Abstract
Background
Transient symptoms in Alzheimer disease (AD) are frequent and include seizures, syncope, and episodes of inattention or confusion. The incidence of seizures in AD and predictors of which patients with AD might be more predisposed to them is based primarily on retrospective studies and is not well established.
Objective
To determine the incidence and predictors of new-onset unprovoked seizures.
Design
Prospective cohort study.
Setting
Three academic centers.
Patients
Four hundred fifty-three patients with probable AD observed prospectively from mild disease stages since 1992.
Main Outcome Measure
Informant interviews every 6 months included questions about whether the patient had a seizure (convulsion, fainting, or “funny” spell) and whether diagnosis or treatment for epilepsy or seizure was made. Two epileptologists independently retrospectively reviewed all available medical records for 52 patients with positive responses to either of these questions, and using a specific checklist form, events were diagnosed as to whether they were unprovoked seizures (intrarater concordance, κ=0.67). Diagnosis of unprovoked seizures constituted the event in survival analyses. Potential predictors included sex, age, race/ethnicity, educational achievement, duration of illness, baseline cognition and function, depression, medical comorbidities, and time-dependent use of cholinesterase inhibitors and neuroleptic agents, apolipoprotein E genotype, and previous electroencephalographic findings.
Results
Over the course of 3518 visit-assessments (per patient: mean, 7.8; maximum, 27), 7 patients (1.5%) developed seizures. Younger age was associated with higher risk (hazard ratio, 1.23; 95% confidence interval, 1.08–1.41; P=.003 for each additional year of age) of seizure incidence. No other predictor was significant. The overall incidence of seizures was low (418 per 100 000 person-years of observation) although significantly higher than expected for idiopathic unprovoked seizures in similar age ranges of the general population (hazard ratio, 8.06; 95% confidence interval, 3.23–16.61).
Conclusions
Unprovoked seizures are uncommon in AD, but they do occur more frequently than in the general population. Younger age is a risk factor for seizures in AD.
Both case-control1 and prospective2–4 previous studies have suggested that Alzheimer disease (AD) may be a risk factor for seizures. Seizures are common in various brain diseases; however, differentiation between nonepileptic and epileptic events may be challenging. Patients with dementia may experience nonepileptic episodes of inattention or confusion as well as syncope or near-syncope and seizures. Hospitalizations may ensue, and anticonvulsant agents are often prescribed. The relationship of epileptic activity to AD is of clinical importance. In addition, it has been proposed to relate to the pathogenesis of AD. For example, according to the findings a recent study by Palop et al,5 spontaneous nonconvulsive seizure activity was noted in a mouse model of AD.
Previous studies in human beings have reported conflicting results about the prevalence of seizures in dementia and AD, with frequency ranging from 5% to 64%.2–4,6–13 Overall, data is scarce about seizures in dementia and AD, and it is not well known which factors might contribute to seizures. Most previous studies have been small, with short follow-up, and have not systematically recorded clinical characteristics that may relate to seizure risk. Individuals with dementia have been enrolled at varying disease stages, and clinical evaluations have typically been infrequent. Few studies have examined the association prospectively. Dementia characterization has not always been comprehensive, and dementia diagnosis has not always been made in academic centers with experience with the disease. Clinical characterization of seizure events has been variable as well, with many reports relying on medical record reviews or assessments by nonepileptologists.
To investigate the issue of seizures in AD, we analyzed data from a large multicenter study of patients with probable AD followed up prospectively on a semiannual basis from the early stages of the disease for up to 14 years. We assessed the incidence of unprovoked seizures and identified characteristics that predict them. A recent publication from our group4 investigated these issues in a small sample (“Predictors Study”14–16 I cohort). This study is an extended evaluation of the incidence of seizures in AD with particular attention to accurate assessment of the nature of possible epileptic events. We used a standardized checklist for review of events. Independent review of all events was performed by 2 epileptologists (H.C. and J.C.). We also considered a wide variety of potential seizure predictors. We markedly increased our sample size by including both the Predictors Study I and II cohorts and also had a longer duration of follow-up to more fully capture any events.
METHODS
PARTICIPANTS
Individuals from 2 Predictors Study cohorts14–16 were included in these analyses. Patients were recruited and studied at 3 sites: Columbia University, New York, New York; The Johns Hopkins University, Baltimore, Maryland; and Massachusetts General Hospital, Harvard University, Boston. The inclusion and exclusion criteria and the evaluation procedures of the Predictors Study are fully described elsewhere.14–17 In brief, patients met Diagnostic and Statistical Manual of Mental Disorders (Third Edition Revised) criteria for primary degenerative dementia of the Alzheimer type and National Institute of Neurological Disorders and Stroke–Alzheimer Disease and Related Disorders Association criteria for probable AD. Enrollment required a modified Mini-Mental State Examination (MMSE) score of 30/57 or higher, which is approximately equivalent to 16/30 or higher on the Folstein MMSE.18,19 The study was approved by the local institutional review boards.
EVALUATION
Neurologic, other clinical, and mental status examinations were conducted at study enrollment and at 6-month intervals thereafter. The cognitive function measure used for the analysis was the modified MMSE,18–20 a 57-point modification of the original MMSE.18 Functional capacity was assessed using the Blessed Dementia Rating Scale, parts 1 and 2.21 Depression recorded using the Columbia University Scale for Psychopathology in Alzheimer’s Disease22 was also used as a predictor.23 At every 6-month visit, medications taken by the patients were recorded. All cholinesterase inhibitors were grouped in a single category and considered as a dichotomous variable in the analyses,16,24 as were all neuroleptic agents.16,24 A modified version15,16 of the Charlson Index of Comorbidity25 (referred to as “comorbidity index”) was calculated and used in a dichotomous form (0 [67%] vs ≥1 [33%]).
When data collection was initiated, apolipoprotein E (APOE) testing was unavailable; however, beginning in the sixth year of the study, the pattern of APOE isoforms was determined26 and considered in the analyses. An electroencephalogram (EEG) was not required but was obtained in a subset of patients as part of the routine dementia workup at or before the baseline visit. The EEG reports were coded dichotomously on the basis of specific criteria for slow dominant rhythm, focal slowing, intermittent rhythmic slowing, other slowing, focal epileptic-form activity, and generalized epileptiform activity.
OUTCOMES
The questions in the original database addressing the occurrence of a seizure were as follows: “In the past 6 months, has the patient been diagnosed or treated for epilepsy or seizures?” and “Has the patient had a seizure (fit, faint, or funny spell) since the last visit?” This information was recorded at baseline and at every 6-month evaluation thereafter. To assess the likelihood of an event being a seizure, 2 epileptologists (H.C. and J.C.) independently retrospectively reviewed the original questionnaires and all available medical records (including clinical and EEG data) for 52 patients with an affirmative answer to either of the 2 questions in any evaluation. Each epileptologist’s conclusion about the epileptic nature of the event (rated dichotomously as “likely in the presence of adequate information vs unlikely or uncertain”) was used for diagnostic agreement calculation. The 2 epileptologists convened and reached a consensus diagnosis if their opinions varied about the likelihood of a seizure. This consensus diagnosis of unprovoked seizures was used for final seizure calculation.
STATISTICAL ANALYSES
We calculated the κ statistic to examine unprovoked seizure diagnostic concordance between the 2 epileptologists. We used the Kaplan-Meier method to calculate survival curves for the development of unprovoked seizures (outcome) in patients who were seizure free at the baseline assessment. The duration between the initial visit and either development of a seizure or last evaluation without a seizure (ie, death or last follow-up) served as the timing variable. Because we did not have a control population without AD, age-specific incidence in 10-year age groups was calculated and compared with age-specific incidence in a referent cohort,27,28 resulting in incidence ratios.
To identify potential predictors of seizure incidence, we calculated Cox proportional hazards models29 with the following predictors: cohort (Predictors Study I vs II cohorts; dichotomous), recruitment center (dummy variable with the New York center as reference), sex (male as reference), age at intake (years), race/ethnicity (dummy variable with white as reference), educational achievement, estimated duration of illness, baseline function (Blessed Dementia Rating Scale), cognition (modified MMSE), depression, medical comorbidities (comorbidity index), and time-dependent use of cholinesterase inhibitors and neuroleptic agents. In supplementary models, we included patient APOE genotype and EEG variables as additional predictors.
RESULTS
Overall, 453 patients with AD were included in the study, with approximately half from each Predictors Study cohort (Table). As dictated by the inclusion criteria, patients were at relatively early stages of AD at initial recruitment (mean MMSE score, 21). They were, on average, well educated and in good general health (approximately two-thirds had a comorbidity index of 0). Most patients were white. About half of the patients reported some symptoms of depression.23
Table 1.
Demographic and Clinical Characteristics of 453 Patients
| Variable | Value |
|---|---|
| Age at study enrollment, mean (SD), y | 74.4 (8.9) |
| Men, No. (%) | 181 (40) |
| Predictors Study cohort I, No. (%) | 247 (55) |
| Recruitment center, No. (%) | |
| New York, New York | 197 (43) |
| Baltimore, Maryland | 139 (31) |
| Boston, Massachusetts | 117 (26) |
| Race/ethnicity, No. (%) | |
| White | 423 (93) |
| Black | 27 (6) |
| Other | 3 (1) |
| Educational achievement, mean (SD), y | 13.7 (3.5) |
| Examination score | |
| Baseline mMMSE, mean (SD) | 38.4 (6.7) |
| Baseline MMSE, mean (SD) | 21.0 (3.3) |
| Baseline BDRS, mean (SD) | 3.6 (2.0) |
| Duration of illness, mean (SD), y | 4.1 (2.4) |
| Comorbidity index 1 (%) | 150 (33) |
| Depression, No. (%) | 208 (46) |
| ε4 Alleles, No. (%) | |
| None | 77 (45) |
| 1–2 | 93 (55) |
| EEG findings,a No. (%) | |
| Slow dominant rhythm | 55 (29) |
| Focal slowing | 22 (12) |
| Intermittent rhythmic slowing | 10 (5) |
| Other slowing | 51 (28) |
| Focal epileptiform | 5 (3) |
| Generalized epileptiform | 2 (1) |
Abbreviations: BDRS, Blessed Dementia Rating Scale; EEG, electroencephalographic; MMSE, Mini-Mental State Examination; mMMSE, modified MMSE.
n=184.
SEIZURE SCREENING QUESTION
Fifty-two of 453 patients (11.5%) gave at least 1 positive response to the seizure-related questions. Compared with 401 patients without any positive response, the 52 with at least 1 positive response were younger (74.9 vs 70.7 years; t=3.3; P=.001). The groups did not differ in recruitment cohort or center, sex, race/ethnicity, educational achievement, baseline cognitive performance, functional status, estimated duration of illness, APOE genotype, mortality or institutionalization risk during follow-up, or presence of depression or medical comorbidities.
Autopsy data were available for 90 patients (75 of the 401 patients without any positive response to the seizure-related questions and 15 of the 52 patients with at least 1 positive response). At autopsy, all 15 patients with at least 1 positive response to the seizure-related questions had AD-type pathologic changes and none had pathologic changes consistent with hippocampal sclerosis. Lewy bodies were present in addition to AD pathologic findings in 27 of 75 patients (36%) without any positive response to the seizure-related questions and in 4 of 15 (27%) with at least 1 positive response to the seizure-related questions (P=.49).
SEIZURE DIAGNOSTICS
Among 52 patients with at least 1 positive response to the seizure-related questions, 7 (13%) were deemed by consensus to have had epileptic events (approximately 1.5% of 453 patients). The EEG reports were available for the epileptologists’ review for 21 of 52 patients (40%). Among those with available EEGs, findings were completely normal in 38%, diffuse slowing was noted in 38%, focal slowing in 20%, and epileptiform activity in 16%. The 2 epileptologists were in diagnostic agreement for 48 of the 52 patients (5 deemed to have and 43 deemed not to have events of an eplipetic nature). There was an initial diagnostic discordance for 4 patients: 2 were considered to have had seizures by 1 epileptologist (J.C.) but not by the other (H.C.), and 2 were considered to have had seizures by 1 epitologist (H.C.) but not by the other (J.C.). The overall κ statistic was 0.67, denoting good diagnostic reproducibility.30 Insofar as seizure semiology, changes in level of attention were noted in all 7 patients, and whole-body convulsion in 6. Two patients demonstrated clinical semiology of lateralized findings; 3 events led to hospitalization, and 5 patients were treated with antiepileptic medications. Four patients had only a single seizure, and 3 had more than 1 seizure. Data only for patients who were seizure free at the baseline assessment were used for seizure incidence calculation. Considering patient-estimated duration of illness before enrollment in the study, seizures occurred at a mean (SD; range) of 8.2 (2.6; 5.1–11.8) years after estimated clinical onset of AD and 3.7 (3.1) years after recruitment into the study.
Among the 45 patients-events not rated by the epileptologists as being of an epileptic nature, 9 (17%) were classified as “very unlikely to be epileptic in the presence of good information for diagnostic assignment,” and 36 (69%) were classified as of “uncertain diagnostic assignment because of inadequate information.” Compared with 45 patients whose events were not convincing of an epileptic nature, the 7 judged to have had seizures were younger (71.9 vs 62.9 years; t=2.85; P=.006). These groups did not differ in recruitment cohort or center, sex, race/ethnicity, educational achievement level, baseline cognitive performance, functional status, estimated duration of illness, APOE genotype, mortality or institutionalization risk during follow-up, or presence of depression or medical comorbidities.
Autopsy data were available for 11 of the 45 patients whose events were not considered of an epileptic nature and for 4 of the 7 subjects deemed to have had seizures (all of the 15 autopsied subjects had AD-type pathologic changes, and none had hippocampal sclerosis). Lewy bodies were present in 3 of 11 patients (27%) with events that were not considered of an epileptic nature and in 1 of 4 patients (25%) with seizures (P=.93).
SEIZURE RATES
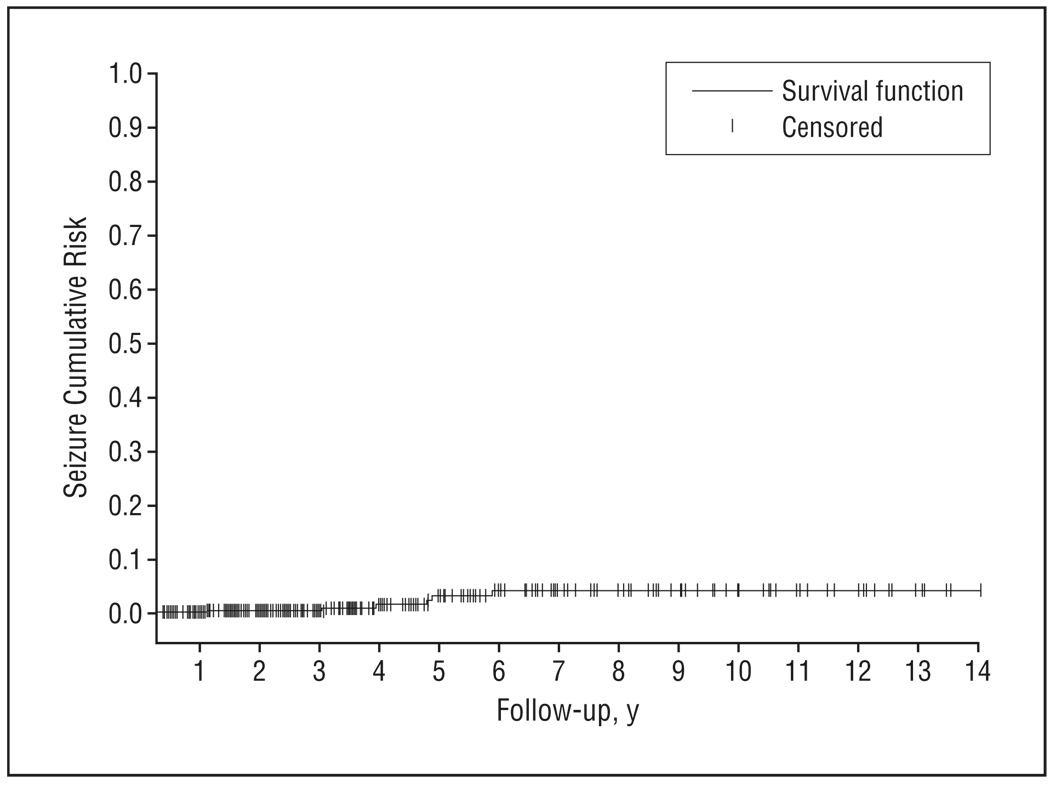
Patients were followed up on average for 3.7 (up to 14) years, during which time there were 3518 visit assessments (on average, 7.8; as many as 27 per patient). During the period that each subject was followed up, missed visits were rare: less than 18% missed more than 1 semiannual visit, and less than 9% missed more than 2 semiannual visits. Cumulative incidence of new-onset seizures (n=7 over 1674 person-years of observation) was 418 per 100 000 person-years of observation (Figure). Assuming a stable rate over time and no increasing risk with increasing severity, this would correspond to approximately 2% of patients with AD developing seizures after 5 years of follow-up. Compared with the rate of unprovoked seizures in the general population of similar age strata,27,28 this incidence was higher than expected (incidence ratio, 8.06; 95% confidence interval [CI], 3.23–16.61).
Figure.
Survival curve based on Kaplan-Meier analysis depicts cumulative seizure incidence.
In sensitivity analyses of 43 patients, we considered as patients with seizures not only the 7 patients with a consensus diagnosis of seizures but also the 36 classified as having uncertain diagnostic assignment because of inadequate information. Cumulative incidence of new-onset seizures (n=43 over 1602 person-years of observation) was 2684 per 100 000 person-years of observation. Assuming a linear risk over time, this would correspond to approximately 13% of patients with AD developing seizures after 5 years of follow-up. Compared with the rate of unprovoked seizures in the general population of similar age strata,27,28 this incidence was higher than expected (incidence ratio, 49.43; 95% CI, 37.58–69.96).
In a Cox model considering cohort, recruitment center, sex, age, race/ethnicity, educational achievement, estimated duration of illness, baseline function, cognition, depression, medical comorbidities, and time-dependent use of cholinesterase inhibitors and neuroleptic agents, only younger age was associated with higher risk of seizures (hazard ratio for each additional year of age, 1.23; 95% CI, 1.08–1.41; P=.003), corresponding to a 23% increased risk. In a supplementary model including patient APOE genotype as an additional predictor (6 incident seizure events in 157 patients), the associations were similar, with younger age being the only predictor of increased seizure risk (hazard ratio, 1.22; 95% CI, 1.01–1.47; P=.04). In a supplementary model considering also the EEG variables as additional predictors (6 incident seizure events in 169 patients), younger age was the only significant predictor of increased seizure risk (for each additional year of age: hazard ratio, 1.39; 95% CI, 1.03–1.85; P=.03).
COMMENT
Our study supports previous reports1–4 that suggested that AD is a risk factor for seizures. However, we also observed that seizures are not common in AD. In our study, only about 1.5% of patients with AD developed seizures over the course of a mean of 3.7 years of follow-up. Most seizures were generalized convulsions and nonrecurrent. The observed incidence corresponds to less than 1 patient with a seizure for every 200 patients with AD followed up over the course of 1 year. Therefore, we conclude that although seizures are more common in patients with AD compared with the general population, they are a quite uncommon feature of AD.
Some studies have concluded that seizures occur in the more advanced stages of the disease.3,9,12 In our study, we did not detect an association between seizures and either estimated disease duration or cognitive or functional performance. Lack of association between patient age at AD onset and seizures3 or higher risk of seizures in younger patients with AD has been noted in other studies, including one from the Predictors Study I cohort.4 Younger patients with AD may have more aggressive disease31 or may be more likely to have a clinical episode recognized; alternatively, the younger brain may be more susceptible to seizure manifestation.
Unlike our group’s previous report from the Predictors Study,4 in the present study, we did not observe an association between seizure risk and either EEG findings or race/ethnicity. Differences between the previous and the present article include the following. The present analyses are much more powerful because we included more than twice as many patients with AD (Predictors Study) with data from additional years of follow-up. We also examined potential seizure events in a more conservative and detailed manner using 2 independent epileptologists and consensus diagnostic procedures. These differences resulted in a lower seizure rate. In addition, we considered in our analyses more potential predictors such as multiple additional EEG variables, additional medical comorbidities, measures of functional status, use of medications such as neuroleptic agents and cholinesterase inhibitors, and autopsy information.
This study has several limitations. Patients with AD were selected from tertiary care university hospitals and specialized diagnostic and treatment centers and, thus, constitute a nonrandom sample of those affected by AD in the population. The percentage of African American and Hispanic patients in our sample was small, and our results might not be generalizable to all racial/ethnic groups. In the absence of internal controls, we used data on incidence of unprovoked seizures from a different population. Routine EEGs were available for only a small percentage of the patients, and the EEG yield insofar as detection of underlying epileptiform activity may vary substantially.32 A large-scale epidemiologic study such as this can only address seizures that are clinically evident but cannot evaluate subclinical epileptic activity. In particular, partial seizures without convulsive components or with more subtle alterations in attention and alertness may have been underrecorded. Despite thorough review of available records, information for reliable diagnostic assignment of events as epileptic or not was missing in a substantial number of subjects. Hence, it is conceivable that considering only patients with convincing clinical information for seizure diagnosis may underestimate the rates of seizure occurrence. In addition, despite expertise in seizure diagnosis, diagnostic concordance between epileptologists was good but not excellent. In the absence of an objective diagnostic marker for seizures, there is always a strong subjective component in seizure characterization, and different diagnostic thresholds may result in different seizure rate estimations (as demonstrated in the sensitivity analyses). Although most of the information was derived by informants, description of epileptic semiology may be less reliable in individuals with dementia; however, even patients with epilepsy without dementia have major difficulties in describing seizure experiences or accurately reporting seizure frequency,33 in particular for complex or generalized seizures. These factors may lead to seizure underestimation. In contrast, fluctuations in alertness and attention and presence of tremor (phenomena common in dementia) may lead to seizure overestimation. For similar reasons, seizure rates in the general population may substantially vary; therefore, the choice of referent population may also affect the calculated incidence ratios. Although patient follow-up and seizure-related screening questions were prospective, clinical evaluation for the epileptic nature of the event was retrospective. Because of the small number of autopsies performed, our power to detect associations with Lewy bodies or hippocampal sclerosis may have been limited.
Confidence in our findings is strengthened by several factors. To our knowledge, this is the largest study of its kind to examine the issue of seizures in AD, supplying enough power for detection and more precise calculation of effects of interest and ability to control for multiple potential confounders. Clinical diagnosis occurred in university hospitals with specific expertise in epilepsy and dementia and was based on uniform application of widely accepted criteria via consensus diagnostic conference procedures for both diseases. In the Predictors Study I and II cohorts, the clinical diagnosis of AD was confirmed in a high percentage (93%) of those evaluated postmortem14,16; it was also confirmed in every autopsy case with positive response to the seizure-related questions. The patients were followed up prospectively, and evaluations were performed semiannually with a high rate of follow-up participation and few missing data. Clinical symptoms and signs of interest were ascertained and coded in a standardized fashion at each visit. Patients with AD were included from relatively early stages of the disease; thus, the cohort captures most of the range of progression over time. We took medication administration into account in a time-dependent manner, which provides higher confidence that the associations are strictly related to the underlying disease process rather than to treatment for it.
Alzheimer disease is an illness with considerable clinical and physiologic heterogeneity.14,16,34–36 Although seizures do occur in AD, the present study indicates that they are not a common phenomenon.
Acknowledgments
Financial Disclosure: Dr Amatniek is an employee of Ortho-McNeil Jannsen Scientific Affairs/Johnson & Johnson and holds stock in Johnson & Johnson.
Funding/Support: This study was supported by grants AG07370, RR00645, and AG 08702 from the National Institutes of Health and by the Henry Panasci Fund (Dr Honig).
Footnotes
Author Contributions: Dr Scarmeas had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Scarmeas, Honig, Cantero, Hauser, and Stern. Acquisition of data: Scarmeas, Honig, Choi, Cantero, Brandt, Blacker, Albert, Bell, and Stern. Analysis and interpretation of data: Scarmeas, Honig, Amatniek, Marder, and Hauser. Drafting of the manuscript: Scarmeas, Honig, and Bell. Critical revision of the manuscript for important intellectual content: Honig, Choi, Cantero, Brandt, Blacker, Albert, Amatniek, Marder, Hauser, and Stern. Statistical analysis: Scarmeas, Honig, Hauser, and Stern. Obtained funding: Honig, Blacker, and Stern. Administrative, technical, and material support: Scarmeas, Honig, Choi, Albert, Amatniek, Bell, and Hauser. Study supervision: Scarmeas.
REFERENCES
- 1.Hesdorffer DC, Hauser WA, Annegers JF, Kokmen E, Rocca WA. Dementia and adult-onset unprovoked seizures. Neurology. 1996;46(3):727–730. doi: 10.1212/wnl.46.3.727. [DOI] [PubMed] [Google Scholar]
- 2.Hauser WA, Morris ML, Heston LL, Anderson VE. Seizures and myoclonus in patients with Alzheimer’s disease. Neurology. 1986;36(9):1226–1230. doi: 10.1212/wnl.36.9.1226. [DOI] [PubMed] [Google Scholar]
- 3.Romanelli MF, Morris JC, Ashkin K, Coben LA. Advanced Alzheimer’s disease is a risk factor for late-onset seizures. Arch Neurol. 1990;47(8):847–850. doi: 10.1001/archneur.1990.00530080029006. [DOI] [PubMed] [Google Scholar]
- 4.Amatniek JC, Hauser WA, DelCastillo-Castaneda C, et al. Incidence and predictors of seizures in patients with Alzheimer’s disease. Epilepsia. 2006;47(5):867–872. doi: 10.1111/j.1528-1167.2006.00554.x. [DOI] [PubMed] [Google Scholar]
- 5.Palop JJ, Chin J, Roberson ED, et al. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer’s disease. Neuron. 2007;55(5):697–711. doi: 10.1016/j.neuron.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sulkava R. Alzheimer’s disease and senile dementia of Alzheimer type: a comparative study. Acta Neurol Scand. 1982;65(6):636–650. doi: 10.1111/j.1600-0404.1982.tb03117.x. [DOI] [PubMed] [Google Scholar]
- 7.Volicer L, Smith S, Volicer BJ. Effect of seizures on progression of dementia of the Alzheimer type. Dementia. 1995;6(5):258–263. doi: 10.1159/000106956. [DOI] [PubMed] [Google Scholar]
- 8.Mendez MF, Catanzaro P, Doss RC, Arguello R, Frey WH., II Seizures in Alzheimer’s disease: clinicopathologic study. J Geriatr Psychiatry Neurol. 1994;7(4):230–233. doi: 10.1177/089198879400700407. [DOI] [PubMed] [Google Scholar]
- 9.Sjögren H. Clinical analysis of morbus Alzheimer and morbus Pick. Acta Psychiatr Scand. 1952;82 suppl:67–115. [PubMed] [Google Scholar]
- 10.Risse SC, Lampe TH, Bird TD, et al. Myoclonus, seizures, and paratonia in Alzheimer disease. Alzheimer Dis Assoc Disord. 1990;4(4):217–225. doi: 10.1097/00002093-199040400-00003. [DOI] [PubMed] [Google Scholar]
- 11.Seltzer B, Sherwin I. A comparison of clinical features in early- and late-onset primary degenerative dementia: one entity or two? Arch Neurol. 1983;40(3):143–146. doi: 10.1001/archneur.1983.04050030037006. [DOI] [PubMed] [Google Scholar]
- 12.Heyman A, Wilkinson WE, Hurwitz BJ, et al. Early-onset Alzheimer’s disease: clinical predictors of institutionalization and death. Neurology. 1987;37(6):980–984. doi: 10.1212/wnl.37.6.980. [DOI] [PubMed] [Google Scholar]
- 13.Samson WN, van Duijn CM, Hop WC, Hofman A. Clinical features and mortality in patients with early-onset Alzheimer’s disease. Eur Neurol. 1996;36(2):103–106. doi: 10.1159/000117218. [DOI] [PubMed] [Google Scholar]
- 14.Scarmeas N, Hadjigeorgiou GM, Papadimitriou A, et al. Motor signs during the course of Alzheimer disease. Neurology. 2004;63(6):975–982. doi: 10.1212/01.wnl.0000138440.39918.0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scarmeas N, Albert M, Brandt J, et al. Motor signs predict poor outcomes in Alzheimer disease. Neurology. 2005;64(10):1696–1703. doi: 10.1212/01.WNL.0000162054.15428.E9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scarmeas N, Brandt J, Albert M, et al. Delusions and hallucinations are associated with worse outcome in Alzheimer disease. Arch Neurol. 2005;62(10):1601–1608. doi: 10.1001/archneur.62.10.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stern Y, Folstein M, Albert M, et al. Multicenter study of predictors of disease course in Alzheimer disease (the “Predictors Study”), I: study design, cohort description, and intersite comparisons. Alzheimer Dis Assoc Disord. 1993;7(1):3–21. doi: 10.1097/00002093-199307010-00002. [DOI] [PubMed] [Google Scholar]
- 18.Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State“: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 19.Stern Y, Sano M, Paulson J, Mayeux R. Modified Mini-Mental State Examination: validity and reliability [abstract] Neurology. 1987;37 suppl 1:179. [Google Scholar]
- 20.Mayeux R, Stern Y, Rosen J, Leventhal J. Depression, intellectual impairment, and Parkinson disease. Neurology. 1981;31(6):645–650. doi: 10.1212/wnl.31.6.645. [DOI] [PubMed] [Google Scholar]
- 21.Blessed G, Tomlinson BE, Roth M. The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. Br J Psychiatry. 1968;114(512):797–811. doi: 10.1192/bjp.114.512.797. [DOI] [PubMed] [Google Scholar]
- 22.Devanand DP, Miller L, Richards M, et al. The Columbia University Scale for Psychopathology in Alzheimer’s Disease. Arch Neurol. 1992;49(4):371–376. doi: 10.1001/archneur.1992.00530280051022. [DOI] [PubMed] [Google Scholar]
- 23.Holtzer R, Scarmeas N, Wegesin DJ, et al. Depressive symptoms in Alzheimer’s disease: natural course and temporal relation to function and cognitive status. J Am Geriatr Soc. 2005;53(12):2083–2089. doi: 10.1111/j.1532-5415.2005.00535.x. [DOI] [PubMed] [Google Scholar]
- 24.Scarmeas N, Brandt J, Blacker D, et al. Disruptive behavior as a predictor in Alzheimer disease. Arch Neurol. 2007;64(12):1755–1761. doi: 10.1001/archneur.64.12.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 26.Hixson JE Pathobiological Determinants of Atherosclerosis in Youth (PDAY) Research Group. Apolipoprotein E polymorphisms affect atherosclerosis in young males. Arterioscler Thromb. 1991;11(5):1237–1244. doi: 10.1161/01.atv.11.5.1237. [DOI] [PubMed] [Google Scholar]
- 27.Hauser WA, Annegers JF, Kurland LT. Incidence of epilepsy and unprovoked seizures in Rochester, Minnesota: 1935–1984. Epilepsia. 1993;34(3):453–468. doi: 10.1111/j.1528-1157.1993.tb02586.x. [DOI] [PubMed] [Google Scholar]
- 28.Hauser WA, Annegers JF, Rocca WA. Descriptive epidemiology of epilepsy: contributions of population-based studies from Rochester, Minnesota. Mayo Clin Proc. 1996;71(6):576–586. doi: 10.4065/71.6.576. [DOI] [PubMed] [Google Scholar]
- 29.Lawless J. Statistical Model and Methods for Lifetime Data. New York, NY: John Wiley & Sons Inc; 1982. [Google Scholar]
- 30.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–174. [PubMed] [Google Scholar]
- 31.Jacobs D, Sano M, Marder K, et al. Age at onset of Alzheimer’s disease: relation to pattern of cognitive dysfunction and rate of decline. Neurology. 1994;44(7):1215–1220. doi: 10.1212/wnl.44.7.1215. [DOI] [PubMed] [Google Scholar]
- 32.Krumholz A, Wiebe S, Gronseth G, et al. Quality Standards Subcommittee of the American Academy of Neurology; American Epilepsy Society. Practice parameter: evaluating an apparent unprovoked first seizure in adults (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology. 2007;69(21):1996–2007. doi: 10.1212/01.wnl.0000285084.93652.43. [DOI] [PubMed] [Google Scholar]
- 33.Corey LA, Kjeldsen MJ, Solaas MH, Nakken KO, Friis ML, Pellock JM. The accuracy of self-reported history of seizures in Danish, Norwegian and U.S. twins. Epilepsy Res. 2009;84(1):1–5. doi: 10.1016/j.eplepsyres.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mayeux R, Stern Y, Spanton S. Heterogeneity in dementia of the Alzheimer type: evidence of subgroups. Neurology. 1985;35(4):453–461. doi: 10.1212/wnl.35.4.453. [DOI] [PubMed] [Google Scholar]
- 35.Scarmeas N, Anderson KE, Hilton J, et al. APOE-dependent PET patterns of brain activation in Alzheimer disease. Neurology. 2004;63(5):913–915. doi: 10.1212/01.wnl.0000137274.93125.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scarmeas N, Brandt J, Albert M, et al. Association between the APOE genotype and psychopathologic symptoms in Alzheimer’s disease. Neurology. 2002;58(8):1182–1188. doi: 10.1212/wnl.58.8.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]