Abstract
Purpose
The role of local excision in treating rectal cancer patients continues to be controversial. The aim of this study was to evaluate the long-term oncological results of local excision for early rectal adenocarcinomas and review the outcomes of salvage therapy on rectal cancer patients.
Materials and Methods
Between March 1992 and September 2005, 35 consecutive patients with early-stage primary rectal adenocarcinomas were treated by local excision with curative intent. The mean tumor distance from the anal verge was 5 cm (range, 1-10 cm).
Results
The median follow-up was 66 months (range, 17-161 months). Pathological examination revealed 23 cases of T1 and 12 cases of T2. Recurrence had developed in 10 patients (6 local recurrences, 4 systemic recurrences). Purely extrapelvic recurrence was observed in only two (5.7%) patients. Of the eight recurrent patients with surgical salvage, five survived with no evidence of disease at the time of this analysis. The 5-year local recurrence-free and disease-free survival rates were 79.6% and 67.9%, respectively.
Conclusion
Local excision alone of early-staged rectal adenocarcinomas, even in the ideal candidate, is followed by a relatively higher local recurrence rate than previously reported and may not be a valid modality. Either the use of adjuvant therapy with local excision, even in patients with T1 lesions or the use of preoperative therapy followed by local excision has good promise.
Keywords: Recurrence, rectal adenocarcinoma, local excision
INTRODUCTION
The potential advantages of local excision for rectal cancer are clear and include minimal morbidity and preservation of anorectal, urinary, and sexual functions. Conventional full-thickness transanal excision has been recommended as the definitive treatment of early rectal cancer, but there have been growing number of concerns and contradictory results regarding the efficacy of local surgery. Many studies have now verified that recurrence after local excision of early rectal cancers is significantly higher than expected.1-4 Furthermore, recurrence after local surgery is generally characterized by advanced disease and poor long-term survival.1,5
We recently reported that local excision after neoadjuvant therapy could probably be an acceptable alternative to conventional radical surgery in selected patients with rectal cancer.6 Those results prompted us to review our experience of transanal excision in patients with rectal cancer. The purpose of this study was to evaluate the long-term oncologic results of transanal local excision for early rectal adenocarcinomas and review the outcomes of salvage therapy.
MATERIALS AND METHODS
We reviewed the records of 54 consecutive patients who were treated by local therapy for rectal cancer at the Gangnam Severance Hospital and Yonsei University Health System between March 1992 and September 2005. Thirty-five patients with pathologic diagnoses of primary T1 or T2 adenocarcinoma of the rectum who had undergone definitive treatment by full-thickness transanal local excision were included in this study. The following were the criteria for patient selection: a mobile tumor, occupying less than one-third of the rectal circumference, well to moderately differentiated, and stage T1-2 on endorectal ultrasonography (ERUS). Nineteen cases were excluded from analysis for the following reasons: laser fulguration (n = 4), stage IV cancer (n = 4), recurred cancer (n = 1), preoperative chemoradiation (n = 9), and follow-up loss (n = 1).
The details of our transanal local excision experience have been previously published.6 Pretreatment assessment included routine physical examination, chest radiography, rigid proctoscopy, colonoscopy, abdominopelvic computed tomography (CT), ERUS, and measurement of serum carcinoembryonic antigen (CEA) levels. Tumor staging was established by considering the combined results of all modalities used. ERUS was performed on all 35 patients, and 21 of their lesions were staged as uT1N0, 13 as uT2N0, and 1 as uT0N0. All tumors were removed by transanal local excision. The lesions were removed using electrocautery to perform a full-thickness excision with approximately a 1-cm margin of normal rectal wall. Two cases of transanal endoscopic microsurgery were included in this analysis.
Patients were followed at 3-month intervals during the first two postoperative years, biannually until the fifth postoperative year, and annually thereafter. At each visit, we took a medical history, measured serum CEA levels, and performed a physical examination (including rectal examination). Additional follow-up examinations including chest X-ray, abdomino-pelvic CT, colonoscopy, and positron emission tomography (PET) scanning were performed on a semiannual basis during the first two postoperative years and then annually, as well as when there was a suspicion of recurrence. Local recurrence was defined as recurrence within the pelvis; and systemic recurrence was defined as disease outside of the pelvis. The main patterns of recurrence were recorded as the first site of detectable failure during the follow-up period. If the patient did not return for observation after one year, the necessary information was obtained by letter or telephone.
Statistical evaluation was performed using the statistical package SPSS for Windows (version 12.0, SPSS Inc., Chicago, IL, USA). Differences between the two groups were tested using the Student's t-test and chi-squared test when appropriate. Kaplan-Meier methodology was used to estimate overall survival, disease-free survival, and local recurrence rates; and the log-rank test was used to assess statistical significance. A value of p < 0.05 was considered statistically significant.
RESULTS
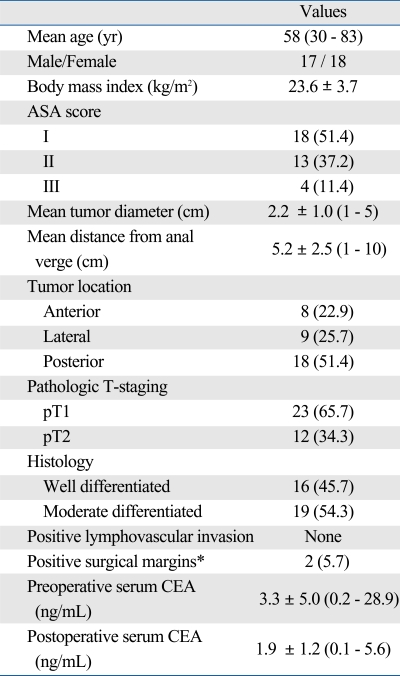
The patient and tumor characteristics for all 35 patients are summarized in Table 1. Patients undergoing transanal excision had a mean tumor diameter of 2.2 cm (range, 1 - 5 cm) and a mean distance from the anal verge of 5.2 cm (range, 1-10 cm). Pathology review confirmed 23 T1 cancers and 12 T2 cancers. All tumors proved to have well-differentiated or moderately-differentiated histology, and there was no case of poorly differentiated or mucinous cancer. There was no evidence of lymphovascular invasion in any case. After transanal excision, two patients with microscopic involvement of the surgical margins received adjuvant radiotherapy due to either the significant medical comorbidity or absolute refusal of a permanent stoma by the patient.
Table 1.
Patient Characteristics (n = 35)
ASA, American Society of Anesthesiologists; CEA, carcinoembryonic antigen.
*Tumor extending to the surgical resection margin (≤ 1 mm).
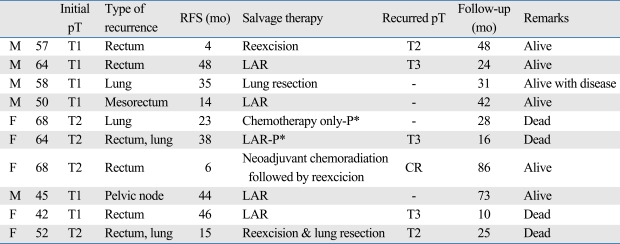
After a median follow-up of 66 months (range, 17-161 months), recurrence was identified in 10 of 35 patients (28.5%). There was no perioperative mortality. The patterns of recurrence, treatment modalities used, and outcome with follow up are shown in Table 2. Local recurrence occurred in 6 patients (17.1%), of whom 5 were T1 and 1 was T2. Two patients (one in T1 and one in T2) had distant metastasis alone (5.7%), and the other two patients (both T2) had distant and local recurrence (5.7%). All metastases were found in the lung, and there was no case of hepatic or peritoneal metastasis. The median recurrence time for the 10 observed recurrences was 29 months (range, 4-48 months).
Table 2.
Patients with Recurrence after Transanal Excision (n = 10)
pT, pathologic T-staging; RFS, recurrence-free survival; LAR, low anterior resection; CR, complete remission.
*P = palliative.
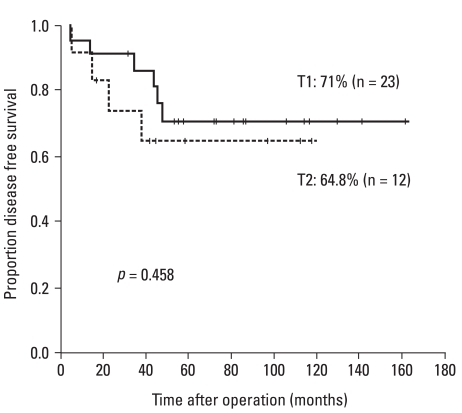
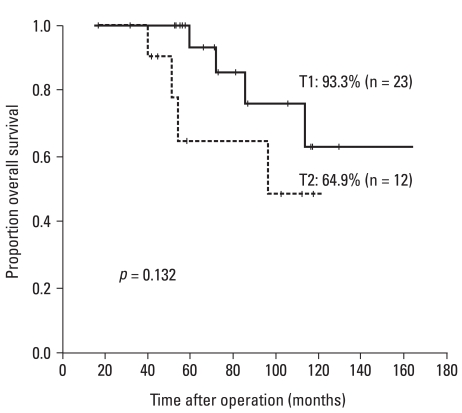
During the follow-up period, there were 8 deaths among the 35 patients. Of these, four died from causes related to cancer recurrence. Seventy-one percent of the T1 patients and 64.8% of the T2 patients were alive with no evidence of disease (Fig. 1). Overall 5-year survival rates were 93.3% and 64.9% for patients with T1 and T2 tumors respectively (Fig. 2). Two of ten patients with recurrent disease received palliative treatment. These two patients had unresectable lung metastases: one received chemotherapy only and the other underwent additional low anterior resection due to obstruction. Both died of their disease. Of the eight recurrent patients with curative surgeries, two died of disease and five were alive with no clinical evidence of recurrent disease at the time of this report. One patient who underwent lung resection was alive despite an additional recurrence that occurred 14 months after salvage surgery. Two patients who received postoperative radiation therapy due to positive resection margins were alive at 42.5 and 45.6 months after surgery with no evidence of disease.
Fig. 1.
Five-year disease free survival rates according to the depth of invasion (T).
Fig. 2.
Five-year overall survival rates according to the depth of invasion (T).
Cox proportional hazards models were used to study the risk factors for recurrence. Gender, age, body mass index, tumor size, location, distance from the anal verge, degree of differentiation, tumor morphology, and preoperative level of serum CEA were examined. None of these variables affected the rate of recurrence independently or in combination with other factors.
DISCUSSION
The role of local excision in the treatment of early rectal cancer continues to be controversial. Local surgery minimizes both morbidity and bowel, bladder, and sexual dysfunction. Local excision of rectal cancer has been accepted for many years as an alternative to radical resection for patients who are unfit to undergo major abdominal surgery. Moreover, with appropriate selection of patients with the lowest chance of recurrence, local excision may be curative.7 Several studies that have demonstrated satisfactory tumor control are heterogeneous in study design, including selection criteria, use of adjuvant therapy, and follow-up periods.8-11
Recent reports with long follow-up periods, however, suggest that this approach is associated with higher-than-expected recurrence rates for early rectal cancer.12-16 Furthermore, the long-term outcome in patients undergoing surgical salvage for recurrent disease appears to be worse than expected.1,5,14 Taylor, et al.12 reported a 30% local recurrence rate for T1 and T2 tumors treated by local excision alone. Garcia-Aguilar, et al.13 found that 18% with T1 tumors and 37% with T2 tumors had recurrence at 54 months of follow up. Madbouly, et al.14 reported a similar overall recurrence rate of a 28.8% in T1 rectal cancers. In the present study, with a median follow up of 66 months, the overall recurrence rate was 28.5% in early rectal tumors with favorable pathologic features, making our results consistent with the most recent reports.12-16
Because of the relatively high rate of local recurrence after local excision, the use of preoperative or postoperative adjuvant therapy with local excision has been evaluated. We recently reported nine highly-selected patients treated with transanal excision after preoperative chemoradiation for locally-advanced rectal cancer,6 and our observation was that a combination of modality transanal excision and preoperative chemoradiation represents an acceptable alternative to conventional radical surgery. Some authors suggest that local excision alone might not be sufficient even for early rectal cancer, and that the potential combination of local excision and postoperative radiation may facilitate good local control with a high survival rate.2,16,17 In this study, two patients who received postoperative radiotherapy were alive at 42.5 and 45.6 months after surgery with no evidence of disease despite positive excision margins. Our series did not include tumors with unfavorable histologic features such as lymphovascular invasion and poor differentiation of mucinous components, except in the two patients with positive excision margins. As a result, the recurrence rates should be lower than reported in series that included unselected patients, nevertheless, we did not observe a lower rate of recurrence in our patients. Interpretation was limited because of the sample size, but we also believe that combining adjuvant therapy and local excision may be a reasonable option to improve oncological outcomes.
Rectal cancer is more likely than colon cancer to be associated with lung metastases without liver metastases.18 The incidence of lung metastases in colorectal cancer ranges from 10% to 20%, depending on the stage of primary tumor.19,20 In our study, all four of the ten patients who had systemic recurrences had pulmonary metastases. This finding suggests a high incidence of lung metastases in patients with local excision for lower rectal cancer. However, there is little available evidence linking the location of rectal cancer and the incidence of pulmonary metastases.
Strict selection criteria are essential when considering local excision, because more favorable outcomes will be expected by proper selection of the patients.21,22 Bretagnol, et al.23 suggested that local excision for cure may be considered in patients with a tumor with no adverse pathologic features, including T1 sm3 or T2, Grades II-III differentiated histology, and lymphovascular invasion. What is the reason for the high local recurrence rates, especially in T1 cancer, in our study? One possible cause is the potential high incidence of deep invasion (sm2 or sm3) in the T1 population. Advanced level of invasion is the well known risk factor in recurrence, but a detailed pathologic evaluation such as a grouping according to the level of invasion (sm1, sm2, sm3) was not performed in this analysis. Our study was a retrospective analysis of patients who underwent local excision according to their pathology report. There are limitations to any retrospective study, including this one. A second possible cause of local failure is the presence of occult, disseminated disease in regional lymphatics. Landmann, et al.3 suggested that nodal disease associated with early rectal lesions tends to be small in size, is very easily missed on preoperative ERUS, and is responsible for pelvic recurrence after local excision. In this study, two of five local recurrences in T1 patients (1 mesorectal and 1 pelvic node recurrence) may be due to inaccurate preoperative staging. Although our study is perhaps not substantial enough to produce definite conclusions, it may still allow us to make valid recommendations.
Local excision alone of early-staged rectal adenocarcinomas, even in the ideal candidate, is followed by a relatively higher local recurrence rate than previously reported and may not be a valid modality. When considering local excision, patients should be informed of the risk of local recurrence. Either the use of adjuvant therapy with local excision, even in patients with T1 lesions, or the use of preoperative therapy followed by local excision has promise, but must be part of a prospective randomized clinical trial before clearer guidelines can be drawn.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Weiser MR, Landmann RG, Wong WD, Shia J, Guillem JG, Temple LK, et al. Surgical salvage of recurrent rectal cancer after transanal excision. Dis Colon Rectum. 2005;48:1169–1175. doi: 10.1007/s10350-004-0930-3. [DOI] [PubMed] [Google Scholar]
- 2.Chakravarti A, Compton CC, Shellito PC, Wood WC, Landry J, Machuta SR, et al. Long-term follow-up of patients with rectal cancer managed by local excision with and without adjuvant irradiation. Ann Surg. 1999;230:49–54. doi: 10.1097/00000658-199907000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Landmann RG, Wong WD, Hoepfl J, Shia J, Guillem JG, Temple LK, et al. Limitations of early rectal cancer nodal staging may explain failure after local excision. Dis Colon Rectum. 2007;50:1520–1525. doi: 10.1007/s10350-007-9019-0. [DOI] [PubMed] [Google Scholar]
- 4.Endreseth BH, Myrvold HE, Romundstad P, Hestvik UE, Bjerkeset T, Wibe A Norwegian Rectal Cancer Group. Transanal excision vs. major surgery for T1 rectal cancer. Dis Colon Rectum. 2005;48:1380–1388. doi: 10.1007/s10350-005-0044-6. [DOI] [PubMed] [Google Scholar]
- 5.Friel CM, Cromwell JW, Marra C, Madoff RD, Rothenberger DA, Garcia-Aguílar J. Salvage radical surgery after failed local excision for early rectal cancer. Dis Colon Rectum. 2002;45:875–879. doi: 10.1007/s10350-004-6320-z. [DOI] [PubMed] [Google Scholar]
- 6.Huh JW, Jung EJ, Park YA, Lee KY, Sohn SK. Preoperative chemoradiation followed by transanal excision for rectal cancer. J Surg Res. 2008;148:244–250. doi: 10.1016/j.jss.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 7.Abir F, Alva S, Longo WE. The management of rectal cancer in the elderly. Surg Oncol. 2004;13:223–234. doi: 10.1016/j.suronc.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 8.Bleday R, Breen E, Jessup JM, Burgess A, Sentovich SM, Steele G., Jr Prospective evaluation of local excision for small rectal cancers. Dis Colon Rectum. 1997;40:388–392. doi: 10.1007/BF02258381. [DOI] [PubMed] [Google Scholar]
- 9.Bailey HR, Huval WV, Max E, Smith KW, Butts DR, Zamora LF. Local excision of carcinoma of the rectum for cure. Surgery. 1992;111:555–561. [PubMed] [Google Scholar]
- 10.Russell AH, Harris J, Rosenberg PJ, Sause WT, Fisher BJ, Hoffman JP, et al. Anal sphincter conservation for patients with adenocarcinoma of the distal rectum: long-term results of radiation therapy oncology group protocol 89-02. Int J Radiat Oncol Biol Phys. 2000;46:313–322. doi: 10.1016/s0360-3016(99)00440-x. [DOI] [PubMed] [Google Scholar]
- 11.Masaki T, Ono M, Muto T. Outcome of local excision for locally invasive rectal carcinomas with special reference to histological features at the invasive margin. Jpn J Clin Oncol. 1998;28:621–625. doi: 10.1093/jjco/28.10.621. [DOI] [PubMed] [Google Scholar]
- 12.Taylor RH, Hay JH, Larsson SN. Transanal local excision of selected low rectal cancers. Am J Surg. 1998;175:360–363. doi: 10.1016/S0002-9610(98)00038-5. [DOI] [PubMed] [Google Scholar]
- 13.Garcia-Aguilar J, Mellgren A, Sirivongs P, Buie D, Madoff RD, Rothenberger DA. Local excision of rectal cancer without adjuvant therapy: a word of caution. Ann Surg. 2000;231:345–351. doi: 10.1097/00000658-200003000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Madbouly KM, Remzi FH, Erkek BA, Senagore AJ, Baeslach CM, Khandwala F, et al. Recurrence after transanal excision of T1 rectal cancer: should we be concerned? Dis Colon Rectum. 2005;48:711–719. doi: 10.1007/s10350-004-0666-0. [DOI] [PubMed] [Google Scholar]
- 15.Mellgren A, Sirivongs P, Rothenberger DA, Madoff RD, García-Aguilar J. Is local excision adequate therapy for early rectal cancer? Dis Colon Rectum. 2000;43:1064–1071. doi: 10.1007/BF02236551. [DOI] [PubMed] [Google Scholar]
- 16.Paty PB, Nash GM, Baron P, Zakowski M, Minsky BD, Blumberg D, et al. Long-term results of local excision for rectal cancer. Ann Surg. 2002;236:522–529. doi: 10.1097/00000658-200210000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coco C, Magistrelli P, Granone P, Roncolini G, Picciocchi A. Conservative surgery for early cancer of the distal rectum. Dis Colon Rectum. 1992;35:131–136. doi: 10.1007/BF02050667. [DOI] [PubMed] [Google Scholar]
- 18.Kirke R, Rajesh A, Verma R, Bankart MJ. Rectal cancer: incidence of pulmonary metastases on thoracic CT and correlation with T staging. J Comput Assist Tomogr. 2007;31:569–571. doi: 10.1097/rct.0b013e318032e8c9. [DOI] [PubMed] [Google Scholar]
- 19.Griffiths EA, Browell DA, Cunliffe WJ. Evaluation of a preoperative staging protocol in the management of colorectal carcinoma. Colorectal Dis. 2005;7:35–42. doi: 10.1111/j.1463-1318.2004.00702.x. [DOI] [PubMed] [Google Scholar]
- 20.Penna C, Nordlinger B. Colorectal metastasis (liver and lung) Surg Clin North Am. 2002;82:1075–1090. doi: 10.1016/s0039-6109(02)00051-8. [DOI] [PubMed] [Google Scholar]
- 21.Maeda K, Maruta M, Sato H, Hanai T, Masumori K, Matumoto M, et al. Outcomes of novel transanal operation for selected tumors in the rectum. J Am Coll Surg. 2004;199:353–360. doi: 10.1016/j.jamcollsurg.2004.05.268. [DOI] [PubMed] [Google Scholar]
- 22.Park KJ, Choi HJ, Roh YH, Shin JS, Lee HS. Local control of local excision for T1/T2 rectal cancer. J Korean Soc Coloproctol. 2007;23:87–92. [Google Scholar]
- 23.Bretagnol F, Rullier E, George B, Warren BF, Mortensen NJ. Local therapy for rectal cancer: still controversial? Dis Colon Rectum. 2007;50:523–533. doi: 10.1007/s10350-006-0819-4. [DOI] [PubMed] [Google Scholar]