohol consumed per day and risk of incident type 2 diabetes among men and women, although a more protective effect of moderate consumption was found for women. For women, the protective effect at moderate consumption and hazardous effect at higher consumption were both statistically significant. For men, the protective effect was statistically significant, but for higher consumption the CI did not exclude the RR 1.
Previous reviews found a protective effect of moderate alcohol consumption but limited evidence for a deleterious effect of heavy consumption. In comparison to the 2 previous meta-analyses, our analysis included 20 cohort studies in total: an additional 6 studies not included by Koppes et al. and an additional 10 not included by Carlsson et al. The meta-analyses by Koppes et al. used a total of 15 studies but included 2 that did not meet our inclusion criteria. That by Carlsson et al. included 13 studies, including 4 that did not meet our criteria. Thus, although there is substantial overlap in the data included in the respective analyses, our article is the most comprehensive in terms of the amount of data contributing to the summary estimates.
Meta-analyses are vulnerable to several biases. Although the gold standard in diabetes ascertainment is the OGTT, the individual studies ascertained diabetes status in various ways including self-report, linkage with national registries, and clinical tests. Additionally, because the diagnostic criteria cutoffs changed during the 1990s, even use of clinical tests was not consistent from study to study. As a result, some misclassification was likely. It is difficult to gauge the extent or type of any misclassification, although it is likely to be nondifferential and hence would attenuate any true causal association. In addition to misclassification due to testing differences, there is also potential misclassification due to the use of self-reporting of diabetes. However, we investigated the potential role of self-reported outcomes versus clinical tests and found no difference in results for women. Lastly, some misclassification of alcohol consumption may have occurred but would likely present as underreporting and not over-reporting of consumption, resulting in a shift of the relationship curve to the left. That is, associations that appear to exist at a given consumption level in our analysis would, in fact, exist at some higher level.
Alcohol consumption, and the resulting health effects, is more complex than mere volume of consumption measured at one point in time. Though several studies measured alcohol more than once (18,19,28,30,33), only one study used more than one alcohol measurement in its main analysis (17). Additional alcohol measurements would add weight to the validity and relevance to the alcohol measure because it is long-term consumption that tends to be of medical and public health concern. Additionally, the way in which alcohol is consumed (i.e., with meals or bingeing on weekends) affects various health outcomes (42). Thus, it may be the case that the risk of diabetes associated with heavy alcohol consumption is due to consumption mainly on the weekend as opposed to the same amount spread over a week. A few studies measured drinking pattern but did not present any analysis or did not present it in an analysis that combined it with volume (17,18,21).
Our meta-analysis addresses the sick quitter effect by making lifetime abstention the reference category. For diabetes, ignoring the sick quitter effect will tend to overestimate the benefit of moderate consumption and underestimate the risk of heavy consumption. However, adjusting for this required some estimate of the proportion of abstainers who are lifetime abstainers. For men, this was based on three studies (19,21,24) in which 37.6% of current abstainers were former drinkers and, for women, four studies (19,21,24,33) in which 49.6% of current abstainers were former drinkers. These estimates are a potential source of bias because the true underlying proportion of former drinkers may be higher or lower. Given the lack of a valid external estimate, we felt our data-driven approach was reasonable.
The included articles varied in adjustment for potential confounders. It was not possible to include only articles thath adjusted for the same factors. The sensitivity analysis in which the most fully adjusted estimates were used resulted in a relationship similar to that found in the main analysis. This consistency of results adds weight to the validity of our findings.
We found significant heterogeneity among men and women, which was expected because of the different methods used in the individual studies. Most studies were conducted in Western countries with the exception of three studies of Japanese and Korean populations; however, this was not a significant source of heterogeneity for men or women (analyses not shown). Study type cannot be a source of heterogeneity because only cohort studies were included, though the variable follow-up time may have contributed. The finding of heterogeneity indicates that there are additional factors such as pattern of consumption that should be considered in future research.
Our findings confirm previous research, both individual studies and summary estimates, of the U-shaped relationship between average alcohol consumption and risk of diabetes in both men and women. Although the biological mechanism responsible for this relationship is still a matter of research, several possibilities exist including increased insulin sensitivity with low levels of alcohol consumption. These factors, together, add weight to the argument for a causal role of alcohol consumption in diabetes. Alcohol consumption in men and women should thus be limited to moderate amounts, and heavy consumption should be discouraged. Moreover, the balance of risk of alcohol consumption on other diseases and health outcomes, even at moderate levels of consumption, may outweigh the positive benefits with regard to diabetes.
Acknowledgments
This work was financially supported by a small contribution of the Global Burden of Disease (GBD) Study to J.R.
No potential conflicts of interest relevant to this article were reported.
We thank the core group of the Comparative Risk Assessment within the GBD 2005 Study for Alcohol for their support and comments on the general methodology and an earlier version of this article: Robin Room, Theo Vos, and Rosana Norman.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2008;31(Suppl. 1):S55–S60 [DOI] [PubMed] [Google Scholar]
- 2. Mayor S: International Diabetes Federation consensus on prevention of type 2 diabetes. Int J Clin Pract 2007;61:1773–1775 [DOI] [PubMed] [Google Scholar]
- 3. Hendriks HF: Moderate alcohol consumption and insulin sensitivity: observations and possible mechanisms. Ann Epidemiol 2007;17(Suppl. 5):S40–S42 [Google Scholar]
- 4. Sarkola T, Iles MR, Kohlenberg-Mueller K, Eriksson CJ: Ethanol, acetaldehyde, acetate, and lactate levels after alcohol intake in white men and women: effect of 4-methylpyrazole. Alcohol Clin Exp Res 2002;26:239–245 [PubMed] [Google Scholar]
- 5. Rimm EB, Williams P, Fosher K, Criqui M, Stampfer MJ: Moderate alcohol intake and lower risk of coronary heart disease: meta-analysis of effects on lipids and haemostatic factors. Br Med J 1999;319:1523–1528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Imhof A, Froehlich M, Brenner H, Boeing H, Pepys MB, Koenig W: Effect of alcohol consumption on systemic markers of inflammation. Lancet 2001;357:763–767 [DOI] [PubMed] [Google Scholar]
- 7. Klatsky AL: Alcohol, cardiovascular diseases and diabetes mellitus. Pharmacol Res 2007;55:237–247 [DOI] [PubMed] [Google Scholar]
- 8. Howard AA, Arnsten JH, Gourevitch MN: Effect of alcohol consumption on diabetes mellitus: a systematic review. Ann Intern Med 2004;140:211–219 [DOI] [PubMed] [Google Scholar]
- 9. Zilkens RR, Puddey IB: Alcohol and cardiovascular disease: more than one paradox to consider. Alcohol and type 2 diabetes: another paradox? J Cardiovasc Risk 2003;10:25–30 [DOI] [PubMed] [Google Scholar]
- 10. Conigrave KM, Rimm EB: Alchol for the prevention of type 2 diabetes mellitus? Treat Endocrinol 2003;2:145–152 [DOI] [PubMed] [Google Scholar]
- 11. Carlsson S, Hammar N, Grill V: Alcohol consumption and type 2 diabetes: meta-analysis of epidemiological studies indicates a U-shaped relationship. Diabetologia 2005;48:1051–1054 [DOI] [PubMed] [Google Scholar]
- 12. Koppes LL, Dekker JM, Hendriks HF, Bouter LM, Heine RJ: Moderate alcohol consumption lowers the risk of type 2 diabetes: a meta-analysis of prospective observational studies. Diabetes Care 2005;28:719–725 [DOI] [PubMed] [Google Scholar]
- 13. Shaper A, Wannamethee G, Walker M: Alcohol and mortality in British men: explaining the U-shaped curve. Lancet 1988;332:1267–1273 [DOI] [PubMed] [Google Scholar]
- 14. World health organization. Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycemia. Geneva, World Health Org., 2006. [Google Scholar]
- 15. Ajani UA, Hennekens CH, Spelsberg A, Manson JE: Alcohol consumption and risk of type 2 diabetes mellitus among US male physicians. Arch Intern Med 2000;160:1025–1030 [DOI] [PubMed] [Google Scholar]
- 16. Beulens JW, Stolk RP, van der Schouw YT, Grobbee DE, Hendriks HF, Bots ML: Alcohol consumption and risk of type 2 diabetes among older women. Diabetes Care 2005;28:2933–2938 [DOI] [PubMed] [Google Scholar]
- 17. Carlsson S, Hammar N, Grill V, Kaprio J: Alcohol consumption and the incidence of type 2 diabetes: a 20-year follow-up of the Finnish twin cohort study. Diabetes Care 2003;26:2785–2790 [DOI] [PubMed] [Google Scholar]
- 18. Conigrave KM, Hu BF, Camargo CA, Jr, Stampfer MJ, Willett WC, Rimm EB: A prospective study of drinking patterns in relation to risk of type 2 diabetes among men. Diabetes 2001;50:2390–2395 [DOI] [PubMed] [Google Scholar]
- 19. Djoussé L, Biggs ML, Mukamal KJ, Siscovick DS: Alcohol consumption and type 2 diabetes among older adults: the Cardiovascular Health Study. Obesity 2007;15:1758–1765 [DOI] [PubMed] [Google Scholar]
- 20. de Vegt F, Dekker JM, Groeneveld WJA, Nijpels G, Stehouwer CDA, Bouter LM, Heine RJ: Moderate alcohol consumption is associated with lower risk for incident diabetes and mortality: the Hoorn Study. Diabetes Res and Clin Pract 2002;57:53–60 [DOI] [PubMed] [Google Scholar]
- 21. Hodge AM, English DR, O'Dea K, Giles GG: Alcohol intake, consumption pattern and beverage type, and the risk of type 2 diabetes. Diabet Med 2006;23:690–697 [DOI] [PubMed] [Google Scholar]
- 22. Holbrook TL, Barrett-Connor E, Wingard DL: A prospective population-based study of alcohol use and non-insulin-dependent diabetes mellitus. Am J Epidemiol 1990;132:902–909 [DOI] [PubMed] [Google Scholar]
- 23. Hu G, Jousilahti P, Peltonen M, Bidel S, Tuomilehto J: Joint association of coffee consumption and other factors to the risk of type 2 diabetes: a prospective study in Finland. Int J Obes 2006;30:1742–1749 [DOI] [PubMed] [Google Scholar]
- 24. Kao WH, Puddey IB, Boland LL, Watson RL, Brancati FL: Alcohol consumption and the risk of type 2 diabetes mellitus: atherosclerosis risk in communities study. Am J Epidemiol 2001;154:748–757 [DOI] [PubMed] [Google Scholar]
- 25. Lee DH, Ha MH, Kim JH, Christiani DC, Gross MD, Steffes M, Blomhoff R, Jacobs DR, Jr: Gamma-glutamyltransferase and diabetes: a 4 year follow-up study. Diabetologia 2003;46:359–364 [DOI] [PubMed] [Google Scholar]
- 26. Lee DH, Folsom AR, Jacobs DR, Jr: Dietary iron intake and type 2 diabetes incidence in postmenopausal women: the Iowa Women's Health Study. Diabetologia 2004;47:185–194 [DOI] [PubMed] [Google Scholar]
- 27. Meisinger C, Thorand B, Schneider A, Stieber J, Döring A, Löwel H: Sex differences in risk factors for incident type 2 diabetes mellitus: the MONICA Augsburg cohort study. Arch Intern Med 2002;162:82–89 [DOI] [PubMed] [Google Scholar]
- 28. Stampfer MJ, Colditz GA, Willett WC, Manson JE, Arky RA, Hennekens CH, Speizer FE: A prospective study of moderate alcohol drinking and risk of diabetes in women. Am J Epidemiol 1988;128:549–558 [DOI] [PubMed] [Google Scholar]
- 29. Strodl E, Kenardy J: Psychosocial and non-psychosocial risk factors for the new diagnosis of diabetes in elderly women. Diabetes Res Clin Pract 2006;74:57–65 [DOI] [PubMed] [Google Scholar]
- 30. Tsumura K, Hayashi T, Suematsu C, Endo G, Fujii S, Okada K: Daily alcohol consumption and the risk of type 2 diabetes in Japanese men: the Osaka Health Survey. Diabetes Care 1999;22:1432–1437 [DOI] [PubMed] [Google Scholar]
- 31. Waki K, Noda M, Sasaki S, Matsumura Y, Takahashi Y, Isogawa A, Ohashi Y, Kadowaki T, Tsugane S. JPHC Study Group. Alcohol consumption and other risk factors for self-reported diabetes among middle-aged Japanese: a population-based prospective study in the JPHC study cohort I. Diabet Med 2005;22:323–331 [DOI] [PubMed] [Google Scholar]
- 32. Wannamethee SG, Shaper AG, Perry IJ, Alberti KG: Alcohol consumption and the incidence of type II diabetes. J Epidemiol Community Health 2002;56:542–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wannamethee SG, Camargo CA, Jr, Manson JE, Willett WC, Rimm EB: Alcohol drinking patterns and risk of type 2 diabetes mellitus among younger women. Arch Intern Med 2003;163:1329–1336 [DOI] [PubMed] [Google Scholar]
- 34. Wei M, Gibbons LW, Mitchell TL, Kampert JB, Blair SN: Alcohol intake and incidence of type 2 diabetes in men. Diabetes Care 2000;23:18–22 [DOI] [PubMed] [Google Scholar]
- 35. World Health Organization. International Guide for Monitoring Alcohol Consumption and Related Harm. Geneva, World Health Org., 2000. [Google Scholar]
- 36. Babor TF, Higgin-Biddle JC, Saunders JB, Monteiro MG; World Health Organization. The Alcohol Use Disorders Identification Test: Guidelines for Use in Primary Care. Geneva, World Health Org., 2001. [Google Scholar]
- 37. Egger M, Davey Smith G, Schneider M, Minder C: Bias in meta-analysis detected by a simple, graphical test. Br Med J 1997;315:629–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Begg CB, Mazumdar M: Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–101 [PubMed] [Google Scholar]
- 39. Cochran WG: The combination of estimates from different experiments. Biometrics 1954;10:101–129 [Google Scholar]
- 40. Higgins JP, Thompson SG: Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–1558 [DOI] [PubMed] [Google Scholar]
- 41. Royston P: A strategy for modelling the effect of a continuous covariate in medicine and epidemiology. Stat Med 2000;19:1831–1847 [DOI] [PubMed] [Google Scholar]
- 42. Rehm J, Gmel G, Sempos CT, Trevisan M: Alcohol-related morbidity and mortality. Alcohol Res Health 2003;27:39–51 [PMC free article] [PubMed] [Google Scholar]