Abstract
Purpose
Adenosine triphosphate-based chemotherapy response assay (ATP-CRA) is a well-documented and validated technology that can individualize chemotherapy for patients with lung, stomach, or breast cancer. This study explored the feasibility of ATP-CRA as a chemosensitivity test in patients with colorectal cancer.
Materials and Methods
A total of 118 patients who underwent surgical resection for colorectal adenocarcinoma were analyzed for chemosensitivity to 6 anticancer drugs using ATP-CRA. We calculated the cell death rate (CDR) by measuring intracellular ATP levels of drug-exposed cells and untreated controls.
Results
Interpretable results were available for 85.5% (118/138) of patients. The mean coefficient of variation for triplicate ATP measurements was 9.2%. The highest CDR was observed in irinotecan (34.0%) and the lowest CDR in etoposide (21.0%). Paclitaxel had the broadest range of CDR (0-86.7%) and 5-FU had the narrowest range of CDR (0-56.8%). The overall highest responsiveness was seen most prevalently in irinotecan (24.7%, 23/93 patients). Irinotecan had the greatest responsiveness in patients with well differentiated and moderately differentiated carcinoma.
Conclusion
Our study suggests that ATP-CRA could be used to identify patients with colorectal cancer who might benefit from treatment with a specific chemotherapeutic agent.
Keywords: Adenosine triphosphate, chemotherapy response assay, colorectal cancer
INTRODUCTION
Colorectal cancer continues to be a challenging clinical problem. It is one of the most common cancers and ranks fourth in frequency in men and third in women worldwide.1 About 70% of all patients diagnosed with colorectal cancer undergo potentially curative surgery, however, half of those present with or develop advanced local disease or metastases. Advanced colorectal cancer has a poor prognosis with a median survival of only a few months range, (6-15 months), despite aggressive chemotherapy and/or surgical resection.2 The heterogeneity of tumor response to chemotherapy is a significant obstacle in cancer treatment, including colorectal cancer. This means that tumors with similar histopathological characteristics may have different clinical outcomes and responsiveness to therapy.3 This explains how the appropriate choice of drug is mandatory for better prognosis and why chemosensitivity testing has been of great interest to oncologists in recent years.
In an attempt to individualize therapy, a number of in vitro chemosensitivity assays have been developed to predict therapeutic response and correlated the test results with clinical response. A review of 12 published in vitro assays by Cortazar and Johnson.4 showed that the mean response rate for patients treated with in vitro-selected therapy was 27% compared to 18% in patients treated with empiric therapy. Although in vitro-selected chemotherapy seems to be at least as good as empiric regimens, this therapy in actuality is not widely used in clinical practice because the potential clinical benefit has not been adequately addressed and various technical problems have been encountered with this assay.5-9
The adenosine triphosphate-based assay (ATP assay) is a sensitive assay that evaluates tumor cell viability by measuring intracellular ATP levels of drug-exposed cells and an untreated control. Furthermore, this assay has been somewhat widely studied because its clinical feasibility was validated in the field of various cancers, including melanoma, breast cancer, stomach cancer, and ovarian cancer.10-15 ATP-CRA is a new method which improved previous ATP assays, thus making it possible to inhibit the proliferation of normal cells in tumor tissue using the ultra-low attachment culture plates, does not require a large amount of specimens, and has a relatively short test turnaround time.16 However, a few studies have applied the ATP assay to patients with colorectal cancer to investigate its clinical utility as a chemosensitivity assay.17-19
Therefore, we performed this preliminary study to explore the feasibility and clinical usefulness of ATP-CRA as a chemosensitivity assay in patients with colorectal cancer, focusing on the success rate, mean coefficient of variation, and turnaround time.
MATERIALS AND METHODS
Between June 2004 and October 2005, we enrolled a total of 138 consecutive patients who underwent surgical resection for colorectal cancer at Gangnam Severance Hospital and the Yonsei University Health System. Eligible patients had histologically confirmed primary adenocarcinoma of the colon and rectum. Patients who had undergone preoperative chemoradiation therapy were excluded from this study. This study was approved by the appropriate Institutional Review Board and informed consent for participation was never denied to any of patients.
ATP-CRA was performed as previously described.16 Tumor tissue specimens were taken at least 0.5 cm3 in size in the operating room and delivered to the laboratory and stored in Hank balanced salt solution (HBSS, Gibco, Rockville, MD, USA) containing 100 IU/mL penicillin (Sigma, St. Louis, MO, USA), 100 µg/mL streptomycin (Sigma), 100 µg/mL gentamicin (Gibco), 2.5 µg/mL amphotericin B (Gibco), and 5% fetal bovine serum (FBS, Gibco). These tissue specimens were washed with ethanol, quantified, and minced before being incubated at 37℃ for 12 to 16 hours with extracellular matrix-degrading enzymes, such as dispase (Sigma) pronase (Sigma), and DNase (Sigma). Cells were harvested using a cell strainer (BD Falcon, Bedford, MA, USA). To eliminate normal cells, cell suspensions were subjected to Ficoll gradient (Histopaque-1077, 1.077 g/mL, Sigma) centrifugation at 400 g for 15 minutes. The viability of isolated cells was tested using trypan blue exclusion.
Separated tumor cells were diluted to 2,000-20,000 viable cells/100 µL using Iscove modified Dulbecco medium (IMDM, Gibco), including 10% FBS, and the cells were then seeded in triplicate onto a 96-well, ultra-low attachment microplate (Costar, Cambridge, MA, USA). These microplates were able to restrict the growth of normal cells such as fibroblasts. In the treated groups, 100 µL of chemotherapeutic agents were added onto the seeded cells and the cells were cultured for 48 hrs at 37℃ in a 5% CO2 incubator. In the untreated control groups, 100 µL of IMDM, without chemotherapeutic agents, was added to 3-6 wells of the microplates. For quality control, a negative control group of 3-6 wells (seeding medium without cells) and 2 positive control groups were included in the culture plate. Each positive control group was composed of 3 wells that contained the minimal (105 pg ATP) and median (280 pg ATP) amounts of ATP as measured in 1,000 tumor cells harvested from tissue. Three test drug concentrations (TDC) were used in triplicate, including 20, 100, and 500% of the plasma peak concentrations determined by training set experiments,20,21 which exhibited a scattered distribution of cell deaths from each specimen (data not shown). Standard 100% TDC values were etoposide (3.6 µg/mL), 5-FU (10.0 µg/mL), gemcitabine (16.9 µg/mL), irinotecan (4.7 µg/mL), oxaliplatin (2.9 µg/mL), and paclitaxel (8.5 µg/mL), which are chemotherapeutic agents studied in several preclinical and research reports,17,22,23 and are also clinically active in colorectal cancer. The successful evaluation at each concentration requires a minimum of 20 mg of tumor tissues. Cells from the untreated control and treated groups were lysed and the amount of ATP in the cell lysates was measured using luciferin and excessive luciferase (Roche, Mannheim, Germany) followed by flash type luminescence measurements on a Victor 3 multi-label counter (PerkinElmer, Boston, MA, USA). Cell death rate (CDR) was calculated as follows:
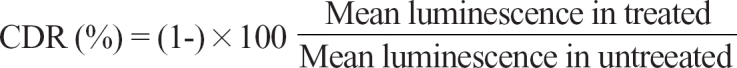
A chemosensitivity index (CSI) was calculated by adding the percentage of CDR at each concentration tested as previously published.15
CSI = 300 - sum (% CDR at 500, 100, and 20% TDC)
To calculate the intra-assay mean coefficient of variation (CV) value, the luminescence values of each specimen were measured 3 times. We then confirmed whether the measured values at 280 pg of ATP were higher than those at 105. If there were contamination of microorganism, inadequate number of cells and an intra-assay mean CV greater than 30, the test was considered a failure. If the measured values in the untreated control were lower than those in the positive group (105 pg of ATP), the specimen was considered to have an unacceptable viability.
Statistical evaluation was carried out using the statistical package SPSS for Windows (Version 11.0; SPSS Inc., Chicago, IL, USA). In vitro drug responsiveness was correlated with tumor histology utilizing the repeated measures analysis of variance. A value of p < 0.05 was considered statistically significant.
RESULTS
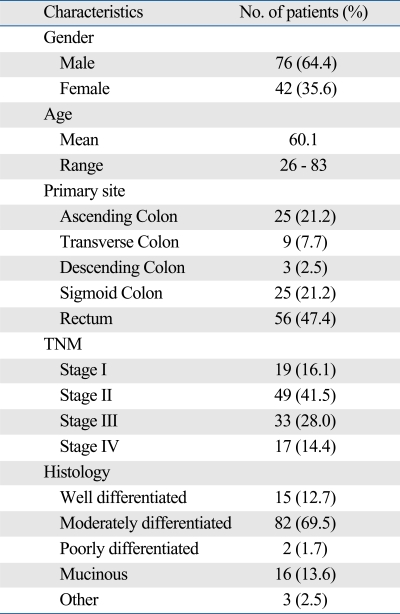
Details of the patients' characteristics are presented in Table 1. The results of ATP-CRA in all cases were reported within 7 days after obtaining the tumor tissues. Interpretable results were abtained in 85.5% (118/138) of the specimens. The intra-assay mean coefficient of variation (CV) for triplicate ATP measurements was 9.2%. The failures were due to microbial contamination (18 cases) and an insufficient amount of viable cells (2 cases). According to the criteria of CDR, 19 (16.1%) assays of the 118 patient samples tested did not produce completely evaluable results from all of the 6 drugs used, and 36 (30.5%) assays did not produce by CSI. This was due to insufficient tissues and excessive CV values. Of the 6 drugs tested, the interpretable mean number of drugs at TDC using 1 tumor tissue was 5.75 (95.8%). Moreover, interpretable results of 3 clinically active drugs (5-FU, oxaliplatin, and irinotecan) were available simultaneously for 94.1% of the total samples (111/118). The difference in interpretable case numbers for all drugs tested between CDR and CSI is shown in Tables 2 and 3, respectively.
Table 1.
Aatient Characteristics (n = 118)
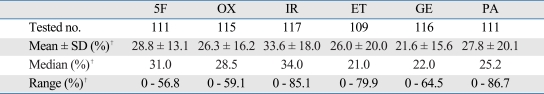
Table 2.
Cell Death Rate at 1X TDC*
5F, 5-FU; OX, oxaliplatin; IR, irinotecan; ET, etoposide; GE, gemcitabine; PA, paclitaxel.
*TDC; is defined as the drug concentration at which tumors show the most heterogeneous inhibition rate.
†Unit is cell death rate.
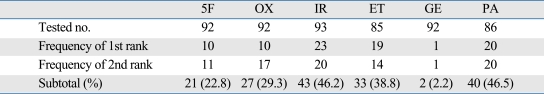
Table 3.
Heterogeneity of Chemosensitivity Index (CSI)*
5F, 5-FU; OX, oxaliplatin; IR, irinotecan; ET, etoposide; GE, gemcitabine; PA, paclitaxel; TDC, test drug concentration.
*CSI = 300 - sum (% CDR at 500, 100, and 20% TDC).
The cytotoxic effects for TDC of the chemotherapeutic agents on cell death ranged from 0 to 86.7% (Table 2). Irinotecan showed the highest median value of CDR (34.0%), while paclitaxel had the widest range of cytotoxic effects range, (0-86.7%). Etoposide achieved the lowest median value of CDR (21.0%) and 5-FU had the narrow-est range of cytotoxic effects range, (0-56.8%).
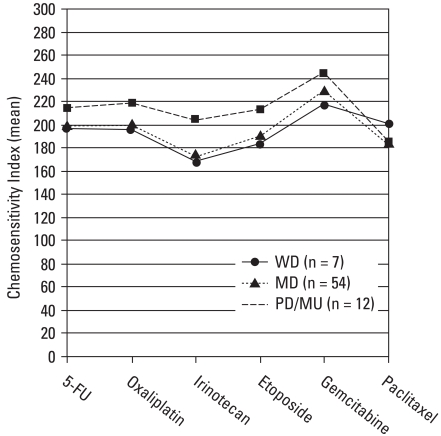
Table 3 demonstrated the marked heterogeneity of CSI to anticancer drugs between the tumors tested. The overall highest responsiveness was observed most prevalently in irinotecan (24.7%, 23/93). In addition, we calculated the mean value of CSI according to the histological type of colorectal cancers (Fig. 1). Irinotecan revealed the greatest responsiveness in patients with well and moderately differentiated carcinoma; whereas paclitaxel achieved the greatest responsiveness in those with poorly differentiated and mucinous carcinoma. However, we found no statistically significant association between the responsiveness and histology (p = 0.144).
Fig. 1.
Mean value of CSI according to the histology (n = 73). Irinotecan had the greatest responsiveness in patients with well differenatiated (WD, n = 7) and moderately differentiated carcinoma (MD, n = 54), while paclitaxel had the greatest responsiveness in those with poorly differentiated and mucinous carcinoma (PD/MU, n = 12). However, there was no statistically significant correlation between responsiveness and histology (p = 0.144). CSI, Chemosensitivity Index.
DISCUSSION
In patients with colorectal cancer, standard adjuvant chemotherapy is combination therapy using 5-FU and leucovorin. This approach has been confirmed by long-term, randomized clinical trials. Since it has long been known that histologically identical tumors may often differ in their responses to treatment, there have been many attempts to design in vitro assays that would be predictive of in vivo response to chemotherapy. The advantages of a successful assay would clearly improve clinical response and reduce side effects, toxicity, and cost.
Various chemosensitivity tests have been developed and studied for the past 20 years, however, they have not yet been adopted on a widespread clinical basis due to a variety of problems: insufficient in vitro-in vivo correlation, long turnaround time (subrenal capsule assay and human tumor clonogenic assay),5,6 low success rate of primary culture, the need for a large amount of specimen assay (methylthiazoletetrazolium (MTT), and histoculture drug response assay (HDRA).7-9 ATP assay measures light production as cellular ATP reacts with the luciferin-luciferase complex. The quantification of the light produced directly corresponds to the number of viable cells.10 The success rate of ATP assay was not significantly different from those of other in vitro studies, such as the MTT assay or the HDRA, although the ATP assay has a higher overall sensitivity and some technical advantages.8,10-15,24
Contrary to the differential staining cytotoxicity (DiSC) assay, which is dependent on the morphologic differences between tumor and normal cells, the measurement of the amount of ATP or enzyme activity (MTT and HDRA) may be affected by contaminated normal cells within the tumor tissue.8,25,26 Kodera, et al.27 recently reported that no significant correlation was observed between the results of an in vitro chemosensitivity test and the survival of patients using 3-dimensional gastric cancer tissue culture systems. However, Iwahashi, et al.28 used another gastric chemosensitivity test to show that overall survival in the test-guided chemotherapy group was significantly better than those in standard and non-chemotherapy groups. One reason for these contradictory results may be that the Iwahashi's group used selective cancer cells, which are different from the 3-dimensional tissue culture systems of the Kodera's group, strongly supporting the notion that the effective elimination and inhibition of the proliferation of normal cells are essential for valid conclusion in the chemosensitivity assay. ATP-CRA, a newly-developed ATP assay, was demonstrated in patients with lung cancer that normal cells were effectively eliminated from the cancer tissue using Ficoll gradient centrifugation.16
The success rates of the ATP assay using tissues of various kinds of cancers (except colorectal cancer) ranged from 85 to 91%.12-16,29-31 In general, colorectal tissues are not easy to culture, because they are fibrous and do not dissociate in the enzymatic solution well. Our study using colorectal cancer tissues showed that the success rate was 85.5%, which was a level similar to the previous reports using non-colorectal cancers tissues.13-15 The mean CV of our test was 9.2%, which was superior to the values obtained by other studies (10.5-13.0%).16,29-32 In the present study, the results of ATP-CRA were reported to physicians within 7 days of specimen collection, therefore, the selection and administration of anticancer drugs could be possible without any delay.
Whitehouse, et al.17 showed that one of the main technical difficulties in cell culture is microbial contamination in ATP assay. In this study, we used a culturing system containing different types of antibiotics at concentrations that would not affect the results from the chemosensitivity test. However, microbial contamination was the major cause of failure in most cases (18/20, 90%). Additional treatments with other antibiotics in future study are needed to minimize experimental failure due to microbial contamination.
There are several methods to obtain cancer tissues for ATP assay, which include surgical resection, endoscopic biopsy, and aspiration of peritoneal or pleural fluid from metastatic lesions.16,17 In our study, we were able to obtain sufficient amounts of tumor tissues from surgical resection in all cases tested. Tumor tissue specimens were taken at least 0.5 cm3 in size in the operating room, which was sufficient quantity for successful assay because an average of 32,196 cancer cells among 1 mg of tissue were isolated. In the present study, we decided to exclude patients who had undergone preoperative radiotherapy because irradiation of colorectal tissues, with its fibrous nature, makes it more difficult to isolate cancer cells.
We tested some experimental chemotherapeutic agents that are not currently used in the treatment of patients with colorectal cancer (e.g., etoposide, gemcitabine, and paclitaxel), but have been investigated in several preclinical and research reports.17,22,23 Nakahara et al.22 used the collagen gel droplet embedded culture sensitivity test and reported that gemcitabine showed the highest efficacy among the 12 anticancer drugs tested in patients with colorectal adenocarcinoma, suggesting that it might be a promising drug for the treatment of human colorectal cancer. Taxanes failed to demonstrate significant clinical benefit in phase II trials in colorectal cancer, but further trials of taxanes may possibly be indicated in patients with chromosomal instability negative colorectal cancer.23 In this study, gemcitabine produced very little growth inhibition in the samples tested. Paclitaxel showed excellent activity in samples with poorly differentiated and mucinous colorectal cancer. These in vitro results appear contradictory to previous reports,22,23 nevertheless, they suggest the possibility of correlation of in vitro ATP-CRA results with clinical response, possibly initiating randomized controlled trials of ATP-CRA directed chemotherapy.
In our study, TDC of 6 anticancer drugs tested were the plasma peak concentrations determined by training set experiments, which exhibited a scattered distribution of cell death from each specimen.20,21 That is, TDC was defined as the drug concentration at which tumors show the most heterogeneous inhibition rate. Based on these results, we established new algorithms of test interpretation and in vitro concentration of drug tested. Our newly developed ATP-CRA method was described and validated in preclinical and clinical studies.16,18,19,33 We previously reported the clinical feasibility of our ATP-CRA in a study on a limited volume of colorectal tumors.18,19 Moon, et al.33 showed that ATP-CRA and clinical outcomes correlated well after assay-guided platinum-based 2-drug chemotherapy for unresectable lung cancer. Our results showed considerable heterogeneous responsiveness between the tumors tested to chemotherapeutic agents at a standard concentration. Generally, the greater the drug effectiveness, the higher the value of CDR and the lower the value of CSI. The median values of CDR were relatively higher with clinically active drugs (e.g., irinotecan, 5-FU, and oxaliplatin) than clinically non-active drugs (e.g., paclitaxel, gemcitabine, and etoposide). Irinotecan had the highest median value and the 2nd widest range of CDR. That is, the conventional 5-FU treatment did not produce better overall growth inhibition compared to that of irinotecan. It is highly possible that a combination of 5-FU and irinotecan or oxaliplatin would show better activity in any samples tested. Modern combination chemotherapy with irinotecan, 5-fluorouracil, and leucovorin or oxaliplatin has demonstrated superior response rates (31-56%) compared with 5% for fluorouracil alone.34,35 This approach is unavailable in our current study, but mandatory for further clinical application. Konecny, et al.15 reported that CSI was superior to other parameters, possibly because this index takes the in vitro tumor growth inhibition of all tested dose levels into account, giving more complete information on drug sensitivity at lower and higher dosages. However, it has not yet been clearly determined which parameter (e.g., CDR vs. CSI) has more accurate information on drug sensitivity using ATP-CRA. Hence, further studies are warranted to identify more sensitive and accurate parameter of ATP-CRA.
We also tested whether histological type was correlated with the effectiveness of all anticancer drugs tested. In samples with moderately differentiated carcinoma appearing the most common histology, irinotecan showed greater responsiveness, followed by paclitaxel and etoposide. In samples with well differentiated carcinoma, the most effective drug was irinotecan, and paclitaxel was the most effective drug in those with poorly differentiated and mucinous carcinoma. Although we found no statistically significant association between responsiveness and histology (p = 0.144), there is a strong possibility that colorectal cancers are heterogeneous in their sensitivity to chemotherapeutic agents, depending on the histological type of carcinoma. Moreover, this in vitro result may enable surgeons to broaden the spectrum of which chemotherapeutic agents are used.
Only randomized trials can demonstrate the superiority of one chemotherapy regimen over another. The ASCO Working Group on Chemotherapy Sensitivity and Resistance Assays29,30 showed that the use of chemosensitivity assays to select chemotherapeutic agents for individual patients is not recommended outside the clinical trial setting. Instead, however, oncologists should recommend chemotherapy treatment on the basis of published clinical trial reports. Since our preliminary study was to primarily define the feasibility of ATP-CRA as a chemosensitivity test in patients with colorectal cancer, a further confirmatory study has to be performed. These include the results of combination chemotherapy, ATP-CRA between initial and recurrent colorectal cancers, ATP-CRA in rectal cancer patients with neoadjuvant therapy, and comparison of ATP-CRA-guided chemotherapy to empirical chemotherapy for advanced colorectal cancer. To define the clinical benefit of assay-guided chemotherapy which is based on the above results, a clinical trial targeting patients with advanced colorectal cancer is underway at our institution. Our results and similar results published in other studies should set the stage for a randomized, prospective trial in the near future.
In conclusion, this preliminary study revealed that ATP-CRA produced varying results that were dependent on individual patients and could be a practical chemosensitivity test in patients with colorectal cancer. However, the clinical use of anticancer drugs with a chemosensitivity test should be performed only in a limited scope because of the lack of prospective randomized studies. Therefore, further long-term follow-up studies with a larger group of patients are warranted to evaluate the results of ATP-CRA.
ACKNOWLEDGEMENTS
The authors would like to thank Sung Ho Choi, Ph.D., for his technical assistance and for providing advice.
Presented in part and awarded The New Jersey Society of Colon and Rectal Surgeons Award at the meeting of The American Society of Colon and Rectal Surgeons, St. Louis, Missouri, June 2 to 6, 2007.
Footnotes
The authors have no financial conflicts of interest.
Part of this study was previously published in J Korean Soc Coloproctol 2007;23:172-9 in Korean.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Goldberg RM, Fleming TR, Tangen CM, Moertel CG, Macdonald JS, Haller DG, et al. Eastern Cooperative Oncology Group; the North Central Center Treatment Gtoup; the Southwest Oncology Group. Surgery for recurrent colon cancer: strategies for identifying resectable recurrence and success rates after resection. Ann Intern Med. 1998;129:27–35. doi: 10.7326/0003-4819-129-1-199807010-00007. [DOI] [PubMed] [Google Scholar]
- 3.Liefers GJ, Tollenaar RA. Cancer genetics and their application to individualised medicine. Eur J Cancer. 2002;38:872–879. doi: 10.1016/s0959-8049(02)00055-2. [DOI] [PubMed] [Google Scholar]
- 4.Cortazar P, Johnson BE. Review of the efficacy of individualized chemotherapy selected by in vitro drug sensitivity testing for patients with cancer. J Clin Oncol. 1999;17:1625–1631. doi: 10.1200/JCO.1999.17.5.1625. [DOI] [PubMed] [Google Scholar]
- 5.Bogden AE, Griffin W, Reich SD, Costanza ME, Cobb WR. Predictive testing with the subrenal capsule assay. Cancer Treat Rev. 1984;11(Suppl A):113–124. doi: 10.1016/0305-7372(84)90050-1. [DOI] [PubMed] [Google Scholar]
- 6.Rozencweig M, Hofmann V, Sanders C, Rombaut W, Früh U, Martz G. In vitro growth of human malignancies in a cloning assay. Recent Results Cancer Res. 1984;94:1–7. doi: 10.1007/978-3-642-82295-7_1. [DOI] [PubMed] [Google Scholar]
- 7.Tanigawa N, Kern DH, Hikasa Y, Morton DL. Rapid assay for evaluating the chemosensitivity of human tumors in soft agar culture. Cancer Res. 1982;42:2159–2164. [PubMed] [Google Scholar]
- 8.Yamaue H, Tanimura H, Tsunoda T, Tani M, Iwahashi M, Noguchi K, et al. Chemosensitivity testing with highly purified fresh human tumour cells with the MTT colorimetric assay. Eur J Cancer. 1991;27:1258–1263. doi: 10.1016/0277-5379(91)90093-s. [DOI] [PubMed] [Google Scholar]
- 9.Vescio RA, Redfern CH, Nelson TJ, Ugoretz S, Stern PH, Hoffman RM. In vivo-like drug responses of human tumors growing in three-dimensional gel-supported primary culture. Proc Natl Acad Sci U S A. 1987;84:5029–5033. doi: 10.1073/pnas.84.14.5029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sevin BU, Peng ZL, Perras JP, Ganjei P, Penalver M, Averette HE. Application of an ATP-bioluminescence assay in human tumor chemosensitivity testing. Gynecol Oncol. 1988;31:191–204. doi: 10.1016/0090-8258(88)90293-4. [DOI] [PubMed] [Google Scholar]
- 11.Petty RD, Sutherland LA, Hunter EM, Cree IA. Comparison of MTT and ATP-based assays for the measurement of viable cell number. J Biolumin Chemilumin. 1995;10:29–34. doi: 10.1002/bio.1170100105. [DOI] [PubMed] [Google Scholar]
- 12.Cree IA, Neale MH, Myatt NE, de Takats PG, Hall P, Grant J, et al. Heterogeneity of chemosensitivity of metastatic cutaneous melanoma. Anticancer Drugs. 1999;10:437–444. doi: 10.1097/00001813-199906000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Cree IA, Kurbacher CM, Untch M, Sutherland LA, Hunter EM, Subedi AM, et al. Correlation of the clinical response to chemotherapy in breast cancer with ex vivo chemosensitivity. Anticancer Drugs. 1996;7:630–635. doi: 10.1097/00001813-199608000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Kawamura H, Ikeda K, Takiyama I, Terashima M. The usefulness of the ATP assay with serum-free culture for chemosensitivity testing of gastrointestinal cancer. Eur J Cancer. 1997;33:960–966. doi: 10.1016/s0959-8049(97)00075-0. [DOI] [PubMed] [Google Scholar]
- 15.Konecny G, Crohns C, Pegram M, Felber M, Lude S, Kurbacher C, et al. Correlation of drug response with the ATP tumorchemosensitivity assay in primary FIGO stage III ovarian cancer. Gynecol Oncol. 2000;77:258–263. doi: 10.1006/gyno.2000.5728. [DOI] [PubMed] [Google Scholar]
- 16.Kang SM, Park MS, Chang J, Kim SK, Kim H, Shin DH, et al. A feasibility study of adenosine triphosphate-based chemotherapy response assay (ATP-CRA) as a chemosensitivity test for lung cancer. Cancer Res Treat. 2005;37:223–227. doi: 10.4143/crt.2005.37.4.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Whitehouse PA, Knight LA, Di Nicolantonio F, Mercer SJ, Sharma S, Cree IA Portsmouth Colorectal Cancer Multidisciplinary Team. Heterogeneity of chemosensitivity of colorectal adenocarcinoma determined by a modified ex vivo ATP-tumor chemosensitivity assay (ATP-TCA) Anticancer Drugs. 2003;14:369–375. doi: 10.1097/00001813-200306000-00008. [DOI] [PubMed] [Google Scholar]
- 18.Huh JW, Park YA, Sohn SK, Choi SH. In-vitro chemosensitivity test for colorectal cancer using an adenosine-triphosphate-based chemotherapy response assay (ATP-CRA) J Korean Soc Coloproctol. 2007;23:172–179. [Google Scholar]
- 19.Huh JW, Park YA, Jung EJ, Lee KY, Kwon JE, Sohn SK. Complete remission of unresectable colon cancer after preoperative chemotherapy selected by adenosine triphosphate-based chemotherapy response assay. J Korean Med Sci. 2008;23:916–919. doi: 10.3346/jkms.2008.23.5.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weisenthal LM, Dill PL, Finklestein JZ, Duarte TE, Baker JA, Moran EM. Laboratory detection of primary and acquired drug resistance in human lymphatic neoplasms. Cancer Treat Rep. 1986;70:1283–1295. [PubMed] [Google Scholar]
- 21.Bird MC, Bosanquet AG, Gilby ED. In vitro determination of tumour chemosensitivity in haematological malignancies. Hematol Oncol. 1985;3:1–10. doi: 10.1002/hon.2900030102. [DOI] [PubMed] [Google Scholar]
- 22.Nakahara T, Sakaeda T, Nakamura T, Tamura T, Nishioka C, Aoyama N, et al. Chemosensitivity assessed by collagen gel droplet embedded culture drug sensitivity test, and MDR1, MRP1, and MRP2 mRNA expression in human colorectal adenocarcinomas. Pharm Res. 2004;21:406–412. doi: 10.1023/B:PHAM.0000019292.03875.3e. [DOI] [PubMed] [Google Scholar]
- 23.Swanton C, Tomlinson I, Downward J. Chromosomal instability, colorectal cancer and taxane resistance. Cell Cycle. 2006;5:818–823. doi: 10.4161/cc.5.8.2682. [DOI] [PubMed] [Google Scholar]
- 24.Furukawa T, Kubota T, Hoffman RM. Clinical applications of the histoculture drug response assay. Clin Cancer Res. 1995;1:305–311. [PubMed] [Google Scholar]
- 25.Maehara Y, Kusumoto H, Kusumoto T, Anai H, Sugimachi K. Tumor tissue is more sensitive to mitomycin C, carboquone, and aclacinomycin A than is adjacent normal tissue in vitro. J Surg Oncol. 1989;40:4–7. doi: 10.1002/jso.2930400104. [DOI] [PubMed] [Google Scholar]
- 26.Reinhold U, Tilgen W. Chemosensitivity testing in oncology. Heidelberg: Springer-Verlag; 2003. pp. 108–145. [Google Scholar]
- 27.Kodera Y, Ito S, Fujiwara M, Mochizuki Y, Ohashi N, Ito Y, et al. In vitro chemosensitivity for paclitaxel, using human gastric carcinoma tissues. Int J Clin Oncol. 2006;11:449–453. doi: 10.1007/s10147-006-0618-x. [DOI] [PubMed] [Google Scholar]
- 28.Iwahashi M, Nakamori M, Nakamura M, Noguchi K, Ueda K, Nakatani Y, et al. Individualized adjuvant chemotherapy guided by chemosensitivity test sequential to extended surgery for advanced gastric cancer. Anticancer Res. 2005;25:3453–3459. [PubMed] [Google Scholar]
- 29.Samson DJ, Seidenfeld J, Ziegler K, Aronson N. Chemotherapy sensitivity and resistance assays: a systematic review. J Clin Oncol. 2004;22:3618–3630. doi: 10.1200/JCO.2004.04.077. [DOI] [PubMed] [Google Scholar]
- 30.Schrag D, Garewal HS, Burstein HJ, Samson DJ, Von Hoff DD, Somerfield MR ASCO Working Group on Chemotherapy Sensitivity and Resistance Assays. American Society of Clinical Oncology Technology Assessment: chemotherapy sensitivity and resistance assays. J Clin Oncol. 2004;22:3631–3638. doi: 10.1200/JCO.2004.05.065. [DOI] [PubMed] [Google Scholar]
- 31.Neale MH, Myatt N, Cree IA, Kurbacher CM, Foss AJ, Hungerford JL, et al. Combination chemotherapy for choroidal melanoma: ex vivo sensitivity to treosulfan with gemcitabine or cytosine arabinoside. Br J Cancer. 1999;79:1487–1493. doi: 10.1038/sj.bjc.6690237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ng TY, Ngan HY, Cheng DK, Wong LC. Clinical applicability of the ATP cell viability assay as a predictor of chemoresponce in platinum-resistant epithelial ovarian cancer using nonsurgical tumor cell samples. Gynecol Oncol. 2000;76:405–408. doi: 10.1006/gyno.1999.5698. [DOI] [PubMed] [Google Scholar]
- 33.Moon YW, Choi SH, Kim YT, Sohn JH, Chang J, Kim SK, et al. Adenosine triphosphate-based chemotherapy response assay (ATP-CRA)-guided platinum-based 2-drug chemotherapy for unresectable nonsmall-cell lung cancer. Cancer. 2007;109:1829–1835. doi: 10.1002/cncr.22601. [DOI] [PubMed] [Google Scholar]
- 34.Goldberg RM, Sargent DJ, Morton RF, Fuchs CS, Ramanathan RK, Williamson SK, et al. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol. 2004;22:23–30. doi: 10.1200/JCO.2004.09.046. [DOI] [PubMed] [Google Scholar]
- 35.Tournigand C, André T, Achille E, Lledo G, Flesh M, Mery-Mignard D, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol. 2004;22:229–237. doi: 10.1200/JCO.2004.05.113. [DOI] [PubMed] [Google Scholar]