Abstract
Neuroanatomical research suggests that interactions between dopamine and glutamate within the mesolimbic dopamine system are involved in both drug-induced locomotor stimulation and addiction. Therefore, genetically determined differences in the locomotor responses to ethanol and cocaine may be related to differences in the effects of these drugs on this system. To test this, we measured drug-induced changes in dopamine and glutamate within the nucleus accumbens (NAcc), a major target of mesolimbic dopamine neurons, using in-vivo microdialysis in selectively bred FAST and SLOW mouse lines, which were bred for extreme sensitivity (FAST) and insensitivity (SLOW) to the locomotor stimulant effects of ethanol. These mice also show a genetically correlated difference in stimulant response to cocaine (FAST > SLOW). Single injections of ethanol (2 g/kg) or cocaine (40 mg/kg) resulted in larger increases in dopamine within the NAcc in FAST compared to SLOW mice. There was no effect of either drug on NAcc glutamate levels. These experiments indicate that response of the mesolimbic dopamine system is genetically correlated with sensitivity to ethanol- and cocaine- induced locomotion. Because increased sensitivity to the stimulating effects of ethanol appears to be associated with greater risk for alcohol abuse, genetically determined differences in the mesolimbic dopamine response to ethanol may represent a critical underlying mechanism for increased genetic risk for alcoholism.
Keywords: alcoholism, alcohol abuse, psychostimulant, selective breeding, behavioral genetics, motor behavior, cocaine, mouse, catecholamine, excitatory amino acid
Introduction
Sensitivity to ethanol-induced stimulation, and insensitivity to ethanol-induced depression, are positively associated with propensity to self-administer ethanol (King et al. 2002; Kulkosky et al. 1993; Risinger et al. 1994; Schuckit 1994; Waller et al. 1986). In humans, sensitivity is measured as alterations in subjective mood (King et al. 2002; Schuckit et al. 2008), or changes in physical activity and body sway after alcohol (Addicott et al. 2007; Schuckit 1994). Locomotor stimulation and depression are used as measures of sensitivity in mice (Moore et al. 1993; Phillips et al. 2002). Through selective breeding, lines of mice were developed that possess genotype-dependent differences in both measures of ethanol sensitivity (Crabbe et al. 1987; Phillips et al. 2002) as a model of similar differences in humans. The line more sensitive to the stimulant and less sensitive to the sedative effects of ethanol (FAST) voluntarily consumes larger amounts of ethanol than does the line of mice (SLOW) with the opposite sensitivity phenotypes (Risinger et al. 1994). These selected lines provide a definitive means of answering questions about the genetic association between sensitivity to ethanol and ethanol-induced neurochemical effects, such as dopaminergic responses.
The mesolimbic dopamine system plays a crucial role in motivated behavior and locomotion (Mogenson & Yang 1991). Ethanol, given systemically or directly into the ventral tegmental area, causes increases in extracellular dopamine levels in the nucleus accumbens (NAcc) (Imperato & Di Chiara 1986; Yim & Gonzales 2000), as do other drugs of abuse (Amalric & Koob 1993; Ikemoto & Panksepp 1999; Tzschentke & Schmidt 2000). Dopamine antagonists block the locomotor stimulant response to ethanol in FAST mice (Shen et al. 1995), and FAST and SLOW mice are differentially sensitive to drugs that activate the mesolimbic dopamine system (Bergstrom et al. 2003).
The FAST and SLOW lines permit a direct test of the hypothesis that magnitude of drug-induced dopamine in the NAcc is genetically related to sensitivity to ethanol stimulation because they were bred for differential sensitivity to this ethanol effect. Also, because ethanol inhibits the N-methyl-D-aspartate subclass of glutamate receptors (Lovinger et al. 1989), has effects on glutamate transmission in the NAcc (Dahchour et al. 2000; Kapasova & Szumlinski 2008; Lominac et al. 2006; Moghaddam & Bolinao 1994; Piepponen et al. 2002), and FAST and SLOW mice are differentially sensitive to glutamatergic drugs (Meyer & Phillips 2003), we hypothesized that ethanol would differentially regulate glutamate transmission in the NAcc in these mouse lines. Finally, since sensitivity to ethanol and cocaine activation are genetically correlated in these mice, and cocaine increases dopamine levels, we predicted that FAST mice would show a greater cocaine-induced NAcc dopamine increase. Repeated cocaine administration elicits sensitization of NAcc glutamate (Pierce et al. 1996), but acute cocaine typically fails to elicit increases in NAcc glutamate, except at higher doses that have large effects on behavior (Smith et al. 1995). Because FAST mice are particularly sensitive to the locomotor stimulant effects of cocaine, we hypothesized that acute cocaine treatment would increase NAcc glutamate in FAST, but not SLOW, mice.
Materials and Methods
Subjects
The FAST and SLOW lines of mice were selectively bred in two replicates from an 8-way cross of genetically divergent inbred strains for extreme sensitivity (FAST-1, FAST-2) and insensitivity (SLOW-1, SLOW-2) to ethanol-induced locomotor stimulation (Crabbe et al. 1987; Phillips et al. 1991; Shen et al. 1995). These mice also exhibit a genetically correlated difference in sensitivity to the depressant effects of ethanol (FAST < SLOW), measured as duration of loss of the righting response after administration of a sedative dose of ethanol (Phillips et al. 2002). In ethanol dose-response (0.5–3.0 g/kg) studies measuring locomotor activity, FAST mice had biphasic dose-response curves with stimulation occurring at most doses, whereas the locomotor activity of SLOW mice was either unaffected or depressed by all doses of ethanol (Palmer et al. 2002a; Shen et al. 1995). Testing occurred between 0800 and 1600 h (the colony lights were on from 06:00 to 18:00). Room temperature was maintained between 20 and 22 °C in the colony and testing rooms. Mice were 50 to 100 days old and weighed 14 to 30 g at the time of surgery. Only males were available for use in these experiments. All procedures were performed in accordance with the Institutional Animal Care and Use Committee and National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. Experiments were designed in such a way as to minimize suffering and utilize the smallest number of mice possible. Final group sizes for each experiment are presented in the results section.
Drugs
Ethanol (Pharmco Products, Brookfield, CT) was diluted from 100% to a final concentration of 20 % (v/v) in 0.9% saline. Mice were injected intraperitoneally (i.p.) with 2 g/kg ethanol. Cocaine HCl (40 mg/kg; Sigma, St. Louis, MO) was injected i.p. at a volume of 10 ml/kg. “Surgical cocktail” was purchased from the Portland Veterans Affairs Medical Center pharmacy, and contained 5 ml ketamine (100 mg/ml), 2.5 ml xylazine (20 mg/ml), 1.5 ml sterile NaCl solution, and 1 ml acepromazine (10 mg/ml). This stock solution was diluted 1:6 in saline for injection, resulting in an anesthetic/hypnotic dose of 140 mg/kg ketamine, 14 mg/kg xylazine and 2.8 mg/kg acepromazine.
We chose to use the single 2 g/kg dose of ethanol because the FAST and SLOW mice were selectively bred based on their response to this dose for the final 32 of 37 total generations of selection (Crabbe et al. 1987; Phillips et al. 1991), and they show their largest locomotor response difference after treatment with this dose (Crabbe et al. 1987; Palmer et al. 2002a; Phillips et al. 1991; Shen et al. 1995). In addition, dose-response studies have suggested that the dopaminergic effect of ethanol peaks near this dose, and does not differ substantially from that seen after treatment with1.5 g/kg and 3 g/kg ethanol doses (Piepponen et al. 2002; Ramachandra et al. 2007; Szumlinski et al. 2007). Similarly, we chose the 40 mg/kg dose of cocaine because FAST and SLOW mice show the largest behavioral differences at this dose and not at other doses (Bergstrom et al. 2003).
Activity monitors
Mice were tested in clear acrylic plastic boxes (40 cm long × 40 cm wide × 30 cm high), that were placed in automated activity monitors (Accuscan Instruments, Columbus, OH). Occlusion of 8 intersecting photobeams were automatically recorded and used by Accuscan software to calculate the distance traveled (in cm) by a mouse during the test sessions. The activity monitors were housed in individual, opaque sound attenuation chambers (Flair Plastics, Portland, OR) that were each illuminated during testing by a 15 W fluorescent bulb, and ventilated by a fan that also masked external noise.
Surgery
The surgical, microdialysis, and high pressure liquid chromatography (HPLC) procedures described below were adapted from previous studies in this and other laboratories (Boehm et al. 2002; McKee & Meshul 2005; Meshul et al. 1999; Olive et al. 2000). After anesthetization with surgical cocktail and placement in a stereotaxic frame (SAS75 Stereotaxic Alignment System, Cartesian Research, Inc, Sandy, OR), a plastic CMA/7 guide cannula (CMA Microdialysis, Stockholm, Sweden) was surgically placed through a hole drilled above the left NAcc so that the tip was positioned above the NAcc, using the following coordinates (relative to bregma): 1.4 mm rostral, 1.0 mm lateral, and 2.9 mm ventral. A stainless steel stylette was inserted into the cannula to prevent clogging. The cannula was secured with dental acrylic and a tethering post (Instech Laboratories, Plymouth Meeting, PA) was also cemented to the skull. Mice were allowed to recover for 3-14 days before microdialysis measures were taken.
Microdialysis
On the evening before testing began (between 15:00 and 20:00 h), one FAST and one SLOW mouse were moved to the testing room, weighed, and lightly anesthetized with a subhypnotic dose of surgical cocktail that provided adequate sedation to allow probe insertion and attachment to the wire tether. CMA/7 concentric microdialysis probes (CMA; 6 kDa cut-off; 0.24 mm outer diameter; 1 mm exposed cuprophane membrane) were inserted into the cannulae. Each mouse was then tethered to a dual-channel microdialysis swivel (Instech) via a wire attached to the tethering post, and the swivel was attached to a counterbalanced lever arm (Instech) mounted on the activity chamber so that the swivel was suspended above the middle of the chamber. The inlet and outlet channel of the swivel were connected to the probe tubing using polyethylene (PE) tubing (0.12 mm inner diameter). Artificial cerebral spinal fluid (aCSF) containing 145 mM NaCl, 2.8 mM KCl, 1.2 mM CaCl, 1.2 mM MgCl2, and 5.4 mM D-glucose, was delivered to the dialysis probe using a 2.5 ml glass syringe (CMA) at a rate of 2 μl/min. Because we have found that aCSF solutions with pHs higher than 5.6 promote the spontaneous oxidation of dopamine (unpublished data), the pH was adjusted to 5.6, consistent with previous studies (Yim & Gonzales 2000). This prevented the oxidation of dopamine in the microdialysis tubing, and has been shown to not alter basal dopamine release in the nucleus accumbens (Thomas & Holman 1989). Once tethered, mice were placed into the activity monitors, and sources of rodent chow and tap water were provided overnight.
Experimental testing and sample collection began 12-17 hours after probe implantation. Food and water were removed, and dialysate and activity data were collected in 15 min epochs for the ethanol study and 20 min epochs for the cocaine study. The shorter sample length was used for the ethanol experiment to ensure that the time course of this relatively fast-acting drug could be observed. Dialysate (30-40 μl) samples were collected in 0.4 ml glass microvials (Agilent Technologies, Palo Alto, CA), sealed with mini crimp-tops (Agilent Technologies) that contained 2 μl of a solution containing 20 mM oxalic acid and 2 M acetic acid (Kankaanpaa et al. 2001), to prevent the spontaneous oxidation of dopamine. Since the sensitivity of the HPLC assay dictates the amount of sample needed, we chose 15-20 min fractions to provide a sufficient amount of sample, while still offering some temporal resolution, and corresponding with ideal times for measuring the locomotor stimulant response to ethanol and cocaine. After one hour of basal activity testing, each mouse was removed from the activity monitors, injected with saline, and returned to the activity monitors. After an hour of post-saline sample collection, each mouse was injected with 2 g/kg ethanol or 40 mg/kg cocaine and returned to the monitors. A high potassium containing aCSF (100 mM KCl) was then perfused for 15-20 min, depending on the experiment, and then normal aCSF was perfused for the remainder of the experiment. This procedure results in the depolarization of terminals within the vicinity of the probe, and was used to determine whether the probe was functioning properly and had been placed within a dopamine-containing area of the brain. Dialysate samples were frozen at -40°C until analyzed by HPLC.
High Pressure Liquid Chromatography (HPLC)
Dopamine levels in the dialysate fractions were measured using HPLC coupled with electrochemical detection, as described previously (Olive et al. 2000). An ESA 582 isocratic solvent delivery system (ESA Inc, North Chelmsford, MA) was used to pump mobile phase (10% Acetonitrile, 90 mM sodium phosphate, 50 mM citric acid, 1.7 mM octanesulfonic acid, 50 μM ethylenediaminetetraacetic acid, pH: 5.6) at a flow rate of 0.34 - 0.6 ml/min. This flow rate was varied from sample to sample to promote separation from other oxidizable substances, and to compensate for changes in elution times, which occurred as a result of gradual column degradation. Under these conditions, dopamine metabolites were not measurable. For electrochemical detection using the ESA electrochemical detector (Model Coulochem III), the reducing electrode was set at −100 mV and the oxidizing electrode was set at either 200 or 280 mV. 20 μl samples were injected onto a C18 column (ESA model MD-150, 3-mm inner diameter, 150 mm long, 3-μm particle size) using an ESA 542 autosampler. The column temperature was between 27-35 °C. Column temperature was varied between samples to promote separation of dopamine from other substances. Dialysate levels of dopamine were calculated from a dopamine standard curve (0.15 to 14 nM), prepared at the time of sample collection. The detection limit of this assay was greater than 50 fmol.
Glutamate concentration in dialysate fractions was determined using a Hewlett Packard 1090 interfaced with a Hewlett Packard 1046A fluorescence detector (Agilent Technologies), as described previously (McKee & Meshul 2005). Samples were derivatized 1 min before injection with o-phthalaldehyde (Sigma) by adding 1 μl of sample, 5 μl of borate buffer (pH 10.4) and 1 μl of o-phthalaldehyde. The entire reaction mixture was injected onto a reverse-phase C18 column (Agilent Technologies) and o-phthalaldehyde derivatives were separated using a 5-min linear gradient (flow rate: 0.45 ml/min) of two mobile phases. Mobile phase A contained 0.018% (v/v) tetraethylammonium, 0.3% (v/v) tetrahydrofuran and 20 mM sodium acetate buffer, pH 7.2. Mobile phase B contained 40% (v/v) acetonitrile, 40% (v/v) methanol and 20% (v/v) 100 mM sodium acetate, (all purchased from Sigma) pH 7.4. The o-phthalaldehyde derivatives of glutamate were detected by fluorescence using an excitation wavelength of 340 nm and an emission wavelength of 450 nm. Standard solutions, prepared at the time of sample collection, contained 0.125 to 5 μM glutamate. The detection limit of this assay was greater than 50 fmol.
Histology
At the end of the experiment, methylene blue (Sigma; 10 mg/ml in saline) was perfused through the probe to provide a marker for accurate histological analysis of probe placement. Brains were removed, frozen, and stained with thionin using standard methods (Boehm et al. 2002). Pictures of the brain sections were taken before and after staining (see Figure 1). If a probe was not at least 50% within the boundaries of the NAcc, the data from that mouse were excluded from all data analyses.
Figure 1.
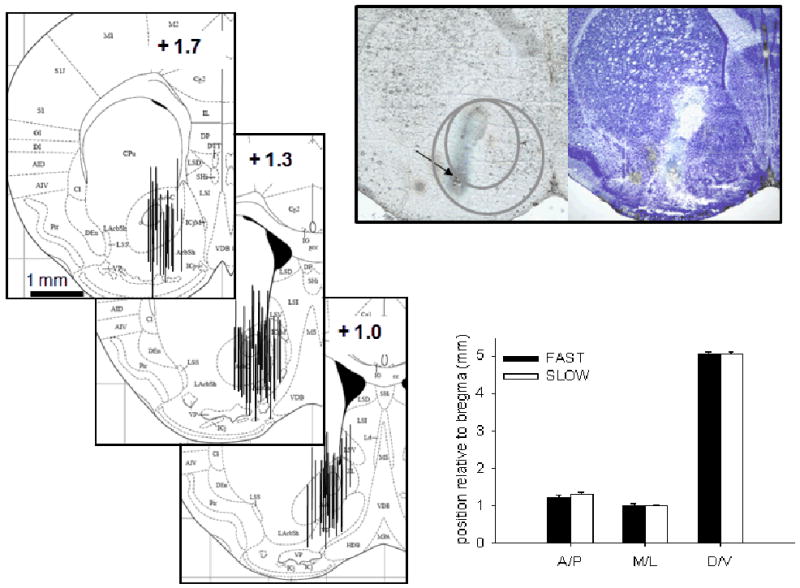
Location of microdialysis probes for both experiments. The figures on the left depict approximate locations of microdialysis probes, according to Paxinos and Franklin (2001). Numbers in the insets depict the distance relative to bregma in mm. The bar graph compares the mean position of the probes, in three spatial dimensions, between FAST and SLOW mice. Mice were S37G74 – S37G80 offspring, where Sxx indicates the selection generation and Gxx indicates the elapsed number of total generations, including those since selection was relaxed. The micrographs depict brain sections, before and after staining with thionin, showing the location of the microdialysis probe as marked by methylene blue (arrow). Inner and outer grey circles denote the approximate boundaries of the NAcc core and shell, respectively. A/P: anterior/posterior; M/L medial/lateral; D/V dorsal/ventral. Data are represented as means ± standard error of the mean (SEM).
Statistics
Dependent measures were the average of the post-saline or post-drug time points for both experiments. For the ethanol experiment, this was the average of the four, 15-min time periods after saline injection and the four after ethanol injection. For the cocaine experiment, this was the average of the three, 20-min time periods after saline injection and the three after cocaine injection. The shorter sample length was used for the ethanol experiment to ensure that the time course of this relatively fast-acting drug could be observed. Locomotor activity data were compared using repeated measures ANOVA (Statistica 6.1, Statsoft, Inc., Tulsa,OK), with Selected Line (FAST, SLOW) and Replicate (1, 2) as between-groups factors and Time (Pre, Post injection) as a within-group factor. For dialysate data, dopamine and glutamate concentrations after drug treatment were expressed as percent relative to the averaged post-saline samples, and analyzed with factorial ANOVA with Selected Line (FAST, SLOW) and Replicate (1, 2) as between groups factors. Main effects and interactions were considered significant at p ≤ 0.05.
Results
Experiment 1: Ethanol-induced increases in NAcc dopamine and glutamate
Activity
Data from 11 mice (six FAST, five SLOW) with undetectable levels of dialysate dopamine or less than 100% increase in dopamine after perfusion of 100 mM potassium were excluded due to dysfunctional probes. In addition, data from three FAST mice and two SLOW mice were incomplete due to technical problems encountered during microdialysis and HPLC analysis. Additional data from two SLOW mice were excluded as statistical outliers (greater than 3 standard deviations above the mean). Thus, the final analysis included data from 13-17 mice within each Selected Line and Replicate category.
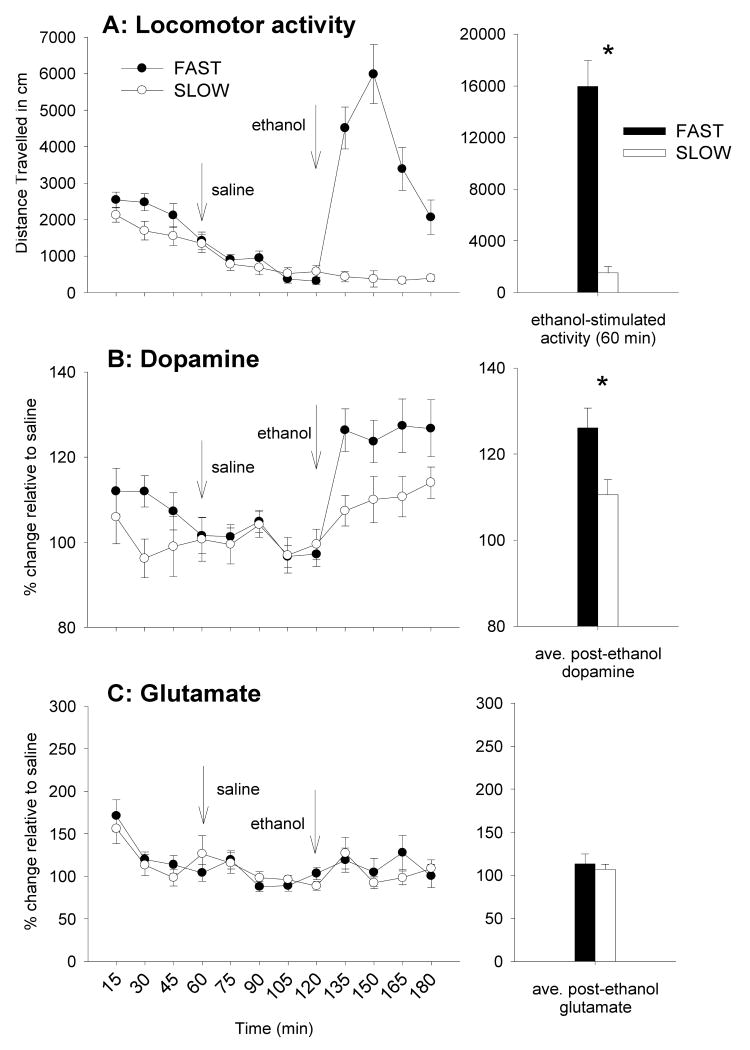
The time courses of basal, post-saline, and post-ethanol activity levels are shown in Figure 2A. The Time by Replicate by Selected Line analysis revealed no systematic interactions with replicate, therefore data are combined for the two replicates within each selected line (n= 31 FAST and 26 SLOW mice). There was a significant interaction of Time and Selected Line [F (1, 55) = 44.5; p < 0.01]. Simple effect analyses indicated that the selected lines did not differ in activity level after saline treatment, but they did after ethanol treatment at all time points (p < 0.01). In addition, FAST mice were significantly stimulated by ethanol (p < 0.01), but SLOW mice were not.
Figure 2.
Behavioral and neurochemical responses to ethanol in FAST and SLOW mice. Panels A, B, and C, left, reflect the time-courses of the locomotor, dopaminergic, and glutamatergic responses to ethanol, respectively. Arrows indicate injections of saline and ethanol at t = 60 and 120 min, respectively. The bar graphs on the right represent the average of the four, 15-min time points after ethanol injection. Asterisks reflect statistically significant differences between the selected lines. Data are represented as means ± standard error of the mean (SEM).
Dopamine
Dialysate dopamine levels during basal, post-saline, post-ethanol, and potassium perfusion periods are shown in Figure 2B. Basal concentrations of dopamine are displayed in Table 1; there were no significant differences between FAST and SLOW mice. Therefore, all data were expressed as percent change relative to the average of the four post-saline time points. FAST mice had a larger dopamine response to ethanol, as reflected by a significant effect of Selected Line [F (1, 55) = 6.7; p < 0.05], that did not interact with replicate; data are shown with the replicates combined. There was no selected line difference in potassium-stimulated increase in dopamine (data not shown), suggesting that the availability of releasable dopamine was not different between FAST and SLOW mice.
Table 1.
Basal levels of dopamine and glutamate during experiments 1 and 2. Values are reported as nM for dopamine and μM for glutamate. Standard errors of the mean are presented in parentheses.
| Exp 1: Dopamine (nM) | Exp 1: Glutamate (μM) | Exp 2: Dopamine (nM) | Exp 2: Glutamate (μM) | |
|---|---|---|---|---|
| FAST mice | 1.47 (0.27) | 1.06 (0.18) | 1.53 (0.25) | 1.03 (0.11) |
| SLOW mice | 1.66 (0.31) | 0.82 (0.12) | 1.62 (0.24) | 1.10 (0.24) |
Glutamate
Dialysate glutamate concentrations are shown in Figure 2C. Basal concentrations of glutamate are displayed in Table 1; there were no significant differences between FAST and SLOW mice or between the two replicates. While there were potassium-stimulated increases in glutamate, there was no difference in the peak glutamate response between FAST and SLOW mice (data not shown).
Histology
Probe placement was 92% accurate in this study; data from three of the five animals with placements that occurred outside of the NAcc had already been removed based on a lack of dialysate dopamine or no response to high potassium aCSF, and the other two poorly placed probes were placed posterior to the NAcc. Data from these mice were removed from all analyses. Microdialysis probe placement is shown in Figure 1. There were no apparent differences in probe placement between FAST and SLOW mice of either replicate. Probes tended to be located within both the core and shell of the NAcc. Sometimes probes encompassed portions of the caudate-putamen or the ventral pallidum.
Experiment 2: Cocaine-induced increases in NAcc dopamine and glutamate
Samples from six mice (two FAST, four SLOW) with undetectable levels of dialysate dopamine or less than 100% increase in dopamine after perfusion of 100 mM potassium were removed from all analyses. Two FAST and two SLOW mice died during testing, and data from two SLOW mice were excluded as statistical outliers (greater than 3 standard deviations above the mean). There were data from 8-12 mice within each Selected Line and Replicate category.
Activity
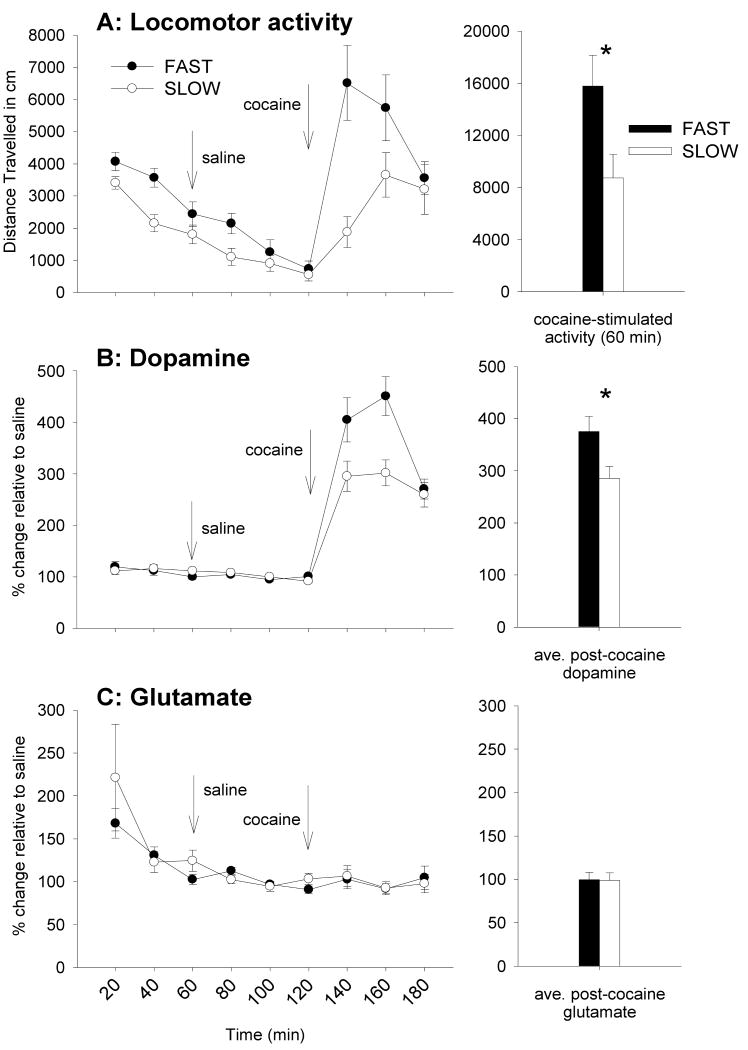
The time courses of basal, post-saline, and post-cocaine activity levels are shown in Figure 3A. The Time by Replicate by Selected Line analysis revealed no interactions with replicate, therefore data are combined for the two replicates within each selected line (n= 24 FAST and 19 SLOW mice). There was a significant interaction of Time and Selected Line [F (1, 41) = 4.1; p = 0.5]. Simple effect analyses indicated that the selected lines did not differ in activity level after saline treatment, but they did after cocaine treatment, during the first post-cocaine time point, with FAST mice showing greater activation (p < 0.05). Both selected lines were significantly stimulated by cocaine (ps < 0.01).
Figure 3.
Behavioral and neurochemical responses to cocaine in FAST and SLOW mice. Panels A, B, and C, left, reflect the time-courses of the locomotor, dopaminergic, and glutamatergic responses to cocaine, respectively. Arrows indicate injections of saline and cocaine at t = 60 and 120 min, respectively. The bar graphs on the right represent the average of the three, 20-min time points after cocaine injection. Asterisks reflect statistically significant differences between the selected lines. Data are represented as means ± standard error of the mean (SEM). Please note that some scales differ from those used in figure 2 to represent better the data.
Dopamine
Basal and post-saline dopamine dialysate concentrations are shown in Table 1, and did not significantly differ between FAST and SLOW mice; therefore, data are expressed as percentages relative to the post-saline period (Figure 3B). There were no differences in cocaine-induced increases between the replicates, so data were analyzed and presented collapsed across replicate. FAST mice showed a significantly larger dopaminergic response to cocaine, compared to SLOW mice [F (1, 41) = 5.4; p < 0.05]. There were no differences in potassium-stimulated increases in dopamine between the selected lines (data not shown).
Glutamate
Basal levels of glutamate are shown in Table 1, and saline and cocaine-induced levels are shown in Figure 3C. There were elevated glutamate levels during the first hour of sampling that had stabilized after the saline injection period. Cocaine did not significantly alter glutamate levels. Infusion of potassium through the microdialysis probes was associated with an increase in glutamate that was similar for the FAST and SLOW lines (data not shown).
Histology
Probe placement was 93% accurate in this study, the data from three mice that had probe placements outside of the NAcc had already been excluded due to lack of dialysate dopamine or no potassium-stimulated increases in dopamine. There were no apparent differences in probe placement between FAST and SLOW mice (Figure 5). The probes typically encompassed both the shell and the core of the NAcc, but sometimes included the striatum and the ventral pallidum as well.
Discussion
Selectively bred lines provided us with a means for testing the hypothesis that heightened sensitivity to the locomotor stimulant effects of ethanol is genetically related to magnitude of dopamine response to ethanol and cocaine in the mesoaccumbens dopamine pathway. Pharmacological studies have suggested that this relationship exists (Jerlhag 2008), and at least one microdialysis study comparing a pair of inbred strains that displays differential sensitivity to ethanol (2 g/kg) stimulation has found this association (Kapasova & Szumlinski 2008), while another has not (Zapata et al. 2006). However, those studies could not differentiate the genetic from non-genetic sources of the association. The FAST and SLOW lines are the only animals that have been specifically bred for behavioral stimulation to ethanol, and a substantial involvement of the basal ganglia in the segregation of the FAST and SLOW lines has been suggested (Demarest et al. 1999). Here, the greater dopamine response to ethanol and to cocaine in FAST compared to SLOW mice that was found in both replicate sets of selected lines offers strong evidence for common genetic regulation of the behavioral and mesoaccumbens dopamine responses to these drugs.
Breeding for differential sensitivity to ethanol-induced locomotion also resulted in a difference in sensitivity to the sedative effects of ethanol, a difference that increased in magnitude across generations of selection (Phillips et al. 2002). Thus, the FAST line represents a model of heightened sensitivity to ethanol-induced stimulation, reduced sensitivity to ethanol-induced sedation and increased dopamine response in at least one reward-related brain region. Inasmuch as the magnitude of increases in accumbal dopamine is associated with the intensity of reinforcement (Phillips et al. 1989), FAST mice may be expected to exhibit increased sensitivity to the rewarding effects of ethanol. FAST mice exhibited increased voluntary ethanol consumption compared to SLOW mice, although the selected lines did not differ when tested for sensitivity to the conditioned rewarding effect of ethanol (0.8-2.0 g/kg) in a place conditioning paradigm (Risinger et al. 1994). This suggests that the genes that influence these two putative measures of ethanol reward are at least partially genetically independent. There have been no studies of the rewarding effects of cocaine in FAST and SLOW mice, but we would expect greater reward sensitivity in FAST mice, given the current post-cocaine dopamine results and the positive association between ethanol and cocaine consumption in lines of mice selected for sensitivity to methamphetamine-induced locomotion (Kamens et al. 2006). However, because measurements of drug reinforcement and reward can be influenced by taste factors, response to novelty, and other variables (Blednov et al. 2008; Blizard & McClearn 2000; Green & Grahame 2008; Orsini et al. 2004), alternative explanations would have to be carefully considered in comparisons of FAST and SLOW mice.
Mesolimbic dopamine as a genetic correlate of ethanol- and cocaine-induced locomotion
A fundamental difference in dopaminergic function between FAST and SLOW mice might explain their differential sensitivity, not just to psychostimulants like cocaine (Bergstrom et al. 2003), but also to morphine (Bergstrom et al. 2003; Holstein et al. 2005), benzodiazepines and barbiturates (Palmer et al. 2002a; Phillips et al. 1992), and ketamine (Meyer & Phillips 2003). While these studies were unable to determine whether there were differences in basal NAcc dopamine levels, data obtained from the potassium perfusion experiments indicated that the FAST and SLOW mice do not differ in the availability of releasable dopamine (Cosford et al. 1994; Ripley et al. 1997). However, the greater sensitivity of FAST compared to SLOW mice to the stimulant effects of cocaine and ethanol corresponded with larger increases in NAcc dopamine. This indicates that the mesolimbic dopamine system was altered during selective breeding for sensitivity to ethanol's locomotor effects. Consistent with this conclusion are data from our recent study of the electrical properties of dopamine neurons. In non-ethanol treated ventral midbrain slices, dopamine neuron pacemaker firing was faster, and ethanol produced a larger increase in spontaneous dopamine neuron firing, in FAST compared to SLOW mice (Beckstead & Phillips, accepted pending revision).
That FAST mice exhibit profound locomotor stimulation to cocaine suggests that they are not engaging in significant stereotyped behaviors; however, greater sensitivity of SLOW mice to stereotypic effects of cocaine could be related to their blunted stimulant response. In addition, because degree of cocaine-induced stereotypy is associated with dopamine uptake inhibition in the NAcc (Budygin 2007), one might expect to see greater dopamine levels after cocaine in the SLOW than FAST mice if the behavioral difference was due to heightened stereotypy in SLOW mice. We recently measured cocaine-induced stereotypy in FAST and SLOW mice (data not shown). In this study, we examined a battery of behaviors including chewing, exophthalmos (eye bulging), circling and line crossing after saline, 10, 20 or 40 mg/kg cocaine, and found little evidence for stereotypic behaviors induced by these doses. For line crossing, only the 40 mg/kg cocaine dose differentiated the FAST from SLOW mice, with FAST showing greater locomotor stimulation than SLOW, consistent with the data shown in Figure 3A.
The quantitative relationship between drug-induced locomotor stimulation and elevated accumbal extracellular dopamine levels does not appear straightforward. In FAST mice there was a substantial difference in the extent to which dopamine was increased by ethanol (∼30%) compared to cocaine (∼350%), whereas the behavioral activation induced by these drugs was similar. SLOW mice displayed behavioral differences to ethanol versus cocaine that were correlated more closely with changes in dopamine. It is possible that degree of behavioral stimulation is limited by a ceiling effect, in which a mouse cannot increase locomotor activity above a certain level. Thus, in the presence of larger increases in dopamine, a larger amount of behavior may not be expressed.
It is well-known that non-dopaminergic processes influence the ethanol response (Heinz et al. 2003; Phillips & Shen 1996; Vengeliene et al. 2008), whereas NAcc dopamine is a primary modulator of cocaine-induced stimulation (Amalric & Koob 1993; Zhang et al. 2001). For example, the stimulant response to ethanol has been associated with its N-methyl-D-aspartate receptor antagonist (Meyer & Phillips 2003) and GABAergic properties (Shen et al. 1998) in FAST and SLOW mice. These properties of ethanol, or another dopamine pathway (e.g., ventral tegmental area to amygdala; Demarest et al. 1999), could be responsible for a portion of the locomotor response. There were increases in dopamine in SLOW mice treated with ethanol, even though these mice did not show stimulation. This suggests dissociation between ethanol-induced activity and ethanol-induced increases in dopamine levels. However, it may be that the neural substrates of ethanol-induced depression mask the behavioral effects of ethanol-induced dopamine in SLOW mice.
There was no evidence for an acute effect of ethanol or cocaine on NAcc glutamate levels in the current studies. A glutamatergic input into the accumbens from other brain areas such as the prefrontal cortex has been demonstrated (LaLumiere & Kalivas 2008; Pennartz et al. 1994), and was confirmed in these studies by the ability of 100 mM potassium-containing aCSF to stimulate increases in glutamate in these mice. Some studies have reported an acute effect of ethanol on NAcc glutamate levels (Dahchour et al. 2000; Kapasova & Szumlinski 2008; Selim & Bradberry 1996) in mice or rats, while at least one has reported no effect (Dahchour et al. 1994) in rats. Changes in glutamate in our study may have been difficult to detect because of rapid uptake or the sampling time of our experiments. However, results from a recent paper are consistent with ours for the same time period following an acute injection of 2 g/kg ethanol (Kapasova & Szumlinski 2008), although increases in glutamate at later time points or after repeated injections of ethanol were seen. At least one study has reported a difference in ethanol-induced changes in glutamate levels in the NAcc in rats selectively bred for high and low sensitivity to the sedative effects of 2 g/kg ethanol (Dahchour et al. 2000), which suggests that the glutamatergic response to ethanol is genetically correlated with this measure of behavioral sensitivity. However, our data do not corroborate this and suggest that glutamate levels in the NAcc are not related to the enhanced sensitivity to ethanol-induced locomotor depression in SLOW mice. These disparities may be due to differences in the effects of ethanol in rats and mice.
Conclusions and Future Directions
The sensitivity of the mesolimbic dopamine system to ethanol and cocaine was altered by selectively breeding for sensitivity to the locomotor stimulant effects of ethanol. These data are unique in that they demonstrate a genetically-determined relationship, but they are in agreement with the growing body of literature that suggests that the ventral tegmental area-NAcc pathway is a common substrate for the locomotor stimulant and reinforcing properties of ethanol and other abused drugs (Amalric & Koob 1993; Di Chiara & Imperato 1986; Rodd-Henricks et al. 2000; Tzschentke & Schmidt 2000). However, additional studies are needed to characterize the nature of the differences between FAST and SLOW mice, such as studies using the no-net-flux method to measure basal neurotransmitter levels and quantitative methods to determine whether transient changes in extracellular dopamine are due to changes in vesicular release or in dopamine uptake and clearance (Chefer et al. 2003). Studies such as these would provide greater insight into the molecular substrates that influence the genetic relationship between ethanol's dopamine-enhancing properties and its ability to induce behavioral stimulation.
While no specific genes have been definitively shown to underlie differences in sensitivity to the locomotor stimulant effects of ethanol, quantitative trait locus analyses have suggested specific chromosomal regions and candidate genes in those regions (Downing et al. 2006; Kamens et al. 2008; Palmer et al. 2002b; Xu et al. 2002). Some of this work recently led us to examine the expression of several nicotinic acetylcholine receptor subunit genes in the FAST and SLOW mice, and to identify a difference in the expression of the α6 and β4 subunit genes (Kamens & Phillips 2008). Whether differences in the expression or function of these genes could influence the dopamine response of FAST and SLOW mice is a question for future exploration.
Acknowledgments
The authors would like to thank Dr. Rueben Gonzales for his invaluable advice and assistance in the development of the microdialysis and HPLC procedures described in this paper. We would also like to thank Noah Gubner for collecting the cocaine stereotypy data. The authors declare that, except for income received from our primary employers, no financial support or compensation has been received from any individual or corporate entity over the past three years for research or professional service and there are no personal financial holdings that could be perceived as constituting a potential conflict of interest. These studies were performed with support from the Department of Veterans Affairs (TJP, CKM), P60 AA10760 (TJP), and F31 AA014070 (PJM).
References
- Addicott MA, Marsh-Richard DM, Mathias CW, Dougherty DM. The biphasic effects of alcohol: comparisons of subjective and objective measures of stimulation, sedation, and physical activity. Alcohol Clin Exp Res. 2007;31:1883–1890. doi: 10.1111/j.1530-0277.2007.00518.x. [DOI] [PubMed] [Google Scholar]
- Amalric M, Koob GF. Functionally selective neurochemical afferents and efferents of the mesocorticolimbic and nigrostriatal dopamine system. Prog Brain Res. 1993;99:209–226. doi: 10.1016/s0079-6123(08)61348-5. [DOI] [PubMed] [Google Scholar]
- Beckstead MJ, Phillips TJ. Mice selectively bred for high or low alcohol-induced locomotion exhibit differences in dopamine neuron function. J Pharmacol Exp Ther. doi: 10.1124/jpet.108.146316. accepted pending revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergstrom HC, Palmer AA, Wood RD, Burkhart-Kasch S, McKinnon CS, Phillips TJ. Reverse selection for differential response to the locomotor stimulant effects of ethanol provides evidence for pleiotropic genetic influence on locomotor response to other drugs of abuse. Alcohol Clin Exp Res. 2003;27:1535–1547. doi: 10.1097/01.ALC.0000091226.18969.B9. [DOI] [PubMed] [Google Scholar]
- Blednov YA, Walker D, Martinez M, Levine M, Damak S, Margolskee RF. Perception of sweet taste is important for voluntary alcohol consumption in mice. Genes Brain Behav. 2008;7:1–13. doi: 10.1111/j.1601-183X.2007.00309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blizard DA, McClearn GE. Association between ethanol and sucrose intake in the laboratory mouse: exploration via congenic strains and conditioned taste aversion. Alcohol Clin Exp Res. 2000;24:253–258. [PubMed] [Google Scholar]
- Boehm SL, 2nd, Piercy MM, Bergstrom HC, Phillips TJ. Ventral tegmental area region governs GABA(B) receptor modulation of ethanol-stimulated activity in mice. Neuroscience. 2002;115:185–200. doi: 10.1016/s0306-4522(02)00378-0. [DOI] [PubMed] [Google Scholar]
- Budygin EA. Dopamine uptake inhibition is positively correlated with cocaine-induced stereotyped behavior. Neurosci Lett. 2007;429:55–58. doi: 10.1016/j.neulet.2007.09.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chefer VI, Zakharova I, Shippenberg TS. Enhanced responsiveness to novelty and cocaine is associated with decreased basal dopamine uptake and release in the nucleus accumbens: quantitative microdialysis in rats under transient conditions. J Neurosci. 2003;23:3076–3084. doi: 10.1523/JNEUROSCI.23-07-03076.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosford RJ, Parsons LH, Justice JB., Jr Effect of tetrodotoxin and potassium infusion on microdialysis extraction fraction and extracellular dopamine in the nucleus accumbens. Neurosci Lett. 1994;178:175–178. doi: 10.1016/0304-3940(94)90753-6. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Young ER, Deutsch CM, Tam BR, Kosobud A. Mice genetically selected for differences in open-field activity after ethanol. Pharmacol Biochem Behav. 1987;27:577–581. doi: 10.1016/0091-3057(87)90371-6. [DOI] [PubMed] [Google Scholar]
- Dahchour A, Hoffman A, Deitrich R, de Witte P. Effects of ethanol on extracellular amino acid levels in high-and low-alcohol sensitive rats: a microdialysis study. Alcohol Alcohol. 2000;35:548–553. doi: 10.1093/alcalc/35.6.548. [DOI] [PubMed] [Google Scholar]
- Dahchour A, Quertemont E, De Witte P. Acute ethanol increases taurine but neither glutamate nor GABA in the nucleus accumbens of male rats: a microdialysis study. Alcohol Alcohol. 1994;29:485–487. [PubMed] [Google Scholar]
- Demarest K, Hitzemann B, Phillips T, Hitzemann R. Ethanol-induced expression of c-Fos differentiates the FAST and SLOW selected lines of mice. Alcohol Clin Exp Res. 1999;23:87–95. [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Preferential stimulation of dopamine release in the nucleus accumbens by opiates, alcohol, and barbiturates: studies with transcerebral dialysis in freely moving rats. Ann N Y Acad Sci. 1986;473:367–381. doi: 10.1111/j.1749-6632.1986.tb23629.x. [DOI] [PubMed] [Google Scholar]
- Downing C, Carosone-Link P, Bennett B, Johnson T. QTL mapping for low-dose ethanol activation in the LXS recombinant inbred strains. Alcohol Clin Exp Res. 2006;30:1111–1120. doi: 10.1111/j.1530-0277.2006.00137.x. [DOI] [PubMed] [Google Scholar]
- Green AS, Grahame NJ. Ethanol drinking in rodents: is free-choice drinking related to the reinforcing effects of ethanol? Alcohol. 2008;42:1–11. doi: 10.1016/j.alcohol.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz A, Schafer M, Higley JD, Krystal JH, Goldman D. Neurobiological correlates of the disposition and maintenance of alcoholism. Pharmacopsychiatry. 2003;36(Suppl 3):S255–258. doi: 10.1055/s-2003-45139. [DOI] [PubMed] [Google Scholar]
- Holstein SE, Pastor R, Meyer PJ, Phillips TJ. Naloxone does not attenuate the locomotor effects of ethanol in FAST, SLOW, or two heterogeneous stocks of mice. Psychopharmacology (Berl) 2005;182:277–289. doi: 10.1007/s00213-005-0066-8. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Panksepp J. The role of nucleus accumbens dopamine in motivated behavior: a unifying interpretation with special reference to reward-seeking. Brain Res Brain Res Rev. 1999;31:6–41. doi: 10.1016/s0165-0173(99)00023-5. [DOI] [PubMed] [Google Scholar]
- Imperato A, Di Chiara G. Preferential stimulation of dopamine release in the nucleus accumbens of freely moving rats by ethanol. J Pharmacol Exp Ther. 1986;239:219–228. [PubMed] [Google Scholar]
- Jerlhag E. The antipsychotic aripiprazole antagonizes the ethanol- and amphetamine-induced locomotor stimulation in mice. Alcohol. 2008;42:123–127. doi: 10.1016/j.alcohol.2007.11.004. [DOI] [PubMed] [Google Scholar]
- Kamens HM, Burkhart-Kasch S, McKinnon CS, Li N, Reed C, Phillips TJ. Ethanol-related traits in mice selectively bred for differential sensitivity to methamphetamine-induced activation. Behav Neurosci. 2006;120:1356–1366. doi: 10.1037/0735-7044.120.6.1356. [DOI] [PubMed] [Google Scholar]
- Kamens HM, McKinnon CS, Li N, Helms ML, Belknap JK, Phillips TJ. The alpha3 Subunit of the Nicotinic Acetylcholine Receptor is a Candidate Gene for Ethanol Stimulation. Genes Brain Behav. 2008 doi: 10.1111/j.1601-183X.2008.00444.x. epub ahead of print, Sep 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kankaanpaa A, Meririnne E, Ariniemi K, Seppala T. Oxalic acid stabilizes dopamine, serotonin, and their metabolites in automated liquid chromatography with electrochemical detection. J Chromatogr B Biomed Sci Appl. 2001;753:413–419. doi: 10.1016/s0378-4347(00)00553-3. [DOI] [PubMed] [Google Scholar]
- Kapasova Z, Szumlinski KK. Strain differences in alcohol-induced neurochemical plasticity: a role for accumbens glutamate in alcohol intake. Alcohol Clin Exp Res. 2008;32:617–631. doi: 10.1111/j.1530-0277.2008.00620.x. [DOI] [PubMed] [Google Scholar]
- King AC, Houle T, de Wit H, Holdstock L, Schuster A. Biphasic alcohol response differs in heavy versus light drinkers. Alcohol Clin Exp Res. 2002;26:827–835. [PubMed] [Google Scholar]
- Kulkosky PJ, Clayborne YJ, Sandoval SL. Cholecystokinin and bombesin inhibit ethanol and food intake in rats selectively bred for ethanol sensitivity. Alcohol Clin Exp Res. 1993;17:545–551. doi: 10.1111/j.1530-0277.1993.tb00797.x. [DOI] [PubMed] [Google Scholar]
- LaLumiere RT, Kalivas PW. Glutamate release in the nucleus accumbens core is necessary for heroin seeking. J Neurosci. 2008;28:3170–3177. doi: 10.1523/JNEUROSCI.5129-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lominac KD, Kapasova Z, Hannun RA, Patterson C, Middaugh LD, Szumlinski KK. Behavioral and neurochemical interactions between Group 1 mGluR antagonists and ethanol: potential insight into their anti-addictive properties. Drug Alcohol Depend. 2006;85:142–156. doi: 10.1016/j.drugalcdep.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Lovinger DM, White G, Weight FF. Ethanol inhibits NMDA-activated ion current in hippocampal neurons. Science. 1989;243:1721–1724. doi: 10.1126/science.2467382. [DOI] [PubMed] [Google Scholar]
- McKee BL, Meshul CK. Time-dependent changes in extracellular glutamate in the rat dorsolateral striatum following a single cocaine injection. Neuroscience. 2005;133:605–613. doi: 10.1016/j.neuroscience.2005.02.020. [DOI] [PubMed] [Google Scholar]
- Meshul CK, Emre N, Nakamura CM, Allen C, Donohue MK, Buckman JF. Time-dependent changes in striatal glutamate synapses following a 6-hydroxydopamine lesion. Neuroscience. 1999;88:1–16. doi: 10.1016/s0306-4522(98)00189-4. [DOI] [PubMed] [Google Scholar]
- Meyer PJ, Phillips TJ. Sensitivity to ketamine, alone or in combination with ethanol, is altered in mice selectively bred for sensitivity to ethanol's locomotor effects. Alcohol Clin Exp Res. 2003;27:1701–1709. doi: 10.1097/01.ALC.0000093602.00193.39. [DOI] [PubMed] [Google Scholar]
- Mogenson GJ, Yang CR. The contribution of basal forebrain to limbic-motor integration and the mediation of motivation to action. Adv Exp Med Biol. 1991;295:267–290. doi: 10.1007/978-1-4757-0145-6_14. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Bolinao ML. Biphasic effect of ethanol on extracellular accumulation of glutamate in the hippocampus and the nucleus accumbens. Neurosci Lett. 1994;178:99–102. doi: 10.1016/0304-3940(94)90299-2. [DOI] [PubMed] [Google Scholar]
- Moore TO, June HL, Lewis MJ. Ethanol-induced stimulation and depression on measures of locomotor activity: effects of basal activity levels in rats. Alcohol. 1993;10:537–540. doi: 10.1016/0741-8329(93)90078-3. [DOI] [PubMed] [Google Scholar]
- Olive MF, Mehmert KK, Hodge CW. Microdialysis in the mouse nucleus accumbens: a method for detection of monoamine and amino acid neurotransmitters with simultaneous assessment of locomotor activity. Brain Res Brain Res Protoc. 2000;5:16–24. doi: 10.1016/s1385-299x(99)00054-9. [DOI] [PubMed] [Google Scholar]
- Orsini C, Buchini F, Piazza PV, Puglisi-Allegra S, Cabib S. Susceptibility to amphetamine-induced place preference is predicted by locomotor response to novelty and amphetamine in the mouse. Psychopharmacology (Berl) 2004;172:264–270. doi: 10.1007/s00213-003-1647-z. [DOI] [PubMed] [Google Scholar]
- Palmer AA, McKinnon CS, Bergstrom HC, Phillips TJ. Locomotor activity responses to ethanol, other alcohols, and GABA-A acting compounds in forward- and reverse-selected FAST and SLOW mouse lines. Behav Neurosci. 2002a;116:958–967. doi: 10.1037//0735-7044.116.6.958. [DOI] [PubMed] [Google Scholar]
- Palmer AA, Miller MN, McKinnon CS, Phillips TJ. Sensitivity to the locomotor stimulant effects of ethanol and allopregnanolone is influenced by common genes. Behav Neurosci. 2002b;116:126–137. doi: 10.1037//0735-7044.116.1.126. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KB. The Mouse Brain in Stereotaxic Coordinates. 2nd. Academic Press; San Diego, CA: 2001. [Google Scholar]
- Pennartz CM, Groenewegen HJ, Lopes da Silva FH. The nucleus accumbens as a complex of functionally distinct neuronal ensembles: an integration of behavioural, electrophysiological and anatomical data. Prog Neurobiol. 1994;42:719–761. doi: 10.1016/0301-0082(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Phillips AG, Blaha CD, Fibiger HC. Neurochemical correlates of brain-stimulation reward measured by ex vivo and in vivo analyses. Neurosci Biobehav Rev. 1989;13:99–104. doi: 10.1016/s0149-7634(89)80017-x. [DOI] [PubMed] [Google Scholar]
- Phillips TJ, Burkhart-Kasch S, Gwiazdon CC, Crabbe JC. Acute sensitivity of FAST and SLOW mice to the effects of abused drugs on locomotor activity. J Pharmacol Exp Ther. 1992;261:525–533. [PubMed] [Google Scholar]
- Phillips TJ, Burkhart-Kasch S, Terdal ES, Crabbe JC. Response to selection for ethanol-induced locomotor activation: genetic analyses and selection response characterization. Psychopharmacology (Berl) 1991;103:557–566. doi: 10.1007/BF02244259. [DOI] [PubMed] [Google Scholar]
- Phillips TJ, Shen EH. Neurochemical bases of locomotion and ethanol stimulant effects. Int Rev Neurobiol. 1996;39:243–282. doi: 10.1016/s0074-7742(08)60669-8. [DOI] [PubMed] [Google Scholar]
- Phillips TJ, Shen EH, McKinnon CS, Burkhart-Kasch S, Lessov CN, Palmer AA. Forward, relaxed, and reverse selection for reduced and enhanced sensitivity to ethanol's locomotor stimulant effects in mice. Alcohol Clin Exp Res. 2002;26:593–602. [PubMed] [Google Scholar]
- Piepponen TP, Kiianmaa K, Ahtee L. Effects of ethanol on the accumbal output of dopamine, GABA and glutamate in alcohol-tolerant and alcohol-nontolerant rats. Pharmacol Biochem Behav. 2002;74:21–30. doi: 10.1016/s0091-3057(02)00937-1. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Bell K, Duffy P, Kalivas PW. Repeated cocaine augments excitatory amino acid transmission in the nucleus accumbens only in rats having developed behavioral sensitization. J Neurosci. 1996;16:1550–1560. doi: 10.1523/JNEUROSCI.16-04-01550.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandra V, Phuc S, Franco AC, Gonzales RA. Ethanol preference is inversely correlated with ethanol-induced dopamine release in 2 substrains of C57BL/6 mice. Alcohol Clin Exp Res. 2007;31:1669–1676. doi: 10.1111/j.1530-0277.2007.00463.x. [DOI] [PubMed] [Google Scholar]
- Ripley TL, Jaworski J, Randall PK, Gonzales RA. Repeated perfusion with elevated potassium in in vivo microdialysis--A method for detecting small changes in extracellular dopamine. J Neurosci Methods. 1997;78:7–14. doi: 10.1016/s0165-0270(97)00129-5. [DOI] [PubMed] [Google Scholar]
- Risinger FO, Malott DH, Prather LK, Niehus DR, Cunningham CL. Motivational properties of ethanol in mice selectively bred for ethanol-induced locomotor differences. Psychopharmacology (Berl) 1994;116:207–216. doi: 10.1007/BF02245064. [DOI] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, McKinzie DL, Crile RS, Murphy JM, McBride WJ. Regional heterogeneity for the intracranial self-administration of ethanol within the ventral tegmental area of female Wistar rats. Psychopharmacology (Berl) 2000;149:217–224. doi: 10.1007/s002139900347. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. Low level of response to alcohol as a predictor of future alcoholism. Am J Psychiatry. 1994;151:184–189. doi: 10.1176/ajp.151.2.184. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Trim R, Kreikebaum S, Hinga B, Allen R. Testing the level of response to alcohol-based model of heavy drinking and alcohol problems in offspring from the San Diego Prospective Study. J Stud Alcohol Drugs. 2008;69:571–579. doi: 10.15288/jsad.2008.69.571. [DOI] [PubMed] [Google Scholar]
- Selim M, Bradberry CW. Effect of ethanol on extracellular 5-HT and glutamate in the nucleus accumbens and prefrontal cortex: comparison between the Lewis and Fischer 344 rat strains. Brain Res. 1996;716:157–164. doi: 10.1016/0006-8993(95)01385-7. [DOI] [PubMed] [Google Scholar]
- Shen EH, Dorow J, Harland R, Burkhart-Kasch S, Phillips TJ. Seizure sensitivity and GABAergic modulation of ethanol sensitivity in selectively bred FAST and SLOW mouse lines. J Pharmacol Exp Ther. 1998;287:606–615. [PubMed] [Google Scholar]
- Shen EH, Harland RD, Crabbe JC, Phillips TJ. Bidirectional selective breeding for ethanol effects on locomotor activity: characterization of FAST and SLOW mice through selection generation 35. Alcohol Clin Exp Res. 1995;19:1234–1245. doi: 10.1111/j.1530-0277.1995.tb01606.x. [DOI] [PubMed] [Google Scholar]
- Smith JA, Mo Q, Guo H, Kunko PM, Robinson SE. Cocaine increases extraneuronal levels of aspartate and glutamate in the nucleus accumbens. Brain Res. 1995;683:264–269. doi: 10.1016/0006-8993(95)00383-2. [DOI] [PubMed] [Google Scholar]
- Szumlinski KK, Diab ME, Friedman R, Henze LM, Lominac KD, Bowers MS. Accumbens neurochemical adaptations produced by binge-like alcohol consumption. Psychopharmacology (Berl) 2007;190:415–431. doi: 10.1007/s00213-006-0641-7. [DOI] [PubMed] [Google Scholar]
- Thomas DN, Holman RB. The effects of pH on amphetamine-induced dopamine release as measured by in vivo dialysis. Life Sci. 1989;45:1299–1305. doi: 10.1016/0024-3205(89)90133-1. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM, Schmidt WJ. Functional relationship among medial prefrontal cortex, nucleus accumbens, and ventral tegmental area in locomotion and reward. Crit Rev Neurobiol. 2000;14:131–142. [PubMed] [Google Scholar]
- Vengeliene V, Bilbao A, Molander A, Spanagel R. Neuropharmacology of alcohol addiction. Br J Pharmacol. 2008;154:299–315. doi: 10.1038/bjp.2008.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waller MB, Murphy JM, McBride WJ, Lumeng L, Li TK. Effect of low dose ethanol on spontaneous motor activity in alcohol-preferring and -nonpreferring lines of rats. Pharmacol Biochem Behav. 1986;24:617–623. doi: 10.1016/0091-3057(86)90567-8. [DOI] [PubMed] [Google Scholar]
- Xu Y, Demarest K, Hitzemann R, Sikela JM. Gene coding variant in Cas1 between the C57BL/6J and DBA/2J inbred mouse strains: linkage to a QTL for ethanol-induced locomotor activation. Alcohol Clin Exp Res. 2002;26:1–7. [PubMed] [Google Scholar]
- Yim HJ, Gonzales RA. Ethanol-induced increases in dopamine extracellular concentration in rat nucleus accumbens are accounted for by increased release and not uptake inhibition. Alcohol. 2000;22:107–115. doi: 10.1016/s0741-8329(00)00121-x. [DOI] [PubMed] [Google Scholar]
- Zapata A, Gonzales RA, Shippenberg TS. Repeated ethanol intoxication induces behavioral sensitization in the absence of a sensitized accumbens dopamine response in C57BL/6J and DBA/2J mice. Neuropsychopharmacology. 2006;31:396–405. doi: 10.1038/sj.npp.1300833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Loonam TM, Noailles PA, Angulo JA. Comparison of cocaine- and methamphetamine-evoked dopamine and glutamate overflow in somatodendritic and terminal field regions of the rat brain during acute, chronic, and early withdrawal conditions. Ann N Y Acad Sci. 2001;937:93–120. doi: 10.1111/j.1749-6632.2001.tb03560.x. [DOI] [PubMed] [Google Scholar]