Abstract
OBJECTIVE
Seasonal environment at birth may influence diabetes incidence in later life. We sought evidence for this effect in a large sample of diabetic youth residing in the U.S.
RESEARCH DESIGN AND METHODS
We compared the distribution of birth months within the SEARCH for Diabetes in Youth Study (SEARCH study) with the monthly distributions in U.S. births tabulated by race for years 1982–2005. SEARCH study participants (9,737 youth with type 1 diabetes and 1,749 with type 2 diabetes) were identified by six collaborating U.S. centers.
RESULTS
Among type 1 diabetic youth, the percentage of observed to expected births differed across the months (P = 0.0092; decreased in October–February and increased in March–July). Their smoothed birth-month estimates demonstrated a deficit in November–February births and an excess in April–July births (smoothed May versus January relative risk [RR] = 1.06 [95% CI 1.02–1.11]). Stratifications by sex or by three racial groups showed similar patterns relating type 1 diabetes to month of birth. Stratification by geographic regions showed a peak-to-nadir RR of 1.10 [1.04–1.16] in study regions from the northern latitudes (Colorado, western Washington State, and southern Ohio) but no birth-month effect (P > 0.9) in study regions from more southern locations. Among type 2 diabetic youth, associations with birth month were inconclusive.
CONCLUSIONS
Spring births were associated with increased likelihood of type 1 diabetes but possibly not in all U.S. regions. Causal mechanisms may involve factors dependent on geographic latitude such as solar irradiance, but it is unknown whether they influence prenatal or early postnatal development.
Diabetes has been found by some investigators to be least common among youth who were born in the fall and/or most common among youth born in the spring. Similar reports have come from several regions of Europe (1–4), from New Zealand (where spring occurs in September–November) (5), and from Israeli Jews (6). This pattern was not demonstrated, however, by some studies elsewhere in Europe (3), in East Asia (7,8), or in Cuba (9). The sole previous publication from the U.S. that tested the birth month and diabetes relationship was restricted to 604 African American diabetic youth who lived in Chicago (10). This report showed that the standardized birth ratio for all participants was reduced for births in October but that this finding was statistically significant only for youth diagnosed with diabetes at 15–17 years of age (versus younger ages) or for youth classified as probably having type 2 diabetes.
Motivated by our interest in the developmental origins of diabetes, we have examined the distribution of birth months in a population-based U.S. sample of youth with diabetes. The calendar date of birth may serve as a useful marker for environmental exposures during the prenatal and early postnatal periods. The date of birth is known precisely for nearly every individual in modern societies, its normative distributions are empirically available wherever births have been widely registered, and it can be categorized in conventional calendar units such as season, month, or week. Knowing the date of birth can also provide a reasonable estimate of the date of conception or of any other developmental “time window.” Thus, critical developmental periods can be associated ecologically with variations in environmental phenomena such as solar exposure, climate, microbial burden, culture, or maternal nutrition. These associations, however, may not be the same in all geographic regions.
RESEARCH DESIGN AND METHODS
The SEARCH for Diabetes in Youth Study (SEARCH study) is a six-center collaboration with the primary goal of ascertaining all cases of physician-diagnosed diabetes, excluding gestational diabetes mellitus, that were recognized at age <20 years in defined U.S. populations. SEARCH study methods were detailed previously (11,12). Diabetic youth were identified in geographically defined populations in eight counties encompassing Cincinnati, Ohio, and five encompassing Seattle, Washington; in South Carolina and Colorado; in managed health care plans in southern California and Hawaii; and in four American Indian populations. These populations represented ∼6% of the U.S. population <20 years old.
To confirm eligibility in the geographic or membership region, SEARCH study participants ≥18 years of age and parents of youth <18 years of age completed a short survey including date of birth, sex, age at diagnosis, and current residence. No information was obtained on the place of birth or the mother's residence during pregnancy. Race and ethnicity were defined from self-reports or medical records for 93.5% of eligible youth and from residential geocoding (with racial and ethnic estimates from the U.S. Bureau of the Census 2000) for the 6.5% of youth who had missing data for these variables. Before implementation of the protocol, the study was reviewed and approved by the local institutional review boards that had jurisdiction over the local study population.
Cases of diabetes were validated by physician report, review of medical records, or self-report of a physician diagnosis of diabetes for a few cases. The clinical diabetes type assigned by the health care provider was recorded as part of the case validation process. We have retained for this analysis those SEARCH study participants categorized as having either type 1 (combining type 1, type 1a, and type 1b) or type 2 diabetes. We excluded participants (<3% of SEARCH study participants) whose diabetes was a hybrid type, maturity-onset diabetes in youth, secondary diabetes, unknown type, other type, or missing type. We made additional exclusions (<5%) for this analysis to eliminate birth-month biases related to the dependence on registrations in the SEARCH study made only within strictly defined calendar years (details and examples are provided in supplementary item I, available in an online appendix at http://care.diabetesjournals.org/cgi/content/full/dc09-0891/DC1).
This analysis includes 11,486 youth with diabetes (6,132 prevalent cases in 2001 and 5,354 incident cases in years 2002–2006). Of these, 9,737 (84.8%) had type 1 diabetes and 1,749 (15.2%) had type 2 diabetes.
Birth-month distributions and statistical methods
Numbers of births by month in the U.S. were obtained from annual tables published by the National Center for Health Statistics. These sources (supplementary item II, available in an online appendix) provided national monthly birth numbers for the total population, as well as monthly birth numbers tabulated separately for whites and blacks. We calculated national birth numbers for “other” racial groups by subtracting the sum for whites and blacks from the total births.
We categorized SEARCH study participants similarly as white, black, and other race in an effort to replicate the classifications provided for the U.S. birth registrations. We designated as “white” those participants who were described as white race and non-Hispanic ethnicity. We designated as “black” those categorized as African American. We designated as “other” those categorized as Hispanic ethnicity, American Indian, Asian, Pacific Islander, “multiple races,” “other race,” and “unknown race.”
Because we were unable to assume that every SEARCH study participant was gestated or born in the same state in which she or he resides, we used the national birth numbers to estimate the race-specific expected proportion of births for a given month and year. These expected monthly proportions were calculated as the total number of children of a given race born in the given month and year divided by the total number of children of that race born in the given year. Thus, for each year, we obtained monthly expected proportions that were also specific for race (white, black, and other). These expected values were merged into the SEARCH study database by race and year of birth. This enhanced database permitted us to calculate for any birth month the ratio of the observed births (among diabetic youth) to the expected proportion of annual births within the corresponding race group and birth year. Our calculation was expressed as the excess (or deficit) in the number of births relative to what would be expected if SEARCH study births had occurred as they did in the U.S. (specific to race and birth year) during the same time interval. The computational details and related tests of significance are presented as supplementary items II and III (available in an online appendix).
The 12 birth-month estimates of diabetes excess were then smoothed using weighted, moving averages (detailed method and examples provided in supplementary item III). The same smoothing process was replicated for analyses stratified by sex, racial groups, age at diabetes diagnosis, birth cohorts, and the geographic regions in which SEARCH study participants resided.
RESULTS
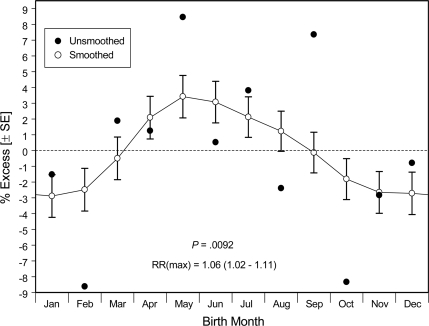
Among our 9,737 youth with type 1 diabetes, the unsmoothed data demonstrated deficits for births occurring in October (mean ± SEM −8.3 ± 3.2%) and February (−8.6 ± 3.4%) and an excess for those occurring in May (+8.5 ± 3.5%) and September (+7.4 ± 3.4%) (Fig. 1, ●). A birth-month association was confirmed (P = 0.0092) by our global, nonparametric test, irrespective of smoothing. After smoothing of these monthly estimates (Fig. 1, ○), the nadir occurred among births in November–February (−2.9 ± 1.4% in January) and the peak occurred for births in approximately May (+3.4 ± 1.3%; May–January relative risk [RR] 1.06 [95% CI 1.02–1.11]).
Figure 1.
Estimates of relative diabetes prevalence associated with month of birth among 9,737 U.S. youth with type 1 diabetes, showing unsmoothed and smoothed (± SEM) data. The P value here and in all figures is derived from a global, nonparametric test of the 12 birth-month estimates, irrespective of smoothing.
Among our 1,749 youth with type 2 diabetes, there was little difference in the relative birth-month percentages across the 12 months. In the absence of a definitive birth-month pattern for type 2 diabetic participants, our further stratified analyses were restricted to the 9,737 youth classified as having type 1 diabetes. These included 6,734 whites, 899 blacks, and 2,104 “others” (1,093 of Hispanic ethnicity, 58 American Indians, 212 Asians/Pacific Islanders, 298 multiple races, 99 other races, and 344 unknown race).
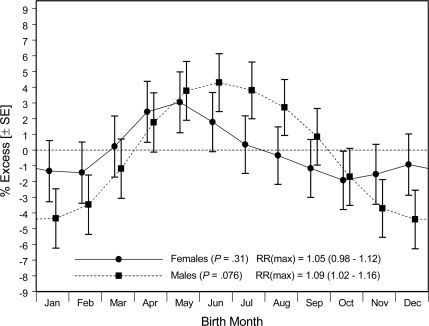
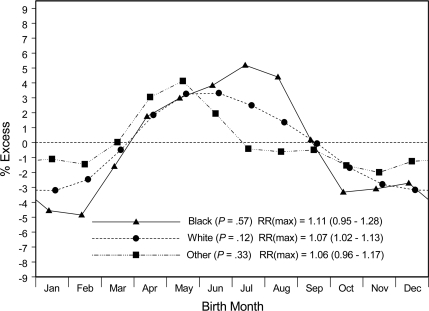
Smoothed estimates for male or female participants with type 1 diabetes demonstrated similar birth-month patterns (Fig. 2). Stratified by three racial groups (Fig. 3), the smoothed distributions all showed nadirs in later fall or winter and peaks in spring or early summer. The relatively large SEMs (not shown for reasons of graphic scale and cluttering) make it difficult to assign the months of uniquely highest or lowest risk with any certainty.
Figure 2.
Smoothed estimates (± SEM) of relative diabetes prevalence associated with month of birth among 4,732 female and 5,005 male U.S. youth with type 1 diabetes.
Figure 3.
Smoothed estimates of relative diabetes prevalence associated with month of birth among 6,734 white, 899 black, and 2,104 other U.S. youth with type 1 diabetes.
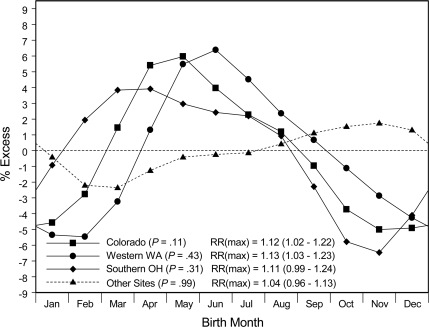
Birth-month patterns for type 1 diabetes stratified by the geographical regions of the SEARCH study demonstrated that three regions (western Washington State, southern Ohio, and Colorado) had similar nadirs for births approximately in November–January and peaks at or near May (Fig. 4). These three regions were located in relatively northern areas (37–49° north latitude) of the U.S. Together these three regions demonstrated a substantial birth-month effect (P = 0.002; peak-to-nadir RR of 1.10 [1.04–1.16]; plot not shown). In contrast, the relatively southern SEARCH study regions (southern California, South Carolina, Hawaii, and American Indian communities of Arizona and New Mexico; 19–36° north latitude) taken together had no significant birth-month effect. This north-south contrast in the birth-month effect was seen clearly among nonwhite SEARCH study participants (i.e., the combination of blacks and others; supplementary Figure A1A, available in an online appendix) and may be present also among the white SEARCH study participants (supplementary Figure A1B).
Figure 4.
Smoothed estimates of relative diabetes prevalence associated with month of birth among U.S. youth with type 1 diabetes who resided in Colorado (n = 2,595), western Washington (n = 2,541), southern Ohio (n = 1,669), or regions of lower latitude (including South Carolina, southern California, Hawaii, and American Indian communities; n = 2,932).
Our comparison of participants with diabetes diagnosed at younger (0–9 years) or older (10–18 years) ages required restricting participants analyzed to those who were born in an earlier birth cohort (1982–1992) because cohorts born after 1992 had no participants with diabetes diagnosed after age 13 and a slightly different racial composition. This stratification of the type 1 diabetic participants by age at diagnosis (0–9 years vs. 10–18 years) (supplementary Figure A2, available in an online appendix) demonstrated similar peaks (summer births) and nadirs (winter births), although the peak-to-nadir contrast may be slightly greater for individuals with diabetes diagnosed at younger ages (RR 1.11 [95% CI 1.02–1.21]) rather than at older ages (1.06 [0.99–1.15]).
Our comparison of participants from two birth cohorts (born in 1982–1992 or in 1993–2005) (supplementary Figure A3, available in an online appendix) required restricting youth analyzed to those with diabetes diagnosed at ages 0–9 years because comparison of older ages at diabetes diagnosis was not possible for youth in the more recent cohort. Among these younger participants, both birth cohorts demonstrated birth-month nadirs at or near December, but the cohort born in 1982–1992 possibly exhibited a delayed birth-month peak that appeared to come in July or August rather than May.
CONCLUSIONS
This observational study of a large, population-based sample of U.S. youth with diabetes confirmed a general pattern in which the lowest risk of developing type 1 diabetes was experienced by those whose births occurred in the months of November–February. At the same time, most subpopulations in our sample experienced their highest risks of developing type 1 diabetes if their births occurred in months around May.
We found that the observed birth-month patterns applied to those participants whom the providers designated as having type 1 diabetes but that the patterns might not be applicable to type 2 diabetes. This absence of a pattern may stem from different pathophysiological mechanisms or, more simply, from the inadequate statistical power of the study because of the relatively small number of youth with type 2 diabetes. A much larger registry of Ukrainian patients with diabetes, presumably type 2, diagnosed after age 40 years recently reported that the peak risk occurred among adults born in May and the minimum risk occurred among those born in December (13). The authors of the Ukrainian study commented that the apparent similarity between birth-month patterns for both types of diabetes has implications for exploring hypotheses based on shared etiological pathways.
The approximately sinusoidal pattern with periodicity of 1 year, together with the absence of a birth-month effect in more equatorial latitudes, is consistent with an effect that could be driven by environmental variations in climate or exposure to sunlight. These local variations might operate either through maternal exposures that are temporally related to fetal periods of pancreatic β-cell development or through postnatal exposures related to infancy time windows of pancreatic maturation. Data from 51 regions of the world suggest that residence in areas with low ultraviolet irradiance (estimated on the date of the winter solstice) is associated with high incidence of type 1 diabetes (14). Ultraviolet radiation contributes substantially to the circulating concentration of vitamin D, and the level of this prohormone may be associated with protection against diabetes through a variety of possible mechanisms (15,16). Ultraviolet penetration into deeper skin layers also contributes to the degradation of circulating folic acid, thus suggesting another mechanism by which variation in solar irradiance during time windows of high developmental plasticity might influence metabolism in later life.
As with the other large studies of birth month and diabetes, the SEARCH study lacks information on where the youth resided during infancy, where they were born, and where their mothers resided during the gestational months. For this reason, some of the youth may be misclassified with regard to their geographic residence during early life. Such misclassifications of early residence locations complicate the interpretation of relationships between details of the local environments in which the diabetic children currently reside and their risk of acquiring diabetes. We cannot be certain how the misclassifications might alter the relationship of diabetes risk to birth month.
Another limitation of this analysis is its classification of participants by the racial groups white, black, and other. We used these coarse categories because they were used in the tabulated U.S. birth registers from which we obtained race-specific, expected distributions. These conventional racial categories do not reflect with certainty any genetic differences or biomedical attributes. Nevertheless, if environmental ultraviolet irradiance plays a strong role in the birth-month effect observed, then slight differences in this effect according to racial group might provide hints about the causal mechanisms of diabetes. Individuals classified as black are likely to have darker skin pigmentation than those classified as white or other, and increased superficial skin pigment reduces the cutaneous penetration of ultraviolet sunlight (17).
If developmental benefits are derived from increased sunlight in June and July (in the northern hemisphere), then the protection associated with being born in November–February might be related to the maternal concentrations of circulating vitamin D being typically at their maximum in June through August (surveyed at 40° north latitude) (18). The fortunate fetus would thus be exposed to the highest levels of vitamin D ∼3–7 months before birth, a time window that might enhance the prenatal development of pancreatic β-cells or protect them during their primary expansion period that follows the first trimester of human pregnancy (19–21). A less fortunate fetus born in May or June would encounter the lowest levels of maternal vitamin D (typically around February-March) (18) during a time window occurring ∼3–4 months before birth. A previous study concerned with dietary sources of vitamin D reported an association of maternal intake of cod liver oil (rich in vitamin D) during pregnancy with a lower risk of type 1 diabetes in offspring, although this protective effect was not observed in the offspring of mothers who took prenatal multivitamin tablets (22). Maternal intake of dietary vitamin D during late pregnancy has been associated with decreased risk of islet autoimmunity among offspring at high risk of type 1 diabetes (23).
Rather than operating during the fetal period, alternative mechanisms of diabetes protection might depend on an infant's postnatal experience with increased concentrations of circulating vitamin D. When a northern-hemisphere baby born in November–February is about 7 months old, the stage at which parents in high-latitude regions would commonly begin to take the infant outside with minimal summertime clothing, the increased solar irradiance of June or July might act to increase the infant's cutaneous production of vitamin D and thus benefit the maturing, postnatal endocrine pancreas. Apart from the possible benefits of increased vitamin D derived from solar irradiance, observational studies have reported that infants' dietary supplementation with vitamin D (24) or intake of cod liver oil (25) during the first year of life was associated with reduced risk of type 1 diabetes. This effect was greater for those starting use of cod liver oil at age 7–12 months than for those using cod liver oil at age 0–6 months (25).
These data from the SEARCH study suggest that environmental factors associated with birth in early winter in the U.S. may protect children and adolescents against the incidence of type 1 diabetes or that factors related to spring birth, approximately during May in northern latitudes, may be associated with an increased diabetes incidence. If the birth-month effect is linked to reduced ultraviolet irradiance during winter months, this geophysical mechanism might be enhanced by related behavioral changes common to colder periods of the year when mothers and infants exercise less outdoors and wear more clothing that blocks sunlight. Beyond mechanisms that involve cutaneous production of vitamin D, other explanations of the birth-month effect may involve seasonal exposures to microbial agents (including the sterilizing effects of ultraviolet irradiance), toxins, or micronutrients that vary with the season of birth or conception and typical ages at which infants are first introduced to environments beyond the immediate household. Clarification of the mechanisms contributing to this seasonal relationship could lead to testable proposals for interventions to reduce the burden of childhood diabetes.
Supplementary Material
Acknowledgments
SEARCH for Diabetes in Youth is funded by the Centers for Disease Control and Prevention (PA number 00097 and DP-05-069) and supported by the National Institute of Diabetes and Digestive and Kidney Diseases. Site contract numbers are as follows: Kaiser Permanente Southern California, U01 DP-000246; University of Colorado Health Sciences Center, U01 DP-000247; Pacific Health Research Institute, U01 DP-000245; Children's Hospital Medical Center (Cincinnati), U01 DP-000248; University of North Carolina at Chapel Hill, U01 DP-000254; University of Washington School of Medicine, U01 DP-000244; and Wake Forest University School of Medicine, U01 DP-000250.
No potential conflicts of interest relevant to this article were reported.
Parts of this study were presented in abstract form at the 68th Scientific Sessions of the American Diabetes Association, San Francisco, California, 6–10 June 2008.
We thank Jennifer Beyer and David Pettitt for helping to identify systematic biases in birth-month data related to certain enrollment periods. George Chaplin provided useful insights into geographic factors associated with ultraviolet irradiance. The SEARCH for Diabetes in Youth Study is indebted to the many youth and their families and health care providers whose participation made this study possible.
Footnotes
The contents of this report are solely the responsibility of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention or the National Institute of Diabetes and Digestive and Kidney Diseases.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Jongbloet PH: Seasonality of birth in patients with childhood diabetes in the Netherlands. Diabetes Care 1998;21:190–191 [DOI] [PubMed] [Google Scholar]
- 2. Samuelsson U, Johansson C, Ludvigsson J: Month of birth and risk of developing insulin dependent diabetes in south east Sweden. Arch Dis Child 1999;81:143–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McKinney PA: Seasonality of birth in patients with childhood type I diabetes in 19 European regions. Diabetologia 2001;44(Suppl. 3):B67–B74 [DOI] [PubMed] [Google Scholar]
- 4. Vaiserman AM, Carstensen B, Voitenko VP, Tronko MD, Kravchenko VI, Khalangot MD, Mechova LV: Seasonality of birth in children and young adults (0–29 years) with type 1 diabetes in Ukraine. Diabetologia 2007;50:32–35 [DOI] [PubMed] [Google Scholar]
- 5. Willis JA, Scott RS, Darlow BA, Lewy H, Ashkenazi I, Laron Z: Seasonality of birth and onset of clinical disease in children and adolescents (0–19 years) with type 1 diabetes mellitus in Canterbury, New Zealand. J Pediatr Endocrinol Metab 2002;15:645–647 [DOI] [PubMed] [Google Scholar]
- 6. Laron Z, Shamis I, Nitzan-Kaluski D, Ashkenazi I: Month of birth and subsequent development of type I diabetes (IDDM). J Pediatr Endocrinol Metab 1999;12:397–402 [DOI] [PubMed] [Google Scholar]
- 7. Ye J, Chen RG, Ashkenazi I, Laron Z: Lack of seasonality in the month of onset of childhood IDDM (0.7–15 years) in Shanghai, China. J Pediatr Endocrinol Metab 1998;11:461–464 [DOI] [PubMed] [Google Scholar]
- 8. Kida K, Mimura G, Ito T, Murakami K, Ashkenazi I, Laron Z: Incidence of type 1 diabetes mellitus in children aged 0–14 in Japan, 1986–1990, including an analysis for seasonality of onset and month of birth: JDS study. The Data Committee for Childhood Diabetes of the Japan Diabetes Society (JDS). Diabet Med 2000;17:59–63 [DOI] [PubMed] [Google Scholar]
- 9. Collado-Mesa F, Diaz-Diaz O, Ashkenazi I, Laron Z: Seasonality of birth and type 1 diabetes onset in children (0–14 years) in Cuba. Diabet Med 2001;18:939–940 [DOI] [PubMed] [Google Scholar]
- 10. Grover V, Lipton RB, Sclove SL: Seasonality of month of birth among African American children with diabetes mellitus in the city of Chicago. J Pediatr Endocrinol Metab 2004;17:289–296 [DOI] [PubMed] [Google Scholar]
- 11. SEARCH for Diabetes in Youth Study Group. D'Agostino RB, Jr, Hamman RF, Kilgo PD, Lawrence JM, Liu LL, Loots B, Linder B, Marcovina S, Rodriguez B, Standiford D, Williams DE: The burden of diabetes mellitus among US youth: prevalence estimates from the SEARCH for Diabetes in Youth Study. Pediatrics 2006;118:1510–1518 [DOI] [PubMed] [Google Scholar]
- 12. Writing Group for the SEARCH for Diabetes in Youth Study Group. Incidence of diabetes in youth in the United States. JAMA 2007;297:2716–2724 [DOI] [PubMed] [Google Scholar]
- 13. Vaiserman A, Khalangot M: Similar seasonality of birth in type 1 and type 2 diabetes patients: a sign for common etiology? Med Hypotheses 2008;71:604–605 [DOI] [PubMed] [Google Scholar]
- 14. Mohr SB, Garland CF, Gorham ED, Garland FC: The association between ultraviolet B irradiance, vitamin D status and incidence rates of type 1 diabetes in 51 regions worldwide. Diabetologia 2008;51:1391–1398 [DOI] [PubMed] [Google Scholar]
- 15. Mathieu C, Gysemans C, Giulietti A, Bouillon R: Vitamin D and diabetes. Diabetologia 2005;48:1247–1257 [DOI] [PubMed] [Google Scholar]
- 16. Luong K, Nguyen LT, Nguyen DN: The role of vitamin D in protecting type 1 diabetes mellitus. Diabetes Metab Res Rev 2005;21:338–346 [DOI] [PubMed] [Google Scholar]
- 17. Armas LAG, Dowell S, Akhter M, Duthuluru S, Huerter C, Hollis BW, Lund R, Heaney RP: Ultraviolet-B radiation increases serum 25-hydroxyvitamin D levels: the effect of UVB dose and skin color. J Am Acad Dermatol 2007;57:588–593 [DOI] [PubMed] [Google Scholar]
- 18. Bodnar LM, Simhan HN, Powers RW, Frank MP, Cooperstein E, Roberts JM: High prevalence of vitamin D insufficiency in black and white pregnant women residing in the northern United States and their neonates. J Nutr 2007;137:447–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Scharfmann R: Control of early development of the pancreas in rodents and humans: implications of signals from the mesenchyme. Diabetologia 2000;43:1083–1092 [DOI] [PubMed] [Google Scholar]
- 20. Piper K, Brickwood S, Turnpenny LW, Cameron IT, Ball SG, Wilson DI, Hanley NA: β Cell differentiation during early human pancreas development. J Endocrinol 2004;181:11–23 [DOI] [PubMed] [Google Scholar]
- 21. Bouwens L, Rooman I: Regulation of pancreatic β-cell mass. Physiol Rev 2005;85:1255–1270 [DOI] [PubMed] [Google Scholar]
- 22. Stene LC, Ulriksen J, Magnus P, Joner G: Use of cod liver oil during pregnancy associated with lower risk of type I diabetes in the offspring. Diabetologia 2000;43:1093–1098 [DOI] [PubMed] [Google Scholar]
- 23. Fronczak CM, Baron AE, Chase HP, Ross C, Brady HL, Hoffman M, Eisenbarth GS, Rewers M, Norris JM: In utero dietary exposures and risk of islet autoimmunity in children. Diabetes Care 2003;26:3237–3242 [DOI] [PubMed] [Google Scholar]
- 24. Hypponen E, Laara E, Reunanen A, Jarvelin MR, Virtanen SM: Intake of vitamin D and risk of type 1 diabetes: a birth-cohort study. Lancet 2001;358:1500–1503 [DOI] [PubMed] [Google Scholar]
- 25. Stene LC, Joner G: Use of cod liver oil during the first year of life is associated with lower risk of childhood-onset type 1 diabetes: a large, population-based, case-control study. Am J Clin Nutr 2003;78:1128–1134 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.