Abstract
Purpose
The clinical features of patients with celiac disease (CD) are variable. In the present study, clinical and laboratory features of 109 patients with CD were retrospectively evaluated.
Materials and Methods
In all cases, diagnosis of CD was made by European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) criteria and clinical and laboratory findings, including hematological and biochemical analyses, immunoglobulin levels, autoantibodies [antinucler antibody (ANA), antidouble stranded DNA (dsDNA), antimitochondrial antibody (AMA), anti-smooth muscle antibody (ASMA), liver kidney antibody (LKM-1), anti thyroid peroxidase (TPO), anti thyroglobulin (Tg)], bone mineral density (BMD), and electroencephalogram were evaluated. The type of CD was recorded.
Results
Of 109 patients with CD, 66 (60.6%) were classical type, 41 (37.6%) were atypical type and 2 (1.8%) were silent type. The mean age was 8.81 ± 4.63 years and the most common symptom was diarrhea (53.2%) followed by failure to thrive, short stature, and abdominal pain. Paleness (40.4%), underweight (34.8%), and short stature (31.2%) were the most common findings. Iron deficinecy anemia (81.6%), zinc deficiency (64.1%), prolonged prothrombin time (35.8%), and elevated transaminase levels (24.7%) were the most common laboratory findings. Eight percent of patients had at least 1 autoantibody, and 28 of 52 patients had low BMD. Four of 38 patients had abnormalty in electroencephalograms. The prevalance of selective immunoglobulin (Ig) A deficiency was 9.1%. Histocompatibility antigen HLA-DQ and/or DQ8 genotypes were found in 91% of patients. Abdominal distention, iron deficiency, prolonged prothrombine time, hypoalbuminemia, and elevated transaminase levels were more significantly frequent in the classical type than atypical type (p < 0.005).
Conclusion
Although classical CD was seen in most patients in the present study, clinical variability of the condition should be kept in mind.
Keywords: Celiac disease, children
INTRODUCTION
CD is an immune-mediated enteropathy caused by permanent sensitivity to gluten in genetically susceptible individuals. Genetic, environmental, and immunolgical factors play a role in pathogenesis. The disease presents different clinical pictures.1-7 Typical clinical pictures of CD during the first 2 years of life are chronic diarrhea and failure to thrive. Atypical features occur in patients with later onset of the disease. Some patients may be asypmtomatic.8,9 It is well known that CD is associated with a number of autoimmune and inflammatory diseases, implicating a common genetic background. Clinical features of Turkish children with CD is still unknown, and there are only a few papers on clinical features from the Middle East.10-12 The aim of this study was to evaluate the clinical and laboratory features of 109 Turkish children with CD at diagnosis.
MATERIALS AND METHODS
In this retrospective study, we reviewed the hospital records of children (n = 109, 0-18 years age) in whom CD was diagnosed at the Department of Pediatric Gastroenterology, Hepatology and Nutrition at Ankara University School of Medicine from January 1998 to December 2006. The study protocol was approved by the local ethics committee. In all cases, diagnosis of CD was made by European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) criteria.13 Patients with gastrointestinal symptoms such as chronic diarrhea and malabsorption were regarded as classical CD, those with unusual intestinal complaints such as recurent abdominal pain and constipation or extraintestinal symptoms such, as anemia and short stature, were classified as atypical CD. Recurrent abdominal pain was defined as abdominal pain which was present at least once a week for at least 3 months before diagnosis and without evidence of inflammatory, anatomic, metabolic, or neoplastic processes to explain the symptoms.14 Constipation was defined as fewer than 3 bowel movements per week or passage of large-diameter stools that may obstruct the toilet or painful defecation during the past 8 weeks.15 CD is defined silent whenever a typical gluten-sensitive enteropathy was found in a subject who was apparently healthy during family screening.
Upon diagnosis of CD, data obtained from each patient included: demographic characteristics, symptoms, associated diseases, physical examination and laboratory findings, including routine hematological and biochemical analysis, serum immunoglobulin, zinc (in 92 patients), iron (in 98 patients), ferritin (in 98 patients), vitamin B12 (in 75 patients), and folic acid (in 82 patients), prothrombin and partial thromboplastine times (in 48 patients), and thyroid function tests (in 65 patients). Bone mineral density (BMD) and parathormon level were evaluated in 52, patients and electroechocardiogram (EEG) in 38 patients. Autoimmune markers including direct coombs, antinucler antibody (ANA), antidouble stranded DNA (dsDNA), antimitochondrial antibody (AMA), anti- smooth muscle antibody (ASMA), liver kidney antibody (LKM-1), thyroid peroxidase (TPOAb) and thyroglobulin autoantibodies (TgAb) were studied in 52 patients. Human leukocyte antigen typing was investigated in 45 patients.
Weight and height were measured in kgs and cms, respectively. HeightSDS and weightSDS were calculated for each child using Tanner-Whitehouse standards according to the following formula: standard deviation score (SDS) = [observed value-mean (value at 50th percentile) for age and gender] standart deviation (SD) for age and gender.16 Short stature was defined as heightSDS < -2.
Body mas index (BMI) was calculated as weight (kg) divided by height (m2). Nutritional status was stated based on the weightSDS and BMI charts from the Centers for Disease Control and Prevention. BMI percentile was evaluated according to the Centers for Disease Control and Prevention's classification criteria.17,18 Normal weight was defined as percentile of BMI for age and gender greater than the 5th and less than the < 85th percentile. Children with a BMI for age and gender below the 5th percentile were classified as underweight; children who were ≥ 85th percentile but < 95th percentile were classified as at risk of being overweight; and children who were ≥ 95th percentile were classified as overweight. Underweight for younger than 2 years old was defined as weight SDS < -2.
Bone mineral density (BMD) and bone mineral content (BMC) were measured at the lumbar spine (L1-4) using dual-energy X-Ray absorptiometry (DEXA). BMD and BMC were expressed in absolute terms (g/cm2 and g/cm), and absolute BMD for each patient was then expressed as a z score when compared with the mean BMD of 143 healthy age and gender matched children. Z score was calculated using following formula:
z score = patient BMD - mean BMD for age-and-gender matched normals SD of BMD of age-and-gender- matched normals.
Osteoporosis was defined if the z score was less than -2, and osteopenia was defined if z score was between -1 and -2.19 Human leukocyte antigen class I and II typing were studied serologically by the standard microlymphocytotoxicity method.20
Statistical analyses
The results were expressed as mean ± SD. The significance of intergroup differences was studied with SPSS 11.0 computer program by chi-squared test, Student's t test and Wilcoxon-signed rank. A p value of < 0.05 was considered significant.
RESULTS
Sixty-four of the 106 children with CD were female. The mean age at diagnosis was 8.81 ± 4.63 years (range 1.5-17 years). Nine patients (8.2%) were younger than 2 years of age and 100 (91.7%) were older than 2 years. Of 109 patients with CD, 66 (60.6%) were classical type, 41 (37.6%) were atypical type and 2 (1.8%) were silent type. The age of children with classical type (7.5 ± 4.3 years) was significantly lower than the age of children with atypical form (10.8 ± 4.3 years) (p = 0.001).
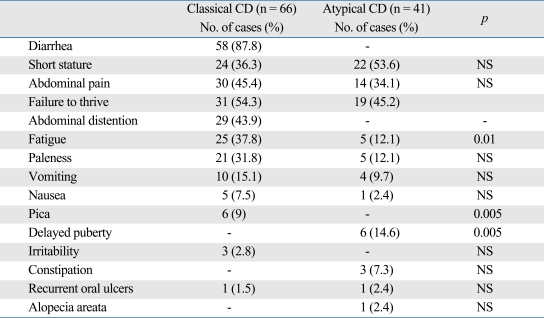
The most frequent symptom was diarrhea (53.2%) followed by failure to thrive (45.9%), short stature (42.2%), abdominal pain (40.4%), abdominal distention (26.6%), fatigue (27.5%), paleness (23.9%), vomiting (12.8%), nausea (5.5%), pica (6.4%), delayed puberty (5.5%), irritability (2.8%), constipation (2.7%), recurrent oral ulcers (1.8%), and alopecia areata (1.8%). Presenting symptoms according to clinical type are shown in Table 1. While delayed puberty was significantly frequent in children with atypical CD (p = 0.005), abdominal distention and pica were significantly frequent in children with classical CD (p = 0.001 and p = 0.005, respectively).
Table 1.
Presenting Symptoms of Patients with CD according to Type
CD, celiac disease.
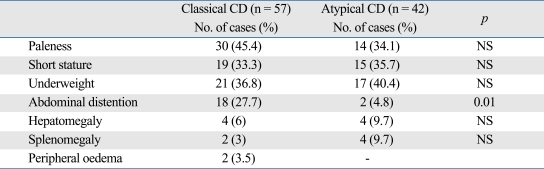
Weight was below the 3rd percentile in 40 children (37%). Height was below the 3rd percentile in 48 children (44%). The most common sign was paleness (40.4%). Underweight (34.8%), short stature (31.2%), abdominal distention (18.3%), hepatomegaly (7.3%), splenomegaly (5.5%), and peripheral odema (1.8%) were other findings on physical examination. Abdominal distention was significantly more frequent in classical type than atypical type (p = 0.01) (Table 2).
Table 2.
Clinical Findings of Patients with CD according to Type
CD, celiac disease.
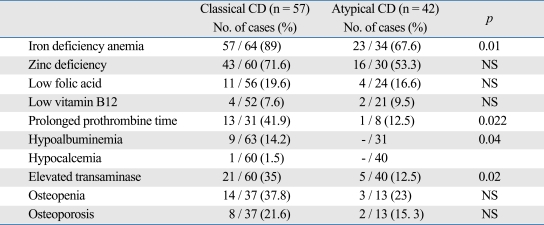
Prevalance of iron deficiency anemia was 81.6%, zinc deficiency 64.1%, low folic acid 18.3%, low vitamin B12 8%, prolonged prothrombin time 35.8%, hypoalbuminea 9.5%, and hypocalcemia 0.9%. Elevated serum transaminase levels were observed in 24.7% of patients. Laboratory findings according to clinical type are shown in Table 3. Prevalance of iron deficiency anemia, prolonged PT, hypoalbuminemia and elevated transaminase levels were significantly higher in classical type than atypical type (p = 0.01, 0.02, 0.04 and 0.02, respectively).
Table 3.
Laboratory Findings of Patients with CD according to Type
CD, celiac disease.
Short stature in 11 patients (10.9%), recurrent iron deficincy anemia in 16 patients (14.6%) and elevated transaminase levels in 1 patient (0.9%) were the only clinical features of CD.
We detected ANA in 5 of 61 patients (8.2%), ASMA in 8 of 52 patients (7.4%), anti-Tg or anti-TPO in 9 of 104 patients (8.6%), and positive direct combs in 1 of 48 patients (2%), whereas anti-dsDNA, AMA and LKM-1 were not detected in any patients. Thyroid dysfunction was detected in 2 of 65 patients (3.1%).
Elevated parathormon levels were detected in 17 of 52 patients (32.6%). Twenty--eight of 52 patients (53.8%) had low bone mineral density. Bone mineral density showed osteopenia in 18 of 52 patients (34.6%) and osteoporosis in 10 of 52 patients (19.2%). Eight of 10 patients with osteoporosis had classical form. There was no significant difference in these parameters between classical and atypical celiac patients.
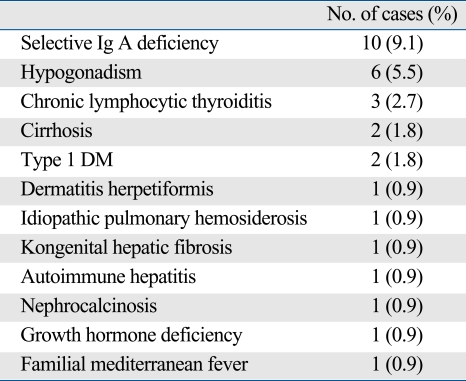
Neurological examination was normal in all patients, and 4 of 38 patients had EEG abnormalities (10.5%). Selective Ig A deficiency was detected in 9.1% of patients. Other disorders associated with CD are shown in Table 4.
Table 4.
Associated Disorders in 109 Patients with CD
CD, celiac disease; Ig, immunoglobulin; DM, diabetes mellitus.
Thirty-six patients (80%) had HLA DQ2, and 2 patients (4.4%) had DQ8. Four patients (8.8%) were positive for both HLA DQ2 and HLA DQ8.
DISCUSSION
We evaluated the features of 109 children with CD. The disease is known to be more frequent among females as shown in our study, with a female-to-male ratio of 1.5 : 1. Clinical features at presentation are very variable. While some develop CD very early in life, others may eat gluten for many years before the disease becomes apparent. In Turkey, as in most countries, the majority of patients are diagnosed with the "classic" CD symptoms. In our study, the most common age of diagnosis was school-age. Although diarrhea was the most frequent symptom and more than half of patients had classical type, CD was diagnosed before 2 years of age in only 8.2% of patients. This may be a late diagnosis.
The clinical picture of CD is very heterogeneous with a broad spectrum of symptoms ranging from malabsorption, chronic diarrhea, and failure to thrive (the classic "triad") to abdominal pain, iron-deficiency anemia, delayed puberty, nonspecific arthritis, depression, ataxia, low bone mineral density, or dental enamel hypoplasia without gastrointestinal complaints.21,22 Paleness and failure to thrive were the most common findings in patients. Failure to thrive may be due to a longer duration of the disease in patients. In our study, short stature appeared to be the most common extraintestinal finding followed by anemia. It is known that short stature can be the only presenting clinical feature of CD, an issue that has recently been reviewed.23 In unselected cases admitted for short stature, the prevalence of CD varies from 2.9 to 8.3%, and CD is by far more common than growth hormone (GH) deficiency or any other organic disorder. When other causes for short stature were excluded, the prevalence could rise to 59%.24 In our study, CD presented with short stature as a sole manifestation in 11 patients (10.9%). Therefore, investigation of CD is recommended in the diagnostic work-up of a short child with no endocrinologic abnormality.
Iron deficiency anemia is a frequent finding in celiac diasease patients, as in our study. It is seen in the majority of patients with 1 or more other findings and can also be the single finding of the disease (25-27). In our study, recurrent iron defiency anemia was the only clinical finding of CD in 16 patients (14.6%). Celiac prevalence in adult patients with iron deficiency anemia varies between 2.6 and 11.8%. Kalayci, et al.28 found that celiac prevalence in children with iron deficiency anaemia was 4.4%. Subclinical CD is an underdiagnosed condition, and physicians should be aware of the significant relation existing between iron deficiency anemia and subclinical coeliac disease.
CD is defined as silent when typical gluten-sensitive enteropathy is found in a patient who is apparently healthy. Large numbers of silent cases of CD have been reported in at-risk groups (such as first-degree relatives and patients with insulin-dependent diabetes) and in general population samples enrolled in screening programs.29 Two patiens had silent CD in the present study. These patients were diagnosed by family screening.
Hypertransaminasemia can be the first finding of CD. Liver histology may reveal steatosis, ranging from chronic hepatitis to more severe lesions.30,31 In our study, there were 2 patients with cirrhosis who were diagnosed with CD. Hypertransaminasemia was observed in 24.7% of patients and it was the presenting symptom in 1 patient. Children with unexplained high transaminase levels and cryptogenic liver disease should be evaluated for CD.
CD is a chronic inflammatory disorder of the small bowel that results in malabsorption of nutrients.32 Because vitamin K is a fat-soluble vitamin absorbed from the small bowel, severe malabsorption leads to vitamin K deficiency, haemocoagulative deficit of the K-dependent factor, and resulting prolonged PT. According to the results of Cavallaro, et al.,33 a prolonged PT was found in 18.5% of adult coeliac patients. They reported that a prolonged PT was only found in a few patients with subclinical CD (0.9%). Ertekin, et al.,34 reported that prevalence of prolonged PT in celiac children in 25%, and that PT was normal in all patients with atypical form. Our results are in accordance with these reports; prolonged PT was found in 14 of 39 patients (35.8%), and only 1 of them was atypical CD.
Celiac disease is associated with IgA deficiency and it is 10-times more frequent in patients with Ig A deficiency than in the general population.35 Serum Ig A deficiency was detected in 9.1% of patients in the present study, which is higher than expected in the normal population. An important associated disease is dermatitis herpetiformis, a dermatological disease also known as "CD of the skin," with a high frequency of CD in adults but with a much lower frequency in childhood CD as in our study.36,37 Another associated disease in our study was idiopathic pulmonary hemosiderosis, a rare condition of unknown autoimmune etiology mainly affecting children and adolescents in which a GFD may be very effective for the regression of the pulmonary hemosiderosis.38 Morever, 1 patient had both FMF and CD, and this is the first description of association of CD and FMF.39
One of the most controversial issues concerning the clinical presentations of CD in pediatrics is the association between the disease and other autoimmune disorders. The 2 most accredited theories propose: 1) this association is secondary to a linkage disequilibrium of genes predisposing for both CD and the associated autoimmune disease or 2) CD leads to the onset of other autoimmune disorders in genetically susceptible individuals.40-43 In our study, there were 6 children with autoimmune diseases, including Type 1 DM, Hashimato's thyroiditis, and autoimmune hepatitis. The prevalance of positivity for ANA, ASMA, and thyroid autoantibodies were found in approximately 8% of patients.
Osteoporosis is 1 of the commoly known complications of untreated CD. Persistent villous atrophy is associated with low bone mineral density. Several clinical and epidemiological studies have been published on the association between CD and osteoporosis. Bone alterations were once thought to derive from calcium and vitamin D deficiency secondary to simple intestinal malabsorption.44-46. Osteopenia was reported in 58-65% of children with newly diagnosed celiac disease.47,48 Prevalance of low bone mineral density was 53.8% in our study.
CD has been associated with neurologic and psychiatric disorders, including cerebellar ataxia, peripheral neuropathy, epilepsy, dementia, and depression. Earlier reports document mainly the involvement of the nervous system as a complication of prediagnosed CD. However, more recent studies have emphasized that a wider spectrum of neurologic syndromes may be the presenting extraintestinal manifestation of gluten sensitivity with or without intestinal pathology. These include migraine, encephalopathy, chorea, brain stem dysfunction, myelopathy, mononeuritis multiplex, Guillain-Barre-like syndrome, and neuropathy with positive antiganglioside antibodies. It remains unclear whether gluten sensitivity contributes to the pathogenesis of these disorders or whether it represents an epiphenomenon.49,50 In our study, involvement of the nervous system was evaluated in 38 children. These children had no neurological symptoms, and neurological examination revealed that all children were normal, but EEG showed slow wave abnormalities in 4 patients.
The major CD-predisposing genes are located in the HLA region, namely the HLA-DQ2 and/or DQ8 genotypes found in at least 98% of patients.8,22 DQ2 and/or DQ8 genotypes were found in 91% of our patients.
In conclusion, the clinical picture of CD is very heterogeneous; therefore, high index of suspicion for CD should be maintained in all developing countries for patients with chronic diarrhea, short stature, or iron-deficiency anemia.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Fasano A, Catassi C. Current approaches to diagnosis and treatment of celiac disease: an evolving spectrum. Gastroenterology. 2001;120:636–651. doi: 10.1053/gast.2001.22123. [DOI] [PubMed] [Google Scholar]
- 2.Catassi C, Fabiani E, Rätsch IM, Coppa GV, Giorgi PL, Pierdomenico R, et al. The coeliac iceberg in Italy. A multicentre antigliadin antibodies screening for coeliac disease in school-age subjects. Acta Paediatr Suppl. 1996;412:29–35. doi: 10.1111/j.1651-2227.1996.tb14244.x. [DOI] [PubMed] [Google Scholar]
- 3.Hill I, Fasano A, Schwartz R, Counts D, Glock M, Horvath K. The prevalence of celiac disease in at-risk groups of children in the United States. J Pediatr. 2000;136:86–90. doi: 10.1016/s0022-3476(00)90055-6. [DOI] [PubMed] [Google Scholar]
- 4.Hoffenberg EJ, MacKenzie T, Barriga KJ, Eisenbarth GS, Bao F, Haas JE, et al. A prospective study of the incidence of childhood celiac disease. J Pediatr. 2003;143:308–314. doi: 10.1067/s0022-3476(03)00282-8. [DOI] [PubMed] [Google Scholar]
- 5.Fasano A, Berti I, Gerarduzzi T, Not T, Colletti RB, Drago S, et al. A multicenter study on the sero-prevalence of coeliac disease in the United States among both at risk and not at risk groups. Arch Int Med. 2003;163:286–292. doi: 10.1001/archinte.163.3.286. [DOI] [PubMed] [Google Scholar]
- 6.Mäki M, Mustalahti K, Kokkonen J, Kulmala P, Haapalahti M, Karttunen T, et al. Prevalence of Celiac disease among children in Finland. N Engl J Med. 2003;348:2517–2524. doi: 10.1056/NEJMoa021687. [DOI] [PubMed] [Google Scholar]
- 7.Catassi C, Fasano A. New developments in childhood celiac disease. Curr Gastroenterol Rep. 2002;4:238–243. doi: 10.1007/s11894-002-0069-0. [DOI] [PubMed] [Google Scholar]
- 8.Fasano A, Catassi C. Celiac disease in children. Best Pract Res Clin Gastroenterol. 2005;19:467–478. doi: 10.1016/j.bpg.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 9.Kagnoff MF. Celiac disease: pathogenesis of a model immunogenetic disease. J Clin Invest. 2007;117:41–49. doi: 10.1172/JCI30253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Demir H, Yüce A, Koçak N, Ozen H, Gürakan F. Celiac disease in Turkish children: presentation of 104 cases. Pediatr Int. 2000;42:483–487. doi: 10.1046/j.1442-200x.2000.01286.x. [DOI] [PubMed] [Google Scholar]
- 11.Ertekin V, Selimoğlu MA, Kardaş F, Aktaş E. Prevalence of celiac disease in Turkish children. J Clin Gastroenterol. 2005;39:689–691. doi: 10.1097/01.mcg.0000174026.26838.56. [DOI] [PubMed] [Google Scholar]
- 12.Masjedizadeh R, Hajiani E, Hashemi J, Shayesteh AA, Moula K, Rajabi T. Celiac disease in South-West of Iran. World J Gastroenterol. 2006;12:4416–4419. doi: 10.3748/wjg.v12.i27.4416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Revised criteria for diagnosis of coeliac disease. Report of Working Group of European Society of Paediatric Gastroenterology and Nutrition. Arch Dis Child. 1990;65:909–911. doi: 10.1136/adc.65.8.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McOmber ME, Shulman RJ. Recurrent abdominal pain and irritable bowel syndrome in children. Curr Opin Pediatr. 2007;19:581–585. doi: 10.1097/MOP.0b013e3282bf6ddc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boccia G, Manguso F, Coccorullo P, Masi P, Pensabene L, Staiano A. Functional defecation disorders in children: PACCT criteria versus Rome II criteria. J Pediatr. 2007;151:394–398.e1. doi: 10.1016/j.jpeds.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 16.Tanner JM, Whitehouse RH. Clinical longitudinal standards for height, weight, height velocity and weight velocity and stages of puberty. Arch Dis Child. 1976;51:170–179. doi: 10.1136/adc.51.3.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barlow SE, Dietz WH. Obesity evaluation and treatment: Expret committee recommendations. The Maternal and Child Health Bureau, Health Resources and Services Administration and the Department of Health and Human Services. Pediatrics. 1998;102:E29. doi: 10.1542/peds.102.3.e29. [DOI] [PubMed] [Google Scholar]
- 18.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, et al. Adv Data. 2000. CDC growth charts: United States; pp. 1–27. [PubMed] [Google Scholar]
- 19.Baroncelli GI, Bertelloni S, Sodini F, Saggese C. Osteoporosis in children and adolescents: etiology and management. Paediatr Drugs. 2005;7:295–323. doi: 10.2165/00148581-200507050-00003. [DOI] [PubMed] [Google Scholar]
- 20.Terasaki PI, Mcclelland JD. Microdroplet assay of human serum cytotoxins. Nature. 1964;204:998–1000. doi: 10.1038/204998b0. [DOI] [PubMed] [Google Scholar]
- 21.Green PH, Jabri B. Coeliac disease. Lancet. 2003;362:383–391. doi: 10.1016/S0140-6736(03)14027-5. [DOI] [PubMed] [Google Scholar]
- 22.Mearin ML. Celiac disease among children and adolescents. Curr Probl Pediatr Adolesc Health Care. 2007;37:86–105. doi: 10.1016/j.cppeds.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 23.Iughetti L, Bulgarelli S, Forese S, Lorini R, Balli F, Bernasconi S. Endocrine aspects of coeliac disease. J Pediatr Endocrinol Metab. 2003;16:805–818. doi: 10.1515/jpem.2003.16.6.805. [DOI] [PubMed] [Google Scholar]
- 24.van Rijn JC, Grote FK, Oostdijk W, Wit JM. Short stature and the probability of coeliac disease, in the absence of gastrointestinal symptoms. Arch Dis Child. 2004;89:882–883. doi: 10.1136/adc.2004.057851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carroccio A, Iannitto E, Cavataio F, Montalto G, Tumminello M, Campagna P, et al. Sideropenic anemia and celiac disease: one study, two points of view. Dig Dis Sci. 1998;43:673–678. doi: 10.1023/a:1018896015530. [DOI] [PubMed] [Google Scholar]
- 26.Bonamico M, Vania A, Monti S, Ballati G, Mariani P, Pitzalis G, et al. Iron deficiency in children with celiac disease. J Pediatr Gastroenterol Nutr. 1987;6:702–706. doi: 10.1097/00005176-198709000-00007. [DOI] [PubMed] [Google Scholar]
- 27.Rossi E. Monosymptomatic forms of celiac disease. Eur J Pediatr. 1982;138:4–5. doi: 10.1007/BF00442319. [DOI] [PubMed] [Google Scholar]
- 28.Kalayci AG, Kanber Y, Birinci A, Yildiz L, Albayrak D. The prevalence of coeliac disease as detected by screening in children with iron deficiency anaemia. Acta Paediatr. 2005;94:678–681. doi: 10.1111/j.1651-2227.2005.tb01964.x. [DOI] [PubMed] [Google Scholar]
- 29.Catassi C, Fabiani E. The spectrum of coeliac disease in children. Baillieres Clin Gastroenterol. 1997;11:485–507. doi: 10.1016/s0950-3528(97)90028-2. [DOI] [PubMed] [Google Scholar]
- 30.Leonardi S, Bottaro G, Patané R, Musumeci S. Hypertransaminasemia as the first symptom in infant celiac disease. J Pediatr Gastroenterol Nutr. 1990;11:404–406. doi: 10.1097/00005176-199010000-00021. [DOI] [PubMed] [Google Scholar]
- 31.Maggiore G, Caprai S. Liver involvement in celiac disease. Indian J Pediatr. 2006;73:809–811. doi: 10.1007/BF02790391. [DOI] [PubMed] [Google Scholar]
- 32.Shamir R. Advances in celiac disease. Gastroenterol Clin North Am. 2003;32:931–947. doi: 10.1016/s0889-8553(03)00061-x. [DOI] [PubMed] [Google Scholar]
- 33.Cavallaro R, Iovino P, Castiglione F, Palumbo A, Marino M, Di Bella S, et al. Prevalence and clinical associations of prolonged prothrombin time in adult untreated coeliac disease. Eur J Gastroenterol Hepatol. 2004;16:219–223. doi: 10.1097/00042737-200402000-00016. [DOI] [PubMed] [Google Scholar]
- 34.Cavallaro R, Iovino P, Castiglione F, Palumbo A, Marino M, Di Bella S, et al. Prevalence and clinical associations of prolonged prothrombin time in adult untreated coeliac disease. Eur J Gastroenterol Hepatol. 2004;16:219–223. doi: 10.1097/00042737-200402000-00016. [DOI] [PubMed] [Google Scholar]
- 35.Cataldo F, Marino V, Ventura A, Bottaro G, Corazza GR. Prevalence and clinical features of selective immunoglobulin A deficiency in coeliac disease: an Italian multicentre study. Italian Society of Paediatric Gastroenterology and Hepatology (SIGEP) and "Club del Tenue" Working Groups on Coeliac Disease. Gut. 1998;42:362–365. doi: 10.1136/gut.42.3.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hervonen K, Hakanen M, Kaukinen K, Collin P, Reunala T. First-degree relatives are frequently affected in coeliac disease and dermatitis herpetiformis. Scand J Gastroenterol. 2002;37:51–55. doi: 10.1080/003655202753387356. [DOI] [PubMed] [Google Scholar]
- 37.Lemberg D, Day AS, Bohane T. Coeliac disease presenting as dermatitis herpetiformis in infancy. J Paediatr Child Health. 2005;41:294–296. doi: 10.1111/j.1440-1754.2005.00614.x. [DOI] [PubMed] [Google Scholar]
- 38.Ertekin V, Selimoglu MA, Gursan N, Ozkan B. Idiopathic pulmonary hemosiderosis in children with celiac disease. Respir Med. 2006;100:568–569. doi: 10.1016/j.rmed.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 39.Kuloğlu Z, Kansu A, Tutar E, Yalçinkaya F, Ensari A, Girgin N. Association of familial Mediterranean fever and celiac disease in a 14-year-old girl with recurrent arthritis. Clin Exp Rheumatol. 2008;26:S131. [PubMed] [Google Scholar]
- 40.Sategna Guidetti C, Solerio E, Scaglione N, Aimo G, Mengozzi G. Duration of gluten exposure in adult coeliac disease does not correlate with the risk for autoimmune disorders. Gut. 2001;49:502–505. doi: 10.1136/gut.49.4.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ziegler AG, Schmid S, Huber D, Hummel M, Bonifacio E. Early infant feeding and risk of developing type 1 diabetes-associated autoantibodies. JAMA. 2003;290:1721–1728. doi: 10.1001/jama.290.13.1721. [DOI] [PubMed] [Google Scholar]
- 42.Ventura A, Magazzù G, Greco L SIGED Study Group for Autoimmune Disorders in Celiac Disease. Duration of exposure to gluten and risk for autoimmune disorders in patients with celiac disease. Gastroenterology. 1999;117:297–303. doi: 10.1053/gast.1999.0029900297. [DOI] [PubMed] [Google Scholar]
- 43.Kumar V, Rajadhyaksha M, Wortsman J. Celiac disease-associated autoimmune endocrinopathies. Clin Diagn Lab Immunol. 2001;8:678–685. doi: 10.1128/CDLI.8.4.678-685.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mora G, Barera G, Beccio S, Menni L, Proverbio MC, Bianchi C, et al. A prospective, longitudinal study of the long-term effect of treatment on bone density in children with celiac disease. J Pediatr. 2001;139:516–521. doi: 10.1067/mpd.2001.116298. [DOI] [PubMed] [Google Scholar]
- 45.Barera G, Mora S, Brambilla P, Ricotti A, Menni L, Beccio S, et al. Body composition in children with celiac disease and the effects of a gluten-free diet: a prospective case-control study. Am J Clin Nutr. 2000;72:71–75. doi: 10.1093/ajcn/72.1.71. [DOI] [PubMed] [Google Scholar]
- 46.Lunt H, Florkowski CM, Cook HB, Whitehead MR. Bone mineral density, type 1 diabetes, and celiac disease. Diabetes Care. 2001;24:791–792. doi: 10.2337/diacare.24.4.791. [DOI] [PubMed] [Google Scholar]
- 47.Tau C, Mautalen C, De Rosa S, Roca A, Valenzuela X. Bone mineral density in children with celiac disease. Effect of a Glutenfree diet. Eur J Clin Nutr. 2006;60:358–363. doi: 10.1038/sj.ejcn.1602323. [DOI] [PubMed] [Google Scholar]
- 48.Kalayci AG, Kansu A, Girgin N, Kucuk O, Aras G. Bone mineral density and importance of a gluten-free diet in patients with celiac disease in childhood. Pediatrics. 2001;108:E89. doi: 10.1542/peds.108.5.e89. [DOI] [PubMed] [Google Scholar]
- 49.Gobbi G, Bouquet F, Greco L, Lambertini A, Tassinari CA, Ventura A, et al. Coeliac disease, epilepsy, and cerebral calcifications. The Italian Working Group on Coeliac Disease and Epilepsy. Lancet. 1992;340:439–443. doi: 10.1016/0140-6736(92)91766-2. [DOI] [PubMed] [Google Scholar]
- 50.Bushara KO. Neurologic presentation of celiac disease. Gastroenterology. 2005;128:S92–S97. doi: 10.1053/j.gastro.2005.02.018. [DOI] [PubMed] [Google Scholar]