Abstract
A diverse array of environmental factors contribute to the overall control of stem cell activity. In particular, new data continues to mount on the influence of the extracellular matrix (ECM) on stem cell fate through physical interactions with cells, such as the control of cell geometry, ECM geometry/topography at the nanoscale, ECM mechanical properties, and the transmission of mechanical or other biophysical factors to the cell. Here we review some of the physical processes by which cues from the ECM can influence stem cell fate, with particular relevance to the use of stem cells in tissue engineering and regenerative medicine.
Keywords: tissue engineering, regenerative medicine, smart biomaterials, matrix elasticity, substrate stiffness, progenitor cell, mechanotransduction
Tissue engineering is a rapidly growing field that seeks to repair or regenerate damaged or diseased tissues and organs through the implantation of combinations of cells, scaffolds, and soluble mediators (Atala, 2008; Vacanti and Langer, 1999). Inherent to this approach is a need for readily available cell sources that, under controlled conditions, can provide the appropriate function, i.e., synthesis of new tissues or paracrine factors. The interest in both adult and embryonic stem cells in the fields of tissue engineering and regenerative medicine has grown tremendously in the past few years [e.g., (Alhadlaq and Mao, 2004; Elisseeff et al., 2006; Kitsberg, 2007; Polak and Bishop, 2006)]. Recent progress in inducing pluripotency from differentiated human cells into embryonic-like cells has only further intensified the need to understand the therapeutic potential of stem cells (Takahashi and Yamanaka, 2006). Stem cells possess critical properties that make them uniquely suited for certain tissue engineering applications. For example, the number of cells needed for certain cellular therapies may be a limiting step for using primary tissue cells; thus, ability of stem cells to replicate in culture while retaining the ability to differentiate into specific lineages can address this issue. Furthermore, the decreased immunogenicity (Nauta and Fibbe, 2007), and potentially “immunosuppressive” properties that have been described in various adult stem cells (Caplan, 2007) may facilitate allogeneic transplantation, providing further advantages as a cell source for regenerative medicine.
With the inherent plasticity and multilineage potential provided by stem cells comes an increased need for regulating cell differentiation, growth, and phenotypic expression. Classically, the control of stem cell fate, either in vivo or in vitro, has been attributed principally to genetic and molecular mediators (e.g., growth factors, transcription factors). However, increasing evidence has revealed that a diverse array of additional environmental factors contribute to the overall control of stem cell activity. In particular, fascinating data continues to mount on the important influence of the “solid-state” environment, i.e., the extracellular matrix (ECM) has on stem cell fate, with particular emphasis on the interactions of ECM ligands with cell surface receptors (Daley et al., 2008). However, it is now clear that ECM-based control of the cell may also occur through multiple physical mechanisms, such as ECM geometry at the micro- and nanoscale, ECM elasticity, or mechanical signals transmitted from the ECM to the cells. An improved understanding of the interaction of these mediators with classical signaling pathways may provide new insights into the regulation of self-renewal and differentiation of stem cells. The ability to better engineer artificial ECMs that can control cell behavior, through physical as well as molecular interactions, may further extend our capabilities in engineering tissue substitutes from adult or embryonic stem cells (Metallo et al., 2007). It is important to note that the majority of studies investigating the influence of physical factors on stem cells have focused on adult stem cell populations such as mesenchymal stem cells (MSCs) or other connective tissue stem cells. These cell types generally represent a heterogeneous population with reduced plasticity in comparison to embryonic or hematopoietic stem cells (Bianco et al., 2008). In the interpretation of these findings, it is relevant to note the responses of MSC populations may reflect culture conditions that select for pre-existing sub-populations of cells, rather than necessarily exhibiting a direct regulatory effect on cell fate. Nonetheless, the restricted lineage potential and ease of accessibility of MSCs has made them an attractive choice as a cell source for tissue engineering and regenerative medicine, and despite the exact mechanism(s) involved, it is clear that physical factors can have a significant influence on the overall behavior of MSC populations.
Cell shape as a regulator of stem cell fate
Cell shape is a potent regulator of cell growth and physiology (Folkman and Moscona, 1978) and many events related to embryonic development and stem cell differentiation are influenced by cell shape. For example, changes in cell shape have been implicated as a potential mechanism that regulates myocardial development (Manasek et al., 1972), while the growth and differentiation of capillary endothelial cells are in part regulated by ECM-induced changes in cell shape (Ingber, 1991). Extending this mechanism to stem cells, a number of studies have shown that stem cell fate can be influenced artificially through control of their shape by artificial extracellular matrices. In addition to physical control of shape, some subset of these effects may result from altered adhesive interactions between the cell and substrate, although many studies have controlled for such effects. That is, the interactions between many extrinsic and intrinsic factors that influence cell shape are varied and complex, and may involve relatively long-term interactions with the cellular microenvironment, as well as more acute changes due to physical factors such as mechanical or osmotic stress (Guilak et al., 1995; Ingber, 2004; Lecuit and Lenne, 2007; McBride and Knothe Tate, 2008) (Figure 1) The effects of cell shape on cellular signaling appear to extend well beyond its influence on adhesion signaling alone (Meyers et al., 2006; Neves et al., 2008).
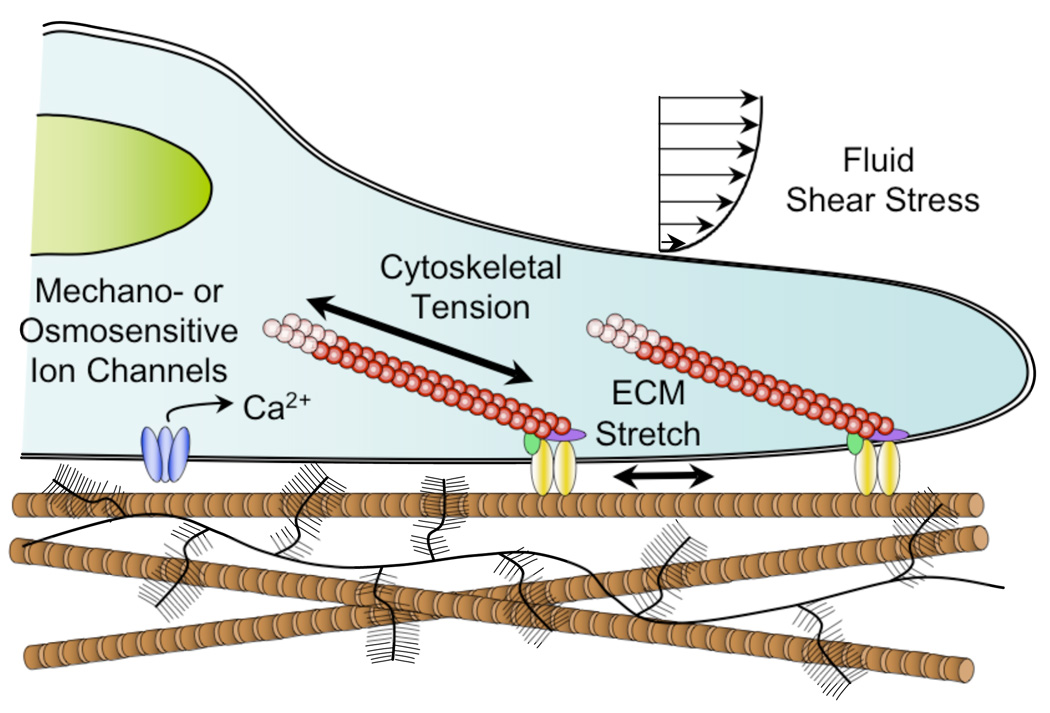
Figure 1. Transduction of mechanical factors and the regulation of stem cell fate.
During development and through life, stem cells may be exposed to a variety of physical signals, including tensile, compressive, shear, osmotic, and fluid stresses, often arising secondarily to biomechanical interactions with their ECM. For example, tension of the ECM can induce stretch of the cytoskeleton, and nucleus through focal adhesions, while compression of the ECM can significantly alter local charge density and ion concentrations, potentially activating osmotically sensitive ion channels. Previous studies have shown that these mechanical stimuli individually can strongly influence stem cell growth and differentiation in vivo and in vitro.
Some of the simplest examples of the influence of cell shape on differentiation have emerged from the development of three-dimensional culture systems, which generally induce a more rounded, spheroidal cell morphology in comparison to standard two-dimensional culture systems. For example, growth of chondrocytes in flattened shape in 2D culture leads to “de-differentiation” and a shift from a chondrocytic phenotype to a more fibroblastic phenotype (Holtzer et al., 1960), while the retention of chondrocytes in a 3D shape using pellet culture (Abbott and Holtzer, 1966) or by encapsulation in a gel such as agarose or alginate retains their normal phenotype (Benya and Shaffer, 1982). Interestingly, the restoration of chondrocyte shape to a rounded morphology by chemical alteration of the actin cytoskeleton also partially restores some of the phenotypic changes (Newman and Watt, 1988; Zanetti and Solursh, 1984). In this light, a number of studies have shown that the differentiation of adult or embryonic stem cells into a chondrocytic phenotype requires a rounded cell shape, either through pellet (e.g., micromass) culture, or through the use of gel-based artificial encapsulation systems (Erickson et al., 2002; Hoben et al., 2008; Johnstone et al., 1998). Indeed, direct comparisons of cell and nuclear shape of bone marrow derived MSCs showed that a more rounded nuclear shape was associated with the greatest expression of molecular markers associated with chondrogenesis (McBride and Knothe Tate, 2008). Similarly, adipose derived stem cells exhibit chondrogenic differentiation within agarose or alginate gel scaffolds, which maintain a spheroidal cell shape. However, under the same medium conditions, the use of fibrin or gelatin as a scaffold, which have similar bulk properties but allow cell attachment and a spread shape in three dimenions, results in a fibrochondrogenic phenotype (Awad et al., 2004). Similarly, murine embryonic stem cell-derived embryoid bodies maintained in three-dimensional culture in poly(ethylene glycol)-based hydrogels showed significant upregulation of cartilage-relevant markers, as compared to a monolayer culture system (Hwang et al., 2006a).
Furthermore, in such artificial systems, the influence of a three-dimensional cell shape can be further modified by the presence of transforming growth factor beta 1 (TGF-β1) (Hwang et al., 2006a) or interactions between cell surface receptors and ECM molecules (Hwang et al., 2006b). For example, human embryonic stem cell-derived cells maintained in three-dimensional culture in arginine-glycine-aspartate-modified hydrogels show significantly greater cartilage-specific gene up-regulation and ECM production than in pellet culture or unmodified poly(ethylene glycol) gels (Hwang et al., 2007). Recent studies have also demonstrated a novel method by which the shape of human MSCs can be dynamically modified in such hydrogels by creating photodegradable poly(ethylene glycol)—based scaffolds (Kloxin et al., 2009). MSCs encapsulated within a densely cross-linked gel exhibited a rounded morphology, but could be induced to a spread shape by reducing the cross-linking density of the gel via photodegradation, allowing controlled temporal changes of the physical interactions between the cells and hydrogel.
The mechanisms by which cell shape influences stem cell fate has been further explored using novel techniques that allow defined “micropatterns” of proteins to be deposited upon a substrate, thereby precisely controlling the area of cell attachment. On small ECM micropatterned islands, cells adopted a poorly spread, rounded morphology, whereas cells adhered to large ECM islands adopt flatten morphologies typical of 2D cultures (Chen et al., 1998) (Figure 2). This shape change from rounded to flattened morphologies profoundly alters the organization of the actin cytoskeleton and the assembly of focal adhesions (Chen et al., 2003). Importantly, this micropatterning approach has revealed that cell shape (i.e. rounded versus flattened morphologies) controls the lineage commitment of MSCs into an adipogenic or osteoblastic phenotype (McBeath et al., 2004) (Figure 2). The complete mechanisms involved in this response remain to be determined, but inhibition of Rho prevented these effects, suggesting that changes in the F-actin cytoskeletal architecture, modulated by Rho/Rock pathways, were involved in the response. These findings are supported by studies showing significant changes in the F-actin cytoskeleton and cellular mechanical properties during differentiation of MSCs into chondrocytes or osteoblasts (Titushkin and Cho, 2007; Yourek et al., 2007).
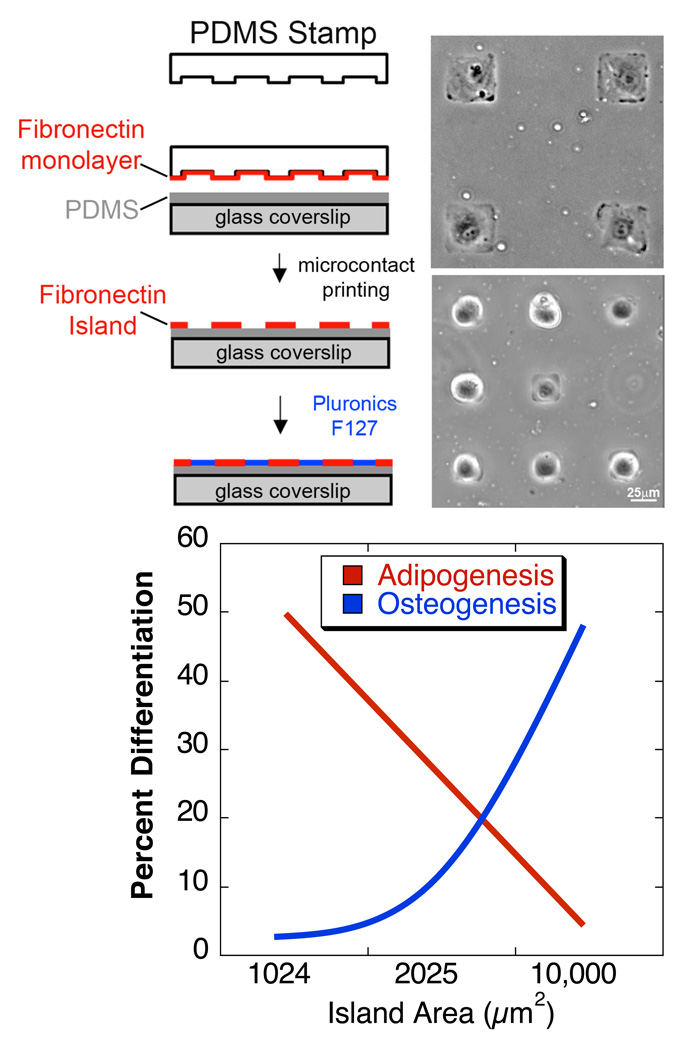
Figure 2. Control of cell shape through microcontact printing controls differentiation of MSCs.
(top) Polydimethylsiloxane (PDMS) stamps with micron-sized features are coated with fibronectin, or other ECM proteins. Fibronectin is transferred from the raised features on the stamp to a PDMS-coated glass coverslip substrate via microcontact printing. Gaps between fibronectin islands are passivated to prevent cell adhesion by adsorption of the non-adhesive, Pluronic F127. By controlling the size of the islands where cells can attach, their shape can be predefined. Phase images (courtesy of R. Desai) of cells patterned on 50×50 or 25×25 µm2 islands. (bottom) human MSCs that were allowed to adhere, flatten, and spread underwent osteogenesis, while unspread, round cells underwent adipogenesis. This switch in lineage commitment was regulated by cell shape through the modulation of endogenous RhoA activity [Data from (McBeath et al., 2004)].
ECM stiffness as a regulator of stem cell fate
In addition to the influence that an artificial ECM may have on cell shape, there is significant evidence that other physical properties of the ECM may also contribute to stem cell fate or lineage commitment. Cells that attach to a substrate have been shown to exert contractile forces, resulting in tensile stresses in the cytoskeleton (Ingber, 2004). Interestingly, the relationship between these forces and the mechanical stiffness, or elasticity, of the ECM can have a major influence on cell behaviors such as migration (Guo et al., 2006; Pelham and Wang, 1997), apoptosis (Wang et al., 2000), and proliferation (Hadjipanayi et al., 2009).
Thus it is not surprising that ECM stiffness can also influence cell differentiation. Early evidence for this phenomenon was observed in studies showing qualitatively that mouse mammary epithelial cells showed increased differentiation when grown on soft collagen gel substrates, as opposed to tissue culture plastic (Emerman et al., 1979). In other studies, the tubulogenesis of human umbilical vein endothelial cells was shown to depend on the mechanical properties of their substrate. Cells grown on soft matrigel or on matrigel co-polymerized with heat-denatured collagen exhibited reduced expression of actin and focal-adhesion plaque as compared to cells remaining in a monolayer pattern on rigid matrigel-coat or on matrigel co-polymerized with type I collagen (Deroanne et al., 2001). However, it is important to note that in these studies, the influence of different ligand densities could not be easily separated from the effects of matrix elasticity (Deroanne et al., 2001; Emerman et al., 1979). In other cell types, myoblasts were cultured on collagen strips attached to glass or polymer gels of differing mechanical stiffnesses. While the fusion of myoblasts into myotubes was found to be independent of substrate stiffness, the development of actin/myosin striations occurred only on gels that had properties similar to those of normal muscle (Engler et al., 2004).
Following these principals, more recent studies have been able to directly test the hypothesis that stem cell lineage specification can be determined by the mechanical properties of the ECM. MSCs grown on variably compliant polyacrylamide gel were found to alter their properties in relation to the stiffness of the substrate (i.e., stiffer substrates induced stiffer cells). Furthermore, the stiffness of the substrate defined the differentiation lineage of the MSC: Soft substrates that mimic the mechanical properties of brain tissue were found to be neurogenic, whereas substrates of intermediate stiffness that mimic muscle were myogenic, and relatively stiff substrates with bone-like properties were found to be osteogenic (Engler et al., 2006). Similarly, the effective stiffness of the underlying substrate has been shown to regulate the differentiation of neural stem cells. Using a synthetic, interfacial hydrogel culture system, adult neural stem cells were grown on substrates varying in moduli between 10 and 10,000 Pa (Saha et al., 2008a). Cell spreading, self-renewal, and differentiation were inhibited on soft substrates (10 Pa), whereas these cells proliferated on substrates with moduli of 100 Pa or greater and exhibited peak levels of a neuronal marker, beta-tubulin III, on substrates that had the approximate stiffness of brain tissue. Softer substrates (~100–500 Pa) promoted neuronal differentiation, whereas stiffer substrates (~1,000–10,000 Pa) led to glial differentiation.
In other studies, it was found that human MSCs could be kept quiescent by growing them on polyacrylamide substrates that mimicked the properties of marrow (Winer et al., 2009). MSCs seeded sparsely on these gels stopped progression through the cell cycle, could be induced to reentered the cell cycle when presented with a stiff substrate. These cells could also be induced into adipogenic or osteogenic pathways when cultured in the appropriate induction medium, suggesting that a soft substrate mimicking the bone marrow niche can maintain MSCs in a quiescent state while preserving their multilineage potential. An important conclusion of this study was that mechanical signals from the elasticity of the ECM may serve as a critical factor in the bone marrow niche that allows the maintenance of MSCs as a reservoir for a long period (Winer et al., 2009). The ability to reproduce these properties in an artificial ECM may provide a novel means of controlling stem cell fate ex vivo (Dellatore et al., 2008; Ghosh and Ingber, 2007).
One caveat to these studies is the fact that multiple tissues may have similar elasticities, and thus it may not be possible to define unique stem cell differentiation by a single set of mechanical properties. This point further emphasizes the potential complexity of the interactions between intrinsic and extrinsic properties of stem cells and their environment in determining their fate (Watt and Hogan, 2000).
Regulation of Stem Cells by Nanotopography of the ECM
As discussed earlier, cell shape can be a potent regulator of growth and differentiation. In addition to overt, macroscopic changes in cell shape, cells have the ability to sense micro- and even nanoscale geometric cues from their environment. Such cues may represent differences in molecular conformation, surface topography or roughness, fiber diameter, or other parameters. For example, neurite outgrowth from neurogenically differentiated stem cells was significantly enhanced when grown within inert but highly porous 3D polystyrene scaffolds, as compared to traditional flat surfaces (Hayman et al., 2005). Similar influences have been observed on cell alignment, where the directional growth and differentiation of adult rat hippocampal progenitors cultured on micropatterned polystyrene substrates chemically modified with laminin exhibited over 75% alignment in the direction of the grooves (13 µm wide and 4 µm high) as well as significantly increased expression of neuronal markers (Recknor et al., 2006). These findings show that the 3D topography of the substrate, in synergy with matrix composition (laminin), can facilitates neuronal differentiation and neurite alignment. Interestingly, the ability of cells to recognize such architectural cues extends to nanoscale topographical features. Human MSCs grown on nanoscale grooves of 350 nm width showed alignment of their cytoskeleton and nuclei of MSCs along the grooves (Yim et al., 2007). A significant up-regulation of neuronal markers such as microtubule-associated protein 2 was observed on these substrates as compared to unpatterned and micropatterned controls. While the combination of such nanotopographic cues with biochemical cues such as retinoic acid further enhanced neurogenesis, nanotopography showed a stronger effect compared to retinoic acid alone on unpatterned surface.
Neuronal progenitor cells appear to show similar responses to electrospun fibers with nanoscale properties. Rat hippocampus-derived adult neural stem cells grown on laminin-coated electrospun polyethersulfone fiber meshes ranging from 283 nm to 1452 nm diameter showed differentiation and proliferation responses that significantly depended on fiber diameter (Christopherson et al., 2009). Cells stretched multi-directionally to follow underlying 283 nm fibers, but when grown on larger fibers, extended along a single fiber axis. With decreasing fiber diameter, a higher degree of proliferation and cell spreading and lower degree of cell aggregation were observed.
The mechanisms by which nanotopographic cues influence stem cell proliferation and differentiation are not well studied, but appear to involve changes in cytoskeletal organization and structure, potentially in response to the geometry and size of the underlying features of the ECM. That is, changes in the feature size of the substrate may influence the clustering of integrins and other cell adhesion molecules, thus altering the number and distribution of focal adhesions (Figure 3a). For example, previous studies have shown that the precise spacing between nanoscale adhesive islands on a substrate can modulate the clustering of the associated integrins, and the formation of focal adhesion and actin stress fibers, and therefore, control the adhesion and spreading of cells (Arnold et al., 2004). In this respect, the nanotopographic features of the ECM have been shown to alter the morphology and proliferation of human embryonic stem cells through cytoskeletal-mediated mechanisms. Polydimethylsiloxane (PDMS) gratings with 600 nm features and spacing were found to induce the alignment and elongation of embryonic stem cells (Gerecht et al., 2007). This study also showed that nanotopographic cues altered the organization of various cytoskeletal components, including F-actin, vimentin, γ-tubulin, and α-tubulin, and the observed changes in proliferation and morphology were abolished by the effect of actin disrupting agents. Alternatively, the influence of nanotopographic features may be mediated through secondary effects, such as alterations in the effective stiffness perceived by the cell (Figure 3b) or differences in protein adsorption due to the structural features of the substrate.
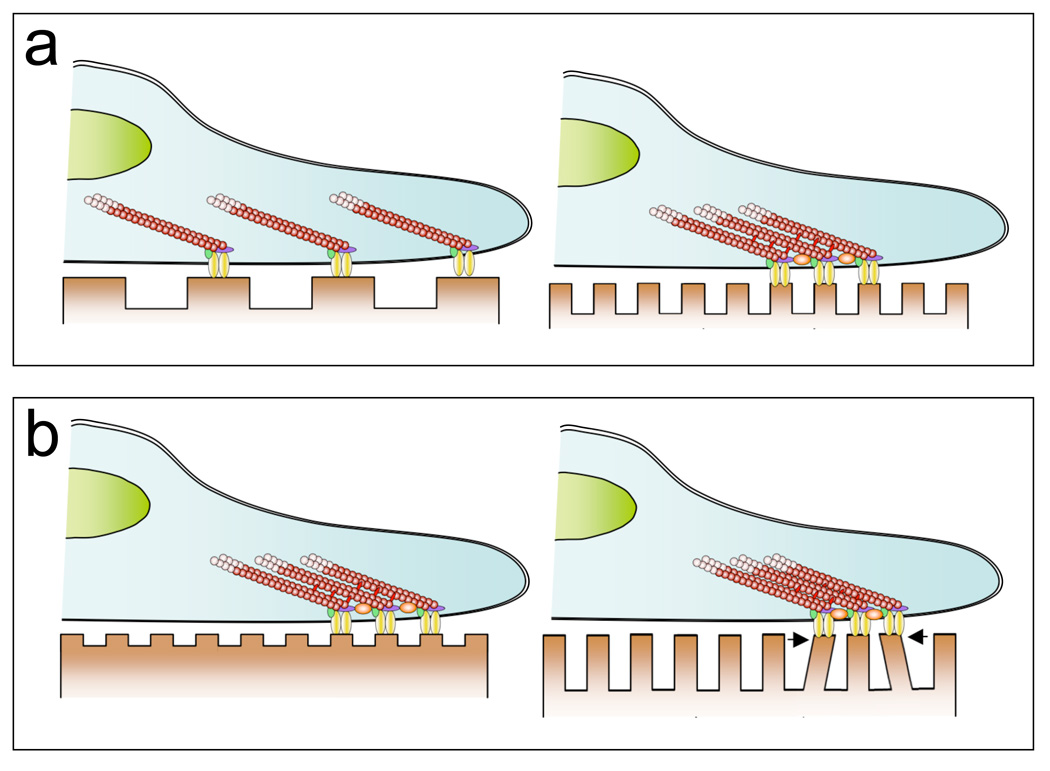
Figure 3. Influence of ECM nanotopography on stem cells.
The nanoscale geometry and size of the features of the ECM may have significant effects on a number of cell properties, such as attachment/adhesion, migration, and proliferation, although the mechanisms responsible for these effects are not well understood. (a) Whereas changes in the feature size of the substrate on the scale of single cells could impact adhesion by altering the degree of cell spreading (Figure 2), varying features at the scale of individual adhesions may alter the clustering of integrins and other cell adhesion molecules. Altered clustering can influence the number and distribution of focal adhesions and subsequently, the structure of the cytoskeleton (Arnold et al., 2004). In turn, these factors may further influence cytoskeletal tension and the transmission and transduction of other molecular and biomechanical signals. (b) Differences in the size and structure (e.g., height) of nanotopographic features may influence cell behavior through secondary effects, such as alterations in the effective stiffness of the substrate (e.g. (Discher et al., 2005; Saha et al., 2008a)).
Mechanical determination of stem cell fate
From the time of development and throughout the life of an organism, cells of the body are constantly exposed to a variety of mechanical stimuli through the actions of muscle forces, gravity, blood flow, and other physical processes. The interactions between cells and mechanical factors are critical to the health and function of various tissues and organs of the body, and are also believed to play an important role in a variety of disease states such as atherosclerosis, osteoarthritis, and osteoporosis (Ingber, 2003). Importantly, there is mounting evidence that mechanical factors can significantly influence the process of development, and may play critical roles in controlling stem cell fate and lineage determination.
As early as the last century, scientists recognized that the mechanical environment could influence development (reviewed in (Estes et al., 2004)). For example, in a series of experiments in the 1930’s and 1940’s, Glücksmann demonstrated that cultured chick rudiments under static compression following displacement of the periosteum and perichondrium resulted in cartilaginous tissue formation whereas tensile stresses promoted bone formation (Glücksmann, 1942). In similar fashion, paralysis of chick embryos resulted in a loss or significant inhibition of cartilage formation, while a mechanical environment in the form of membranous bone articulation resulted in secondary cartilage formation (Fang and Hall, 1995; Hall and Herring, 1990; Murray and Drachman, 1969). The common thread in all of these early studies was an emphasis on the undeniable influence of the mechanical environment during development.
Despite such in vivo evidence, little was known regarding the biomechanical and biochemical mechanisms by which such mechanical factors could affect gene expression and the determination of stem cell fate. One major difficulty in studying such interactions has been the complexity in determining the precise nature of mechanical “signals” perceived by stem cells in vivo. For example, simple mechanical loading of tissues results in complex physical environments that consist of time-varying stress, strain, fluid flow and pressure, and potentially, other biophysical changes such as osmotic pressure or electric fields that are generated by the ubiquitous presence of fixed and mobile electric charge on biological molecules (Guilak et al., 1997). In principle, these changes in the microenvironment may significantly alter the structure of ECM proteins and the activity of soluble growth factors and cytokines. As such, it is difficult to isolate the effects of mechanical force in vivo from indirect effects associated with mechanically-driven changes in adhesive cues and/or paracrine signaling, and as described earlier, subsequent changes in cell shape. Nonetheless, mechanical forces can directly affect cellular function, and the transduction processes by which cells “sense” applied physical stimuli are only recently being uncovered (Liedtke and Kim, 2005).
Importantly, cells are not simply passive biomaterials with constant mechanical properties, but rather use signals from the ECM to “tune” their mechanical properties by dynamically remodeling their cytoskeletal networks. Thus cellular responses to mechanical perturbations are not only a function of the input stimuli, but are also determined by the coupling of these stimuli to mechanosensitive changes in the cytoskeletal organization, interaction with the ECM, and cellular force production. In some experimental systems, these cell-generated forces may be necessary or sufficient to influence stem cell differentiation (Engler et al., 2006; McBeath et al., 2004). In this context, the field of “mechanobiology” has exploded in the past few years in characterizing the response of stem cells to more highly controlled mechanical (and other physical) loading, and in determining the biophysical mechanisms and biochemical signal transduction pathways that regulate lineage commitment (Wang and Thampatty, 2008). As the biophysical signals to which stem cells respond may involve a variety of secondary factors that are engendered in the ECM subsequent to initial mechanical loading, several studies have attempted to isolate the influence of specific physical stimuli such as cell stretch (i.e., tension), compression, or fluid shear stress on stem cell behavior.
For example, the influence of cyclic strain on MSC phenotype has been studied extensively in vitro for vascular tissue engineering. MSCs cultured on various protein coated flexible membranes and subjected to 5% or 10% cyclic uniaxial stretch demonstrated commitment toward a myogenic phenotype as noted by the expression of smooth muscle actin (SMA) among other factors (Gong and Niklason, 2008; Hamilton et al., 2004; Park et al., 2004; Yang et al., 2000). However, strains of 1% or 15% failed to either induce commitment to the myogenic lineage or caused a decrease in smooth muscle cell markers, pointing to the importance of the magnitude of strain during differentiation (Yang et al., 2000). The strain-induced myogenic phenotype was dependent on the protein to which the cells were attached; not coating the substrate resulted in loss of strain-induced myogenesis (Gong and Niklason, 2008). The effect of the mechanical environment is also dependent on cell type, as adipose-derived stem cells subjected to a similar uniaxial strain protocol (10% uniaxial cyclic strain at 1Hz for 7 days) showed decreased expression of myogenic markers (Lee et al., 2007).
Uniform biaxial strain has also been applied in vitro to MSCs to enhance osteogenic differentiation as noted by increases in specific osteogenic markers, namely Runx2, osterix, alkaline phosphatase, and calcium deposition (Sen et al., 2008; Simmons et al., 2003; Thomas and el Haj, 1996; Yoshikawa et al., 1997). Also, for bone tissue engineering, pulsatile fluid flow has been shown to upregulate osteogenic markers in adipose-derived stem cells, but only after some degree of osteogenesis had occurred (Knippenberg et al., 2005) further alluding to the potential differential effects the mechanical environment may have dependent on both the cell type and differentiated phenotype of the cell.
In other studies, cyclic mechanical strain has been shown to induce mouse embryonic stem cell differentiation into vascular smooth muscle cells (Shimizu et al., 2008). Flk-1-positive (Flk-1+) embryonic stem cells subjected to cyclic strain (4–12% strain, 1 Hz, 24 h) showed significant increases in proliferation and reoriented perpendicular to the direction of strain, exhibiting dose-dependent increases in smooth muscle alpha-actin and smooth muscle-myosin heavy chain at both the protein and gene level. Interestingly, inhibition of platelet-derived growth factor receptor beta completely blocked the mechanically-induced differentiation of embryonic stem cells, suggest that activation of the receptor by cyclic strain plays a critical role in vascular smooth muscle cell differentiation from Flk-1+ ES cells.
Mechanical strain has also been shown to inhibit the differentiation of human embryonic stem cells, while promoting self-renewal without selecting against survival of differentiated or undifferentiated cells (Saha et al., 2006). Interestingly, stem cells cultured while being cyclically strained retained pluripotency, evidenced by their ability to differentiate to cell lineages in all three germ layers. The influence of mechanical strain appeared to involve the TGF-b/activin/nodal pathway, as strain was shown to upregulate TGF-β1, Activin A, Nodal, and SMAD2/3 phosphorylation in undifferentiated embryonic stem cells (Saha et al., 2008b), whereas inhibition of the TGFβ/Activin/Nodal receptor stimulated differentiation.
Cyclic unconfined compression has also been shown to alter the phenotype of mesenchyme-derived stem cells. For example, the chondrogenic induction of stage 23/24 chick limb bud cells embedded in an agarose matrix was significantly enhanced by dynamic mechanical unconfined compression as a function of applied frequency, specifically noting an approximate doubling in the cartilage nodule density and a greater than 2-fold increase in proteoglycan synthesis rates compared to static compression (Elder et al., 2001; Elder et al., 2000). MSCs encapsulated in a 2% agarose matrix and subjected to unconfined dynamic compression exhibited increased aggrecan and collagen II transcript levels over non-loaded controls, which also translated into enhanced protein deposition in the matrix (Huang et al., 2004; Mauck et al., 2007). Additionally, intermittent hydrostatic pressure positively affected chondrogenically induced MSC aggregates resulting in significantly higher levels of collagen and proteoglycan content compared to unloaded controls (Angele et al., 2003).
As might be expected, the influence of mechanical loading on stem cell response appears to depend on the type of stem cell as well as the state of (pre-)differentiation. For example, dynamic mechanical compression can significantly increase the chondrocytic expression (e.g., Sox-9, type II collagen, and aggrecan) of bone marrow-derived MSCs encapsulated in a hydrogel, irrespective of the presence of chondrogenic growth factors (Terraciano et al., 2007). Under the same conditions, embryonic stem cell-derived embryoid bodies exhibit significant downregulation of cartilage-specific genes in response to mechanical compression. Following chondrogenic differentiation with TGFβ-1, however, these cells showed significant increases in the expression of cartilage-specific genes when exposed to mechanical compression (Terraciano et al., 2007), suggesting that the mechanosensitivity of different types of stem cells is highly dependent on their state of differentiation. Taken together, it is that clear physical signals, in part, regulate differentiation but also that the ensuing phenotype is dependent on a myriad of factors including, but not limited to, the biochemical environment, biomaterials that are being employed, the differentiated state of the cell, and the precise mechanical loading protocol.
Mechanisms of mechanical signal transduction
In order to elucidate the biological mechanisms involved in the mechanotransduction pathways that control stem cell differentiation in these previous examples, investigators have begun to explore how mechanical forces are transduced into biochemical signals that can in turn regulate, synergize, and modulate signaling cascades induced by other stimuli [e.g., (Cohen and Chen, 2008; Ghosh and Ingber, 2007)]. Such studies have examined a number of different mechanisms, but as of now, the role of the actin cytoskeleton and the activity and expression of transcription factors and chromatin remodeling enzymes directly involved in gene expression have been found to play a significant role in mechanical signal transduction.
For example, mechanical stretch has been shown to regulate the expression and localization of a set of novel chromatin remodeling enzymes called TIPs (tension-induced proteins) (Jakkaraju et al., 2005). Specifically, an applied static 5% axial stretch is sufficient to trigger TIP1 expression and nuclear localization in cultured embryonic lung MSCs, and such expression is similarly observed in vivo in lung mesenchymal stem cells undergoing smooth muscle myogenesis, a tension-dependent process (Yang et al., 2000). Importantly, TIP1 expression is both necessary for stretch-induced myogenesis in vitro, and TIP1 expression is sufficient to phenocopy the effect of mechanical stretch on the expression of differentiation-specific genes (Jakkaraju et al., 2005). While TIPs have not yet been studied in other stem cells, mechanical stretch has been shown to regulate other lineage commitment events, including osteogenic versus adipogenic fate decision. In a model mesenchymal stem cell line (C3H10T1/2), stretch inhibits adipogenic commitment, and promotes osteogenesis, by regulating the expression and function of the PPAR-γ transcription factor, a master regulator of adipogenesis (David et al., 2007). Stretch appears to antagonize PPAR-γ through altered responsiveness of PPAR-γ promoters, possibly through reduced accessibility/recruitment of activated PPAR-γ protein. This stretch-regulated adipogenic-to-osteogenic switch is therefore consistent with mechanically-sensitive chromatin remodeling, and possibly involving TIPs.
These studies and others implicate the role of the actin cytoskeleton, and in particular, the RhoA pathway to regulate stem cell differentiation. Manipulation of RhoA, and its downstream effector, Rho kinase (ROCK), appear sufficient to direct commitment of mesenchymal stem cells towards osteogenic (high RhoA signaling) versus adipogenic (low RhoA signaling) fates (McBeath et al., 2004). Importantly, the ability of RhoA-ROCK signaling to stimulate osteogenesis required myosin II activity, suggesting that this differentiation response is dependent on the ability of cells to generate contractile forces and cytoskeletal tension. The contribution of the RhoA pathway to lineage commitment decisions is further evidenced by the p190RhoGAP knockout mouse. The p190RhoGAP mouse exhibits a perinatal lethality phenotype associated with deficiencies in insulin/IGF signaling and CREB activity (Sordella et al., 2002). Moreover, the loss of p190RhoGAP, which normally restricts RhoA activity through GTP hydrolysis, results in a concomitant upregulation of RhoA activity and increase in myogenic differentiation (at the expense of adipogenesis) both in vitro and in vivo (Sordella et al., 2003). Although this adipogenic-myogenic switch is dependent on RhoA-mediated activation of ROCK, it is not yet clear to what extent ROCK activity regulates adipogenic differentiation strictly through effects on insulin signaling (Sordella et al., 2003) versus through its effects on cytoskeletal tension (McBeath et al., 2004). However, it is worth noting the Rho-ROCK-myosin signaling regulates the activity of the SRF transcription factor (Fan et al., 2007; Mack et al., 2001), and SRF and its co-factors are intimately associated with myogenic differentiation (Wang and Olson, 2004).
As such, cell-generated forces/tension are likely to contribute to Rho-mediated myogenic differentiation. Although it is not yet clear why RhoA activation promotes myogenic differentiation in one context (Sordella et al., 2003) and osteogenic differentiation in another (McBeath et al., 2004), these studies pinpoint RhoA signaling as a pivotal molecular player in the lineage commitment of MSCs in response to both mechanical (cell spreading, intracellular tension) and soluble (insulin/IGF1) factors.
In addition to the regulation of stem cell response to mechanical factors through cytoskeletal tension/RhoA/ROCK, there is new evidence for the potential role of mechano- and osmotically-sensitive ion channels in the regulation of stem cell differentiation. Several classes of ion channels in the Transient Receptor Potential (TRP) family of cation channels have been recently discovered that appear to serve as the primary sensory channels in the body that transduce physical signals such as temperature, osmolarity, and stretch into intracellular Ca2+ signaling [e.g., (Caterina and Julius, 2001; Christensen and Corey, 2007; Liedtke, 2007; Mutai and Heller, 2003; Nilius and Voets, 2004; Pedersen and Nilius, 2007). Of particular interest is the finding that such transduction channels can play important roles in the growth and differentiation of various stem cells. For example, the canonical TRP channel TRPC1, which can be gated by mechanical stretch, play roles in the processes of neuronal development, including neural stem cell proliferation, cerebellar granule cell survival, axon path finding, and neuronal morphogenesis (Fiorio Pla et al., 2005; Tai et al., 2008). In other studies, functional gene screening has identified TRPV4, a mechanically and osmotically sensitive ion channel (Gao et al., 2003; Liedtke et al., 2003), as a regulator of the chondrogenic differentiation of C3H10T1/2 MSCs (Muramatsu et al., 2007). In CD133+ adipose tissue stem cells, TRPC3 was found to be responsible for high levels of VEGF-induced Ca2+ entry that was preferentially detected in rim areas of expanding colonies, and TRPC3 function was found to be essential in determining the fate of CD133+ progenitor-derived colonies (Poteser et al., 2008). Growing evidence suggests direct links between RhoA mediated actin organization and TRP channel-mediated signaling (Barnes et al., 2005; Beech, 2005; DeWire et al., 2007; Mehta et al., 2003; Zhang and Bourque, 2008), opening the intriguing possibility that the physical signaling pathways regulated by cytoskeletal tension and mechanosensitive ion channels may in fact be linked.
Controlling the Physical Microenvironment of Stem Cells for Tissue Engineering
These studies and others clearly show that physical interactions with the ECM significantly influence stem cell behavior, and can interact with chemical (i.e., composition), molecular (i.e., soluble mediators), or genetic (cell type) factors to regulate cell fate. In studying the stem cell niche, focus was originally placed on cell-cell interactions as a primary regulator of stem cell fate, with growing interest more recently on the role of the molecular composition of the niche as well (Scadden, 2006; Watt and Hogan, 2000). The protein adhesion molecules contributing to asymmetric stem cell division, which include β1 integrin, CD146, and E-cadherin, have begun to be identified within the niche environment of hair follicle, intestinal epithelial, limbic, and spermatogonial stem cells, respectively (Baharvand et al., 2007; Kanatsu-Shinohara et al., 2008; Oatley and Brinster, 2008; Ohyama et al., 2006; Tanentzapf et al., 2007). In addition to the regulation of normal stem cell function, there has been growing interest in the concept that niche has a “dynamic” nature that can change properties under certain conditions (Adams and Scadden, 2008). Such alterations in a stem cell niche could not only lead to aberrant growth or differentiation of stem cells, but also provide a potential therapeutic target for regenerative medicine. For example, cell-cell interactions in the stem cell niche can be strongly influenced by paracrine signaling by hormones, as has been shown in multiple organ systems, such as in bone/marrow (Calvi et al., 2003; Kronenberg, 2007), breast (Brisken and Duss, 2007), or prostate (Kasper, 2008). However, an understanding of how physical signals may influence or be influenced by traditional receptor-ligand signaling in the niche remains to be determined.
In this regard, the control of ECM composition in engineered constructs has proven to be a valuable tool in guiding the development and commitment of stem cells during neo-tissue formation. For example, mouse embryonic stem cell-derived embryoid bodies cultured in semi-interpenetrating polymer networks, made of collagen, fibronectin, or laminin show significantly different differentiation and viability depending on the composition of the ECM (Battista et al., 2005). Importantly, the ability to engineer artificial ECMs that, through physical as well as molecular interactions, enable directed control of stem cell behavior may further extend our capabilities in engineering functional tissue substitutes. By controlling the nanotopography, mechanical properties, and mechanical loading environment of tissue engineering scaffolds, we may further improve the regulation of stem cell fate in bioartificial systems. Despite significant advances shown in these in vitro studies, the ultimate success of such approaches will require in vivo demonstrations of functional engraftment and tissue regeneration. Such findings emphasize the importance of a multi-disciplinary approach to the use of stem cells in the development of engineered tissue substitutes, involving the coalescence of many disciplines such as cell and molecular biology, materials science, biomedical engineering, medicine, and surgery.
Acknowledgments
This study was supported in part NIH grants AG15768, AR048852, AR48182, AR50245, GM74048, EB00262, EB001046, the Coulter Foundation, and the Duke Translational Research Institute.
References
- Abbott J, Holtzer H. The loss of phenotypic traits by differentiated cells. 3. The reversible behavior of chondrocytes in primary cultures. J Cell Biol. 1966;28:473–487. doi: 10.1083/jcb.28.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams GB, Scadden DT. A niche opportunity for stem cell therapeutics. Gene Ther. 2008;15:96–99. doi: 10.1038/sj.gt.3303063. [DOI] [PubMed] [Google Scholar]
- Alhadlaq A, Mao JJ. Mesenchymal stem cells: isolation and therapeutics. Stem Cells Dev. 2004;13:436–448. doi: 10.1089/scd.2004.13.436. [DOI] [PubMed] [Google Scholar]
- Angele P, Yoo JU, Smith C, Mansour J, Jepsen KJ, Nerlich M, Johnstone B. Cyclic hydrostatic pressure enhances the chondrogenic phenotype of human mesenchymal progenitor cells differentiated in vitro. J Orthop Res. 2003;21:451–457. doi: 10.1016/S0736-0266(02)00230-9. [DOI] [PubMed] [Google Scholar]
- Arnold M, Cavalcanti-Adam EA, Glass R, Blummel J, Eck W, Kantlehner M, Kessler H, Spatz JP. Activation of integrin function by nanopatterned adhesive interfaces. Chemphyschem. 2004;5:383–388. doi: 10.1002/cphc.200301014. [DOI] [PubMed] [Google Scholar]
- Atala A. Advances in tissue and organ replacement. Curr Stem Cell Res Ther. 2008;3:21–31. doi: 10.2174/157488808783489435. [DOI] [PubMed] [Google Scholar]
- Awad HA, Wickham MQ, Leddy HA, Gimble JM, Guilak F. Chondrogenic differentiation of adipose-derived adult stem cells in agarose, alginate, and gelatin scaffolds. Biomaterials. 2004;25:3211–3222. doi: 10.1016/j.biomaterials.2003.10.045. [DOI] [PubMed] [Google Scholar]
- Baharvand H, Heidari M, Ebrahimi M, Valadbeigi T, Salekdeh GH. Proteomic analysis of epithelium-denuded human amniotic membrane as a limbal stem cell niche. Mol Vis. 2007;13:1711–1721. [PubMed] [Google Scholar]
- Barnes WG, Reiter E, Violin JD, Ren XR, Milligan G, Lefkowitz RJ. beta-Arrestin 1 and Galphaq/11 coordinately activate RhoA and stress fiber formation following receptor stimulation. J Biol Chem. 2005;280:8041–8050. doi: 10.1074/jbc.M412924200. [DOI] [PubMed] [Google Scholar]
- Battista S, Guarnieri D, Borselli C, Zeppetelli S, Borzacchiello A, Mayol L, Gerbasio D, Keene DR, Ambrosio L, Netti PA. The effect of matrix composition of 3D constructs on embryonic stem cell differentiation. Biomaterials. 2005;26:6194–6207. doi: 10.1016/j.biomaterials.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Beech DJ. TRPC1: store-operated channel and more. Pflugers Arch. 2005;451:53–60. doi: 10.1007/s00424-005-1441-3. [DOI] [PubMed] [Google Scholar]
- Benya PD, Shaffer JD. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell. 1982;30:215–224. doi: 10.1016/0092-8674(82)90027-7. [DOI] [PubMed] [Google Scholar]
- Bianco P, Robey PG, Simmons PJ. Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell. 2008;2:313–319. doi: 10.1016/j.stem.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisken C, Duss S. Stem cells and the stem cell niche in the breast: an integrated hormonal and developmental perspective. Stem Cell Rev. 2007;3:147–156. doi: 10.1007/s12015-007-0019-1. [DOI] [PubMed] [Google Scholar]
- Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, Bringhurst FR, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- Caplan AI. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol. 2007;213:341–347. doi: 10.1002/jcp.21200. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Julius D. The vanilloid receptor: a molecular gateway to the pain pathway. Annu Rev Neurosci. 2001;24:487–517. doi: 10.1146/annurev.neuro.24.1.487. [DOI] [PubMed] [Google Scholar]
- Chen CS, Alonso JL, Ostuni E, Whitesides GM, Ingber DE. Cell shape provides global control of focal adhesion assembly. Biochem Biophys Res Commun. 2003;307:355–361. doi: 10.1016/s0006-291x(03)01165-3. [DOI] [PubMed] [Google Scholar]
- Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Micropatterned surfaces for control of cell shape, position, and function. Biotechnol Prog. 1998;14:356–363. doi: 10.1021/bp980031m. [DOI] [PubMed] [Google Scholar]
- Christensen AP, Corey DP. TRP channels in mechanosensation: direct or indirect activation? Nat Rev Neurosci. 2007;8:510–521. doi: 10.1038/nrn2149. [DOI] [PubMed] [Google Scholar]
- Christopherson GT, Song H, Mao HQ. The influence of fiber diameter of electrospun substrates on neural stem cell differentiation and proliferation. Biomaterials. 2009;30:556–564. doi: 10.1016/j.biomaterials.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Cohen DM, Chen CS. Melton D, editor. Mechanical control of stem cell differentiation. Stembook. 2008 ( http://www.stembook.org/node/516). [PubMed] [Google Scholar]
- Daley WP, Peters SB, Larsen M. Extracellular matrix dynamics in development and regenerative medicine. J Cell Sci. 2008;121:255–264. doi: 10.1242/jcs.006064. [DOI] [PubMed] [Google Scholar]
- David V, Martin A, Lafage-Proust MH, Malaval L, Peyroche S, Jonoes DB, Vico L, Guignandon A. Mechanical loading down-regulates peroxisome proliferator-activated receptor gamma in bone marrow stromal cells and favors osteoblastogenesis at the expense of adipogenesis. Endocrinology. 2007;148:2553–2562. doi: 10.1210/en.2006-1704. [DOI] [PubMed] [Google Scholar]
- Dellatore SM, Garcia AS, Miller WM. Mimicking stem cell niches to increase stem cell expansion. Curr Opin Biotechnol. 2008;19:534–540. doi: 10.1016/j.copbio.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deroanne CF, Lapiere CM, Nusgens BV. In vitro tubulogenesis of endothelial cells by relaxation of the coupling extracellular matrix-cytoskeleton. Cardiovasc Res. 2001;49:647–658. doi: 10.1016/s0008-6363(00)00233-9. [DOI] [PubMed] [Google Scholar]
- DeWire SM, Ahn S, Lefkowitz RJ, Shenoy SK. Beta-arrestins and cell signaling. Annu Rev Physiol. 2007;69:483–510. doi: 10.1146/annurev.physiol.69.022405.154749. [DOI] [PubMed] [Google Scholar]
- Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- Elder SH, Goldstein SA, Kimura JH, Soslowsky LJ, Spengler DM. Chondrocyte differentiation is modulated by frequency and duration of cyclic compressive loading. Ann Biomed Eng. 2001;29:476–482. doi: 10.1114/1.1376696. [DOI] [PubMed] [Google Scholar]
- Elder SH, Kimura JH, Soslowsky LJ, Lavagnino M, Goldstein SA. Effect of compressive loading on chondrocyte differentiation in agarose cultures of chick limb-bud cells. J Orthop Res. 2000;18:78–86. doi: 10.1002/jor.1100180112. [DOI] [PubMed] [Google Scholar]
- Elisseeff J, Ferran A, Hwang S, Varghese S, Zhang Z. The role of biomaterials in stem cell differentiation: applications in the musculoskeletal system. Stem Cells Dev. 2006;15:295–303. doi: 10.1089/scd.2006.15.295. [DOI] [PubMed] [Google Scholar]
- Emerman JT, Burwen SJ, Pitelka DR. Substrate properties influencing ultrastructural differentiation of mammary epithelial cells in culture. Tissue Cell. 1979;11:109–119. doi: 10.1016/0040-8166(79)90011-9. [DOI] [PubMed] [Google Scholar]
- Engler AJ, Griffin MA, Sen S, Bonnemann CG, Sweeney HL, Discher DE. Myotubes differentiate optimally on substrates with tissue-like stiffness: pathological implications for soft or stiff microenvironments. J Cell Biol. 2004;166:877–887. doi: 10.1083/jcb.200405004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- Erickson GR, Gimble JM, Franklin DM, Rice HE, Awad H, Guilak F. Chondrogenic potential of adipose tissue-derived stromal cells in vitro and in vivo. Biochem Biophys Res Commun. 2002;290:763–769. doi: 10.1006/bbrc.2001.6270. [DOI] [PubMed] [Google Scholar]
- Estes BT, Gimble JM, Guilak F. Mechanical signals as regulators of stem cell fate. Curr Top Dev Biol. 2004;60:91–126. doi: 10.1016/S0070-2153(04)60004-4. [DOI] [PubMed] [Google Scholar]
- Fan L, Sebe A, Peterfi Z, Masszi A, Thirone AC, Rotstein OD, Nakano H, McCulloch CA, Szaszi K, Mucsi I, et al. Cell contact-dependent regulation of epithelial-myofibroblast transition via the rho-rho kinase-phospho-myosin pathway. Mol Biol Cell. 2007;18:1083–1097. doi: 10.1091/mbc.E06-07-0602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang J, Hall BK. Differential expression of neural cell adhesion molecule (NCAM) during osteogenesis and secondary chondrogenesis in the embryonic chick. Int J Dev Biol. 1995;39:519–528. [PubMed] [Google Scholar]
- Fiorio Pla A, Maric D, Brazer SC, Giacobini P, Liu X, Chang YH, Ambudkar IS, Barker JL. Canonical transient receptor potential 1 plays a role in basic fibroblast growth factor (bFGF)/FGF receptor-1-induced Ca2+ entry and embryonic rat neural stem cell proliferation. J Neurosci. 2005;25:2687–2701. doi: 10.1523/JNEUROSCI.0951-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkman J, Moscona A. Role of cell shape in growth control. Nature. 1978;273:345–349. doi: 10.1038/273345a0. [DOI] [PubMed] [Google Scholar]
- Gao X, Wu L, O'Neil RG. Temperature-modulated diversity of TRPV4 channel gating: activation by physical stresses and phorbol ester derivatives through protein kinase C-dependent and -independent pathways. J Biol Chem. 2003;278:27129–27137. doi: 10.1074/jbc.M302517200. [DOI] [PubMed] [Google Scholar]
- Gerecht S, Bettinger CJ, Zhang Z, Borenstein JT, Vunjak-Novakovic G, Langer R. The effect of actin disrupting agents on contact guidance of human embryonic stem cells. Biomaterials. 2007;28:4068–4077. doi: 10.1016/j.biomaterials.2007.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh K, Ingber DE. Micromechanical control of cell and tissue development: implications for tissue engineering. Adv Drug Deliv Rev. 2007;59:1306–1318. doi: 10.1016/j.addr.2007.08.014. [DOI] [PubMed] [Google Scholar]
- Glücksmann A. The role of mechanical stresses in bone formation in vitro. J Anat. 1942;76:132–139. [PMC free article] [PubMed] [Google Scholar]
- Gong Z, Niklason LE. Small-diameter human vessel wall engineered from bone marrow-derived mesenchymal stem cells (hMSCs) FASEB J. 2008;22:1635–1648. doi: 10.1096/fj.07-087924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilak F, Ratcliffe A, Mow VC. Chondrocyte deformation and local tissue strain in articular cartilage: a confocal microscopy study. J Orthop Res. 1995;13:410–421. doi: 10.1002/jor.1100130315. [DOI] [PubMed] [Google Scholar]
- Guilak F, Sah RL, Setton LA. Physical regulation of cartilage metabolism. In: Mow VC, Hayes WC, editors. Basic Orthopaedic Biomechanics. Philadelphia: Lippincott-Raven; 1997. pp. 179–207. [Google Scholar]
- Guo WH, Frey MT, Burnham NA, Wang YL. Substrate rigidity regulates the formation and maintenance of tissues. Biophys J. 2006;90:2213–2220. doi: 10.1529/biophysj.105.070144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjipanayi E, Mudera V, Brown RA. Close dependence of fibroblast proliferation on collagen scaffold matrix stiffness. J Tissue Eng Regen Med. 2009;3:77–84. doi: 10.1002/term.136. [DOI] [PubMed] [Google Scholar]
- Hall BK, Herring SW. Paralysis and growth of the musculoskeletal system in the embryonic chick. J Morphol. 1990;206:45–56. doi: 10.1002/jmor.1052060105. [DOI] [PubMed] [Google Scholar]
- Hamilton DW, Maul TM, Vorp DA. Characterization of the response of bone marrow-derived progenitor cells to cyclic strain: implications for vascular tissue-engineering applications. Tissue Eng. 2004;10:361–369. doi: 10.1089/107632704323061726. [DOI] [PubMed] [Google Scholar]
- Hayman MW, Smith KH, Cameron NR, Przyborski SA. Growth of human stem cell-derived neurons on solid three-dimensional polymers. J Biochem Biophys Methods. 2005;62:231–240. doi: 10.1016/j.jbbm.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Hoben GM, Koay EJ, Athanasiou KA. Fibrochondrogenesis in two embryonic stem cell lines: effects of differentiation timelines. Stem Cells. 2008;26:422–430. doi: 10.1634/stemcells.2007-0641. [DOI] [PubMed] [Google Scholar]
- Holtzer H, Abbott J, Lash J, Holtzer S. The Loss of Phenotypic Traits by Differentiated Cells in Vitro, I. Dedifferentiation of Cartilage Cells. Proc Natl Acad Sci U S A. 1960;46:1533–1542. doi: 10.1073/pnas.46.12.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CY, Hagar KL, Frost LE, Sun Y, Cheung HS. Effects of cyclic compressive loading on chondrogenesis of rabbit bone-marrow derived mesenchymal stem cells. Stem Cells. 2004;22:313–323. doi: 10.1634/stemcells.22-3-313. [DOI] [PubMed] [Google Scholar]
- Hwang NS, Kim MS, Sampattavanich S, Baek JH, Zhang Z, Elisseeff J. Effects of three-dimensional culture and growth factors on the chondrogenic differentiation of murine embryonic stem cells. Stem Cells. 2006a;24:284–291. doi: 10.1634/stemcells.2005-0024. [DOI] [PubMed] [Google Scholar]
- Hwang NS, Varghese S, Elisseeff J. Cartilage tissue engineering: Directed differentiation of embryonic stem cells in three-dimensional hydrogel culture. Methods Mol Biol. 2007;407:351–373. doi: 10.1007/978-1-59745-536-7_24. [DOI] [PubMed] [Google Scholar]
- Hwang NS, Varghese S, Zhang Z, Elisseeff J. Chondrogenic differentiation of human embryonic stem cell-derived cells in arginine-glycine-aspartate-modified hydrogels. Tissue Eng. 2006b;12:2695–2706. doi: 10.1089/ten.2006.12.2695. [DOI] [PubMed] [Google Scholar]
- Ingber D. Extracellular matrix and cell shape: potential control points for inhibition of angiogenesis. J Cell Biochem. 1991;47:236–241. doi: 10.1002/jcb.240470309. [DOI] [PubMed] [Google Scholar]
- Ingber DE. Mechanobiology and diseases of mechanotransduction. Ann Med. 2003;35:564–577. doi: 10.1080/07853890310016333. [DOI] [PubMed] [Google Scholar]
- Ingber DE. The mechanochemical basis of cell and tissue regulation. Mech Chem Biosyst. 2004;1:53–68. [PubMed] [Google Scholar]
- Jakkaraju S, Zhe X, Pan D, Choudhury R, Schuger L. TIPs are tension-responsive proteins involved in myogenic versus adipogenic differentiation. Dev Cell. 2005;9:39–49. doi: 10.1016/j.devcel.2005.04.015. [DOI] [PubMed] [Google Scholar]
- Johnstone B, Hering TM, Caplan AI, Goldberg VM, Yoo JU. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res. 1998;238:265–272. doi: 10.1006/excr.1997.3858. [DOI] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M, Takehashi M, Takashima S, Lee J, Morimoto H, Chuma S, Raducanu A, Nakatsuji N, Fassler R, Shinohara T. Homing of mouse spermatogonial stem cells to germline niche depends on beta1-integrin. Cell Stem Cell. 2008;3:533–542. doi: 10.1016/j.stem.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Kasper S. Exploring the origins of the normal prostate and prostate cancer stem cell. Stem Cell Rev. 2008;4:193–201. doi: 10.1007/s12015-008-9033-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitsberg D. Human embryonic stem cells for tissue engineering. Methods Mol Med. 2007;140:33–65. doi: 10.1007/978-1-59745-443-8_3. [DOI] [PubMed] [Google Scholar]
- Kloxin AM, Kasko AM, Salinas CN, Anseth KS. Photodegradable hydrogels for dynamic tuning of physical and chemical properties. Science. 2009;324:59–63. doi: 10.1126/science.1169494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knippenberg M, Helder MN, Doulabi BZ, Semeins CM, Wuisman PI, Klein-Nulend J. Adipose tissue-derived mesenchymal stem cells acquire bone cell-like responsiveness to fluid shear stress on osteogenic stimulation. Tissue Eng. 2005;11:1780–1788. doi: 10.1089/ten.2005.11.1780. [DOI] [PubMed] [Google Scholar]
- Kronenberg HM. PTH regulates the hematopoietic stem cell niche in bone. Adv Exp Med Biol. 2007;602:57–60. doi: 10.1007/978-0-387-72009-8_7. [DOI] [PubMed] [Google Scholar]
- Lecuit T, Lenne PF. Cell surface mechanics and the control of cell shape, tissue patterns and morphogenesis. Nat Rev Mol Cell Biol. 2007;8:633–644. doi: 10.1038/nrm2222. [DOI] [PubMed] [Google Scholar]
- Lee WC, Maul TM, Vorp DA, Rubin JP, Marra KG. Effects of uniaxial cyclic strain on adipose-derived stem cell morphology, proliferation, and differentiation. Biomech Model Mechanobiol. 2007;6:265–273. doi: 10.1007/s10237-006-0053-y. [DOI] [PubMed] [Google Scholar]
- Liedtke W. TRPV channels' role in osmotransduction and mechanotransduction. Handb Exp Pharmacol. 2007:473–487. doi: 10.1007/978-3-540-34891-7_28. [DOI] [PubMed] [Google Scholar]
- Liedtke W, Kim C. Functionality of the TRPV subfamily of TRP ion channels: add mechano-TRP and osmo-TRP to the lexicon! Cell Mol Life Sci. 2005;62:2985–3001. doi: 10.1007/s00018-005-5181-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liedtke W, Tobin DM, Bargmann CI, Friedman JM. Mammalian TRPV4 (VR-OAC) directs behavioral responses to osmotic and mechanical stimuli in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2003;100 Suppl 2:14531–14536. doi: 10.1073/pnas.2235619100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack CP, Somlyo AV, Hautmann M, Somlyo AP, Owens GK. Smooth muscle differentiation marker gene expression is regulated by RhoA-mediated actin polymerization. J Biol Chem. 2001;276:341–347. doi: 10.1074/jbc.M005505200. [DOI] [PubMed] [Google Scholar]
- Manasek FJ, Burnside MB, Waterman RE. Myocardial cell shape change as a mechanism of embryonic heart looping. Dev Biol. 1972;29:349–371. doi: 10.1016/0012-1606(72)90077-2. [DOI] [PubMed] [Google Scholar]
- Mauck RL, Byers BA, Yuan X, Tuan RS. Regulation of cartilaginous ECM gene transcription by chondrocytes and MSCs in 3D culture in response to dynamic loading. Biomech Model Mechanobiol. 2007;6:113–125. doi: 10.1007/s10237-006-0042-1. [DOI] [PubMed] [Google Scholar]
- McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell. 2004;6:483–495. doi: 10.1016/s1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- McBride SH, Knothe Tate ML. Modulation of stem cell shape and fate A: the role of density and seeding protocol on nucleus shape and gene expression. Tissue Eng Part A. 2008;14:1561–1572. doi: 10.1089/ten.tea.2008.0112. [DOI] [PubMed] [Google Scholar]
- Mehta D, Ahmmed GU, Paria BC, Holinstat M, Voyno-Yasenetskaya T, Tiruppathi C, Minshall RD, Malik AB. RhoA interaction with inositol 1,4,5-trisphosphate receptor and transient receptor potential channel-1 regulates Ca2+ entry. Role in signaling increased endothelial permeability. J Biol Chem. 2003;278:33492–33500. doi: 10.1074/jbc.M302401200. [DOI] [PubMed] [Google Scholar]
- Metallo CM, Mohr JC, Detzel CJ, de Pablo JJ, Van Wie BJ, Palecek SP. Engineering the stem cell microenvironment. Biotechnol Prog. 2007;23:18–23. doi: 10.1021/bp060350a. [DOI] [PubMed] [Google Scholar]
- Meyers J, Craig J, Odde DJ. Potential for control of signaling pathways via cell size and shape. Curr Biol. 2006;16:1685–1693. doi: 10.1016/j.cub.2006.07.056. [DOI] [PubMed] [Google Scholar]
- Muramatsu S, Wakabayashi M, Ohno T, Amano K, Ooishi R, Sugahara T, Shiojiri S, Tashiro K, Suzuki Y, Nishimura R, et al. Functional gene screening system identified TRPV4 as a regulator of chondrogenic differentiation. J Biol Chem. 2007;282:32158–32167. doi: 10.1074/jbc.M706158200. [DOI] [PubMed] [Google Scholar]
- Murray PDF, Drachman BD. The role of movement in the development of joints and related structures: the head and neck in the chick embryo. J Embryo Exp Morphol. 1969;22:349–371. [PubMed] [Google Scholar]
- Mutai H, Heller S. Vertebrate and invertebrate TRPV-like mechanoreceptors. Cell Calcium. 2003;33:471–478. doi: 10.1016/s0143-4160(03)00062-9. [DOI] [PubMed] [Google Scholar]
- Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;0110:3499–3506. doi: 10.1182/blood-2007-02-069716. [DOI] [PubMed] [Google Scholar]
- Neves SR, Tsokas P, Sarkar A, Grace EA, Rangamani P, Taubenfeld SM, Alberini CM, Schaff JC, Blitzer RD, Moraru, et al. Cell shape and negative links in regulatory motifs together control spatial information flow in signaling networks. Cell. 2008;133:666–680. doi: 10.1016/j.cell.2008.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman P, Watt FM. Influence of cytochalasin D-induced changes in cell shape on proteoglycan synthesis by cultured articular chondrocytes. Exp Cell Res. 1988;178:199–210. doi: 10.1016/0014-4827(88)90391-6. [DOI] [PubMed] [Google Scholar]
- Nilius B, Voets T. Diversity of TRP channel activation. Novartis Found Symp. 2004;258:140–149. discussion 149–159, 263–146. [PubMed] [Google Scholar]
- Oatley JM, Brinster RL. Regulation of spermatogonial stem cell self-renewal in mammals. Annu Rev Cell Dev Biol. 2008;24:263–286. doi: 10.1146/annurev.cellbio.24.110707.175355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohyama M, Terunuma A, Tock CL, Radonovich MF, Pise-Masison CA, Hopping SB, Brady JN, Udey MC, Vogel JC. Characterization and isolation of stem cell-enriched human hair follicle bulge cells. J Clin Invest. 2006;116:249–260. doi: 10.1172/JCI26043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JS, Chu JS, Cheng C, Chen F, Chen D, Li S. Differential effects of equiaxial and uniaxial strain on mesenchymal stem cells. Biotechnol Bioeng. 2004;88:359–368. doi: 10.1002/bit.20250. [DOI] [PubMed] [Google Scholar]
- Pedersen SF, Nilius B. Transient receptor potential channels in mechanosensing and cell volume regulation. Methods Enzymol. 2007;428:183–207. doi: 10.1016/S0076-6879(07)28010-3. [DOI] [PubMed] [Google Scholar]
- Pelham RJ, Jr., Wang Y. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc Natl Acad Sci U S A. 1997;94:13661–13665. doi: 10.1073/pnas.94.25.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polak JM, Bishop AE. Stem cells and tissue engineering: past, present, and future. Ann N Y Acad Sci. 2006;1068:352–366. doi: 10.1196/annals.1346.001. [DOI] [PubMed] [Google Scholar]
- Poteser M, Graziani A, Eder P, Yates A, Machler H, Romanin C, Groschner K. Identification of a rare subset of adipose tissue-resident progenitor cells, which express CD0133 and TRPC3 as a VEGF-regulated Ca2+ entry channel. FEBS Lett. 2008;582:2696–2702. doi: 10.1016/j.febslet.2008.06.049. [DOI] [PubMed] [Google Scholar]
- Recknor JB, Sakaguchi DS, Mallapragada SK. Directed growth and selective differentiation of neural progenitor cells on micropatterned polymer substrates. Biomaterials. 2006;27:4098–4108. doi: 10.1016/j.biomaterials.2006.03.029. [DOI] [PubMed] [Google Scholar]
- Saha K, Keung AJ, Irwin EF, Li Y, Little L, Schaffer DV, Healy KE. Substrate modulus directs neural stem cell behavior. Biophys J. 2008a;95:4426–4438. doi: 10.1529/biophysj.108.132217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha S, Ji L, de Pablo JJ, Palecek SP. Inhibition of human embryonic stem cell differentiation by mechanical strain. J Cell Physiol. 2006;206:126–137. doi: 10.1002/jcp.20441. [DOI] [PubMed] [Google Scholar]
- Saha S, Ji L, de Pablo JJ, Palecek SP. TGFbeta/Activin/Nodal pathway in inhibition of human embryonic stem cell differentiation by mechanical strain. Biophys J. 2008b;94:4123–4133. doi: 10.1529/biophysj.107.119891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scadden DT. The stem-cell niche as an entity of action. Nature. 2006;441:1075–1079. doi: 10.1038/nature04957. [DOI] [PubMed] [Google Scholar]
- Sen B, Xie Z, Case N, Ma M, Rubin C, Rubin J. Mechanical strain inhibits adipogenesis in mesenchymal stem cells by stimulating a durable beta-catenin signal. Endocrinology. 2008;149:6065–6075. doi: 10.1210/en.2008-0687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu N, Yamamoto K, Obi S, Kumagaya S, Masumura T, Shimano Y, Naruse K, Yamashita JK, Igarashi T, Ando J. Cyclic strain induces mouse embryonic stem cell differentiation into vascular smooth muscle cells by activating PDGF receptor beta. J Appl Physiol. 2008;104:766–772. doi: 10.1152/japplphysiol.00870.2007. [DOI] [PubMed] [Google Scholar]
- Simmons CA, Matlis S, Thornton AJ, Chen S, Wang CY, Mooney DJ. Cyclic strain enhances matrix mineralization by adult human mesenchymal stem cells via the extracellular signal-regulated kinase (ERK1/2) signaling pathway. J Biomech. 2003;36:1087–1096. doi: 10.1016/s0021-9290(03)00110-6. [DOI] [PubMed] [Google Scholar]
- Sordella R, Classon M, Hu KQ, Matheson SF, Brouns MR, Fine B, Zhang L, Takami H, Yamada Y, Settleman J. Modulation of CREB activity by the Rho GTPase regulate00s cell and organism size during mouse embryonic development. Dev Cell. 2002;2:553–565. doi: 10.1016/s1534-5807(02)00162-4. [DOI] [PubMed] [Google Scholar]
- Sordella R, Jiang W, Chen GC, Curto M, Settleman J. Modulation of Rho GTPase signaling regulates a switch between adipogenesis and myogenesis. Cell. 2003;113:147–158. doi: 10.1016/s0092-8674(03)00271-x. [DOI] [PubMed] [Google Scholar]
- Tai Y, Feng S, Du W, Wang Y. Functional roles of TRPC channels in the developing brain. Pflugers Arch. 2008 doi: 10.1007/s00424-008-0618-y. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Tanentzapf G, Devenport D, Godt D, Brown NH. Integrin-dependent anchoring of a stem-cell niche. Nat Cell Biol. 2007;9:1413–1418. doi: 10.1038/ncb1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terraciano V, Hwang N, Moroni L, Park HB, Zhang Z, Mizrahi J, Seliktar D, Elisseeff J. Differential response of adult and embryonic mesenchymal progenitor cells to mechanical compression in hydrogels. Stem Cells. 2007;25:2730–2738. doi: 10.1634/stemcells.2007-0228. [DOI] [PubMed] [Google Scholar]
- Thomas GP, el Haj AJ. Bone marrow stromal cells are load responsive in vitro. Calcif Tissue Int. 1996;58:101–108. doi: 10.1007/BF02529731. [DOI] [PubMed] [Google Scholar]
- Titushkin I, Cho M. Modulation of cellular mechanics during osteogenic differentiation of human mesenchymal stem cells. Biophys J. 2007;93:3693–3702. doi: 10.1529/biophysj.107.107797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacanti JP, Langer R. Tissue engineering: the design and fabrication of living replacement devices for surgical reconstruction and transplantation. Lancet. 1999;354 Suppl 1:SI32–SI34. doi: 10.1016/s0140-6736(99)90247-7. [DOI] [PubMed] [Google Scholar]
- Wang DZ, Olson EN. Control of smooth muscle development by the myocardin family of transcriptional coactivators. Curr Opin Genet Dev. 2004;14:558–566. doi: 10.1016/j.gde.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Wang HB, Dembo M, Wang YL. Substrate flexibility regulates growth and apoptosis of normal but not transformed cells. Am J Physiol Cell Physiol. 2000;279:C1345–C1350.00. doi: 10.1152/ajpcell.2000.279.5.C1345. [DOI] [PubMed] [Google Scholar]
- Wang JH, Thampatty BP. Mechanobiology of adult and stem cells. Int Rev Cell Mol Biol. 2008;271:301–346. doi: 10.1016/S1937-6448(08)01207-0. [DOI] [PubMed] [Google Scholar]
- Watt FM, Hogan BL. Out of Eden: stem cells and their niches. Science. 2000;287:1427–1430. doi: 10.1126/science.287.5457.1427. [DOI] [PubMed] [Google Scholar]
- Winer JP, Janmey PA, McCormick ME, Funaki M. Bone marrow-derived human mesenchymal stem cells become quiescent on soft substrates but remain responsive to chemical or mechanical stimuli. Tissue Eng Part A. 2009;15:147–154. doi: 10.1089/ten.tea.2007.0388. [DOI] [PubMed] [Google Scholar]
- Yang Y, Beqaj S, Kemp P, Ariel I, Schuger L. Stretch-induced alternative splicing of serum response factor promotes bronchial myogenesis and is defective in lung hypoplasia. J Clin Invest. 2000;106:1321–1330. doi: 10.1172/JCI8893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yim EK, Pang SW, Leong KW. Synthetic nanostructures inducing differentiation of human mesenchymal stem cells into neuronal lineage. Exp Cell Res. 2007;313:1820–1829. doi: 10.1016/j.yexcr.2007.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa T, Peel SA, Gladstone JR, Davies JE. Biochemical analysis of the response in rat bone marrow cell cultures to mechanical stimulation. Biomed Mater Eng. 1997;7:369–377. [PubMed] [Google Scholar]
- Yourek G, Hussain MA, Mao JJ. Cytoskeletal changes of mesenchymal stem cells during differentiation. Asaio J. 2007;53:219–228. doi: 10.1097/MAT.0b013e31802deb2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanetti NC, Solursh M. Induction of chondrogenesis in limb mesenchymal cultures by disruption of the actin cytoskeleton. J Cell Biol. 1984;99:115–123. doi: 10.1083/jcb.99.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Bourque CW. Amplification of transducer gain by angiotensin II-mediated enhancement of cortical actin density in osmosensory neurons. J Neurosci. 2008;28:9536–9544. doi: 10.1523/JNEUROSCI.1495-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]