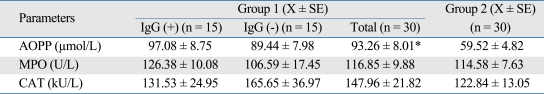Abstract
Purpose
Helicobacter pylorus (HP) is a Gram-negative spiral-shaped microaerophilic bacterium, which colonizes in the gastric mucosa of humans. The gastric human pathogen HP causes chronic gastritis and ulcers, and has a strong relationship with gastric cancer. The aim of this study was to determine advanced oxidation protein products (AOPP) levels, activities of myeloperoxidase (MPO) and catalase (CAT) in two groups.
Materials and Methods
For this aim, one group included 30 patients with gastric cancer (Group 1) and the other included 30 subjects with non-gastric cancer and Anti-HP immunoglobulin (Ig) G antibody positive (group 2). Anti-HP IgG antibody test values were found as positive in fifty percent of group 1 and all of the group 2 patients.
Results
Significantly increased AOOP levels were found in group 1 (p < 0.05) compared to group 2. There were no significant differences between the groups in regard to activities of MPO and CAT. In addition, AOPP level, MPO and CAT activities were similar among the Anti-HP IgG positive and negative subgroups of group 1 patients.
Conclusion
The result of this study indicated that gastric cancer patients were characterized by increased protein oxidation, whereas there was no significant difference in oxidative stress parameters and antioxidant enzyme activity between the Anti-HP IgG positive and negative gastric cancer patients.
Keywords: Gastric cancer, helicobacter pylorus, oxidative stress
INTRODUCTION
Helicobacter pylorus (HP) is a Gram-negative spiral-shaped microaerophilic bacterium, which colonized in the gastric mucosa of humans.1 HP infection is a worldwide pathogenic condition in the development of infection, causing different problems in the gastric mucosa.2 In addition, some chronic infections, acquired early in life, confer an increased risk of gastric cancer.3,4 Although more than 50% of the human population are infected with H. pylori, only a subset develops disease. This is considered to be due to both pathogen-inherent virulence factors and the type and intensity of the oxidative stress that is induced by the inflammation.5
It has been convincingly demonstrated that HP-induced mucosal inflammation is accompanied by formation of reactive oxygen species (ROS) by the invading polymorphonuclear cells and macrophages. However, free oxygen radicals, like superoxide, do not cause any major oxidative damage to the gastric epithelium per se.6 The severity of active inflammation of HP (+) gastritis has been shown to be directly correlated to the presence of high concentrations of free radicals which disappear completely with resolution of active inflammation after eradication.7,8 Free radicals, however, do not provoke oxidative damage on the gastric epithelium directly, but through the formation of peroxinitrite,9 and the levels of nitrotyrosine, a specific marker of peroxinitrite,10 have been reported to be significantly higher in patients with HP (+) gastritis than in those with HP (-) gastritis.11,12
Oxidative stress causes damage to important biological structures such as proteins, carbohydrates, lipids and nucleic acids, and may enhance inflammatory response. New compounds and modified structures are formed, and one of them is advanced oxidation protein products (AOPP).13 Plasma AOPP concentrations may be a useful marker in protein oxidative damage, measuring highly oxidized proteins, especially albumin.14 Neutrophils are considered to be major effectors cells in the tissue damage that occurs in inflammatory disease. One of the major granule proteins is the enzyme myeloperoxidase (MPO), a heme protein that accounts for 5% of the total neutrophil protein.15 The release of ROS by neutrophils may result in lipid and protein peroxidation, but protein oxidation has not been extensively studied in clinical settings.16 An antioxidant enzyme catalase (CAT) is located in peroxisomes, and decomposes hydrogen peroxide to water and oxygen.17
The aim of the present study was to investigate and compare the serum enzyme protein levels of MPO, CAT, and AOPP in patients with gastric cancer and Anti-HP IgG antibody positive non-gastric cancer controls. This study is the first report to describe an association between protein oxidation and H. pylori infected patients.
MATERIALS AND METHODS
The thirty patients with gastric cancer (12 female, 18 male; range, 38-83 years) were included in the study as group 1, and Anti-HP IgG antibody test was detected as positive in 50% (15/30) of patients with carcinoma. All of the patients in this group underwent upper gastrointestinal endoscopies because of various indications (dyspeptic symptoms, recurrent abdominal pain, and chronic vomiting). Routine regular biopsies (duodenum, antrum, corpus, and esophagus) were collected, and the diagnosis of gastric cancer was made by histopathological examination. Histopathology showed adenocarsinoma in 28 samples (93%) and lymphoma in 2 (7%) of group 1. Of the 28 adenocarsinoma, 8 were classified as intestinal (7 male and 1 female; mean age 53.25 years, range 45-66 years), and 3 as diffuse (2 male and 1 female; mean age 44.33 years, range 38-53 years). The other 17 samples were reported as adenocarcinoma (7 male and 10 female; mean age 63.77 years, range 46-83 years).
The control group also included 30 subjects (18 female and 12 male, range; 32-65 years) and all of the subjects had positive Anti-HP IgG antibody (group 2). Exclusion criteria included subjects who had been taking other drugs, known drug allergy to the study drugs, gastric cancer, gastro duodenal ulcer, liver cirrhosis, renal failure, severe concomitant disease, pregnancy, or lactation and previous gastric surgery in group 2. The institutional ethics committee approved the study and all participants gave written informed consent.
Venous blood samples were obtained from all patients to measure the levels of AOPP, MPO, CAT, and other biochemical parameters. The tubes were centrifuged for 10 min at 3,000 r.p.m and all samples were stored at -70℃ until analyzed.
An anti-HP IgG antibody test, ELISA (R-Biopharm AG, Germany), was conducted for detecting HP-infected participants.
Determination of AOPP was based on a spectrophotometric assay according to Witko-Sarsat, et al.18 AOPP levels were expressed in µmol of chloramine-T equivalents per litre of serum (µmol/L).
Serum MPO activity was determined by the method of Bradley et al.,19 and was based on kinetic measurement of the formation rate of the yellowish-orange product of the oxidation of o-dianisidne with MPO in the presence of hydrogen peroxide (H2O2) at 460 nm. One unit of MPO was defined as that degrading 1 µmol of H2O2 per minute at 25℃. A molar extinction coefficient of 1.3×104 M-1 cm-1 of oxidized 0-dianisidine was used for the calculation. MPO activity was expressed in units per liter of plasma (U/L).
The serum CAT activity was determined by Goth's colorimetric method,20 in which serum was incubated with H2O2, and the enzyme reactions were stopped by the addition of ammonium molybdate. Serum CAT activity was expressed as kU/L.
Biochemical measurements were performed using a Hitachi PP Modular Automatic analyzer (Tokyo, Japan) with Roche original reagents. CRP level was measured by nepholometric method using Cardio Phase hs CRP reagents (Dade Behring), and hematological parameters were also evaluated in the both groups using Coulter (USA) automated cell counter.
Statistical analysis
The results are expressed as mean (X) ± standard error (SE). Kolmogorov-Smirnov normality test was applied for all variables. Then, groups of data (both subgroups in group 1 and groups 1-2) were compared with an analysis of independent student's t test.
RESULTS
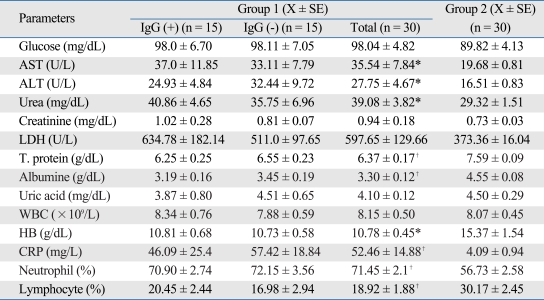
Mean age of the group 1 was significantly higher than that in group 2 (58.12 ± 1.92, 44.32 ± 1.44, respectively) (p < 0.01).
Significantly increased AOOP level was found in group 1 as compared to group 2 (Means of the groups are 93.26 ± 8.01 and 59.52 ± 4.82 µmol/L, respectively) (p < 0.05). Activities of MPO (group 1: 116.85 ± 9.88, group 2: 114.58 ± 7.63 U/L) and CAT (group 1: 147.96 ± 21.83, group 2: 122.84 ± 13.05 kU/L) were found statistically insignificant between the groups. In addition, AOPP level [Anti-HP IgG (+): 97.08 ± 8.75, Anti-HP IgG(-): 89.44 ± 7.98 µmol/L), MPO (Anti-HP IgG (+): 126.38 ± 10.08, Anti-HP IgG (-): 106.59 ± 17.45 U/L] and CAT [Anti-HP IgG (+): 131.53 ± 24.95, Anti-HP IgG (-): 165.65 ± 36.97 kU/L] activities were similar between the Anti-HP IgG positive and negative subgroups of group 1 patients. Both the Spearman rank correlation (p = 0.711, r = -0.078) and analyse of variance (ANOVA) (F = 0.002, p = 0.964) showed no significant relationship between the stage and AOPP levels in group 1.
However, significantly increased AST, and ALT activities, urea, CRP, and neutrophil levels, and decreased protein, albumin, hemoglobin, and lymphocyte levels were also found in group 1, compared to group 2.
DISCUSSION
Although the association between HP colonization and gastric noncardia adenocarcinoma is strong, estimates of the magnitude of the association vary, presumably because of differences in study design, length of follow-up, patient age at diagnosis, histologic subtype of cancer, HP strain, and host characteristics.21
During the process of colonizing the host, HP induces a strong inflammatory response from host cells infiltrated. This defense is mediated by neutrophils and macrophages, culminating in generation of large amounts of ROS, which is presented to the persistent pathogen. Production of ROS by gastric cells,22 and phagocytes induced by HP has been shown in vitro, and increased levels of ROS in the gastric mucosa have been measured in HP-infected patients.23,24 Wang, et al.25 reported that the accumulation of lipid peroxides in HP cells could be one of the major outcomes of oxidative stress imposed by the host. It is well known that lipid peroxidation contributes to cell injury by altering the basic physical properties and structural organization of membrane components.26 Unsaturated fatty acids are rarely produced in H. pylori cells, but they can be taken up from the environment.27 The growth of HP displays added sensitivity to unsaturated free fatty acids due to their incorporation into phospholipids of the membrane, leading to membrane dysfunction.28 The lipid hydroperoxide (LOOH), lipid radical, epoxyllylic peroxyl radical, and peroxyl radicals are the intermediates products of free radical chain reaction and these radicals are considered to be highly genotoxic.29 It was also reported that at least two enzymes of the Prx family in H. pylori have activity in reducing LOOH: an alkyl hydroperoxide reductase (AhpC) and a bacterioferritin-comigratory protein (BCP). Both proteins play significant roles in resisting oxidative stress and contribute to the efficient colonization of the host, suggesting that prevention of lipid peroxidation may be important for HP in vivo survival.30 It is well documented that both AhpC and BCP confer protection from unsaturated fatty acids-mediated toxicity, and that the mutant cells defective in AhpC, BCP, or in both enzymes contain increased amounts of LOOH. The source of lipid peroxidation is probably the unsaturated fatty acids in the blood-based medium for growth of HP cells.29 Recent studies have also shown that the oxidative stress response of HP is much more vast, adaptable, and interconnected than previously appreciated. Furthermore, mutations affecting almost all of these newly described enzymes appear to be as important for survival in the host as the well-studied traditional oxidative stress combating enzymes like CAT and superoxide dismutase (SOD).31
Gastric carcinoma is one of the most common neoplasms in the world, and most are adenocarcinomas. According to the Lauren classification, two epidemiologically distinct types exist. Intestinal-type carcinoma (tubular carcinoma, TC) is associated with HP infection, chronic gastritis, atrophy, intestinal metaplasia, and dysplasia that evolve as a multi-step process into this type of cancer. Diffuse-type carcinoma, which occurs more often in younger patients, is also associated with HP infection, but not with atrophy and intestinal metaplasia, and is associated with a worse prognosis. Mixed polymorphous carcinoma encompasses tumors showing both glandular and diffuse components.32 Although approximately half of the world population is infected with HP, a cancerous outcome of infection is very rare. The reasons for this phenomenon are unknown. Gastric cancer is usually diagnosed after the 5th decade of life, but HP infection usually occurs in childhood and remains active lifelong unless treated. A cohort study has shown a link between early infection and increased cancer risk.2,33 In the present study, the level of AOOP was increased significantly in the gastric cancer patients as compared with non-gastric cancer patients. However, there were no significant differences between the Anti-HP IgG positive and negative patients with gastric cancer. The subjects with gastric cancer had advanced clinical stage of disease (27% in stage 2, and 73% in stage 3) in this study. And, there was no significant correlation between the AOPP levels and clinical stages of gastric cancer. To our best knowledge, this is the first study that investigated the AOPP levels in patients with HP. Oxidative stress has been suggested to play a major role in carcinogenesis, but the mechanisms involved still remain unclear.32 Spontaneous non-enzymatic modifications of protein are commonly reported in tissues with slow turnover and they are considered by several authors as a possible common mechanism involved in the progression of many pathological conditions.34 Among the non-enzymatic processes, oxidative stress and glycation have aroused a particular interest in recent years.35 It was also recently demonstrated that lipid peroxides per se can enhance the process of protein glycation.36 Biochemical effects of free radicals also include oxidative modification of proteins,37 however, protein oxidation has not been extensively studied in clinical settings until recent years because of lack of easily accessible methods to detect protein damage. The observed increase in plasma AOPP levels in patients with gastric cancer which is seemingly associated with cellular damage resulting from reactive oxygen metabolites includes lipid peroxidation, protein oxidation, and oxidative DNA damage. All of these oxidative products can result in biochemical changes, leading to cancer. A positive association has been demonstrated between HP infection and gastric adenocarcinoma with increased oxidative stress.38 Therefore, we think that appropriate treatment to reduce oxidative stress would be expected to prevent subsequent gastric carcinogenesis through lessening HP-associated inflammation.
Myeloperoxidase is a lysosomal enzyme in polymorphonuclear leukocytes and monocytes, and produces hypochlorous acid which has microbicidal activity against a wide range of organisms, resulting in tissue inflammation. It was shown that water extract of HP can activate neutrophils and enhance the secretion of MPO.39 CAT is a ubiquitous, well-studied enzyme that catalyses the decomposition of hydrogen peroxide (H2O2) into water and oxygen to protect cells from the damaging effects of H2O2.24 HP has been shown to produce CAT, but the reported amount of this enzyme secreted would not be sufficient to scavenge extra cellular oxidants.40 Almost all the previous studies on the association of with HP in adult population were performed in gastric mucosa.12,21,23 Some previous studies have reported that no CAT activity was found in most gastric juices, probably because of their low pH levels, and in some antral tissue specimens.41,42 Akcan, et al.2 reported no unchanged of MPO and SOD activity in gastric mucosa of childhood period with HP (+) and (-) subjects, and Bulbuloglu, et al.42 also reported no unchanged of CAT activity in antral mucosal between HP (+) and (-) subjects. In the present study, MPO and CAT activities were similar between the subjects included in the study. There are varying results on antioxidant enzyme activities in patients with gastric cancer.43-45 However, recent results are similar to present result. Al-Shukaili, et al.43 reported that no change of MPO activity in the serum of patients with gastric cancer compared to healthy controls. Dursun, et al.44 reported decreased CAT activity in patients with esophageal and gastric cancer compared with controls. However, Dincer, et al.46 reported decreased glutathione peroxidase (GSH-Px) and increased SOD activity in gastric cancer patients when compared to healthy subjects. Generally, antioxidants are involved directly in the conversion of ROS to less-reactive species, however, antioxidant protection therapy against free radicals should be used with caution since its effects depend on the stage at which it is introduced. When used during the progression stage of cancer, it might actually stimulate growth of tumors through enhanced survival of tumor cells. Another important issue which should be taken into consideration is a pro-oxidant character of some antioxidants which may occur depending on the concentration and environment (oxygen pressure) in which they act.47 Therefore, further investigations are needed to elucidate the importance of antioxidant enzymes in gastric cancer patients.
In conclusion, the results of our study showed that gastric cancer patients revealed increased protein oxidation. However, myeloperoxidase and catalase activities in gastric cancer patients were not different from those in H. pylori IgG positive non-gastric cancer subjects. However, further clinical studies are necessary to explain the relationship between protein oxidation and antioxidant enzymes activities in gastric cancer and H. pylori infection.
Table 1.
Biochemical and Hematological Features of the Subjects Included in the Study
Significant different as compared to group 2.
*p < 0.05.
†p < 0.01.
Table 2.
Levels of AOPP and Activities of MPO and CAT of the Subjects Included in the Study
Significant different as compared to group 2.
*p < 0.05.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Godlewska R, Dzwonek A, Mikuła M, Ostrowski J, Pawłowski M, Bujnicki JM, et al. Helicobacter pylori protein oxidation influences the colonization process. Int J Med Microbiol. 2006;296:321–324. doi: 10.1016/j.ijmm.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 2.Akcam M, Elmas O, Yilmaz A, Cağlar S, Artan R, Gelen T, et al. Myeloperoxidase, xanthine oxidase and superoxide dismutase in the gastric mucosa of Helicobacter pylori positive and negative pediatric patients. Mol Cell Biochem. 2006;290:125–130. doi: 10.1007/s11010-006-9176-9. [DOI] [PubMed] [Google Scholar]
- 3.Nardone G, Morgner A. Helicobacter pylori and gastric malignancies. Helicobacter. 2003;8(Suppl 1):44–52. doi: 10.1046/j.1523-5378.2003.00160.x. [DOI] [PubMed] [Google Scholar]
- 4.Isakov V, Malfertheiner P. Helicobacter pylori and nonmalignant diseases. Helicobacter. 2003;8(Suppl 1):36–43. doi: 10.1046/j.1523-5378.2003.00169.x. [DOI] [PubMed] [Google Scholar]
- 5.Tummala S, Keates S, Kelly CP. Update on the immunologic basis of Helicobacter pylori gastritis. Curr Opin Gastroenterol. 2004;20:592–597. doi: 10.1097/00001574-200411000-00015. [DOI] [PubMed] [Google Scholar]
- 6.Elfvin A, Bölin I, Lönroth F, Fändriks L. Gastric expression of inducible nitric oxide synthase and myeloperoxidase in relation to nitrotyrosine in Helicobacter pylori-infected Mongolian gerbils. Scand J Gastroenterol. 2006;41:1013–1018. doi: 10.1080/00365520600633537. [DOI] [PubMed] [Google Scholar]
- 7.Farkas R, Selmeci L, Tulassay Z, Pronai L. Superoxide-dismutase activity of the gastric mucosa in patients with Helicobacter pylori infection. Anticancer Res. 2003;23:4309–4312. [PubMed] [Google Scholar]
- 8.Felley CP, Pignatelli B, Van Melle GD, Crabtree JE, Stolte M, Diezi J, et al. Oxidative stress in gastric mucosa of asymptomatic humans infected with Helicobacter pylori: effect of bacterial eradication. Helicobacter. 2002;7:342–348. doi: 10.1046/j.1523-5378.2002.00107.x. [DOI] [PubMed] [Google Scholar]
- 9.Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci U S A. 1990;87:1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Halliwell B. What nitrates tyrosine? Is nitrotyrosine specific as a biomarker of peroxynitrite formation in vivo? FEBS Lett. 1997;411:157–160. doi: 10.1016/s0014-5793(97)00469-9. [DOI] [PubMed] [Google Scholar]
- 11.Kimura A, Tsuji S, Tsujii M, Sawaoka H, Iijima H, Kawai N, et al. Expression of cyclooxygenase-2 and nitrotyrosine in human gastric mucosa before and after Helicobacter pylori eradication. Prostaglandins Leukot Essent Fatty Acids. 2000;63:315–322. doi: 10.1054/plef.2000.0220. [DOI] [PubMed] [Google Scholar]
- 12.Sakaguchi AA, Miura S, Takeuchi T, Hokari R, Mizumori M, Yoshida H, et al. Increased expression of inducible nitric oxide synthase and peroxynitrite in Helicobacter pylori gastric ulcer. Free Radic Biol Med. 1999;27:781–789. doi: 10.1016/s0891-5849(99)00124-0. [DOI] [PubMed] [Google Scholar]
- 13.Kalousová M, Zima T, Tesar V, Dusilová-Sulková S, Skrha J. Advanced glycoxidation end products in chronic diseases-clinical chemistry and genetic background. Mutat Res. 2005;579:37–46. doi: 10.1016/j.mrfmmm.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 14.Witko-Sarsat V, Gausson V, Nguyen AT, Touam M, Drüeke T, Santangelo F, et al. AOPP-induced activation of human neutrophil and monocyte oxidative metabolism: a potential target for N-acetylcysteine treatment in dialysis patients. Kidney Int. 2003;64:82–91. doi: 10.1046/j.1523-1755.2003.00044.x. [DOI] [PubMed] [Google Scholar]
- 15.Pullar JM, Vissers MC, Winterbourn CC. Living with a killer: the effects of hypochlorous acid on mammalian cells. IUBMB Life. 2000;50:259–266. doi: 10.1080/713803731. [DOI] [PubMed] [Google Scholar]
- 16.Matteucci E, Biasci E, Giampietro O. Advanced oxidation protein products in plasma: stability during storage and correlation with other clinical characteristics. Acta Diabetol. 2001;38:187–189. doi: 10.1007/s592-001-8077-3. [DOI] [PubMed] [Google Scholar]
- 17.Winterbourn CC. Superoxide as an intracellular radical sink. Free Radic Biol Med. 1993;14:85–90. doi: 10.1016/0891-5849(93)90512-s. [DOI] [PubMed] [Google Scholar]
- 18.Witko-Sarsat V, Friedlander M, Capeillére-Blandin C, Nguyen-Khoa T, Nguyen AT, Zingraff J, et al. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int. 1996;49:1304–1313. doi: 10.1038/ki.1996.186. [DOI] [PubMed] [Google Scholar]
- 19.Bradley PP, Priebat DA, Christensen RD, Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol. 1982;78:206–209. doi: 10.1111/1523-1747.ep12506462. [DOI] [PubMed] [Google Scholar]
- 20.Góth L. A simple method for determination of serum catalase activity and revision of reference range. Clin Chim Acta. 1991;196:143–151. doi: 10.1016/0009-8981(91)90067-m. [DOI] [PubMed] [Google Scholar]
- 21.Kamangar F, Dawsey SM, Blaser MJ, Perez-Perez GI, Pietinen P, Newschaffer CJ, et al. Opposing risks of gastric cardia and noncardia gastric adenocarcinomas associated with Helicobacter pylori seropositivity. J Natl Cancer Inst. 2006;98:1445–1452. doi: 10.1093/jnci/djj393. [DOI] [PubMed] [Google Scholar]
- 22.Bagchi D, Bhattacharya G, Stohs SJ. Production of reactive oxygen species by gastric cells in association with Helicobacter pylori. Free Radic Res. 1996;24:439–450. doi: 10.3109/10715769609088043. [DOI] [PubMed] [Google Scholar]
- 23.Ramarao N, Gray-Owen SD, Meyer TF. Helicobacter pylori induces but survives the extracellular release of oxygen radicals from professional phagocytes using its catalase activity. Mol Microbiol. 2000;38:103–113. doi: 10.1046/j.1365-2958.2000.02114.x. [DOI] [PubMed] [Google Scholar]
- 24.Baik SC, Youn HS, Chung MH, Lee WK, Cho MJ, Ko GH, et al. Increased oxidative DNA damage in Helicobacter pylori-infected human gastric mucosa. Cancer Res. 1996;56:1279–1282. [PubMed] [Google Scholar]
- 25.Wang G, Conover RC, Benoit S, Olczak AA, Olson JW, Johnson MK, et al. Role of a bacterial organic hydroperoxide detoxification system in preventing catalase inactivation. J Biol Chem. 2004;279:51908–51914. doi: 10.1074/jbc.M408450200. [DOI] [PubMed] [Google Scholar]
- 26.Jacob RF, Mason RP. Lipid peroxidation induces cholesterol domain formation in model membranes. J Biol Chem. 2005;280:39380–39387. doi: 10.1074/jbc.M507587200. [DOI] [PubMed] [Google Scholar]
- 27.Scherer C, Müller KD, Rath PM, Ansorg RA. Influence of culture conditions on the fatty acid profiles of laboratory-adapted and freshly isolated strains of Helicobacter pylori. J Clin Microbiol. 2003;41:1114–1117. doi: 10.1128/JCM.41.3.1114-1117.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun CQ, O'Connor CJ, Roberton AM. Antibacterial actions of fatty acids and monoglycerides against Helicobacter pylori. FEMS Immunol Med Microbiol. 2003;36:9–17. doi: 10.1016/S0928-8244(03)00008-7. [DOI] [PubMed] [Google Scholar]
- 29.Wang G, Hong Y, Johnson MK, Maier RJ. Lipid peroxidation as a source of oxidative damage in Helicobacter pylori: protective roles of peroxiredoxins. Biochim Biophys Acta. 2006;1760:1596–1603. doi: 10.1016/j.bbagen.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 30.Wang G, Olczak AA, Walton JP, Maier RJ. Contribution of the Helicobacter pylori thiol peroxidase bacterioferritin comigratory protein to oxidative stress resistance and host colonization. Infect Immun. 2005;73:378–384. doi: 10.1128/IAI.73.1.378-384.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang G, Alamuri P, Maier RJ. The diverse antioxidant systems of Helicobacter pylori. Mol Microbiol. 2006;61:847–860. doi: 10.1111/j.1365-2958.2006.05302.x. [DOI] [PubMed] [Google Scholar]
- 32.Bancel B, Esteve J, Souquet JC, Toyokuni S, Ohshima H, Pignatelli B. Differences in oxidative stress dependence between gastric adenocarcinoma subtypes. World J Gastroenterol. 2006;12:1005–1012. doi: 10.3748/wjg.v12.i7.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blaser MJ, Chyou PH, Nomura A. Age at establishment of Helicobacter pylori infection and gastric carcinoma, gastric ulcer, and duodenal ulcer risk. Cancer Res. 1995;55:562–565. [PubMed] [Google Scholar]
- 34.Choe YH, Kim SK, Son BK, Lee DH, Hong YC, Pai SH. Randomized placebo-controlled trial of Helicobacter pylori eradication for iron-deficiency anemia in preadolescent children and adolescents. Helicobacter. 1999;4:135–139. doi: 10.1046/j.1523-5378.1999.98066.x. [DOI] [PubMed] [Google Scholar]
- 35.Vijayan G, Sundaram RC, Bobby Z, Hamide A, Selvaraj N, Dasse NR. Increased plasma malondialdehyde and fructosamine in anemic H pylori infected patients: effect of treatment. World J Gastroenterol. 2007;13:796–800. doi: 10.3748/wjg.v13.i5.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Selvaraj N, Bobby Z, Sathiyapriya V. Effect of lipid peroxides and antioxidants on glycation of hemoglobin: an in vitro study on human erythrocytes. Clin Chim Acta. 2006;366:190–195. doi: 10.1016/j.cca.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 37.Davies KJ. Protein damage and degradation by oxygen radicals. I. general aspects. J Biol Chem. 1987;262:9895–9901. [PubMed] [Google Scholar]
- 38.Park S, Kim WS, Choi UJ, Han SU, Kim YS, Kim YB, et al. Amelioration of oxidative stress with ensuing inflammation contributes to chemoprevention of H. Pylori-associated gastric carcinogenesis. Antioxid Redox Signal. 2004;6:549–560. doi: 10.1089/152308604773934305. [DOI] [PubMed] [Google Scholar]
- 39.Takemura T, Granger DN, Evans DJ, Jr, Evans DG, Graham DY, Anderson DC, et al. Extract of Helicobacter pylori induces neutrophils to injure endothelial cells and contains antielastase activity. Gastroenterology. 1996;110:21–29. doi: 10.1053/gast.1996.v110.pm8536858. [DOI] [PubMed] [Google Scholar]
- 40.Mori M, Suziki H, Suzuki M, Kai A, Miura S, Ishii H. Catalase and superoxide dimutase secreted from Helicobacter pylori. Helicobacter. 1997;2:100–105. doi: 10.1111/j.1523-5378.1997.tb00067.x. [DOI] [PubMed] [Google Scholar]
- 41.Durak I, Ormeci N, Akyol O, Canbolat O, Kavutçu M, Bulbül M. Adenosine deaminase, 5'-nucleotidase, xanthine oxidase, superoxide dismutase, and catalase activities in gastric juices from patients with gastric cancer, ulcer, and atrophic gastritis. Dig Dis Sci. 1994;39:721–728. doi: 10.1007/BF02087413. [DOI] [PubMed] [Google Scholar]
- 42.Bulbuloglu E, Inanc F, Bakaris S, Kantarceken B, Cetinkaya A, Cağlar R, et al. Association of adenosine deaminase, superoxide dismutase, and catalase activities with Helicobacter pylori. Dig Dis Sci. 2005;50:2296–2299. doi: 10.1007/s10620-005-3050-6. [DOI] [PubMed] [Google Scholar]
- 43.Al-Shukaili A, Al-Jabri AA, Al-Moundhri MS. Prognostic value of auto-antibodies in the serum of Omani patients with gastric cancer. Saudi Med J. 2006;27:1873–1877. [PubMed] [Google Scholar]
- 44.Dursun H, Bilici M, Uyanik A, Okcu N, Akyüz M. Antioxidant enzyme activities and lipid peroxidation levels in erythrocytes of patients with oesophageal and gastric cancer. J Int Med Res. 2006;34:193–199. doi: 10.1177/147323000603400209. [DOI] [PubMed] [Google Scholar]
- 45.Starzyñska T, Malfertheiner P. Helicobacter and digestive malignancies. Helicobacter. 2006;11:32–35. doi: 10.1111/j.1478-405X.2006.00431.x. [DOI] [PubMed] [Google Scholar]
- 46.Dincer Y, Himmetoglu S, Akcay T, Ersoy EY, Gunes KN, Tortum O. Prognostic significances of oxidative DNA damage evaluated by 8-hydroxy-deoxyguanosine and antioxidant enzymes in patients undergoing resection of gastric and colon carcinoma. Neoplasma. 2007;54:131–136. [PubMed] [Google Scholar]
- 47.Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact. 2006;160:1–40. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]