Abstract
Background/Objective
Adult survivors of childhood cancer can have altered social functioning. We sought to identify factors that predict marriage and divorce outcomes in this growing population.
Methods
Retrospective cohort study of 8,928 ≥ five-year adult survivors of childhood malignancy and 2,879 random sibling controls participating in the Childhood Cancer Survivor Study. Marital status, current health, psychological status, and neurocognitive functioning were determined from surveys and validated instruments.
Results
Survivors were more likely to be never-married than siblings (relative risk (RR) = 1.21; 95% confidence interval (CI) 1.15–1.26) and the U.S. population (RR=1.25; 95% CI= 1.21 – 1.29), after adjusting for age, gender, and race. Patients with central nervous system (CNS) tumors were at greatest risk for not marrying (RR=1.50; 95% CI= 1.41–1.59). Married survivors divorced at frequencies similar to controls. In multivariable regression analysis, non-marriage was most associated with cranial radiation (RR=1.15; 95% CI=1.02–1.31 for >2400 centigray). In analysis of neurobehavioral functioning, non-marriage was associated with worse task efficiency (RR=1.27; 95% CI=1.20–1.35), but not with emotional distress, or problems with emotional regulation, memory, or organization. Physical conditions predictive of non-marriage included short stature (RR=1.27; 95% CI=1.20–1.34) and poor physical function (RR=1.08; 95% CI=1.00–1.18). Structural equation modeling suggested that cranial radiation influenced marriage status through short stature, cognitive problems, and poor physical function.
Conclusions
Childhood cancer survivors married at lower frequencies compared to peers. Patients with CNS tumors, cranial radiation, impaired processing efficiency, and short stature were more likely to never marry. Divorce patterns in survivors were similar to peers.
Keywords: Survivorship, Cancer, Predictors, Marriage, Divorce
Introduction
Approximately 80% of children with cancer will survive five or more years from diagnosis of their disease (1). The impact of cancer treatment on physical health, during the first several decades following diagnosis and treatment, has been well-characterized, and survivors are known to be at increased risk for second neoplasms (2–4), cardiovascular disease (5, 6), endocrine dysfunction (6), and early death (7, 8). Several psychological sequelae have been described as well, with sub-groups of survivors reporting depression (9–11), anxiety (10), and post-traumatic stress symptoms (12–14).
In addition to physical and mental health, attention must be given to the overall functioning of survivors in society. For instance, survivors have been shown to experience lower educational attainment (15), higher rates of unemployment (16, 17), and difficulty obtaining health insurance (16, 18). Although the institution of marriage has undergone many changes in modern times, it represents another social outcome that can be used to gauge the adaptation of survivors to life after cancer since it represents an aspiration for the majority of young adults in today’s society (19). Relationships are challenging for all adults, but may be especially difficult for survivors, who struggle with the burdens of past disease. In one study, 29% of childhood cancer survivors cited disability or prior illness as a barrier to marriage (20). Uncertainty about future health may also impact survivor relationships (21–23).
The available literature on marriage outcomes after childhood cancer is characterized by inconsistent findings, likely resulting from the limited size and/or distinct composition of the study populations (24–29). Moreover, many of the earlier reports did not assess the underlying causes of observed patterns or have appropriate comparisons to non-cancer populations. Most recently in 2007, Frobisher et al. reported reduced marriage frequencies in 9,954 British childhood cancer survivors diagnosed from 1940 to 1991 compared to those expected from the general population and concluded that survivors were less likely to get married (30). While this study was large, there was limited measurement of emotional and cognitive functioning as potential mediators of decreased marriage frequencies. Also, a key marital outcome, divorce, was not examined.
The Childhood Cancer Survivor Study (CCSS) provides a unique opportunity to add to our understanding of marriage outcomes because of the size and characterization of the cohort, as well as the availability of a sibling comparison group. In this paper, we 1) describe marriage and divorce frequencies in childhood cancer survivors from the CCSS cohort, with comparison to both a sibling cohort and data from the U.S. Census; and 2) identify patient and treatment factors that predict marital status, including psychosocial distress and neurocognitive impairment.
Methods
Study Population
CCSS Cohort
The CCSS is a 26-institution retrospective cohort of survivors of childhood cancer designed to study the late effects of cancer therapy. Eligibility criteria included: 1) diagnosis of leukemia, central nervous system (CNS) tumor, Hodgkin’s lymphoma, non-Hodgkin Disease (HD), Wilms tumor, neuroblastoma, soft tissue sarcoma, or bone tumor; 2) diagnosis and initial treatment at a participating center; 3) diagnosis between January 1, 1970, and December 31, 1986; 4) age <21 years at diagnosis; and 5) survival of ≥ 5 years after diagnosis. The methodology has been previously described (31) and study documents are available.1 Each participating center’s institutional review board reviewed and approved the CCSS protocol and contact documents.
Starting in August, 1994, participants completed an extensive baseline questionnaire which included demographic characteristics marital status, and health history. Two subsequent surveys were administered (2000 Survey: beginning in May, 2000, and 2003 Survey: beginning in November, 2002) to obtain updated information. Trained data abstractors reviewed participants’ medical records for detailed cancer diagnosis and treatment information.
Of the 20,691 patients eligible for participation, 14,363 completed the baseline questionnaire; 3,058 were lost to follow-up; and 3,205 refused participation. Of the 14,363 initial participants, 10,366 completed the first follow-up questionnaire (2000 Survey), and 9,308 completed the second follow-up questionnaire (2003 Survey). Cases were excluded from the current analysis if they were younger than 15 years (n=3) or if they were married prior to diagnosis of malignancy (n=75), yielding 9,230 individuals, of whom 8,928 had known marital status.
Siblings
A random sample of participating survivors (n= 6,005) was asked to contact their sibling closest in age for participation in the study. Of these, 3,839 siblings completed the baseline (enrollment) survey, 2,540 completed the 2000 Survey, and 2,951 completed the 2003 Survey. For the current analysis, siblings were restricted to those subjects age 15 years and older, alive, and in follow-up as of 2003 Survey, resulting in 2,789 siblings with known marital status. See Table 1 for case characteristics compared to siblings.
Table 1.
Characteristics of Childhood Cancer Survivor Study (CCSS) Cases and Siblings
| CCSS Cohort |
Sibling Cohort |
|||||
|---|---|---|---|---|---|---|
| N | % | N | % | p (chi-sq) | ||
| Diagnosis | ||||||
| Leukemia | 3159 | 34.2 | . | . | N/A*. | |
| Central Nervous Tumor | 1171 | 12.7 | . | . | . | |
| Hodgkin Disease | 1151 | 12.5 | . | . | . | |
| Non-Hodgkin Lymphoma | 697 | 7.6 | . | . | . | |
| Kidney (Wilms) | 868 | 9.4 | . | . | . | |
| Neuroblastoma | 629 | 6.8 | . | . | . | |
| Soft tissue sarcoma | 809 | 8.8 | . | . | . | |
| Bone cancer | 746 | 8.1 | . | . | . | |
| Sex | ||||||
| Male | 4695 | 50.9 | 1315 | 46.1 | <.0001 | |
| Female | 4535 | 49.1 | 1537 | 53.9 | . | |
| Age at last contact (years) | ||||||
| 15–19 | 338 | 3.7 | 147 | 5.2 | <.0001 | |
| 20–24 | 1742 | 18.9 | 415 | 14.6 | . | |
| 25–29 | 1982 | 21.5 | 522 | 18.3 | . | |
| 30–34 | 2131 | 23.1 | 531 | 18.6 | . | |
| 35–39 | 1640 | 17.8 | 510 | 17.9 | . | |
| 40+ | 1397 | 15.1 | 727 | 25.5 | . | |
| Race/ethnicity | ||||||
| White non-Hispanic | 7895 | 85.9 | 2532 | 91.9 | <.0001 | |
| Other | 1299 | 14.1 | 222 | 8.1 | . | |
| Education | ||||||
| Did not complete high school | 458 | 5.0 | 122 | 4.3 | <.0001 | |
| Completed high school | 4814 | 52.7 | 1313 | 46.2 | . | |
| College graduate | 3859 | 42.3 | 1408 | 49.5 | . | |
| Household income | ||||||
| < $40,000 | 2905 | 32.4 | 637 | 22.9 | <.0001 | |
| >= $40,000 | 6062 | 67.6 | 2149 | 77.1 | . | |
| Personal income | ||||||
| < $40,000 | 6625 | 75.0 | 1574 | 63.1 | <.0001 | |
| >= $40,000 | 2205 | 25.0 | 921 | 36.9 | . | |
| Employment status | ||||||
| Unemployed | 428 | 4.7 | 69 | 2.4 | <.0001 | |
| Disabled | 708 | 7.8 | 37 | 1.3 | . | |
| Employed or retired | 7895 | 87.4 | 2718 | 96.2 | . | |
N/A= Not applicable
U.S. Population
Data on marital status of the U.S. population were obtained from the 2002 Current Population Survey (CPS), as issued by the Bureau of Census. The report includes marital status, stratified by gender, current age (15 years and older), education and race.2
Measures
On each CCSS survey questionnaire, participants categorized themselves as “single/never married,” “married,” “living as married,” “widowed,” “divorced,” or “separated/no longer living as married.” Reponses were grouped into three outcomes: “never-married,” “currently-divorced,” and “ever-divorced.” “Never-married” was available from the 2003 Survey. Subjects responding “divorced” or “separated” on 2003 Survey were defined as “currently-divorced,” consistent with past studies (24, 32). Cases who reported “divorced” or “separated” on any survey were classified as “ever-divorced.” It is possible that an individual responding “married” on consecutive surveys may in fact be divorced and remarried. We anticipate that the number of divorce cases missed in this manner will be negligible, given a median time of 5 years between the baseline and 2000 Survey, and about 2 years between the 2000 and 2003 surveys.
In the 2002 CPS, “never-married” and “currently-divorced” were clearly defined; “ever-divorced“ was not available. Also, the CPS did not include a “living with partner as married” category. Therefore, when drawing comparison to the general population, cohort members in the “living with partner as married” category as of 2003 Survey were considered “never-married.”
Data from the 2003 Survey were used for variables that change with time including education, income, employment status, and height. Diminished height was defined as height below the tenth percentile for age, gender, and ethnicity, as reported by the Centers for Disease Control and Prevention (CDC).3 Perceived infertility was defined as “yes” to the question, “Has a doctor ever told you that you might have trouble having children?”
Psychological health was evaluated on the baseline and the 2003 Survey with the Brief Symptom Inventory-18 (BSI-18), an 18-item checklist that measures symptoms of anxiety, depression, and somatic distress (33). Responses were scored to generate a Global Severity Index (GSI) score (34). In our analysis, subjects with GSI elevations ≥ 50 on either of two BSI-18 administrations were classified as having a positive history of psychological distress, consistent with a previous validation study in cancer survivors by Recklitis et al.(35).
Neurocognitive functioning, including executive skills, was evaluated with the Childhood Cancer Survivorship Study Neurocognitive Questionnaire (CCSS-NCQ), a 25-item instrument that is predominantly a subset of items from an early investigational version of the Behavior Rating Inventory of Executive Functioning-Adult version. Krull et al identified four domains that demonstrated good internal consistency: task efficiency, emotional regulation, organization, and memory skills.(36) Subjects were classified as “high risk” for neurocognitive dysfunction if the response on any of the questions for the respective factor was “often a problem” consistent with validation studies of this instrument.
Analyses
Frequencies of “never-married” and “currently-divorced” were described in CCSS cases and compared to frequencies for siblings and the U.S. population (as of the 2002 CPS), overall and in a stratified fashion, by age and gender. The “currently-divorced” proportion was calculated as the number “divorced” or “separated,” divided by the total number “married,” “widowed,” “divorced,” or “separated.” Likelihood ratio tests were used to determine the statistical significance of differences between groups. Survivors were compared to U.S. population data and to the sibling comparison group. Generalized estimating equation formulations of the model and significance tests were utilized to account for the intra-family correlation between survivors and siblings (37)
Among survivors, case-case comparisons were conducted with respect to the outcomes, “never-married” and “ever-divorced.” The analysis of “ever-divorced” was restricted to those subjects who had been married at least once and who had reported marital status on all three surveys. Log-binomial regression models were used to evaluate associations between explanatory variables and each outcome. These models allow direct calculation of age-adjusted RRs with 95% confidence intervals to compare the probability of outcomes between survivor sub-groups and were selected over logistic regression due to the high prevalence of the outcome (38). Multivariable regression models, including factors marginally significant in the unadjusted analysis (p < 0.2), were created to determine the independent role of each variable, adjusted for age at diagnosis, gender, and educational status. Potential confounders and interactions were also evaluated
Structural equation models of the observed data (weighted least-square parameter estimates [delta parameterization]) were analyzed using Mplus 5.2 software (39). All variables were directly observed measures; there were no latent variables. Never married (N=2616) and Ever Married (N=3924) sub-samples at the Follow-up 2 survey with complete data comprised the final sample for the SEM analysis. We chose to use samples with complete data rather than to use data imputation in order to avoid potentially distorting coefficients of association and correlation relating variables (40). The best-fitting model was determined according to the following criteria: 1) conceptually sound; 2) statistically significant parameter estimates (PE) that represent the strength of the path between two variables (read as standardized regression coefficients); 3) meets the established SEM fit criteria (non-significant χ2 statistic (P > 0.05); 4) root mean square error of approximation (RMSEA) ≤ 0.05; 5) weighted root mean square residual (WRMR) <1.0 (41, 42)]; 6) comparative fit index (CFI) and Tucker Lewis index (TLI) ≥ 0.90 (43); and 7) the highest percentage of explained variance for the outcome.
Results
Marital Status of CCSS Cohort at Last Contact
At last contact, 42.4 % (n = 3,783) of survivors were currently married, 7.3 % (n = 654) were divorced or separated, 0.2 % (n = 20) were widowed, and 46.4 % (n = 4,141) had never been married. Of those never-married (n=3698), 90% were living as single and 10% lived with a partner outside of marriage.
Comparison of Survivor Marital Status with Siblings and the U.S. Population
Survivors were significantly more likely to never have married than siblings (RR= 1.21; 95% CI 1.15–1.26) and the U.S. population. (RR=1.25; 95% CI= 1.21 – 1.29), after adjusting for age, gender, and race (See Table 2 and Table 3). The trend was apparent across all age groups 25 years old and older. It was particularly marked for those in the 35–44 year and 45+ year age groups, where survivors were 1.90 (95% CI 1.55 – 2.32) and 2.35 (95% CI 1.29 – 4.28) times more likely than siblings to be never-married, after adjusting for gender and race (data not shown). Cases with a history of CNS tumor (RR=1.49; 95% CI= 1.40 – 1.58) and leukemia (RR=1.19; 95% CI=1.12 – 1.25) had the greatest likelihood of never marrying. Upon further stratification, the probability of never marrying remained elevated in leukemia patients who received cranial radiation (RR 1.25; 95% CI= 1.18–1.32), but not in those treated with chemotherapy only (RR=1.03; 95% CI=0.96–1.10).
Table 2.
Frequencies of Never-Married Status – Comparison of Survivors with Siblings and the U.S. Census Population, adjusted for race
| Survivors | Siblings | U.S Census | ||||
|---|---|---|---|---|---|---|
| N | % | N | % | % | ||
|
All ages 18–54 years |
||||||
| Total | 3856 | 46.1 | 821 | 31.7† | 32.4* | |
| Male | 2083 | 49.2 | 421 | 35.3† | 36.5* | |
| Female | 1773 | 43.0 | 400 | 28.6† | 24.7* | |
| Age 18–24 years | ||||||
| Total | 1444 | 85.3 | 371 | 83.7 | 84.4 | |
| Male | 726 | 89.9 | 183 | 88.4 | 86.5 | |
| Female | 718 | 81.1 | 188 | 79.7 | 79.7 | |
| Age 25–34 years | ||||||
| Total | 1841 | 48.2 | 338 | 34.7† | 37.2* | |
| Male | 1028 | 52.4 | 186 | 39.9† | 41.8* | |
| Female | 813 | 43.7 | 152 | 29.9† | 27.5* | |
| Age 35–44 years | ||||||
| Total | 524 | 21.5 | 99 | 11.0† | 15.9* | |
| Male | 304 | 24.1 | 46 | 11.2† | 19.4* | |
| Female | 220 | 18.7 | 53 | 10.9† | 9.7* | |
| Age 45–54 years | ||||||
| Total | 47 | 11.5 | 13 | 4.8† | 10.0* | |
| Male | 25 | 12.0 | 6 | 5.6 | 11.9 | |
| Female | 22 | 11.1 | 7 | 4.3† | 6.9* | |
Indicates p < 0.05, survivors vs. siblings
Indicates p < 0.05, survivors vs. U.S. Census
Table 3.
Relative risk of Never-Married and Ever-Divorced in CCSS cases compared to sibling comparison group, overall and stratified by cancer diagnosis, adjusted by age at evaluation, gender, and race (please note, there is no referent within the diagnostic category: every row is compared to the sibling group)
| Never-married | Ever Divorced | |||||||
| N | % | RR (95%CI) | p-value | N | % | RR (95%CI) | p-value | |
| All Cancers | 3698 | 41.4 | 1.21 (1.15–1.26) | <0.0001 | 981 | 21.4 | 1.08 (0.96–1.21) | 0.20 |
| Leukemia | 1508 | 49.2 | 1.19 (1.12–1.25) | <0.0001 | 282 | 20.8 | 1.09 (0.94–1.27) | 0.26 |
| Central Nervous Tumor | 698 | 62.5 | 1.49 (1.40–1.58) | <0.0001 | 78 | 21.5 | 1.07 (0.86–1.34) | 0.553 |
| Non-Hodgkin Lymphoma | 204 | 29.6 | 1.09 (0.98–1.22) | 0.13 | 104 | 24.4 | 1.19 (0.97–1.46) | 0.10 |
| Kidney (Wilms) | 377 | 46.2 | 0.97 (0.90–1.04) | 0.41 | 56 | 14.4 | 0.80 (0.60–1.07) | 0.14 |
| Neuroblastoma | 333 | 59.9 | 1.14 (1.06–1.22) | 0.0002 | 38 | 19.8 | 1.17 (0.86–1.60) | 0.32 |
| Soft tissue sarcoma | 270 | 33.9 | 1.11 (1.02–1.21) | 0.02 | 87 | 19.4 | 0.93 (0.75–1.15) | 0.50 |
| Bone cancer | 149 | 20.1 | 1.02 (0.87–1.19) | 0.02 | 120 | 22.6 | 1.03 (0.85–1.24) | 0.78 |
| Hodgkin Disease | 159 | 13.9 | 1.05 (0.91–1.21) | 0.54 | 216 | 24.4 | 1.13 (0.96–1.33) | 0.13 |
| Sibling Comparison Group | 776 | 27.8 | Ref | N/A | 304 | 19.9 | Ref | N/A |
Survivors divorced at similar frequencies to siblings (RR= 1.08; 95% CI= 0.96–1.21) and to population controls (RR = 0.96; 95% CI 0.89 – 1.03, p=0.23). No cancer diagnosis group had an elevated risk of divorce (Table 3). No statistically significant differences in divorce frequencies were observed across age or gender groups (data not shown).
Predictors of Never-Married Status in Survivors
Univariate analysis adjusted for gender and age at last contact and gender indicated that age <13 years at diagnosis (RR=1.52; 1.34–1.72) and cranial radiation >2400 centigray (RR=1.28 compared to no cranial radiation; 95% CI=1.14–1.43) were the strongest predictors of non-marriage among treatment factors (Table 4). The following medical and neuropsychological conditions were significantly associated with never marrying (Table 4): short stature, history of tumor recurrence, poor self-reported physical functioning, emotional distress, problems with task efficiency, problems with organization, and problems with memory. Report of a perceived fertility problem was associated with a lower likelihood of not marrying (RR=0.91; 95% CI= 0.87–0.95).
Table 4.
Univariate analysis of the association of patient factors, cancer treatment, and medical conditions with marital status, adjusted for gender and age at last contact
| Characteristic | Never-married | Ever-divorced | ||||||
|---|---|---|---|---|---|---|---|---|
| N | % | RR (95%CI) | p-value | N | % | RR (95%CI) | p-value | |
| Age at diagnosis (years) | ||||||||
| <13 | 3338 | 50.6 | 1.52 (1.34–1.72) | <.0001 | 580 | 20.5 | 1.12 (0.98–1.28) | 0.10 |
| 13–20 | 360 | 15.4 | 1.00 | . | 401 | 22.7 | 1.00 | . |
| Gender | ||||||||
| Female | 1692 | 38.5 | 1.00 | . | 524 | 22.0 | 1.00 | . |
| Male | 2006 | 44.2 | 1.16 (1.12–1.21) | <.0001 | 457 | 20.7 | 0.92 (0.82–1.03) | 0.13 |
| Diagnosis | ||||||||
| Leukemia | 1508 | 49.2 | 1.87 (1.60–2.17) | <.0001 | 282 | 20.8 | 0.97 (0.82–1.15) | 0.73 |
| Central Nervous Tumor | 698 | 62.5 | 2.22 (1.90–2.59) | <.0001 | 78 | 21.5 | 0.96 (0.76–1.20) | 0.70 |
| Non-Hodgkin Lymphoma | 204 | 29.6 | 1.56 (1.31–1.86) | <.0001 | 104 | 24.4 | 1.07 (0.87–1.31) | 0.55 |
| Kidney (Wilms) | 377 | 46.2 | 1.59 (1.35–1.88) | <.0001 | 56 | 14.4 | 0.73 (0.55–0.97) | 0.03 |
| Neuroblastoma | 333 | 59.9 | 1.96 (1.67–2.30) | <.0001 | 38 | 19.8 | 1.00 (0.73–1.38) | 0.99 |
| Soft tissue sarcoma | 270 | 33.9 | 1.69 (1.42–2.00) | <.0001 | 87 | 19.4 | 0.84 (0.68–1.05) | 0.14 |
| Bone cancer | 149 | 20.1 | 1.33 (1.09–1.62) | 0.005 | 120 | 22.6 | 0.94 (0.77–1.14) | 0.54 |
| Hodgkin Disease | 159 | 13.9 | 1.00 | . | 216 | 24.4 | 1.00 | . |
| Cranial radiation | ||||||||
| >2400 | 89 | 54.6 | 1.28 (1.14–1.43) | <.0001 | 9 | 14.5 | 0.68 (0.37–1.26) | 0.23 |
| >0 and ≤2400 | 726 | 49.6 | 1.10 (1.04–1.17) | 0.001 | 128 | 19.5 | 1.01 (0.83–1.22) | 0.92 |
| 0 | 1150 | 42.5 | 1.00 | . | 273 | 19.8 | 1.00 | . |
| Stem cell Transplant | ||||||||
| Yes | 37 | 44.0 | 0.96 (0.81–1.15) | 0.70 | 7 | 16.7 | 0.81 (0.41–1.59) | 0.53 |
| No | 3355 | 41.6 | 1.00 | . | 867 | 20.8 | 1.00 . | |
| Treatment duration | ||||||||
| ≥ 2 years | 1884 | 45.1 | 1.06 (1.01–1.10) | 0.01 | 409 | 20.1 | 0.98 (0.87–1.10) | 0.69 |
| <2 years | 1386 | 37.6 | 1.00 | . | 434 | 21.2 | 1.00 | . |
| Perceived fertility problem |
||||||||
| Yes | 1208 | 33.8 | 0.91 (0.87–0.95) | <.0001 | 481 | 23.2 | 1.14 (1.01–1.27) | 0.03 |
| No | 2262 | 45.8 | 1.00 | . | 466 | 19.7 | 1.00 | . |
| Short stature | ||||||||
| Yes | 978 | 57.2 | 1.20 (1.16–1.24) | <.0001 | 150 | 23.7 | 1.16 (0.99–1.35) | 0.07 |
| No | 2571 | 37.0 | 1.00 | . | 810 | 20.9 | 1.00 | . |
| Subsequent Malignant Neoplasm |
||||||||
| Yes | 220 | 27.8 | 1.05 (0.97–1.15) | 0.24 | 114 | 21.6 | 0.91 (0.77–1.09) | 0.31 |
| No | 3478 | 42.7 | 1.00 | . | 867 | 21.3 | 1.00 | . |
| Recurrence | ||||||||
| Yes | 415 | 45.8 | 1.11 (1.06–1.17) | <.0001 | 93 | 21.7 | 0.98 (0.82–1.19) | 0.87 |
| No | 3283 | 40.9 | 1.00 | . | 888 | 21.3 | 1.00 | . |
| Poor physical function (SF-36 T-score <40) |
||||||||
| Yes | 375 | 42.9 | 1.19 (1.14–1.24) | <.0001 | 141 | 31.7 | 1.52 (1.30–1.78) | <.0001 |
| No | 2717 | 40.9 | 1.00 | . | 687 | 19.6 | 1.00 | . |
| Emotional Distress (GSI T-score ≥ 50) | ||||||||
| Yes | 1613 | 39.9 | 1.08 (1.03–1.12) | 0.0007 | 544 | 25.3 | 1.40 (1.25–1.57) | <.0001 |
| No | 1513 | 36.8 | 1.00 | . | 412 | 18.0 | 1.00 | . |
| NCQ: problems with task efficiency |
||||||||
| Yes | 935 | 49.4 | 1.17 (1.12–1.22) | <.0001 | 220 | 25.4 | 1.37 (1.20–1.58) | <.0001 |
| No | 1637 | 35.6 | 1.00 | . | 500 | 18.5 | 1.00 | . |
| NCQ: problems with organization |
||||||||
| Yes | 550 | 44.4 | 1.10 (1.04–1.15) | 0.0002 | 141 | 22.7 | 1.13 (0.96–1.32) | 0.15 |
| No | 2022 | 38.5 | 1.00 | . | 579 | 19.7 | 1.00 | . |
| NCQ: problems with memory |
||||||||
| Yes | 581 | 43.5 | 1.06 (1.01–1.12) | 0.02 | 174 | 25.9 | 1.34 (1.16–1.56) | 0.0001 |
| No | 1991 | 38.6 | 1.00 | . | 546 | 18.9 | 1.00 | . |
| NCQ: problems with emotional regulation |
||||||||
| Yes | 666 | 41.8 | 0.99 (0.94–1.04) | 0.69 | 205 | 24.6 | 1.32 (1.15–1.52) | 0.0001 |
| No | 1906 | 38.9 | 1.00 | . | 515 | 18.9 | 1.00 | . |
| Educational Attainment Did not complete high school |
193 | 50.4 | 1.04 (0.96–1.13) | 0.40 | 45 | 31.7 | 2.15 (1.66–2.78) | <.0001 |
| Completed high school | 2139 | 46.2 | 1.04 (1.00–1.09) | 0.08 | 579 | 26.8 | 1.80 (1.60–2.03) | <.0001 |
| College graduate | 1323 | 34.4 | 1.00 | . | 352 | 15.5 | 1.00 | . |
Table 5–Table 7 displays the results of three separate multivariable models (that all included gender, age at last contact, age at diagnosis, and educational attainment) divided into disease and treatment factors, neurobehavioral functioning, and physical functioning factors. Significant disease and treatment predictors of non-marriage included cranial radiation >2400 centigray (RR=1.15 compared to no radiation; 95% CI=1.02–1.31) and history of recurrence (RR=1.10; 95% CI=1.00–1.20). Impaired task efficiency was the only neurobehavioral condition significantly associated with not being married in adjusted analysis (RR= 1.27; 95%=1.20–1.35). Problems with emotional regulation were associated with a greater likelihood of getting married. In terms of physical conditions, short stature (RR=1.27; 95% CI=1.2–1.34) and poor self-reported physical functioning (RR=1.08; 95% CI=1.00–1.18) were associated with not ever marrying. Perceived fertility problem was not included in the adjusted model because the direction of the association suggested that fertility status likely was determined after marriage.
Table 5.
Multivariable regression model of the association between disease and treatment factors with marital status, adjusted for gender and age at last contact
| Characteristic | Never-married | Ever-divorced | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N | % | RR (95%CI) | p-value | N | % | RR (95%CI) | p-value | ||
| Age at diagnosis |
<13 (years) | 3338 | 50.6 | 1.38 (1.14–1.67) | 0.0008 | 580 | 20.5 | 1.28 (1.03–1.60) | 0.03 |
| 13–20 | 360 | 15.4 | 1.00 | . | 401 | 22.7 | 1.00 | . | |
| Gender | Female | 1692 | 38.5 | 1.00 | <.0001 | 524 | 22.0 | 1.00 | 0.04 |
| Male | 2006 | 44.2 | 1.13 (1.06–1.19) | . | 457 | 20.7 | 0.83 (0.70–0.99) | . | |
| Educational Attainment |
Did not complete high school |
193 | 50.4 | 1.14 (1.00–1.30) | 0.04 | 45 | 31.7 | 2.58 (1.80–3.70) | <.0001 |
| Completed high school |
2139 | 46.2 | 1.12 (1.05–1.20) | 0.0003 | 579 | 26.8 | 1.79 (1.49–2.17) | <.0001 | |
| College graduate |
1323 | 34.4 | 1.00 | . | 352 | 15.5 | 1.00 | . | |
| Cranial radiation (Centigray) |
>2400 | 89 | 54.6 | 1.15 (1.02–1.31) | 0.03 | 9 | 14.5 | 0.60 (0.32–1.11) | 0.10 |
| >0 and ≤2400 | 726 | 49.6 | 1.03 (0.97–1.11) | 0.33 | 128 | 19.5 | 0.91 (0.73–1.13) | 0.40 | |
| 0 | 1150 | 42.5 | 1.00 | . | 273 | 19.8 | 1.00 | . | |
| Stem cell Transplant |
Yes | 37 | 44.0 | 1.05 (0.82–1.34) | 0.71 | 7 | 16.7 | 1.6 (0.61–4.19) | 0.33 |
| No | 3355 | 41.6 | 1.00 | . | 867 | 20.8 | 1.00 | . | |
| Treatment duration (years) |
≥ 2 years | 1884 | 45.1 | 1.04 (0.97–1.12) | 0.29 | 409 | 20.1 | 1.05 (0.85–1.30) | 0.67 |
| <2 years | 1386 | 37.6 | 1.00 | . | 434 | 21.2 | 1.00 | . | |
| Recurrence | Yes | 415 | 45.8 | 1.10 (1.01–1.2) | 0.04 | 93 | 21.7 | 0.96 (0.70–1.32) | 0.82 |
| No | 3283 | 40.9 | 1.00 | . | 888 | 21.3 | 1.00 | . | |
Table 7.
Multivariable regression model of the association between physical conditions with marital status, adjusted for age at last contact
| Characteristic | Never-married | Ever-divorced | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N | % | RR (95%CI) | p-value | N | % | RR (95%CI) | p-value | ||
| Age at diagnosis (years) |
<13 | 3338 | 50.6 | 1.4 0(1.21–1.62) | <.0001 | 580 | 20.5 | 1.08 (0.91–1.26) | 0.38 |
| 13–20 | 360 | 15.4 | 1.00 | . | 401 | 22.7 | 1.00 | . | |
| Gender | Female | 1692 | 38.5 | 1.20 (1.13–1.26) | <.0001 | 524 | 22.0 | 0.95 (0.83–1.09) | 0.45 |
| Male | 2006 | 44.2 | 1.00 | . | 457 | 20.7 | 1.00 | . | |
| Educational Attainment |
Did not complete high school |
193 | 50.4 | 1.10 (1.04–1.17) | 0.0009 | 45 | 31.7 | 1.68 (1.46–1.93) | <.0001 |
| Completed high school |
2139 | 46.2 | 1.00 | . | 579 | 26.8 | 1.00 | . | |
| College graduate |
1323 | 34.4 | 1.40 (1.21–1.62) | <.0001 | 352 | 15.5 | 1.08 (0.91–1.26) | 0.38 | |
| Short stature |
Yes | 978 | 57.2 | 1.27 (1.2–1.34) | <.0001 | 150 | 23.7 | 1.13 (0.94–1.36) | 0.18 |
| No | 2571 | 37.0 | 1.00 | . | 810 | 20.9 | 1.00 | . | |
| Poor physical function (SF-36 T- score <40) |
Yes | 375 | 42.9 | 1.08 (1.00–1.18) | 0.05 | 141 | 31.7 | 1.4 (1.18–1.67) | 0.0001 |
| No | 2717 | 40.9 | 1.00 | . | 687 | 19.6 | 1.00 | . | |
Male gender and younger age at diagnosis were consistently associated with greater likelihood of not getting married, in adjusted analyses. No differences were noted upon further stratification of the significant factors identified in multivariable analysis by gender or cranial radiation.
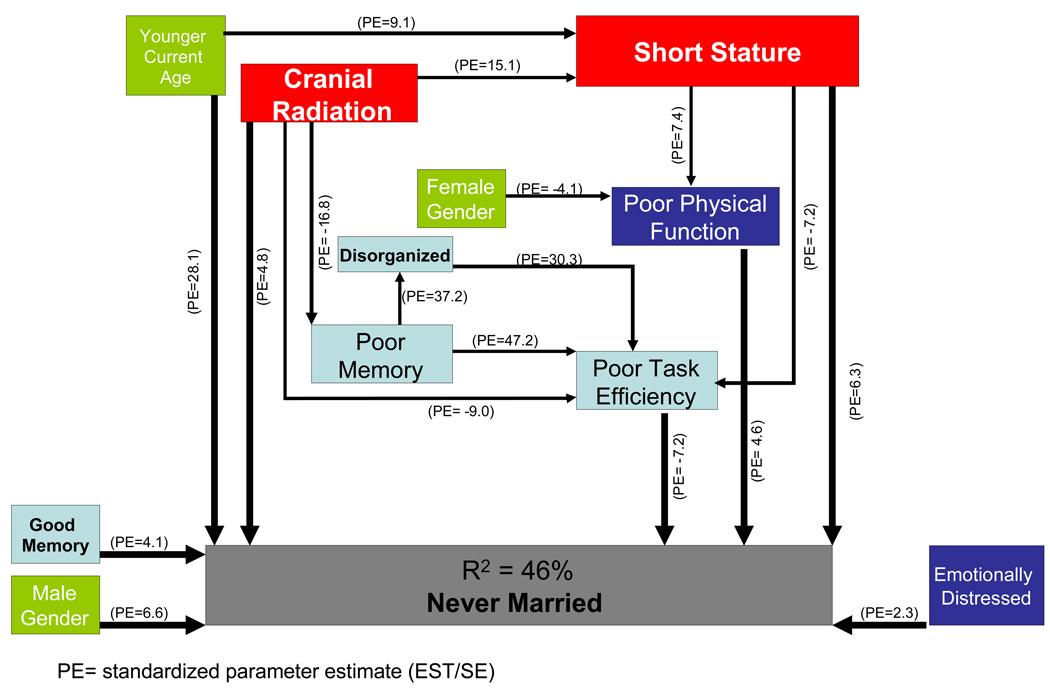
Table 8 and the Figure collectively present the results of the structural equation modeling. Table 8 provides a description of all significant variables and their contribution to the model, including the estimated regression coefficients (EST) for each parameter, the standard error of the parameter estimates (SE), the coefficient divided by the standard error (EST/SE, or z-score), the standardized coefficients (STDYX), and the p-value for the path between the two variables. For binary dependent variables, the regression coefficients produced are logistic regression coefficients. Figure 1 represents a simplified graphic version of the complete SEM results. A well fitting model (χ2=21.91, df=14, P=0.08; CFI=0.999; TLI =0.998; RMSEA=0.009; WRMR = 0.557) explained 45.6% of the variance in survivors’ never having been married. The strongest predictor of never having married, based on the weight of the standardized coefficients, was younger current age followed by short stature, poor task efficiency, male gender, history of CNS radiation, better memory, poor physical function, and poor emotional functioning.
Table 8.
Structural Equation Model for Predictors of Never-Married Status in CCSS Cases (corresponding to Figure)
| Estimate (EST) |
Standard Error (SE) |
EST/SE | STD YX Estimate |
P-Value | |
|---|---|---|---|---|---|
| Younger Current Age | 0.091 | 0.003 | 28.06 | 0.518 | <0.0001 |
| Short Stature | 0.225 | 0.035 | 6.34 | 0.198 | <0.0001 |
| Poor Task Efficiency | −0.047 | 0.007 | −7.17 | −0.154 | <0.0001 |
| Male Gender | 0.233 | 0.035 | 6.62 | 0.090 | <0.0001 |
| CNS Radiation | 0.219 | 0.045 | 4.83 | 0.079 | <0.0001 |
| Better Memory | 0.046 | 0.011 | 4.06 | 0.078 | <0.0001 |
| Poor Physical Function | 0.004 | 0.001 | 4.63 | 0.074 | <0.0001 |
| Poor Emotional Function | 0.003 | 0.001 | 2.32 | 0.040 | 0.020 |
Figure 1.
Graphic representation of structural equation modeling of predictors of never-married status in CCSS cases
History of CNS radiation was an indirect influence on never having married through 1) short stature (P=<0.0001), 2) poor memory (P=<0.0001), 3) poor physical function (P= <0.0001)] ; and 4) poor task efficiency (P=<0.001). History of CNS radiation also was a direct influence on never having married, presumably through factors that we did not measure in this study. Short stature was an indirect influence on never having married through poor task efficiency (P=<0.0001), and poor physical function (P=<0.0001). The indirect impact of poor task efficiency on never having married through by poor memory; the indirect impact of poor physical function was through by poor task efficiency.
Predictors of Ever-Divorced Status in Survivors
Among ever-married survivors, after adjusting for gender and age at last contact, factors found to be significantly associated with history of divorce were poor physical functioning (RR=1.52; 95% CI=1.30–1.78), perceived fertility problem (RR=1.14; 95% CI=1.01–1.27), emotional distress (RR=1.40; 95% CI= 1.25–1.57), problems with task efficiency (RR=1.37; 95% CI=1.20–1.58), impaired working memory (RR=1.34; 95% CI=1.16–1.56), and problems with emotional regulation (RR=1.32; 1.15–1.52) as displayed in Table 4. No significant treatment factor was identified.
Multivariable models were examined for divorce (Table 5–Table 7). An age younger than 13 years at diagnosis (RR=1.28: 95% CI=1.03–1.60), emotional distress (RR=1.33; 95% CI=1.15–1.54), and self-report of poor physical functioning (RR=1.40; 95% CI=1.18–1.67) were independently predictive of divorce. Interactions were examined; there were no difference in the association between risk factors and divorce status between males and females and by cranial radiation status.
Discussion
In this large, multi-site cohort of adult survivors of childhood cancer, we concluded that survivors were 1.21 times more likely to be unmarried than the sibling comparison group and 1.25 times more likely to be unmarried than the U.S. Census population, after adjusting for age, gender, and race. Our risk estimates are similar to that of the 2007 report by Frobisher et al. based on the 9,954 member British Cancer Survivor Study (BCCSS) (30). Younger age at diagnosis and history of cranial radiation were the most important predictors of never getting married among cases. From structural equation modeling, we found that cranial radiation exposure was an indirect influence on never having married mediated by short stature, impaired memory, worse processing speed, and poor physical function. Emotional distress among survivors is a direct influence of never getting married, separate from cranial radiation exposure. Our other major finding was that divorce patterns among childhood cancer survivors are similar to that of the general population and a sibling comparison group. This reassuring conclusion is contrary to an older report by Byrne et al. in 1989 (24). Ours is the largest study to our knowledge that examines divorce outcomes.
Our results should be further compared and contrasted with that of the other large, recent cohort study by Frobisher et al. in the BCSS. The BCCSS study only compared cases to population data and no summary relative risk statistic was reported. However, marriage frequencies stratified by age and gender from the Frobisher publication suggested that survivors were 1.1–1.6 times more likely to be unmarried. These estimates are similar to our own verified with both sibling and general population comparison groups. Both the CCSS and the BCSS studies identified males, history of CNS tumor, exposure to CNS radiation, and poor physical function as predictors of non-marriage.
Our CCSS study of marriage was unique in that we also included standardized measures specific to emotional and cognitive functioning to understand why certain patient groups were less likely to marry. In the CCSS cohort, structural equation modeling helped to elucidate that cranial radiation indirectly influenced never getting married through worse cognitive processing difficulties and short stature, as well as poor physical function. In the childhood cancer survivor population, short stature is usually due to decreased pituitary function as a result of CNS radiation. In the general population, diminished height is a known to be associated with lower marriage rates (44), and bachelors are significantly shorter (45). In 1996, a meta-analytic review concluded that females are more romantically attracted to taller males (46). In a more recent large study of responses to personal advertisements, males with higher education and taller height had significantly more responses (47). Pawlowski speculates that “male height is an important trait on the mate market” because it is an indicator of reproductive potential, while education and intelligence are proxies for economic status (47). There is evidence that taller males father more children (45) and are perceived as healthier (48).
Structural equation analysis suggests that cranial radiation also has a direct influence on non-marriage, presumably mediated through some factor that we did not measure in this study. Future studies should examine the potential role of factors such as social intelligence, attractiveness to the other sex, altered sexual maturation, and libido. Emotional distress and male gender were other factors directly associated with never getting married.
Cranial radiation has been associated with social difficulties in past studies. Pui et al. found cranial radiation to predict non-marriage in female survivors of acute lymphoblastic leukemia (ALL) (49). In a study of adolescent survivors, Barrera et al. concluded that those treated with cranial radiation were less likely to have close friends than survivors treated without cranial radiation (50). Thus, it seems that the negative effects of cranial radiation on social integration begin at an early age and persist into adulthood.
The current study has some methodological characteristics that should be considered in the interpretation of the results. Due to the time elapsed between surveys and the nature of the question about marital status, it is possible that some cases of divorce were missed. As a result, we may have under-estimated the risk of being ever-divorced. The CCSS participants were diagnosed between 1970 and 1986 in an earlier era, and thus may not be directly generalizable to more recently treated cohorts of pediatric cancer survivors Finally, although the size of the CCSS cohort is a strength, it also limits the nature of contact with participants to standardized questionnaires. Thus, while we can state that survivors marry less frequently than controls of similar age and gender, we do not have data directly relating to the thoughts, desires, and motivations underlying this behavior.
The CCSS is a valuable resource for survivorship studies because of the multi-site design, large sample size, and high participation rates (51)]. For the baseline CCSS survey, 69% of the total eligible population participated (15% could not be located and 15% declined participation). Participation rates on the follow-up surveys have ranged from 77–81%. Comparisons of available demographic and cancer-related characteristics between participants and non-participants at the initial baseline questionnaire showed that the only significant difference between these groups was vital status. That is, the next-of-kin relatives of patients who died more than 5 years after diagnosis were less likely to have participated than patients who were still alive. Comparisons have also been done between participants and non-participant at subsequent questionnaires (52)]. While differences are moderate in size (<10% differences), the study retains more female, white race, college-educated, higher-income, and older participants. In our current analysis, we adjust for gender, race, age, and socio-economic status.
Marriage and divorce patterns are objective measures that can be used to gauge social integration and success of intimate relationships among childhood cancer survivors. While it can be debated whether marriage is a desirable outcome, marriage is generally an expected developmental goal in our society to the extent that most adults in the U.S. are married by the age of 30 years. Our large cohort study confirms that childhood cancer survivors are less likely to be married compared to their non-cancer peers. Among survivors, patients with CNS tumors or a history of cranial radiation were most likely not to marry. Cranial radiation influenced marriage status through short stature, cognitive processing difficulties, and poor physical function. Except for those with reduced physical function, there was no increased risk of divorce among survivors who did marry. Studies such as ours are important to understand how the growing population of childhood cancer survivors functions in our society. Separate analyses are underway in the CCSS to better understand factors that contribute to other adult benchmarks such as living independently, achieving higher education, and personal income.
Table 6.
Multivariable regression model of the association between neurobehavioral conditions with marital status, adjusted for age at last contact
| Characteristic | Never-married | Ever-divorced | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N | % | RR (95%CI) | p-value | N | % | RR (95%CI) | p-value | ||
| Age at diagnosis (years) |
<13 | 3338 | 50.6 | 1.44 (1.24–1.66) | <.0001 | 580 | 20.5 | 1.06 (0.9–1.24) | 0.50 |
| 13–20 | 360 | 15.4 | 1.00 | . | 401 | 22.7 | 1.00 | . | |
| Gender | Female | 1692 | 38.5 | 1.15 (1.09–1.22) | <.0001 | 524 | 22.0 | 0.93 (0.81–1.07) | 0.31 |
| Male | 2006 | 44.2 | 1.00 | . | 457 | 20.7 | 1.00 | . | |
| Educational Attainment |
Did not complete high school |
193 | 50.4 | 1.06 (0.92–1.22) | 0.41 | 45 | 31.7 | 1.86 (1.34–2.59) | 0.0002 |
| Completed high school |
2139 | 46.2 | 1.11 (1.05–1.18) | 0.0002 | 579 | 26.8 | 1.67 (1.45–1.91) | <.0001 | |
| College graduate |
1323 | 34.4 | 1.00 | . | 352 | 15.5 | 1.00 | . | |
| Emotional Distress (GSI T-score ≥ 50) |
Yes | 1613 | 39.9 | 1.03 (0.97–1.08) | 0.39 | 544 | 25.3 | 1.33 (1.15–1.54) | 0.0001 |
| No | 1513 | 36.8 | 1.00 | . | 412 | 18.0 | 1.00 | . | |
| NCQ: problems task efficiency |
Yes | 935 | 49.4 | 1.27 (1.2–1.35) | <.0001 | 220 | 25.4 | 1.14 (0.97–1.35) | 0.12 |
| No | 1637 | 35.6 | 1.00 | . | 500 | 18.5 | 1.00 | . | |
| NCQ: problems organization |
Yes | 550 | 44.4 | 1.05 (0.98–1.12) | 0.17 | 141 | 22.7 | 0.95 (0.8–1.14) | 0.60 |
| No | 2022 | 38.5 | 1.00 | . | 579 | 19.7 | 1.00 | . | |
| NCQ: problems with memory |
Yes | 581 | 43.5 | 0.97 (0.91–1.04) | 0.46 | 174 | 25.9 | 1.12 (0.94–1.33) | 0.20 |
| No | 1991 | 38.6 | 1.00 | . | 546 | 18.9 | 1.00 | . | |
| NCQ: problems emotional regulation |
Yes | 666 | 41.8 | 0.9 (0.85–0.97) | 0.003 | 205 | 24.6 | 1.11 (0.95–1.31) | 0.19 |
| No | 1906 | 38.9 | 1.00 | . | 515 | 18.9 | 1.00 | . | |
APPENDIX
The Childhood Cancer Survivor Study (CCSS) is a collaborative, multi-institutional project, funded as a resource by the National Cancer Institute, of individuals who survived five or more years after diagnosis of childhood cancer. CCSS is a retrospectively ascertained cohort of 20,346 childhood cancer survivors diagnosed before age 21 between 1970 and 1986 and approximately 4,000 siblings of survivors, who serve as a control group. The cohort was assembled through the efforts of 26 participating clinical research centers in the United States and Canada. The study is currently funded by a U24 resource grant (NCI grant # U24 CA55727) awarded to St. Jude Children’s Research Hospital. Currently, we are in the process of expanding the cohort to include an additional 14,000 childhood cancer survivors diagnosed before age 21 between 1987 and 1999. For information on how to access and utilize the CCSS resource, visit www.stjude.org/ccss
APPENDIX
| CCSS Institutions and Investigators | |
|---|---|
| St. Jude Children’s Research Hospital, Memphis, TN | Leslie L. Robison, Ph.D.#‡, Melissa Hudson, M.D.*‡ |
| Greg Armstrong, M.D. ‡, Daniel M. Green, M.D. ‡ | |
| Children's Healthcare of Atlanta/Emory University Atlanta, GA |
Lillian Meacham, M.D. *, Ann Mertens, Ph.D. ‡ |
| Children's Hospitals and Clinics of Minnesota Minneapolis St. Paul, MN |
Joanna Perkins, M.D.* |
| Children’s Hospital and Medical Center, Seattle, WA | Douglas Hawkins, M.D.*, Eric Chow, M.D. ‡ |
| Children’s Hospital, Denver, CO | Brian Greffe, M.D.* |
| Children’s Hospital Los Angeles, CA | Kathy Ruccione, RN, MPH* |
| Children’s Hospital, Oklahoma City, OK | John Mulvihill, M.D.‡ |
| Children’s Hospital of Philadelphia, PA | Jill Ginsberg, M.D.*, Anna Meadows, M.D. ‡ |
| Children’s Hospital of Pittsburgh, PA | Jean Tersak, M.D. *, |
| Children’s National Medical Center, Washington, DC | Gregory Reaman, M.D.*, Roger Packer, M.D.‡ |
| Cincinnati Children’s Hospital Medical Center | Stella Davies, M.D., Ph.D.‡ |
| City of Hope- Los Angeles, CA | Smita Bhatia, M.D. *‡ |
| Dana-Farber Cancer Institute/Children’s Hospital Boston, MA |
Lisa Diller, M.D.*†, |
| Fred Hutchinson Cancer Research Center, Seattle, WA | Wendy Leisenring, Sc.D.*‡ |
| Hospital for Sick Children, Toronto, ON | Mark Greenberg, MBChB.*, Paul C. Nathan, M.D. *‡ |
| International Epidemiology Institute, Rockville, MD | John Boice, Sc.D.‡ |
| Mayo Clinic, Rochester, MN | Vilmarie Rodriguez, M.D. * |
| Memorial Sloan-Kettering Cancer Center New York | Charles Sklar, M.D.*‡, Kevin Oeffinger, M.D.‡ |
| Miller Children’s Hospital | Jerry Finklestein, MD † |
| National Cancer Institute, Bethesda, MD | Roy Wu, Ph.D.†, Nita Sibel, M.D. †, |
| Preetha Rajaraman, Ph.D. † | |
| Nationwide Children's Hospital, Columbus, Ohio | Amanda Termuhlen, M.D.*, Sue Hammond, M.D.‡ |
| Riley Hospital for Children, Indianapolis, IN | Terry A. Vik, M.D.* |
| Roswell Park Cancer Institute, Buffalo, NY | Martin Brecher, M.D. * |
| St. Louis Children’s Hospital, MO | Robert Hayashi, M.D.* |
| Stanford University School of Medicine, Stanford, CA | Neyssa Marina, M.D. *, Sarah S. Donaldson, M.D. ‡, |
| Texas Children’s Hospital, Houston, TX | Zoann Dreyer, M.D.* |
| University of Alabama, Birmingham, AL | Kimberly Whelan, M.D., MSPH* |
| University of Alberta, Edmonton, AB | Yutaka Yasui, Ph.D.‡ |
| University of California-Los Angeles, CA | Jacqueline Casillas, MD MSHS*, Lonnie Zeltzer, M.D. †‡ |
| University of California-San Francisco, CA | Robert Goldsby, M.D.* |
| University of Michigan, Ann Arbor, MI | Raymond Hutchinson, M.D.* |
| University of Minnesota, Minneapolis, MN | Joseph Neglia, M.D., MPH‡*, |
| University of Southern California | Dennis Deapen, Dr. P.H. ‡ |
| UT-Southwestern Medical Center at Dallas, TX | Dan Bowers, M.D.* |
| U.T.M.D. Anderson Cancer Center, Houston, TX | Louise Strong, M.D.*‡, Marilyn Stovall, MPH, Ph.D.‡ |
Institutional Principal Investigator
Former Institutional Principal Investigator
Member CCSS Steering Committee
Project Principal Investigator (U24 CA55727)
Footnotes
National Cancer Institute (U24-CA55727, L.L. Robison, Principal Investigator) and support to St. Jude Children’s Research Hospital from American Lebanese Syrian Associated Charities (ALSAC).
Grant Number KL2 RR024138 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research.
Conflict of Interest: none
References
- 1.Horner M, Ries L, Krapcho M, et al. SEER Cancer Statistics Review 1975–2006. Bethesda, MD: National Cancer Institute; 2009 http://seercancergov/csr/1975_2006/ based on November 2008 SEER data submission, posted to the SEER web site.
- 2.Neglia JP, Friedman DL, Yasui Y, et al. Second malignant neoplasms in five-year survivors of childhood cancer: childhood cancer survivor study. J Natl Cancer Inst. 2001;93:618–629. doi: 10.1093/jnci/93.8.618. [DOI] [PubMed] [Google Scholar]
- 3.Bassal M, Mertens AC, Taylor L, et al. Risk of selected subsequent carcinomas in survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2006;24:476–483. doi: 10.1200/JCO.2005.02.7235. [DOI] [PubMed] [Google Scholar]
- 4.Davies SM. Subsequent malignant neoplasms in survivors of childhood cancer: Childhood Cancer Survivor Study (CCSS) studies. Pediatr Blood Cancer. 2007 doi: 10.1002/pbc.21113. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz CL. Long-term survivors of childhood cancer: the late effects of therapy. Oncologist. 1999;4:45–54. [PubMed] [Google Scholar]
- 6.Gurney JG, Kadan-Lottick NS, Packer RJ, et al. Endocrine and cardiovascular late effects among adult survivors of childhood brain tumors: Childhood Cancer Survivor Study. Cancer. 2003;97:663–673. doi: 10.1002/cncr.11095. [DOI] [PubMed] [Google Scholar]
- 7.Mertens AC, Yasui Y, Neglia JP, et al. Late mortality experience in five-year survivors of childhood and adolescent cancer: the Childhood Cancer Survivor Study. J Clin Oncol. 2001;19:3163–3172. doi: 10.1200/JCO.2001.19.13.3163. [DOI] [PubMed] [Google Scholar]
- 8.Mertens AC. Cause of mortality in 5-year survivors of childhood cancer. Pediatr Blood Cancer. 2007 doi: 10.1002/pbc.21114. [DOI] [PubMed] [Google Scholar]
- 9.Lansky SB, List MA, Ritter-Sterr C. Psychosocial consequences of cure. Cancer. 1986;58:529–533. doi: 10.1002/1097-0142(19860715)58:2+<529::aid-cncr2820581320>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 10.Chang PN. Psychosocial needs of long-term childhood cancer survivors: a review of literature. Pediatrician. 1991;18:20–24. [PubMed] [Google Scholar]
- 11.Zeltzer LK, Chen E, Weiss R, et al. Comparison of psychologic outcome in adult survivors of childhood acute lymphoblastic leukemia versus sibling controls: a cooperative Children's Cancer Group and National Institutes of Health study. J Clin Oncol. 1997;15:547–556. doi: 10.1200/JCO.1997.15.2.547. [DOI] [PubMed] [Google Scholar]
- 12.Rourke MT, Hobbie WL, Schwartz L, Kazak AE. Posttrauamatic stress disorder (PTSD) in young adult survivors of childhood cancer. Pediatr Blood Cancer. 2006 doi: 10.1002/pbc.20942. [DOI] [PubMed] [Google Scholar]
- 13.Hobbie WL, Stuber M, Meeske K, et al. Symptoms of posttraumatic stress in young adult survivors of childhood cancer. J Clin Oncol. 2000;18:4060–4066. doi: 10.1200/JCO.2000.18.24.4060. [DOI] [PubMed] [Google Scholar]
- 14.Langeveld NE, Grootenhuis MA, Voute PA, de Haan RJ. Posttraumatic stress symptoms in adult survivors of childhood cancer. Pediatr Blood Cancer. 2004;42:604–610. doi: 10.1002/pbc.20024. [DOI] [PubMed] [Google Scholar]
- 15.Mitby PA, Robison LL, Whitton JA, et al. Utilization of special education services and educational attainment among long-term survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Cancer. 2003;97:1115–1126. doi: 10.1002/cncr.11117. [DOI] [PubMed] [Google Scholar]
- 16.Teta MJ, Del Po MC, Kasl SV, Meigs JW, Myers MH, Mulvihill JJ. Psychosocial consequences of childhood and adolescent cancer survival. J Chronic Dis. 1986;39:751–759. doi: 10.1016/0021-9681(86)90158-x. [DOI] [PubMed] [Google Scholar]
- 17.de Boer AG, Verbeek JH, van Dijk FJ. Adult survivors of childhood cancer and unemployment: A metaanalysis. Cancer. 2006;107:1–11. doi: 10.1002/cncr.21974. [DOI] [PubMed] [Google Scholar]
- 18.Park ER, Li FP, Liu Y, et al. Health insurance coverage in survivors of childhood cancer: the Childhood Cancer Survivor Study. J Clin Oncol. 2005;23:9187–9197. doi: 10.1200/JCO.2005.01.7418. [DOI] [PubMed] [Google Scholar]
- 19.Cherlin AJ. American marriage in the early twenty-first century. Future Child. 2005;15:33–55. doi: 10.1353/foc.2005.0015. [DOI] [PubMed] [Google Scholar]
- 20.Holmes HA, Holmes FF. After ten years, what are the handicaps and life styles of children treated for cancer? An examination of the present status of 124 such survivors. Clin Pediatr (Phila) 1975;14:819–823. doi: 10.1177/000992287501400906. [DOI] [PubMed] [Google Scholar]
- 21.Kokkonen J, Vainionpaa L, Winqvist S, Lanning M. Physical and psychosocial outcome for young adults with treated malignancy. Pediatr Hematol Oncol. 1997;14:223–232. doi: 10.3109/08880019709009492. [DOI] [PubMed] [Google Scholar]
- 22.Stam H, Grootenhuis MA, Last BF. The course of life of survivors of childhood cancer. Psychooncology. 2005;14:227–238. doi: 10.1002/pon.839. [DOI] [PubMed] [Google Scholar]
- 23.Zebrack BJ, Chesler M. Health-related worries, self-image, and life outlooks of long-term survivors of childhood cancer. Health Soc Work. 2001;26:245–256. doi: 10.1093/hsw/26.4.245. [DOI] [PubMed] [Google Scholar]
- 24.Byrne J, Fears TR, Steinhorn SC, et al. Marriage and divorce after childhood and adolescent cancer. Jama. 1989;262:2693–2699. [PubMed] [Google Scholar]
- 25.Green DM, Zevon MA, Hall B. Achievement of life goals by adult survivors of modern treatment for childhood cancer. Cancer. 1991;67:206–213. doi: 10.1002/1097-0142(19910101)67:1<206::aid-cncr2820670134>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 26.Langeveld NE, Ubbink MC, Last BF, Grootenhuis MA, Voute PA, De Haan RJ. Educational achievement, employment and living situation in long-term young adult survivors of childhood cancer in the Netherlands. Psychooncology. 2003;12:213–225. doi: 10.1002/pon.628. [DOI] [PubMed] [Google Scholar]
- 27.Li FP, Stone R. Survivors of cancer in childhood. Ann Intern Med. 1976;84:551–553. doi: 10.7326/0003-4819-84-5-551. [DOI] [PubMed] [Google Scholar]
- 28.Nagarajan R, Neglia JP, Clohisy DR, et al. Education, employment, insurance, and marital status among 694 survivors of pediatric lower extremity bone tumors: a report from the childhood cancer survivor study. Cancer. 2003;97:2554–2564. doi: 10.1002/cncr.11363. [DOI] [PubMed] [Google Scholar]
- 29.Pastore G, Mosso ML, Magnani C, Luzzatto L, Bianchi M, Terracini B. Physical impairment and social life goals among adult long-term survivors of childhood cancer: a population-based study from the childhood cancer registry of Piedmont, Italy. Tumori. 2001;87:372–378. doi: 10.1177/030089160108700603. [DOI] [PubMed] [Google Scholar]
- 30.Frobisher C, Lancashire ER, Winter DL, Jenkinson HC, Hawkins MM. Long-term population-based marriage rates among adult survivors of childhood cancer in Britain. Int J Cancer. 2007;121:846–855. doi: 10.1002/ijc.22742. [DOI] [PubMed] [Google Scholar]
- 31.Robison LL, Mertens AC, Boice JD, et al. Study design and cohort characteristics of the Childhood Cancer Survivor Study: a multi-institutional collaborative project. Med Pediatr Oncol. 2002;38:229–239. doi: 10.1002/mpo.1316. [DOI] [PubMed] [Google Scholar]
- 32.Rauck AM, Green DM, Yasui Y, Mertens A, Robison LL. Marriage in the survivors of childhood cancer: a preliminary description from the Childhood Cancer Survivor Study. Med Pediatr Oncol. 1999;33:60–63. doi: 10.1002/(sici)1096-911x(199907)33:1<60::aid-mpo11>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 33.Derogatis LR. BSI-18 Administration, Scoring, and Procedures Manual. Minneapolis, MN: National Computer Systems; 2000. [Google Scholar]
- 34.Zabora J, BrintzenhofeSzoc K, Jacobsen P, et al. A new psychosocial screening instrument for use with cancer patients. Psychosomatics. 2001;42:241–246. doi: 10.1176/appi.psy.42.3.241. [DOI] [PubMed] [Google Scholar]
- 35.Recklitis CJ, Rodriguez P. Screening childhood cancer survivors with the brief symptom inventory-18: classification agreement with the symptom checklist-90-revised. Psychooncology. 2007;16:429–436. doi: 10.1002/pon.1069. [DOI] [PubMed] [Google Scholar]
- 36.Krull KR, Gioia G, Ness KK, et al. Reliability and validity of the Childhood Cancer Survivor Study Neurocognitive Questionnaire. Cancer. 2008 doi: 10.1002/cncr.23809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–130. [PubMed] [Google Scholar]
- 38.Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol. 2005;162:199–200. doi: 10.1093/aje/kwi188. [DOI] [PubMed] [Google Scholar]
- 39.Muthen K, Muthen B. Mplus User's Guide. Fourth ed. Los Angeles: Muthen & Muthen; 2007. [Google Scholar]
- 40.Kalton G, Kasprzyk D. The treatment of missing survey data. Survey Methodology. 1986;12:1–16. [Google Scholar]
- 41.Browne M, Cudeck R. Alternative ways of assessing model fit. In: Bollen KA, Long JA, editors. Testing structural equation models. Newbury Park, CA: Sage; 1993. pp. 136–162. [Google Scholar]
- 42.Hu L, Bentler P. Cutoff criteria for fit indices in covariance structure analysis: conventional criteria versus new alternatives. Structural Equation Modeling. 1999;6:1–55. [Google Scholar]
- 43.Bollen K. Overall fit in covariance structure models: two types of sample size effects. Psychology Bulletin. 1990;107:256–259. [Google Scholar]
- 44.Herpin N. Love, careers, and heights in France, 2001. Econ Hum Biol. 2005;3:420–449. doi: 10.1016/j.ehb.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 45.Pawlowski B, Dunbar RI, Lipowicz A. Tall men have more reproductive success. Nature. 2000;403:156. doi: 10.1038/35003107. [DOI] [PubMed] [Google Scholar]
- 46.Pierce C. Body height and romantic attraction: a meta-analytic test of the male-taller norm. Social Behavior and Personality. 1996;24:143–150. [Google Scholar]
- 47.Pawlowski B, Koziel S. The impact of traits offeredin personal advertisements on response rates. Evolution and Human Behavior. 2002;23:139–149. [Google Scholar]
- 48.Mueller U, Mazur A. Evidence of unconstrained directional selction for male tallness. Behavioral Ecology and Sociobiology. 2001;50:302–311. [Google Scholar]
- 49.Pui CH, Cheng C, Leung W, et al. Extended follow-up of long-term survivors of childhood acute lymphoblastic leukemia. N Engl J Med. 2003;349:640–649. doi: 10.1056/NEJMoa035091. [DOI] [PubMed] [Google Scholar]
- 50.Barrera M, Shaw AK, Speechley KN, Maunsell E, Pogany L. Educational and social late effects of childhood cancer and related clinical, personal, and familial characteristics. Cancer. 2005;104:1751–1760. doi: 10.1002/cncr.21390. [DOI] [PubMed] [Google Scholar]
- 51.Robison LL, Armstrong GT, Boice JD, et al. The Childhood Cancer Survivor Study: a National Cancer Institute-supported resource for outcome and intervention research. J Clin Oncol. 2009;27:2308–2318. doi: 10.1200/JCO.2009.22.3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leisenring WM, Mertens AC, Armstrong GT, et al. Pediatric cancer survivorship research: experience of the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27:2319–2327. doi: 10.1200/JCO.2008.21.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]