Abstract
Cyclic mechanical loading of articular cartilage results in a complex biomechanical environment at the scale of the chondrocytes that strongly affects cellular metabolic activity. Under dynamic loading conditions, the quantitative relationships between macroscopic loading characteristics and solid and fluid mechanical variables in the local cellular environment are not well understood. In this study, an axisymmetric multiscale model of linear biphasic cell-matrix interactions in articular cartilage was developed to investigate the cellular microenvironment in an explant subjected to cyclic confined compressive loading. The model was based on the displacement-velocity-pressure (u-v-p) mixed penalty weighted residual formulation of linear biphasic theory that was implemented in the COMSOL Multiphysics software package. The microscale cartilage environment was represented as a three-zone biphasic region consisting of a spherical chondrocyte with encapsulating pericellular matrix (PCM) that was embedded in a cylindrical extracellular matrix (ECM) subjected to cyclic confined compressive loading boundary conditions. Biphasic material properties for the chondrocyte and the PCM were chosen based on previous in vitro micropipette studies of cells or chondrons isolated from normal or osteoarthritic cartilage. Simulations performed at four loading frequencies in the range 0.01–1.0 Hz supported the hypothesized dual role of the PCM as both a protective layer for the cell and a mechanical transducer of strain. Time varying biphasic variables at the cellular scale were strongly dependent on relative magnitudes of the loading period, and the characteristic gel diffusion times for the ECM, the PCM and the chondrocyte. The multiscale simulations also indicated that axial strain was significantly amplified in the range 0.01–1.0Hz, with a decrease in amplification factor and frequency insensitivity at the higher frequencies. Simulations of matrix degradation due to osteoarthritis indicated that strain amplification factors were more significantly altered when loss of matrix stiffness was exclusive to the PCM. The findings of this study demonstrate the complex dependence of dynamic mechanics in the local cellular environment of cartilage on macroscopic loading features and material properties of the ECM and the chondron.
Keywords: chondron, poroelastic, collagen, proteoglycan, finite element method
1. INTRODUCTION
Articular cartilage is the primary load-bearing soft tissue in diarthrodial joints and exhibits apparent viscoelastic responses including creep, stress relaxation, and hysteresis under cyclic loading. The cartilage extracellular matrix (ECM) is maintained by the metabolic activity of the chondrocytes, which are dispersed throughout the ECM and comprise 1–10% of the tissue volume in adult cartilage [1]. In addition to genetic and environmental factors, it is now well established that mechanical factors, such as tensile, compressive, and shear stresses and strains, fluid pressurization and electrokinetic effects, strongly influence chondrocyte activity (reviewed in [2]). The sequence of mechanobiological events by which chondrocytes convert extracellular “signals” into intracellular responses, which serve to maintain or alter cartilage ECM, is complex and not well understood. A detailed characterization of the local environment of the chondrocyte necessitates the development of biomechanical models that can quantify relationships between applied loading at the macroscopic (mm) scale of a joint or tissue explant, and microscopic (micron) scale mechanical variables near the cells.
Many experimental studies have investigated the effects of dynamic loading on cell metabolic activity in cartilage explants [3–11], tissue-engineered cartilage [12–20], and chondrocyte cell cultures [21, 22], as well as effects on chondrocyte differentiation in cell-seeded scaffolds [23, 24]. In vitro studies of cartilage explants have demonstrated that static loading generally inhibits glycosaminoglycan (GAG) synthesis [6], while cyclic loading can increase or suppress GAG synthesis, depending primarily on mechanical factors such as the applied loading frequency [4–6, 8, 10, 11], amplitude [3, 4, 9, 11] and duration of load application [4, 8], as well as the radial position in unconfined cylindrical explants [5]. In tissue engineering studies for cell-seeded scaffolds, the effects of the frequency of dynamic loading [12, 14, 15, 19, 20] have been evaluated over a wide range of frequencies, in conjunction with factors such as cell seeding density [16], loading configuration [17], or the presence of a cell-associated matrix [25] to measure ECM regeneration over the culture time period. Taken together, these studies indicate that macroscopic dynamic mechanical loading of articular cartilage strongly influences regulatory pathways by which chondrocytes respond to their surroundings [26, 27].
Under dynamic compression, the diffusive drag force as interstitial fluid flows past cartilage ECM is an important contributor to the apparent viscoelastic response of the tissue. This fundamental biomechanical mechanism can be captured within the framework of biphasic theory [28], which models articular cartilage as an incompressible continuum mixture of fluid and solid phases. Macroscopic loading on the millimeter-scale of a cartilage layer or explant induces nonuniform deformation [29–32] on the micron-scale of the chondrocyte, as well as stress, strain, pressure and flow fields that can be simulated via biphasic models. Such models have the potential to aid in delineation of specific components of the biphasic cellular microenvironment that are involved in cell metabolic regulatory responses to mechanical load. Previously, mathematical solutions for dynamic compressive loading of a linear, homogeneous, biphasic cartilage layer have been developed for the cases of confined compression [33] and unconfined compression [34].
A more detailed description of biphasic mechanics in the local cellular environment of cartilage necessitates a multiscale extension of these homogeneous models to include inhomogeneous inclusions representing the chondrocyte and its encapsulating pericellular matrix (PCM) which, together, have been termed the chondron [35]. The PCM of articular cartilage is known to be rich in proteoglycans and is distinguished from ECM by the presence of type VI collagen [36, 37]. With osteoarthritis, chondron size is enlarged and occurs in conjunction with an increase in cell proliferation [38]. In vitro micropipette aspiration studies of chondrons isolated from human and canine tissue indicate that the pericellular matrix is several orders magnitude stiffer than the chondrocyte in the middle and deep zones [39], and that its elastic and biphasic mechanical properties exhibit significant alterations with osteoarthritis [40, 41]. These studies suggest that the PCM plays a critical biomechanical role in regulating cell-matrix interactions in articular cartilage [42].
Previously, multiscale finite element models have been developed and applied to simulate biphasic cell-matrix interactions for spheroidal inclusions representing chondrocytes [43] or a chondron embedded in cartilage ECM under steady state loading [42] or transient stress relaxation in unconfined compression [44]. In these cases, the inclusion of a biphasic region representing the PCM that surrounds the chondrocyte strongly influenced the mechanical environment of the cell. In this study, an axisymmetric multiscale finite element model of linear biphasic cell-matrix interactions under cyclic confined compressive loading is considered. The microscale environment of single chondrocyte is modeled as a three-zone region consisting of a spherical cell with a surrounding pericellular layer that is embedded in a cylindrical ECM. A macroscopic theoretical solution for dynamic confined compressive loading of a linear biphasic layer [33] is employed to formulate boundary conditions for the microscale domain. The resulting multiscale dynamic biphasic model is used to analyze the effects of loading frequencies in the range 0.01–1.0 Hz on biphasic mechanics in the local cellular environment. In particular, simulations for the transmission of axial strain to the cellular level are motivated by the potential role of strain as a sensitive mechanical variable that may be involved in cellular signal transduction. The objective of this study was to develop a biphasic model of cell-matrix interactions under cyclic loading conditions that can aid in correlation of fluid and solid mechanics in the cellular microenvironment with chondrocyte biosynthetic activity in tissue explants and tissue engineered constructs.
2. COMPUTATIONAL MODEL AND METHODS
Biphasic model of cyclic confined compression
Linear biphasic theory was used to model articular cartilage as a continuum mixture of fluid and solid phases [28]. The governing mass and momentum equations are
| (1) |
where α= s, f denotes the solid and fluid phases, respectively, vα is the velocity, φα is the volume fraction, σα is the partial Cauchy stress and πα is the momentum exchange vector. Under the assumptions of a linear isotropic solid phase, an inviscid fluid phase, and diffusive fluid-solid drag with constant hydraulic permeability k, the constitutive relations are
| (2) |
where p is the pore pressure, es = [(∇us) + (∇us)T]/2 is the solid phase infinitesimal strain, us is the solid displacement, and λs,μs are the solid phase Lame coefficients. Suh et al. [33] developed an analytical series solution of Eqs. 1–2 for uniaxial cyclic confined compression of a linear biphasic layer (0 ≤ z ≤ h). In this simplified geometry, the governing Eqs. 1–2 reduce to a single diffusion equation for the axial displacement component ws (z,t). Specifically Suh et al. [33] derived a theoretical solution for the (loading) boundary conditions:
| (3) |
where f is the loading frequency. Since a primary focus of the current study was on strain mechanotransduction, similar mathematical techniques were used to derive a theoretical solution for the (displacement) boundary conditions
| (4) |
The boundary conditions in Eq. 4 correspond to an applied, exclusively compressive, deformation at peak engineering strain −ε0 starting at t = 1/(2 f) and repeating periodically every 1/f seconds. The resulting theoretical solutions for solid phase displacement and pore pressure are, respectively:
| (5) |
| (6) |
where ω = 2π f, αn = k(λs + 2μs)(nπ/h)2,βn = (−1)n+1ε0ω/(nπ) and the axial fluid phase velocity is determined from Eq. 5, via the formula
| (7) |
Multiscale model
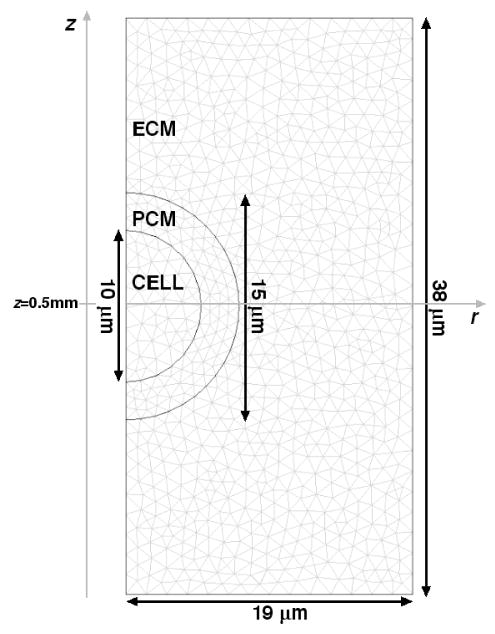
Chondrons, which consist of the chondrocyte and its surrounding PCM, were modeled as biphasic inclusions within a biphasic ECM. Alexopoulos et al. [41] developed a linear biphasic finite element model for in vitro micropipette aspiration and applied it to measure biphasic PCM properties for chondrons isolated from non-degenerate human cartilage, assuming previously measured mechanical properties for chondrocytes [45, 46]. These measured cell and PCM material property values were employed in the current study, along with representative values for articular cartilage ECM (Table 1). The chondrocyte and chondron were modeled as spherical inclusions with a cell radius of 5μm and a pericellular layer thickness of 2.5 μm [44], and the dimensions of the microscale cylindrical domain were a radius of 19 μm and a height of 38 μm (Fig. 1). This geometry corresponds to a chondrocyte volume fraction of roughly 1%, when the tissue is idealized as a periodic arrangement of cubes with side length equal to the diameter of the microscale cylindrical domain, and is consistent with the small volume fraction of these cells in articular cartilage [1].
Table I.
Biphasic material parameter values for simulation of microscale mechanics, based on Alexopoulos et al (2005) [41].
| Chondrocyte | PCM (Normal) | PCM (OA) | ECM (Normal) | ECM (OA) | |
|---|---|---|---|---|---|
| Es | 0.35 kPa | 38.7 kPa | E=23.5kPa | 1000 kPa | 600kPa |
| νs | 0.43 | 0.04 | 0.03 | 0.04 | 0.04 |
| k | 1.00e-16 m4/Ns | 4.19e-17 m4/Ns | 10.2e-17 m4/Ns | 1.00e-15 m4/Ns | 2.00e-15m4/Ns |
Figure 1.
Geometry and FEM mesh for simulation of microscale mechanical interactions between the chondron and ECM.
Based on previous finite element modeling studies of cell-matrix interactions [44], a multiscale modeling approach was employed. Specifically, the macroscopic (mm-scale) biphasic deformation of cartilage in confined compression subject to the boundary conditions of Eq. 4 was modeled via the theoretical series solution Eqs. 5–7. This solution was employed to formulate boundary conditions for axial solid displacement (Eq. 5), pressure (Eq. 6) and axial fluid displacement (Eq. 7) (both radial displacements were set to zero) to simulate microscopic (μm -scale) biphasic deformation of the chondron inclusion problem (Fig. 1).
Finite element method
The chondron inclusion problem was solved numerically via a custom implementation of the axisymmetric mixed-penalty linear biphasic finite element formulation [47] in the software package COMSOL Multiphysics (CM) (version 3.2b, COMSOL, Inc., Burlington, MA). In particular, the mixed-penalty method replaces the mixture incompressibility Eq. 1a, with its penalty form
| (8) |
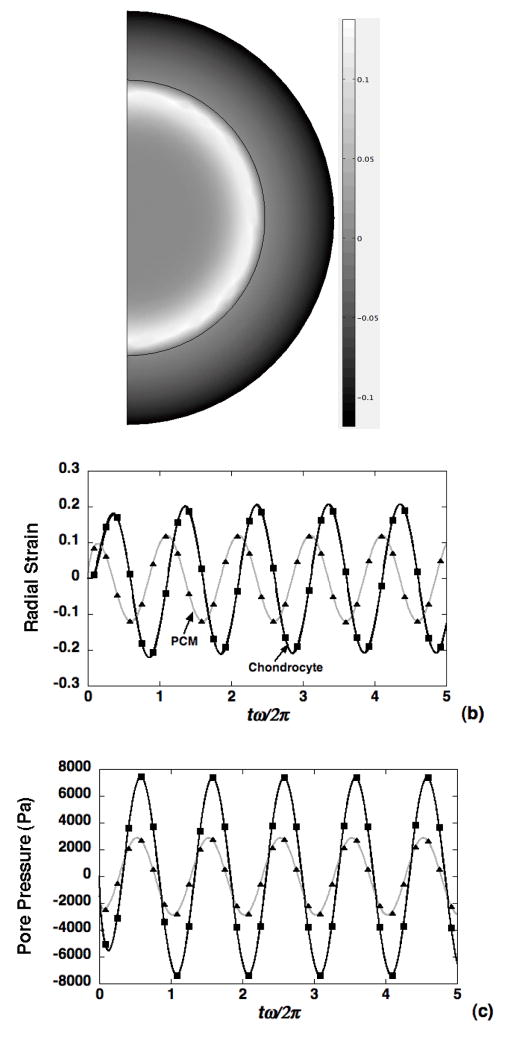
where β is a user-specified penalty parameter that is chosen to be several orders of magnitude larger than typical pressures in the tissue. A conforming mesh consisting of triangular elements was employed (Fig. 1), and the set of five dependent variables was comprised of the pore pressure, and the radial and axial components of the solid and fluid displacement. All displacements were approximated by Lagrange quadratic elements, and pressure was approximated via Lagrange linear elements. The corresponding discretized system of equations comprised a linear system of first-order ordinary differential equations that were solved using the “Time dependent” solver in CM. This solver views the discretized equations as a differential-algebraic system, employing a version of the DASPK solver, and is thus an implicit scheme that employs variable-order variable stepsize backward differentiation formulas [48]. At each time-step, the spatial linear system was solved using the CM option “Direct (UMFPACK)”, which employs the unsymmetric-pattern multifrontal method and direct LU factorization with the COLAMD and AMD approximate minimum degree preordering algorithms to minimize fill-in [49]. Accuracy of the finite element implementation was established via simulation of one-dimensional biphasic deformation of a spherical chondron subjected to a cyclic displacement at 0.1Hz applied normal to its boundary, where previous solutions are available [50] (Fig. 2).
Figure 2.
Validation of the biphasic FEM model (symbols) against a previous theoretical solution for cyclic radial deformation (0.1Hz) in a spherical chondron [50]. (a) Spatial strain profile at t=5sec, radial strain (b) and pore pressure (c) at the chondron boundary and cell-PCM interface. Cell and PCM parameters were chose based on the Table I (normal case).
Parameter values
Loading frequencies in the range f=0.01– 1.0Hz were considered and the macroscopic engineering strain (Eq. 4) was taken as ε0 = 0.01. The center of the micro-scale domain (Fig. 1) was taken at a depth of z/h= 0.5, where h = 1mm, to represent mid-zone articular cartilage corresponding to depths of 0.519mm and 0.481mm at the top and bottom of the microscale domain, respectively. To establish numerical convergence, the mesh resolution and penalty parameter β were varied in the case where all three zones were assigned material properties equal to those of the ECM (Table I). The resulting numerical solutions for axial strain, axial solid stress and pore pressure were compared to the macroscopic theoretical solution Eqs. 5–7 to establish the number of elements (Fig. 1) and magnitude of the penalty parameter (β = 1e20) required to achieve numerical convergence. Subsequently, simulations were performed with the distinct material property values for the cell, PCM, and ECM listed in Table I. For all results shown, it was verified that the chosen mesh resolution (Fig. 1) was sufficient for stabilization of stress and strain computations via successive mesh refinement. Results were also analyzed to ensure that, for the chosen domain size, solutions did not exhibit boundary layers near the domain edges. The presence of such boundary layers would preclude the inherent assumption in the model that microscale perturbations in the macroscopic biphasic deformation (due to inclusion of the chondron) vanish in the far field, i.e. where the macroscopic boundary conditions are prescribed.
3. RESULTS
The multiscale model described in the previous section was used to simulate the effects of macroscopic loading frequency on linear biphasic cell-matrix interactions in articular cartilage.
Force transmission
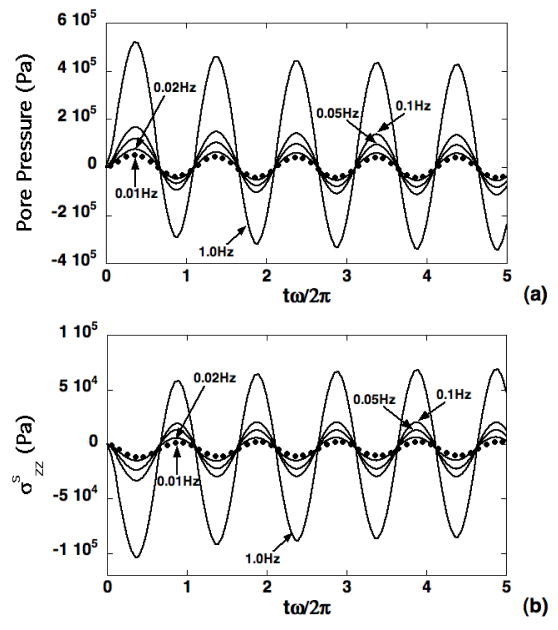
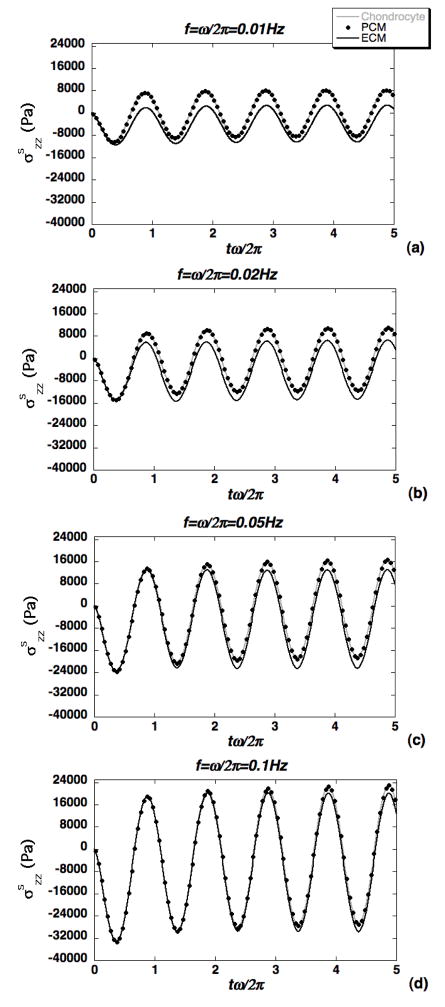
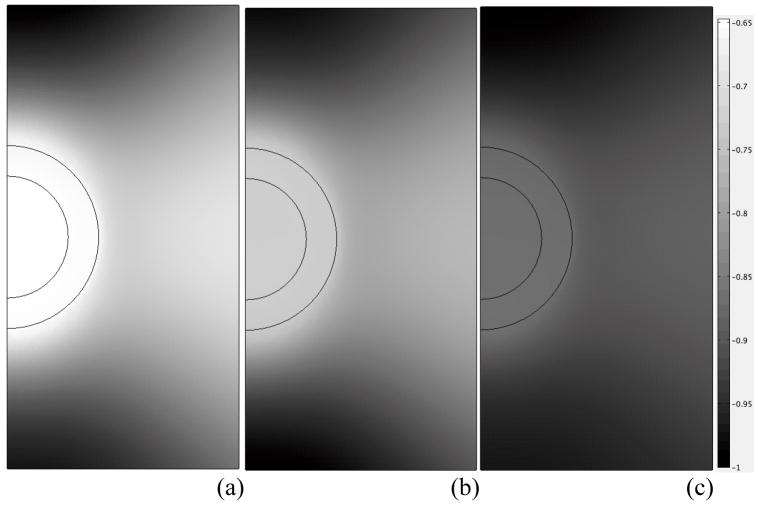
Using the theoretical solution (Eqs. 5–6), the effects of loading frequency f on macroscopic transmission of forces via normal ECM to the microscopic domain were evaluated via the axial solid stress and pore pressure at z=0.519mm with advancing load cycles (Fig. 3). Amplitudes of transmitted axial solid stress and pressure were observed to increase with loading frequency, with both compressive and tensile forces exhibited at separate intervals of the loading cycles. Force transmission within the microscale domain was evaluated along the symmetry axis via a representative pericellular axial solid stress at 0.25μm below the PCM-ECM interface, and a representative chondrocyte axial solid stress at 0.5 μm below the cell-PCM interface (Fig. 4). These point locations correspond to a distance from the cell center (z=0.5mm) equal to 90% of the cell radius, and equal to the cell radius plus 90% of the PCM layer thickness. The ECM axial solid stress was evaluated at the top of the microscale domain. In all cases, microscale axial stress amplitude increased with increasing loading frequency, and the PCM axial solid stress response was indistinguishable from that of the chondrocyte. Relative to the ECM, axial stress in the chondron exhibited larger tensile amplitude and smaller compressive amplitude, with more pronounced differences at lower frequencies (Fig. 4). To quantify the spatial distribution of forces on the chondrocyte, a solid phase “spherical stress” determined as the projection of the solid phase traction vector in the direction normal to the boundary of the chondron was also evaluated. The effect of increasing frequency on these spatial profiles is shown after 4.5 loading cycles, where the stress has been normalized to its prescribed value at the upper boundary (Fig. 5). It is observed that the presence of the biphasic inclusion representing the chondron leads to reduction in solid stress magnitude of up to 35% at the lowest frequency f=0.01Hz (Fig. 5a), and that this effect diminishes significantly with increasing loading frequency (Fig. 5c).
Figure 3.
Simulations of pore pressure (a) and solid stress (b) transmitted to the microscale domain via the ECM based on the theoretical solution in Eqs. 5–6. Responses are shown at z=0.519mm for four different loading frequencies in the range f=0.01–1.0Hz.
Figure 4.
Illustration of force transmission at three points along the symmetry axis located at 90% of the cell radius (chondrocyte), 90% of the PCM thickness and at the top of the microscale domain (ECM). Axial solid stress is shown at four loading frequencies: (a) f=0.01Hz, (b) f=0.02Hz, (c) f=0.05Hz and (d) f=0.1Hz.
Figure 5.
Spatial profiles of the solid phase “spherical stress” shown after 4.5 loading cycles for loading frequencies: (a) f=0.01Hz, (b) f=0.02Hz and (c) f=0.1Hz.
Strain transmission
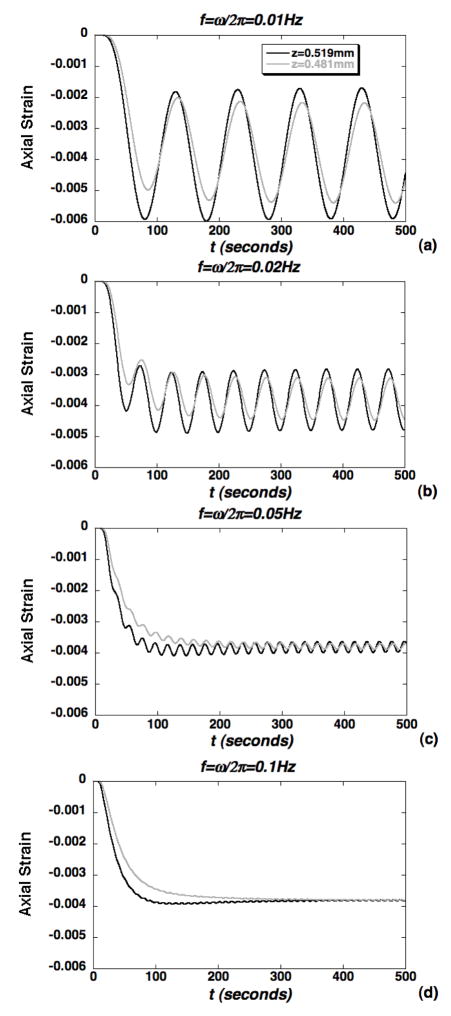
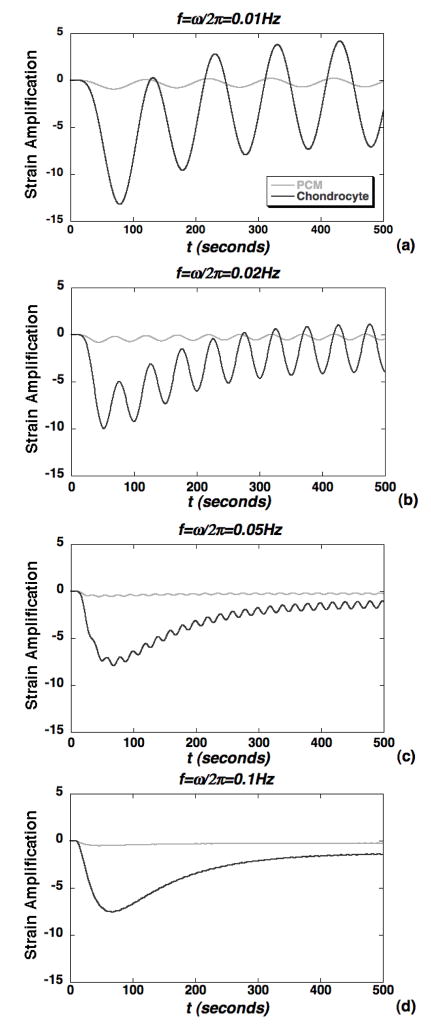
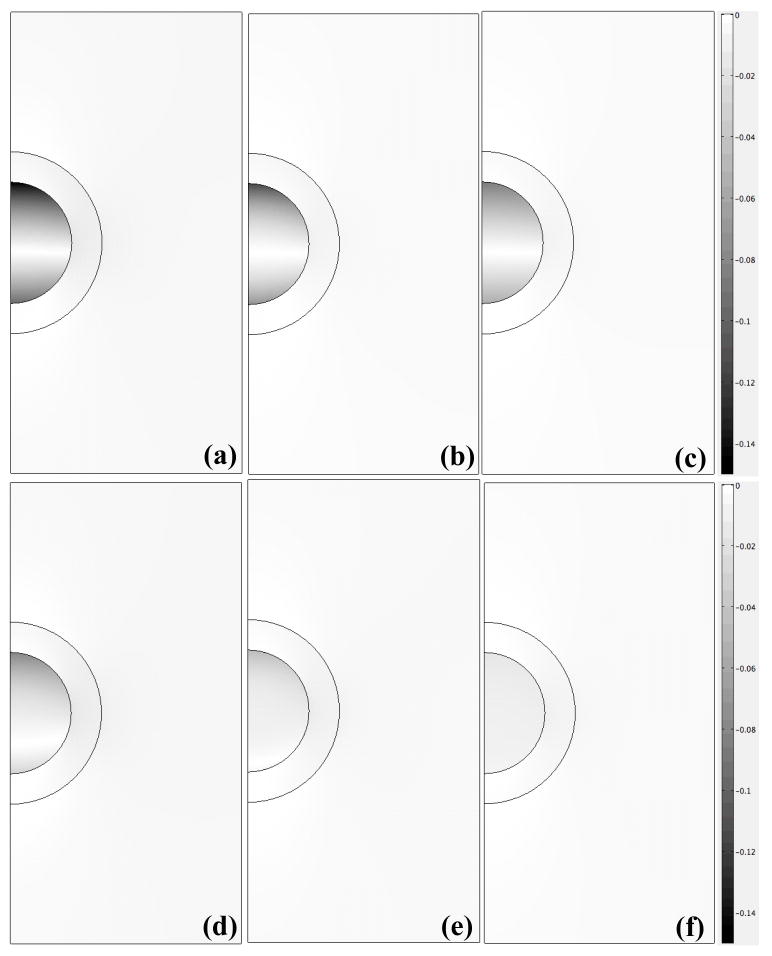
The magnitude of axial strain transmitted to the microscale chondrocyte environment via the ECM was also evaluated using the macroscopic theoretical solution (Eqs. 5–6) (Fig. 6). It was generally observed that the axial strain, macroscopically prescribed at an engineering strain ε0 = 1%, was diminished significantly in mechanical transduction to 50% depth but remained exclusively compressive. At the lower frequencies (0.01–0.02Hz), the microscale strain exhibited a two-scale response with oscillations at the loading frequency enveloped in a slower exponentially varying curve governing the transition to a steady oscillation (Fig. 6a–b). As the frequency was increased to 0.1Hz, the oscillations were diminished and the response tends to a frequency-insensitive curve governing the, slower, exponentially varying transition to equilibrium (Fig. 6c–d). Strain amplification in the chondron was also simulated via evaluation of the axial strain, normalized to ε0 = 1%, at representative points that were 0.25μm below the PCM-ECM interface and 0.5 μm below the cell-PCM interface (Fig. 7). Over the entire course of loading (500s), the peak cellular strain amplification factor was roughly 13 at the lowest frequency of 0.01Hz (Fig. 7a) and decreased to roughly 7.5 at 0.1Hz, where the response becomes insensitive to frequency (Figs. 7c–d). Similarly, the steady state strain amplification factor was roughly 7 at 0.01Hz and decreased to roughly 1.5 at the higher frequencies. At times corresponding to peak strain amplification, the chondrocyte exhibits nonuniform spatial distributions of axial strain (Fig. 8a–c). While strain amplification was also observed in the steady regime, chondrocyte profiles were more spatially uniform (Fig. 8d–f).
Figure 6.
Simulations of axial strain transmitted to the microscale domain via the ECM shown at the top and bottom of the microscale domain for loading frequencies: (a) f=0.01Hz, (b) f=0.02Hz, (c) f=0.05Hz and (d) f=0.1Hz.
Figure 7.
Simulations of axial strain amplification within the chondron via representative points located at 90% of the cell radius (chondrocyte), 90% of the PCM thickness. Amplification factors are normalized to the macroscopic engineering strain ε0=1% and shown for the loading frequencies: (a) f=0.01Hz, (b) f=0.02Hz, (c) f=0.05Hz and (d) f=0.1Hz.
Figure 8.
Spatial profiles of axial strain magnitude at times corresponding to: (a–c) peak strain amplification for f=0.01Hz [(a), t=80s], f=0.02Hz [(b), t=50s] and f=0.05Hz [(c), t=70s], and (d–f) steady state amplification at local minima for f=0.01Hz [(d), t=480s], f=0.02Hz [(e), t=450s] and f=0.05Hz [(f), t=490s].
OA effects on strain transmission
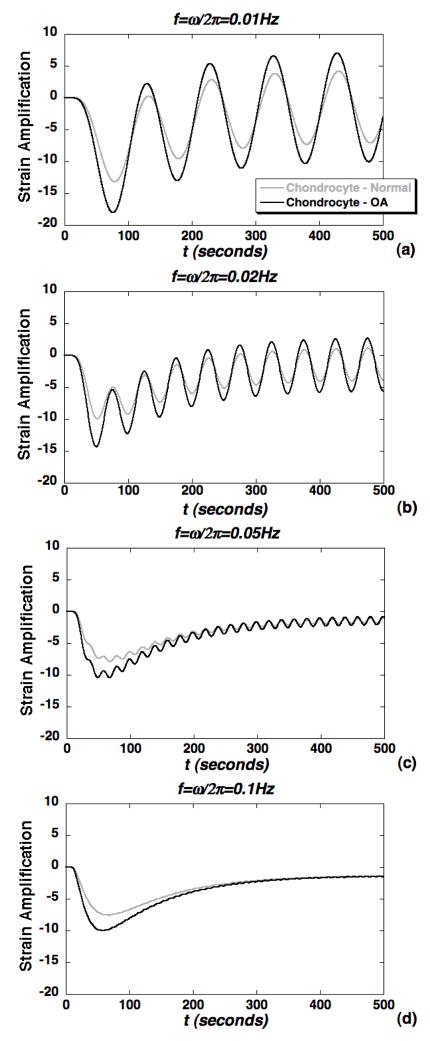
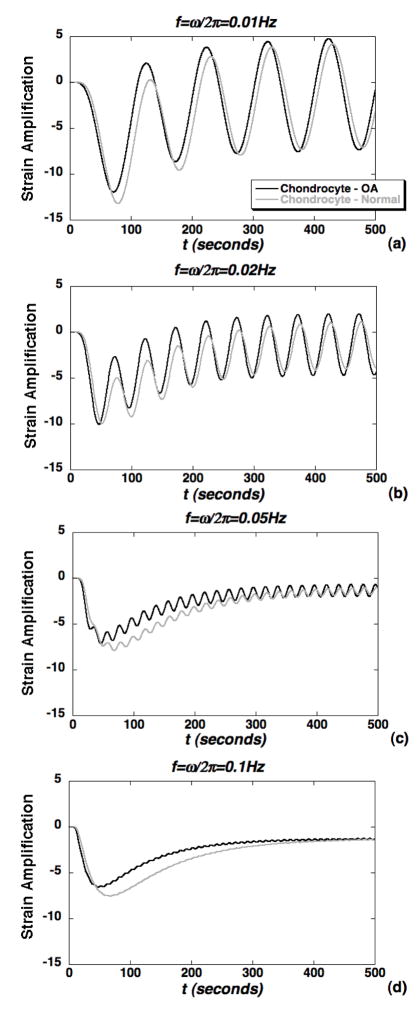
To model potential alterations in the cellular microenvironment due to OA, simulations of strain amplification were repeated with biphasic parameter values representing two models of OA (Table I). In the first case (Fig. 9) loss of matrix stiffness was assumed to be exclusive to the PCM while, in the second case (Fig. 10) loss of both PCM and ECM stiffness was simulated. For the OA PCM model, alterations in biphasic PCM properties resulted in a significant increase in peak strain amplification factors for all cases in the frequency range 0.01–1.0Hz (Fig. 9). In the steady regime, strain amplification factors were significantly altered with an OA PCM only at the lower frequencies 0.01–0.02Hz (Fig. 9a–b). For the OA PCM-ECM model, peak strain amplification factors were slightly lower in the OA case, but not significantly altered in the steady regime (Fig. 10). The alterations in peak amplification were less pronounced at lower frequencies as compared to the OA PCM model (Fig. 10a–b). Overall, strain measures within the chondron were observed to exhibit complex spatial and time-varying responses that varied with both macroscopic loading frequency and biphasic PCM properties.
Figure 9.
Simulations of axial strain amplification within the chondrocyte via a representative point located at 90% of the cell radius comparing cases of normal PCM&ECM and OA exclusive to the PCM (see Table I). Amplification factors are normalized to the macroscopic engineering strain ε0=1% and shown for the loading frequencies: (a) f=0.01Hz, (b) f=0.02Hz, (c) f=0.05Hz and (d) f=0.1Hz.
Figure 10.
Simulations of axial strain amplification within the chondrocyte via a representative point located at 90% of the cell radius comparing cases of normal PCM&ECM and OA PCM&ECM (see Table I). Amplification factors are normalized to the macroscopic engineering strain ε0=1% and shown for the loading frequencies: (a) f=0.01Hz, (b) f=0.02Hz, (c) f=0.05Hz and (d) f=0.1Hz.
4. DISCUSSION
The multiscale biphasic model of cyclic confined compressive loading presented in this study demonstrates the complex dependence of dynamic mechanics in the microscopic cellular environment on features of the applied macroscopic loading and material properties of the ECM and chondron. The time-varying responses for mechanical variables such as axial strain exhibit a multiscale character that is governed, in large part, by relative magnitudes of the macroscopic loading period f−1, and characteristic gel diffusion times for the ECM, the PCM and the chondrocyte. For example, in the case of ECM mechanotransduction, there are two characteristic time scales, i.e. the loading period and ECM gel diffusion time. The macroscopic analytical solution (Eqs. 5–7) for biphasic deformation at 50% ECM depth under strain-controlled deformation (Eq. 4) is consistent with the finding in the force-controlled case (Eq. 3) that the ECM is a low-pass filter for transmission of strain (Fig. 6), and a high-pass filter for transmission of pressure and axial solid stress (Fig. 3)[33].
For the microscopic biphasic inclusion problem considered in this study, an additional time scale is introduced that is governed by the material and geometric properties of the chondron. At lower frequencies, the protective role of the PCM in shielding the chondrocyte from solid stress is enhanced (Fig. 5). The simulations presented in this study suggest that, at lower frequencies, the loading period and the cell, PCM and ECM gel diffusion times are on comparable scales for mechanical interaction, thus leading to significant energy dissipation by the PCM (Fig. 5a). As the loading frequency is increased, coupling between these microscale biphasic mechanisms is diminished and the axial stress exhibits a more spatially uniform response (Fig. 5c) that is insensitive to loading frequency (Fig. 4). The relatively uniform axial solid stress and pressure distributions within the chondron (Figs. 4–5) are likely due to continuity of these quantities along biphasic interfaces [51]. Similarly, axial solid stress amplitudes in the chondrocyte microenvironment increase substantially with increasing frequency (Fig. 4), most likely due to insignificant recovery time for PCM diffusive drag to contribute to the overall mechanical response in this loading regime.
The multiscale simulations of axial strain presented in this study focus on the potential role of intracellular strain as one biophysical variable in the process of mechanical signal transduction in articular cartilage, although there is evidence that chondrocytes respond to a variety of other physical signals [2]. For the frequency range considered in this study (0.01–1.0Hz), axial strain exhibited time-varying (Fig. 7) and nonuniform strain profiles (Fig. 8) within the chondrocyte. At lower frequencies, axial strain in the chondrocyte is highly amplified and frequency-dependent (Figs. 7a–c) suggesting that a spectrum of intracellular biophysical transduction mechanisms may be involved in this loading regime. At higher frequencies (Fig. 7d), the strain response tends to that of a non-oscillatory creep curve, most likely associated with intermittent loading at the applied strain magnitude. In this frequency range, these simulations suggest that the small loading period inhibits the ability of the tissues to exhibit fast viscoelastic recovery prior to the subsequent load cycle. Together, these findings suggest that confined compressive loading in the frequency range 0.01–0.1Hz induces a complex time and spatially varying intracellular biophysical state that can be exploited to induce desired spatio-temporal characteristics of specific biomechanical stimuli within a tissue or construct. Taken together with experimental studies of the spatial variations in cellular metabolism [5, 52], such studies can provide further insights into the role of specific biophysical parameters in regulating the mechanobiology of chondrocytes.
The aforementioned relative magnitudes of loading and gel diffusion time scales also depend on chondron and ECM biphasic mechanical properties. Based on in vitro micropipette aspiration of isolated human chondrons, Alexopoulos et al. [41] determined that biphasic material properties of human PCM were significantly altered with OA (Table I). Simulations using the multiscale biphasic model indicate that alterations in PCM biphasic properties result in significant changes in cellular strain amplification profiles at all frequencies, particularly in the regime of peak amplification at earlier times (Fig. 9). These findings suggest that a model of early-stage OA, in which matrix degradation is exclusive to the PCM, will induce significant changes in intracellular chondrocyte deformation in both space and time. If degenerative changes are modeled in both the PCM and ECM (Fig. 10), the effects of these changes on the cellular microenvironment are reduced. Since the PCM properties chosen for the simulations were based on in vitro analysis of isolated chondrons, future studies examining the in situ deformation of chondrons within the ECM of cartilage explants (e.g., [30]) will facilitate development of more realistic models of the effects of OA on the mechanical environment of the chondrocyte.
It should be noted that the present model assumes that macroscopic and microscopic strains are infinitesimal and, as such, application is limited to experimental loading at small amplitudes. In particular, as macroscopic strain levels are increased, the small strain assumption may cease to be valid in the cell since it is much softer than the ECM and may exhibit finite strain and a water volume fraction that varies significantly with deformation. As the cell-to-tissue volume fraction is significantly increased, a potential limitation of the model is the appearance of coupled effects near the boundaries of the microscopic domain due to reduction in the size of the microscale domain. Extension to this case would necessitate a more complex simulation of biphasic interactions between many cells and their surrounding matrix, which is beyond the scope of the current study. Similarly, increasing cell and PCM volume fractions decreases accuracy of our assumption that intrinsic ECM mechanical properties are those associated with cartilage explant tests that treat the sample as a homogeneous material. It is noted that recent confocal microscopy techniques for fluorescence imaging of in situ chondrocyte and PCM deformation within an explant [30] show promise for development of models that facilitate simultaneous determination of intrinsic cell, PCM and ECM mechanical properties. The biphasic model also neglects mechano-chemical coupling arising from the negative fixed charge density associated with proteoglycans in the ECM and PCM and, thus, measured properties and responses are associated with an isotonic external environment mimicking osmolality of the synovial fluid in diarthrodial joints. Additionally, the assumption of axisymmetric geometry is a model simplification that facilitated the parametric analysis performed in this study. In reality, chondrocytes within cartilage ECM can exhibit non-spheroidal geometry, off-axis alignment, and small clusters of cells can be surrounded by a single PCM. These factors, combined with the limitation of the axisymmetric model to chondrons located near the symmetry axis, indicate that extension to three dimensions is required for broader application of the multiscale biphasic model to simulating cell-matrix interactions in articular cartilage. In summary, the multiscale biphasic simulations presented in this study provide an initial model of dynamic cell-matrix interactions with potential application to correlating fluid and solid mechanical variables in the cartilage microenvironment to experimentally measure chondrocyte biosynthetic activity in cartilage explants and cell-gel constructs.
Acknowledgments
Supported in part by the Whitaker Foundation (RG-020933), the NSF (DMS-0636590), the Arthritis Foundation, and NIH grants AR50245, AG15768, and AR48182.
References
- 1.Stockwell RA. Biology of Cartilage Cells. Cambridge University Press; Cambridge: 1979. pp. 126–148. [Google Scholar]
- 2.Guilak F, Sah RL, Setton LA. In: Physical regulation of cartilage metabolism,” Basic Orthopaedic Biomechanics. Mow VC, Hayes WC, editors. Lippincott-Raven; Philadelphia: 1997. pp. 179–207. [Google Scholar]
- 3.Torzilli PA, Grigiene R, Huang C, Friedman SM, Doty SB, Boskey AL, Lust G. Characterization of cartilage metabolic response to static and dynamic stress using a mechanical explant test system. Journal of Biomechanics. 1997;30(1):1–9. doi: 10.1016/s0021-9290(96)00117-0. [DOI] [PubMed] [Google Scholar]
- 4.Steinmeyer J, Knue S. The proteoglycan metabolism of mature bovine articular cartilage explants superimposed to continuously applied cyclic mechanical loading. Biochemical and Biophysical Research Communications. 1997;240(1):216–221. doi: 10.1006/bbrc.1997.7641. [DOI] [PubMed] [Google Scholar]
- 5.Buschmann MD, Kim YJ, Wong M, Frank E, Hunziker EB, Grodzinsky AJ. Stimulation of aggrecan synthesis in cartilage explants by cyclic loading is localized to regions of high interstitial fluid flow. Archives of Biochemistry & Biophysics. 1999;366(1):1–7. doi: 10.1006/abbi.1999.1197. [DOI] [PubMed] [Google Scholar]
- 6.Li KW, Williamson AK, Wang AS, Sah RL. Growth responses of cartilage to static and dynamic compression. Clinical Orthopaedics and Related Research. 2001;391:S34–S48. doi: 10.1097/00003086-200110001-00005. [DOI] [PubMed] [Google Scholar]
- 7.Blain EJ, Gilbert SJ, Wardale RJ, Capper SJ, Mason DJ, Duance VC. Up-regulation of matrix metalloproteinase expression and activation following cyclical compressive loading of articular cartilage in vitro. Archives of Biochemistry and Biophysics. 2001;396(1):49–55. doi: 10.1006/abbi.2001.2575. [DOI] [PubMed] [Google Scholar]
- 8.Sauerland K, Raiss RX, Steinmeyer J. Proteoglycan metabolism and viability of articular cartilage explants as modulated by the frequency of intermittent loading. Osteoarthritis and Cartilage. 2003;11(5):343–350. doi: 10.1016/s1063-4584(03)00007-4. [DOI] [PubMed] [Google Scholar]
- 9.Piscoya JL, Fermor B, Kraus VB, Stabler TV, Guilak F. The influence of mechanical compression on the induction of osteoarthritis-related biomarkers in articular cartilage explants. Osteoarthritis Cartilage. 2005;13(12):1092–1099. doi: 10.1016/j.joca.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 10.Palmoski MJ, Brandt KD. Effects of static and cyclic compressive loading on articular cartilage plugs in vitro. Arthritis & Rheumatism. 1984;27(6):675–681. doi: 10.1002/art.1780270611. [DOI] [PubMed] [Google Scholar]
- 11.Sah RL, Kim YJ, Doong JY, Grodzinsky AJ, Plaas AH, Sandy JD. Biosynthetic response of cartilage explants to dynamic compression. Journal of Orthopaedic Research. 1989;7(5):619–636. doi: 10.1002/jor.1100070502. [DOI] [PubMed] [Google Scholar]
- 12.Waldman SD, Couto DC, Grynpas MD, Pilliar RM, Kandel RA. A single application of cyclic loading can accelerate matrix deposition and enhance the properties of tissue-engineered cartilage. Osteoarthritis and Cartilage. 2006;14(4):323–330. doi: 10.1016/j.joca.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 13.Chowdhury TT, Bader DL, Lee DA. Dynamic compression counteracts IL-1 beta induced iNOS and COX-2 activity by human chondrocytes cultured in agarose constructs. Biorheology. 2006;43(3–4):413–429. [PubMed] [Google Scholar]
- 14.Lee DA, Noguchi T, Frean SP, Lees P, Bader DL. The influence of mechanical loading on isolated chondrocytes seeded in agarose constructs. Biorheology. 2000;37(1–2):149–161. [PubMed] [Google Scholar]
- 15.Davisson T, Kunig S, Chen A, Sah R, Ratcliffe A. Static and dynamic compression modulate matrix metabolism in tissue engineered cartilage. Journal of Orthopaedic Research. 2002;20(4):842–848. doi: 10.1016/S0736-0266(01)00160-7. [DOI] [PubMed] [Google Scholar]
- 16.Mauck RL, Seyhan SL, Ateshian GA, Hung CT. Influence of seeding density and dynamic deformational loading on the developing structure/function relationships of chondrocyte-seeded agarose hydrogels. Annals of Biomedical Engineering. 2002;30(8):1046–1056. doi: 10.1114/1.1512676. [DOI] [PubMed] [Google Scholar]
- 17.Chowdhury TT, Bader DL, Shelton JC, Lee DA. Temporal regulation of chondrocyte metabolism in agarose constructs subjected to dynamic compression. Archives of Biochemistry and Biophysics. 2003;417(1):105–111. doi: 10.1016/s0003-9861(03)00340-0. [DOI] [PubMed] [Google Scholar]
- 18.Hung CT, Mauck RL, Wang CCB, Lima EG, Ateshian GA. A paradigm for functional tissue engineering of articular cartilage via applied physiologic deformational loading. Annals of Biomedical Engineering. 2004;32(1):35–49. doi: 10.1023/b:abme.0000007789.99565.42. [DOI] [PubMed] [Google Scholar]
- 19.Waldman SD, Spiteri CG, Grynpas MD, Pilliar RM, Kandel RA. Long-term intermittent compressive stimulation improves the composition and mechanical properties of tissue-engineered cartilage. Tissue Engineering. 2004;10(9–10):1323–1331. doi: 10.1089/ten.2004.10.1633. [DOI] [PubMed] [Google Scholar]
- 20.Lee DA, Bader DL. Compressive strains at physiological frequencies influence the metabolism of chondrocytes seeded in agarose. Journal of Orthopaedic Research. 1997;15(2):181–188. doi: 10.1002/jor.1100150205. [DOI] [PubMed] [Google Scholar]
- 21.Waldman SD, Spiteri CG, Grynpas MD, Pilliar RM, Hong J, Kandel RA. Effect of biomechanical conditioning on cartilaginous tissue formation in vitro. Journal of Bone and Joint Surgery. 2003;85A:101–105. doi: 10.2106/00004623-200300002-00013. [DOI] [PubMed] [Google Scholar]
- 22.Suh JK, Baek GH, Aroen A, Malin CM, Niyibizi C, Evans CH, Westerhausen-Larson A. Intermittent sub-ambient interstitial hydrostatic pressure as a potential mechanical stimulator for chondrocyte metabolism. Osteoarthritis and Cartilage. 1999;7(1):71–80. doi: 10.1053/joca.1998.0163. [DOI] [PubMed] [Google Scholar]
- 23.Angele P, Schumann D, Angele M, Kinner B, Englert C, Hente R, Fuchtmeier B, Nerlich M, Neumann C, Kujat R. Cyclic, mechanical compression enhances chondrogenesis of mesenchymal progenitor cells in tissue engineering scaffolds. Biorheology. 2004;41(3–4):335–346. [PubMed] [Google Scholar]
- 24.Elder SH, Goldstein SA, Kimura JH, Soslowsky LJ, Spengler DM. Chondrocyte differentiation is modulated by frequency and duration of cyclic compressive loading. Annals of Biomedical Engineering. 2001;29(6):476–482. doi: 10.1114/1.1376696. [DOI] [PubMed] [Google Scholar]
- 25.Kelly TA, Wang CC, Mauck RL, Ateshian GA, Hung CT. Role of cell-associated matrix in the development of free-swelling and dynamically loaded chondrocyte-seeded agarose gels. Biorheology. 2004;41(3–4):223–237. [PubMed] [Google Scholar]
- 26.Grodzinsky AJ, Levenston ME, Jin M, Frank EH. Cartilage tissue remodeling in response to mechanical forces. Annual Review of Biomedical Engineering. 2000;2:691–713. doi: 10.1146/annurev.bioeng.2.1.691. [DOI] [PubMed] [Google Scholar]
- 27.Wilkins RJ, Browning JA, Urban JPG. Chondrocyte regulation by mechanical load. Biorheology. 2000;37(1–2):67–74. [PubMed] [Google Scholar]
- 28.Mow VC, Kuei SC, Lai WM, Armstrong CG. Biphasic creep and stress relaxation of articular cartilage in compression: Theory and experiments. Journal of Biomechanical Engineering. 1980;102(1):73–84. doi: 10.1115/1.3138202. [DOI] [PubMed] [Google Scholar]
- 29.Guilak F, Ratcliffe A, Mow VC. Chondrocyte deformation and local tissue strain in articular cartilage: a confocal microscopy study. Journal of Orthopaedic Research. 1995;13(3):410–421. doi: 10.1002/jor.1100130315. [DOI] [PubMed] [Google Scholar]
- 30.Choi JB, Youn I, Cao L, Leddy HA, Gilchrist CL, Setton LA, Guilak F. Zonal changes in the three-dimensional morphology of the chondron under compression: The relationship among cellular, pericellular, and extracellular deformation in articular cartilage. Journal of Biomechanics. 2007;40(12):2596–2603. doi: 10.1016/j.jbiomech.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chahine NO, Hung CT, Ateshian GA. In-situ measurements of chondrocyte deformation under transient loading. Eur Cell Mater. 2007;13:100–111. doi: 10.22203/ecm.v013a11. [DOI] [PubMed] [Google Scholar]
- 32.Schinagl RM, Gurskis D, Chen AC, Sah RL. Depth-dependent confined compression modulus of full-thickness bovine articular cartilage. Journal of Orthopaedic Research. 1997;15(4):499–506. doi: 10.1002/jor.1100150404. [DOI] [PubMed] [Google Scholar]
- 33.Suh JK, Li Z, Woo SL. Dynamic behavior of a biphasic cartilage model under cyclic compressive loading. J Biomech. 1995;28(4):357–364. doi: 10.1016/0021-9290(94)00103-b. [DOI] [PubMed] [Google Scholar]
- 34.Suh JK. Dynamic unconfined compression of articular cartilage under a cyclic compressive load. Biorheology. 1996;33(4–5):289–304. doi: 10.1016/0006-355x(96)00023-6. [DOI] [PubMed] [Google Scholar]
- 35.Poole CA, Flint MH, Beaumont BW. Chondrons in cartilage: ultrastructural analysis of the pericellular microenvironment in adult human articular cartilages. Journal of Orthopaedic Research. 1987;5(4):509–522. doi: 10.1002/jor.1100050406. [DOI] [PubMed] [Google Scholar]
- 36.Poole CA, Wotton SF, Duance VC. Localization of type IX collagen in chondrons isolated from porcine articular cartilage and rat chondrosarcoma. Histochemical Journal. 1988;20(10):567–574. doi: 10.1007/BF01002611. [DOI] [PubMed] [Google Scholar]
- 37.Poole CA, Ayad S, Schofield JR. Chondrons from articular cartilage: I. Immunolocalization of type VI collagen in the pericellular capsule of isolated canine tibial chondrons. Journal of Cell Science. 1988;90(Pt 4):635–643. doi: 10.1242/jcs.90.4.635. [DOI] [PubMed] [Google Scholar]
- 38.Lee GM, Paul TA, Slabaugh M, Kelley SS. The pericellular matrix is enlarged in osteoarthritic cartilage. Transactions of the Orthopaedic Research Society. 1997;22:495. [Google Scholar]
- 39.Guilak F, Alexopoulos LG, Haider MA, Ting-Beall HP, Setton LA. Zonal uniformity in mechanical properties of the chondrocyte pericellular matrix: micropipette aspiration of canine chondrons isolated by cartilage homogenization. Ann Biomed Eng. 2005;33(10):1312–1318. doi: 10.1007/s10439-005-4479-7. [DOI] [PubMed] [Google Scholar]
- 40.Alexopoulos LG, Haider MA, Vail TP, Guilak F. Alterations in the mechanical properties of the human chondrocyte pericellular matrix with osteoarthritis. J Biomech Eng. 2003;125(3):323–333. doi: 10.1115/1.1579047. [DOI] [PubMed] [Google Scholar]
- 41.Alexopoulos LG, Williams GM, Upton ML, Setton LA, Guilak F. Osteoarthritic changes in the biphasic mechanical properties of the chondrocyte pericellular matrix in articular cartilage. J Biomech. 2005;38(3):509–517. doi: 10.1016/j.jbiomech.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 42.Alexopoulos LG, Setton LA, Guilak F. The biomechanical role of the chondrocyte pericellular matrix in articular cartilage. Acta Biomater. 2005;1(3):317–325. doi: 10.1016/j.actbio.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 43.Wu JZ, Herzog W. Analysis of the mechanical behavior of chondrocytes in unconfined compression tests for cyclic loading. J Biomech. 2006;39(4):603–616. doi: 10.1016/j.jbiomech.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 44.Guilak F, Mow VC. The mechanical environment of the chondrocyte: A finite element model of cell-matrix interactions in articular cartilage. Journal of Biomechanics. 2000;33:1663–1673. [PubMed] [Google Scholar]
- 45.Trickey WR, Lee GM, Guilak F. Viscoelastic properties of chondrocytes from normal and osteoarthritic human cartilage. Journal of Orthopaedic Research. 2000;18:891–898. doi: 10.1002/jor.1100180607. [DOI] [PubMed] [Google Scholar]
- 46.Trickey WR, Baaijens FP, Laursen TA, Alexopoulos LG, Guilak F. Determination of the Poisson’s ratio of the cell: recovery properties of chondrocytes after release from complete micropipette aspiration. J Biomech. 2006;39(1):78–87. doi: 10.1016/j.jbiomech.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 47.Spilker R, Maxian TA. A mixed-penalty finite element formulation of the linear bipasic theory for soft tissues. IJNME. 1990;30:1063–1082. [Google Scholar]
- 48.Brown PN, Hindmarsh AC, Petzold LR. Using Krylov methods in the solution of large-scale differential algebraic systems. SIAM J Sci Comput. 1994;15:1467–1488. [Google Scholar]
- 49.Davis TA. A column pre-ordering strategy for the unsymmetric-patterns multifrontal method. ACM Transactions on Mathematical Software. 2004;20:165–195. [Google Scholar]
- 50.Haider MA. A radial biphasic model for local cell-matrix mechanics in articular cartilage. SIAM Journal on Applied Mathematics. 2004;64:1588–1608. [Google Scholar]
- 51.Hou JS, Holmes MH, Lai WM, Mow VC. Boundary conditions at the cartilage-synovial fluid interface for joint lubrication and theoretical verifications. Journal of Biomechanical Engineering. 1989;111(1):78–87. doi: 10.1115/1.3168343. [DOI] [PubMed] [Google Scholar]
- 52.Ng KW, Mauck RL, Statman LY, Lin EY, Ateshian GA, Hung CT. Dynamic deformational loading results in selective application of mechanical stimulation in a layered, tissue-engineered cartilage construct. Biorheology. 2006;43(3–4):497–507. [PubMed] [Google Scholar]