Abstract
OBJECTIVE
Foot ulceration remains a major health problem for diabetic patients and has a major impact on the cost of diabetes treatment. We tested a hyperspectral imaging technology that quantifies cutaneous tissue hemoglobin oxygenation and generated anatomically relevant tissue oxygenation maps to assess the healing potential of diabetic foot ulcers (DFUs).
RESEARCH DESIGN AND METHODS
A prospective single-arm blinded study was completed in which 66 patients with type 1 and type 2 diabetes were enrolled and followed over a 24-week period. Clinical, medical, and diabetes histories were collected. Transcutaneous oxygen tension was measured at the ankles. Superficial tissue oxyhemoglobin (oxy) and deoxyhemoglobin (deoxy) were measured with hyperspectral imaging from intact tissue bordering the ulcer. A healing index derived from oxy and deoxy values was used to assess the potential for healing.
RESULTS
Fifty-four patients with 73 ulcers completed the study; at 24 weeks, 54 ulcers healed while 19 ulcers did not heal. When using the healing index to predict healing, the sensitivity was 80% (43 of 54), the specificity was 74% (14 of 19), and the positive predictive value was 90% (43 of 48). The sensitivity, specificity, and positive predictive values increased to 86, 88, and 96%, respectively, when removing three false-positive osteomyelitis cases and four false-negative cases due to measurements on a callus. The results indicate that cutaneous tissue oxygenation correlates with wound healing in diabetic patients.
CONCLUSIONS
Hyperspectral imaging of tissue oxy and deoxy may predict the healing of DFUs with high sensitivity and specificity based on information obtained from a single visit.
Diabetes is a major global disease that affects 194 million people worldwide and is expected to increase in prevalence to 344 million by the year 2030 (1). One major complication of diabetes is foot ulceration, which occurs in as many as 15–25% of type 1 and type 2 diabetic patients over their lifetimes (2–4). Studies show that between 2 and 6% of diabetic patients will develop a foot ulcer every year (5,6). The feet of patients with diabetes are at risk for ulceration due to a wide range of pathological conditions, the major three being peripheral neuropathy, foot deformity, and trauma, which may be exacerbated by comorbid peripheral vascular disease (4,7). If left untreated, foot ulcers lead to infection and deep-tissue necrosis (8).
Foot pathology is a major source of morbidity in patients with diabetes and is a leading cause of hospitalization. Infected and/or ischemic diabetic foot ulcers (DFUs) account for about 25% of all hospital visits among patients with diabetes. Previous studies have shown that a DFU preceeds roughly 85% of all lower-extremity amputations in patients with diabetes (9,10), and more than 88,000 amputations are performed annually on diabetic patients (11). The cost to manage foot disorders is estimated at several billion dollars annually (5,12). Successful clinical management of DFUs not only has the potential to reduce the cost of caring for these patients but also to improve quality of life by reducing comorbidities.
Current treatment options for DFUs include offloading to reduce pressure on the wound, wound care to prevent infections, and wound debridement to remove necrotic debris and restimulate the wound healing process (11,13,14). Even with these measures, some wounds fail to heal. Having a means to assess healing potential may help triage wounds earlier to more aggressive therapies, thereby avoiding infections and amputations.
Clinical measurements of microvascular function may be an important part of DFU assessment (15–17). Hyperspectral imaging (HSI) was developed as a novel noninvasive diagnostic tool to quantify tissue oxygenation and generate anatomically relevant maps of microcirculatory changes seen in diabetic patients (18). HSI generates a map of regions of interest based on local molecular composition. With proper wavelength selection, spatial maps of molecules such as oxyhemoglobin (oxy) and deoxyhemoglobin (deoxy) can be acquired.
A pilot study of 10 type 1 diabetic patients with 21 DFU sites showed that HSI identified changes in tissue oxygenation in the diabetic foot that were predictive of ulcer healing (18). The sensitivity, specificity, and positive predictive value of the healing index were 93, 86, and 93%, respectively. The goal of the current study was to test the accuracy of HSI in evaluating the healing potential of DFUs in a large number of type 1 and 2 diabetic patients.
RESEARCH DESIGN AND METHODS
This was a prospective observational study conducted at three centers: University of California Los Angeles/Olive View Medical Center, Cleveland Clinic, and University of Pennsylvania School of Medicine. Patients were followed for 11 visits over 24 weeks. The primary end point was to establish the effectiveness of hyperspectral tissue oxygenation mapping (HTOM) for predicting whether DFUs in both type 1 and 2 diabetic patients would heal. Healing was defined as complete re-epithelialization at 24 weeks. The study was designed based on the preliminary data obtained from the pilot study (18).
Inclusion criteria
Patients aged 21–85 years diagnosed with type 1 or type 2 diabetes with at least one DFU were eligible. The diagnosis of type 1 and type 2 diabetes was established according to the recommendations of the American Diabetes Association Expert Committee on the Diagnosis and Classification of Diabetes Mellitus (19).
Exclusion criteria
Exclusion criteria included heart failure with consequent lower-extremity edema, stroke or ischemic attack with residual nerve dysfunction, uncontrolled hypertension, end-stage renal disease/renal transplant, peripheral arterial disease that was severe enough to require surgery, severe peripheral edema, any other serious chronic disease that can affect wound healing, treatment with antineoplastic drugs or glucocorticoids, and pregnant or lactating women.
Data collection
Studies were performed according to a uniformed study protocol that was approved by the institutional review boards at each center. After receiving a description of the protocol and asking questions, all patients agreeing to participate signed an approved informed consent form.
Medical and family histories were collected from each patient. Clinical evaluation included age, sex, ethnicity/race, weight, height, BMI, systolic and diastolic blood pressure, ankle brachial index (ABI), A1C, diabetes type, and diabetes duration. Neuropathy was graded according to the Neuropathic Symptom Score and Neuropathy Disability Score (NDS) (20,21).
Transcutaneous oxygen tension (tcPO2) was measured at the ankle of both legs using a transcutaneous oxygen monitor (Model PF-5000; Perimed, North Royalton, OH). The oxygen-monitoring electrodes were coupled to the skin using adhesive fixation rings, and an electrolytic solution was used and set to maintain a temperature of 44°C. The solution was allowed to equilibrate for 15 min prior to recording.
HTOM and skin temperature at the center of the image were collected with a commercial HSI system (OxyVu; HyperMed, Burlington, MA). The HSI system obtains multiple images at discrete wavelengths, providing a diffuse reflectance spectrum for each pixel in the image. The system uses wavelengths between 500 and 660 nm to include oxy and deoxy absorption peaks. Tissue oxygenation images or maps were constructed from oxy and deoxy values determined from each pixel in the image. Skin temperature was monitored with an infrared remote temperature sensor.
Prior to imaging, the system was calibrated to a reflectance card. Patients were imaged supine on a standard examination table or in a reclining chair and were allowed to rest for 10 min to minimize systemic vascular effects. Dorsal foot and periwound tissues were imaged. A fiducial target was placed to facilitate image realignment correcting for patient movement.
Image registration, processing, and quality assessment were conducted following the procedure. For wounds larger than 1 cm in diameter, mean oxy and deoxy values were extracted from a 1-cm radial border consisting of intact skin in the periwound region while avoiding any hyperkeratotic tissue. For wounds less than 1 cm in diameter, a 0.5-cm border was used.
Spectral decomposition was used to extract relative values of tissue oxy and deoxy from the diffuse reflectance spectra by comparing with standard transmission spectra from solutions (22). Oxy and deoxy units represent relative concentrations of oxy and deoxy found in the tissue volume measured by the HSI system (approximately the effective pixel size of the object [0.1 mm × 0.1 mm] multiplied by the penetration depth of light into tissue [∼1–2 mm in this wavelength range]). Tissue oxygen saturation (StO2), the fraction of oxygenated hemoglobin in superficial (predominantly subpapillary plexus) blood vessels, was calculated as the percentage of oxy over the sum of oxy and deoxy.
Ulcers were classified into one of two groups: ulcers that healed within 24 weeks or ulcers that did not heal within 24 weeks. Ulcers with complete re-epithelialization and no exudates at the last visit (∼24 weeks) were classified as healed. A healing index was then derived to best separate healed from nonhealed ulcers. The healing index was calculated as the distance between the point defined by the oxy and deoxy values and the linear discriminant decision line that best separated healed ulcers from nonhealed ulcers. A positive healing index was more likely to heal, whereas a negative healing index was more likely not to heal.
Patients received regular care by their doctors, including offloading and debridement when required. The treating physicians were blinded to the HSI data. No criteria for wound size or duration were used to select patients. Clinical and HSI data were captured on case report forms and uploaded into a central database and central file server, respectively.
Statistics
Statistical analyses were performed by an independent biostatistician. The data were analyzed to detect differences between patients with DFUs that healed and those with DFUs that did not heal. For categorical factors such as sex, differences between the healed and nonhealed proportions were compared with the χ2 test. For continuous factors such as oxy, deoxy, and StO2, differences between the means of the two groups were compared with the Student's t test using the more conservative test assuming unequal variances. Values were reported as means ± SD. A P value <0.05 was considered significant. Sensitivity, specificity, and positive predictive values for healing were calculated using standard definitions. Linear discriminant analysis was used to develop the threshold for separating the healed and nonhealed groups.
RESULTS
A total of 66 type 1 and type 2 diabetic patients (58 male and 8 female) with DFUs were enrolled in the study. The mean age was 50 years (range 25–68). Twelve patients were excluded from the study because they either failed to complete the study (n = 10) or required amputation prior to the 24-week visit (n = 2). The 54 patients who completed the study and presented with 73 ulcers comprise the data in this report. Fifty-four ulcers healed while 19 ulcers did not.
No significant differences in demographics or clinical characteristics were detected when comparing patients with DFUs that healed (n = 38) and patients with DFUs that did not heal (n = 16). Both groups were found to be well matched for sex, age, and other patient demographics and clinical characteristics (Table 1). No differences were seen in the level of neuropathy when comparing feet with DFUs that healed and feet with DFUs that did not heal. The mean NDS was 6.7 ± 4.9 and 7.7 ± 3.4, respectively (P = 0.39).
Table 1.
Patient demographics and clinical characteristics
| Patients with healed ulcers | Patients with any nonhealed ulcers | P | |
|---|---|---|---|
| n | 38 | 16 | |
| Age (years) | 51 (34–68) | 52 (25–63) | 0.73 |
| Sex (m/f) | 35/3 | 14/2 | 0.34 |
| Diabetes (type 1/type 2) | 15/23 | 8/8 | 0.19 |
| Diabetes duration (years) | 13 ± 10 | 12 ± 8 | 0.77 |
| A1C (%) | 9.7 ± 2.6 | 9.5 ± 2.4 | 0.83 |
| BMI (kg/m2) | 34 ± 10 | 31 ± 12 | 0.41 |
| Systolic BP (mmHg) | 135 ± 24 | 142 ± 21 | 0.28 |
| Diastolic BP (mmHg) | 76 ± 13 | 79 ± 9 | 0.38 |
| NSS | 5.3 ± 3.3 | 4.9 ± 3.0 | 0.65 |
| NDS | 7.7 ± 3.4 | 6.7 ± 4.9 | 0.39 |
Data are n, means ± SD, and median (range). BP, blood pressure; NSS, Neuropathy Symptom Score.
The ABI was similar for limbs with healed and nonhealed ulcers (Table 2). All ABIs were 0.78 or greater, which is consistent with mild peripheral arterial disease, at most, in a few subjects. As expected for a high-risk diabetic group, a large fraction of limbs (47%) were noncompressible, having an ABI >1.2. Mean tcPO2 at the ankle was 48 ± 15 and 46 ± 18 mmHg, respectively (P = 0.61). In addition, no differences were seen in the level of tissue oxygenation. Mean StO2 taken from the dorsal foot had mean values of 53 ± 13% for feet with DFUs that healed and 47 ± 12% for feet with DFUs that did not heal (P = 0.16).
Table 2.
Lower-limb assessment
| Foot with nonhealed ulcers (n = 19) | Foot with healed ulcers (n = 54) | P | |
|---|---|---|---|
| At the ankle | |||
| ABI <0.4 | 0 | 0 | — |
| ABI 0.4–0.69 | 0 | 0 | — |
| ABI 0.70–0.89 | 3 | 4 | — |
| ABI 0.90–1.19 | 6 | 16 | — |
| ABI >1.2 | 5 | 19 | — |
| ABI, NR | 5 | 15 | — |
| tcPO2 (mmHg) | 46 ± 16 | 48 ± 15 | 0.61 |
| At the foot | |||
| Oxy (dorsum) (AU) | 42 ± 18 | 44 ± 19 | 0.72 |
| Deoxy (dorsum) (AU) | 44 ± 13 | 37 ± 13 | 0.081 |
| StO2 (dorsum) (%) | 47 ± 12 | 53 ± 13 | 0.16 |
| At the ulcer border | |||
| Ulcer size (cm2) | 5.8 ± 6.2 | 3.2 ± 3.9 | 0.10 |
| Oxy (AU) | 64 ± 22 | 85 ± 21 | 0.0013 |
| Deoxy (AU) | 41 ± 12 | 44 ± 14 | 0.47 |
| StO2 (%) | 60 ± 10 | 66 ± 9 | 0.024 |
| Healing index | −0.15 ± 18 | 0.15 ± 19 | <0.0001 |
| Temperature (°C) | 33 ± 3 | 33 ± 3 | 0.83 |
Data are n and means ± SD. Data in bold are statistically significant. AU, arbitary units; NR, not recorded.
When evaluating mean HTOM values in the periwound area, significant differences were observed when comparing DFUs that healed with DFUs that did not heal (Table 2). Higher oxy values were noted around DFUs that healed (85 ± 21 vs. 64 ± 22, P = 0.0013). Mean StO2 was also higher in healing ulcers (66 ± 9% vs. 60 ± 10%, P = 0.024). The temperature around the ulcers was not found to be different. A difference in ulcer size was detected when comparing DFUs that did not heal with DFUs that did heal. The mean areas were 5.8 ± 6.2 and 3.2 ± 3.9 cm2, respectively. However, based on the more conservative Student's t test using unequal variances, this difference was not significant (P < 0.10).
No major difference was seen when comparing anatomical site distributions for the healed ulcers with those of the nonhealed ulcers. Twenty-nine ulcers were located on the plantar metatarsal phalangeal joints; 20 healed, and 9 did not heal. Fifteen ulcers were located on the plantar phalangeal joints; 10 healed, and 5 did not heal. Eight ulcers were located on the plantar arch; seven healed, one did not heal. Seven ulcers were located on the dorsal metatarsal phalangeal joints; five healed, and two did not heal. The remaining ulcers were located on the heel (n = 5), lateral foot (n = 4), dorsal phalangeal joints (n = 3), and distal ankle (n = 2).
HTOM as a predictor for DFU healing
Hyperspectral and visual images from a patient at baseline with a DFU that healed and corresponding images from a patient with a DFU that did not heal are shown in Fig. 1. In the healed DFU case (top panels), the mean values for oxy, deoxy, and StO2 were 75, 34, and 69%, respectively; while in the nonhealed case (bottom panels), tissue oxygenation was lower with values of 60, 53, and 53%, respectively.
Figure 1.
Visible and hyperspectral image of a healing DFU taken with the HTOM system. The top panels show a healed DFU case. HTOM values are 75, 34, and 69% for oxy, deoxy, and StO2, respectively. The bottom panels show a nonhealed DFU case. HTOM values are 60, 53, and 53% for oxy, deoxy, and StO2, respectively. Tissue oxygenation is higher in the healed ulcer as seen by the more purplish tone compared with the more cyan/green tone. Mean oxy and deoxy values were determined for each ulcer from an approximate 1-cm–thick band drawn within the periwound area.
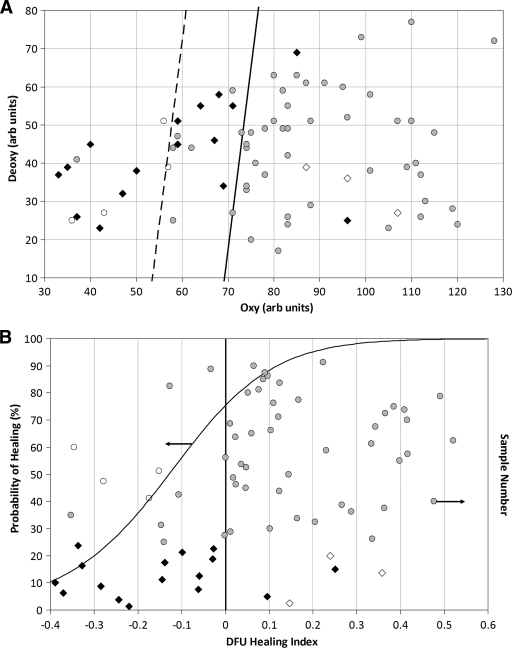
A scatter plot of mean oxy and deoxy values, measured from periwound tissue for all ulcers during the initial visit, is shown in Fig. 2A. The data were grouped according to the healing status at 24 weeks. The linear discriminant decision line best separating healed ulcers from nonhealed ulcers is shown. HTOM points to the left of the decision line best represent ulcers that did not heal and would have a negative healing index, while points to the right of the decision line best represent ulcers that did heal and would have a positive healing index. The mean healing index for ulcers that did not heal in 24 weeks is significantly different from that for ulcers that healed within 24 weeks (−0.15 vs. 0.15, P < 0.0001).
Figure 2.
A: Oxy and deoxy values for healed and nonhealed DFUs. The diagonal solid line represents the decision line for a healing algorithm based on oxy and deoxy values. Ninety percent of points lying to the right of the line healed. The diagonal dashed line represents a second decision line where 87% of the points (seven of eight excluding calluses) lying to the left of the line did not heal. B: Probability of healing based on HTOM healing index for healed and nonhealed DFUs. An ulcer with a positive healing index has a higher likelihood to heal. ♢, Ulcers with underlying osteomyelitis that did not heal; ♦, ulcers that did not heal at 24 weeks; ○, ulcers that healed and were surrounded by callus;  , ulcers that healed at 24 weeks. arb, arbitrary.
, ulcers that healed at 24 weeks. arb, arbitrary.
A plot of the healing index is shown in Fig. 2B. The healing index indicated that the sensitivity for healing was 80% (43 of 54), the specificity was 74% (14 of 19), and the predictive positive value was 90% (43 of 48). The sigmoidal line represents the probability of healing based on the healing index from the two groups. Of the five patients with ulcers that did not heal and had a positive healing index, three had underlying osteomyelitis and one refused to wear proper foot gear. The remaining 70 ulcer sites did not have any clinical signs of osteomyelitis. Of the 10 ulcers that did heal and had a negative healing index, the tissue surrounding 4 of the ulcers were callused.
When selecting borders around the wound, the thickness of the border was important. The ability to separate healing from nonhealing ulcers was optimal when using a border thickness between 0.5 and 1 cm. Reduced discrimination was observed when increasing the border thickness to 2 cm.
CONCLUSIONS
In this multicenter 24-week study, we show that HTOM provides a local assessment of microvascular oxygenation status that is predictive of ulcer healing in type 1 and type 2 diabetic patients. HTOM offers high sensitivity (81%) and specificity (74%) in determining healing potential. Additionally, the data show that HSI can assess healing capacity with a 90% positive predictive value. Higher ulcer healing predictions are possible if care is taken to avoid evaluating ulcers with underlying osteomyelitis and those with overlying calluses. The sensitivity, specificity, and positive predictive values increase to 86, 88, and 96%, respectively, when removing three false-positive osteomyelitis cases and four false-negative cases due to measurements on calluses.
The results of this study not only confirm preliminary results obtained in an earlier pilot study (18) but also firmly establish that HTOM serves as a clinically relevant technique for predicting DFU healing based on an evaluation of 54 patients and 73 ulcers. HTOM allows the physician to identify DFUs at risk of not healing much earlier because data are collected and assessed from the first visit (i.e., without having to wait for 6 months). These results demonstrate that HSI can measure tissue oxygenation with spatial resolutions of 100 microns without coming into contact with the patient's foot.
Adequate tissue microvascular perfusion is an essential element for wound healing. The HSI system used in this study was designed to evaluate tissue oxygenation at the superficial microvascular level. HTOM values predicted healing status better when evaluating tissue close to the wound margins, thus demonstrating periwound changes in microvessels that can be used for assessing the healing capacity of a DFU. Healed DFUs demonstrate increased microvascular oxygenation as evidenced by an increase in oxy and StO2 when compared with nonhealed DFUs. A similar increase in StO2 has been noted previously with another independently established technique (23).
Based on the NDS, 65% of the patients had moderate to severe foot neuropathy (NDS ≥5). HTOM taken from the dorsal foot showed that 93% of patients had a StO2 that was greater than 30%, while 87% of patients had a tcPO2 at the ankle that was greater than 30 mmHg. Both oxygenation techniques indicate the foot was reasonably well oxygenated in the basal state, and differences between healing and nonhealing ulcers only surfaced once the wound was present and the tissue was either able or not able to respond to the injury.
In summary, HTOM can accurately predict wound healing in advance e.g., several months before the wound heals. Physicians are now able to identify which DFUs are at risk for delayed healing based on reduced oxygenation levels resulting in elected triage to therapies designed to increase oxygen delivery to tissue. Based on these results assessing microvascular perfusion, HTOM may be used in three main areas of patient care: DFU management, surgical planning, and monitoring of therapy. The addition of quantitative oxygenation information to the current available tools and treatment could allow for more targeted therapy and could potentially accelerate current case resolutions.
Ultimately, HTOM has the potential to screen for lower-extremity complications due to diabetes. This is based on the ability of HTOM to quantify risk in a specific area of tissue and the ability to observe ischemic complications before they would otherwise be visible to the naked eye. HTOM provides the diabetes caregiver with the information necessary to treat and monitor foot complications faster and more specifically than would be possible with current available methods, which lack adequate sensitivity, specificity, and spatial localization to assess the microvascular status of the foot.
Acknowledgments
This research was funded in part through a grant from the National Institute of Diabetes and Digestive and Kidney Diseases (R42-DK069871) and in part at the Cleveland Clinic by the National Institutes of Health (NIH), National Center for Research Resources (CTSA 1UL1RR024989). E.M.'s salary is partially funded via the NIH National Heart, Lung, and Blood Institute (grant K12 HL083772-01).
No potential conflicts of interest relevant to this article were reported.
We thank Dr. Robert Lew from Boston University's Statistics Department for his help with the statistical analysis. We also thank Wendy Arriaga, Gustavo Chavez, and Martha Cornejo from Olive View and Samantha Keevey from the Cleveland Clinic who have been extremely helpful with data collection.
Footnotes
Clinical trial reg. no. NCT00617916, clinicaltrials.gov.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Wild S, Roglic G, Green A, Sicree R, King H: Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004;27:1047–1053 [DOI] [PubMed] [Google Scholar]
- 2. Reiber GE: The epidemiology of diabetic foot problems. Diabet Med 1996; 13(Suppl. 1):S6–S11 [PubMed] [Google Scholar]
- 3. Palumbo PJ, Melton IJ: Peripheral vascular disease and diabetes. In Diabetes in America. 1st ed. Harris MI, Hamman RF. Eds. Washington, DC, U.S. Govt. Printing Office, 1985, p. 401–408 (DHHS publ. no. 85-1468) [Google Scholar]
- 4. Boulton AJM, Armstrong DG, Albert SF, Frykberg RG, Hellman R, Kirkman MS, Lavery LA, LeMaster JW, Mills JL, Sr, Mueller MJ, Sheehan P, Wukich DK: Comprehensive foot examination and risk assessment: a report of the Task Force of the Foot Care Interest Group of the American Diabetes Association, with endorsement by the American Association of Clinical Endocrinologists. Diabetes Care 2008;31:1679–1685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ramsey SD, Newton K, Blough D, McCulloch DK, Sandhu N, Reiber GE, Wagner EH: Incidence, outcomes, and cost of foot ulcers in patients with diabetes. Diabetes Care 1999;22:382–387 [DOI] [PubMed] [Google Scholar]
- 6. Abbott CA, Carrington AL, Ashe H, Bath S, Every LC, Griffiths J, Hann AW, Hussein A, Jackson N, Johnson KE, Ryder CH, Torkington R, Van Ross ER, Whalley AM, Widdows P, Williamson S, Boulton AJ; the North-West Diabetes Foot Care Study. The North-West Diabetes Foot Care Study: incidence of, and risk factors for, new diabetic foot ulceration in a community-based patient cohort. Diabet Med 2002;19:377–384 [DOI] [PubMed] [Google Scholar]
- 7. Reiber GE, Vileikyte L, Boyko EJ, del Aguila M, Smith DG, Lavery LA, Boulton AJM: Causal pathways for incident lower-extremity ulcers in patients with diabetes from two settings. Diabetes Care 1999;22:157–162 [DOI] [PubMed] [Google Scholar]
- 8. Lavery LA, Armstrong DG, Wunderlich RP, Mohler MJ, Wendel CS, Lipsky BA: Risk factors for foot infections in individuals with diabetes. Diabetes Care 2006;29:1288–1293 [DOI] [PubMed] [Google Scholar]
- 9. Pecoraro RE, Reiber GE, Burgess EM: Pathways to diabetic limb amputation: basis for prevention. Diabetes Care 1990;13:513–521 [DOI] [PubMed] [Google Scholar]
- 10. Reiber GE, Boyko EJ, Smith DC: Lower extremity foot ulcers and amputations in diabetes. In Diabetes in America. 2nd ed. Harris MI, Cowie CC, Stern MP, Boyko EJ, Reiber GE, Bennet PH. Eds. Washington, DC, U.S. Govt. Printing Office, 1995, p. 402–428 [Google Scholar]
- 11. Frykberg RG, Armstrong DG, Giurini J, Edwards A, Kravette M, Kravitz S, Ross C, Stavosky J, Stuck R, Vanore J; American College of Foot and Ankle Surgeons. Diabetic foot disorders: a clinical practice guideline. J Foot Ankle Surg 2000;39(Suppl. 5):S1–S60 [PubMed] [Google Scholar]
- 12. Harrington C, Zagari MJ, Corea J, Klitenic J: A cost analysis of diabetic lower-extremity ulcers. Diabetes Care 2000;23:1333–1338 [DOI] [PubMed] [Google Scholar]
- 13. Sumpio BE: Foot ulcers. N Engl J Med 2000;343:787–793 [DOI] [PubMed] [Google Scholar]
- 14. Frykberg RG: Diabetic foot ulcers: pathogenesis and management. Am Fam Physician 2002;66:1655–1662 [PubMed] [Google Scholar]
- 15. Cobb J, Claremont D: Noninvasive measurement techniques for monitoring of microvascular function in the diabetic foot. Int J Low Extrem Wounds 2002;1:161–169 [DOI] [PubMed] [Google Scholar]
- 16. Zimny S, Dessel F, Ehren M, Pfohl M, Schatz H: Early detection of microcirculatory impairment in diabetic patients with foot at risk. Diabetes Care 2001;24:1810–1814 [DOI] [PubMed] [Google Scholar]
- 17. Ubbink DT, Spincemaille GH, Reneman RS, Jacobs MJ: Prediction of imminent amputation in patients with non-reconstructible leg ischemia by means of microcirculatory investigations. J Vasc Surg 1999;30:114–121 [DOI] [PubMed] [Google Scholar]
- 18. Khaodhiar L, Dinh T, Schomacker KT, Panasyuk SV, Freeman JE, Lew R, Vo T, Panasyuk AA, Lima C, Giurini JM, Lyons TE, Veves A: The use of medical hyperspectral technology to evaluate microcirculatory changes in diabetic foot ulcers and to predict clinical outcomes. Diabetes Care 2007;30:903–910 [DOI] [PubMed] [Google Scholar]
- 19. Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2003;26(Suppl. 1):S5–S20 [DOI] [PubMed] [Google Scholar]
- 20. American Diabetes Association and American Academy of Neurology. Report and recommendations of the San Antonio Conference on Diabetic Neuropathy (Consensus Statement). Diabetes Care 1988;11:592–597 [DOI] [PubMed] [Google Scholar]
- 21. Pham H, Armstrong DG, Harvey C, Harkless LB, Giurini JM, Veves A: Screening techniques to identify people at high risk for diabetic foot ulceration: a prospective multicenter trial. Diabetes Care 2000;23:606–611 [DOI] [PubMed] [Google Scholar]
- 22. Zuzak KJ, Schaeberle MD, Lewis EN, Levin IW: Visible reflectance hyperspectral imaging: characterization of a noninvasive, in vivo system for determining tissue perfusion. Anal Chem 2002;74:2021–2028 [DOI] [PubMed] [Google Scholar]
- 23. Beckert S, Witte MB, Königsrainer A, Coerper S: The impact of the Micro-Lightguide O2C for the quantification of tissue ischemia in diabetic foot ulcers. Diabetes Care 2004;27:2863–2867 [DOI] [PubMed] [Google Scholar]