Abstract
The increasing frequency at which poorly soluble new chemical entities are being discovered raises concerns in the pharmaceutical industry about drugability associated with erratic dissolution and low bioavailability of these hydrophobic compounds. Nanonization provides a plausible pharmaceutical basis for enhancing oral bioavailability and therapeutic effectiveness of these compounds by increasing their surface area. This paper surveys methods available to pharmaceutical manufacturing nanoparticles, including wet chemical processes, media milling, high pressure homogenization, gas-phase synthesis, and form-in-place processes, and elaborates physicochemical rational and gastrointestinal physiological basis upon which nano-drugs can be readily absorbed. Relevant examples are illustrated to show that nano-drugs permeate Caco-2 cell monolayer fast and are well absorbed into animal systemic circulation with high Tmax and AUC, resulting in oral bioavailability higher than their counterpart micro-drugs. The size-dependent permeability and bioavailability should be given particular consideration in the development of potent and selective drug candidates with poor aqueous solubility.
Keywords: Nanoparticle formulation, Nanonization, Bioavailability
INTRODUCTION
The first industrial production of nanomaterials occurred in the early 20th century with the production of carbon black (CAS# 1333-86-4) and later, in the 1940s, fumed silica. However, it was not until the latter half of the 20th century that the scientific understanding of materials in ultrafine particles really developed and people recognized that significant improvements to material properties could be achieved by nanonization. The term “nanotechnology” was then coined to define the application of nanoscience knowledge for practical purposes to benefit society.
The emerging field of nanotechnology seeks to exploit distinct technological advantages of nanoscience. It is not only about the realization of devices, constructs, methods, and techniques at this size scale, but also about the functional enhancement gains over conventional technology. DNA nanotechnology is writing a new chapter in the history of the molecule by directing the assembly of highly structured materials with specific DNA nanoscale features and DNA computation [1–3]. The booming nanotechnologies are supported by massive state investments in many countries [4]. Companies worldwide are producing consumer products with growing “nano”-content. Pharmaceutical involvement is now in an exponential growth and covers from low molecular weight drugs to macromolecules like proteins and plasmid DNA. It was estimated that about 30% nanomaterial-directed companies will focus on the medical and pharmaceutical nanoparticle development [5]. Significant effort has been devoted to develop nanotechnology for controlled drug delivery to offer a suitable means of delivering bioactive agents. In this respect, nanotechnology focuses on formulating bioactive agents in biocompatiable nanosystems such as nanoparticles, nanocapsules, micellar systems, and conjugates. The unusual properties of nanoparticles are already employed in a number of applications.
The fact that nanoparticles exist in the same size domain as proteins makes nanomaterials suitable for biological tagging or labeling. In order to use nanoparticles as biological tags, a biological or molecular coating or layer acting as a bioinorganic interface should be attached to the nanoparticle. Examples of biological coatings may include antibodies, biopolymers like collagen [17], or monolayers of small molecules that make the nanoparticles biocompatible [18]. In addition, as optical detection techniques are wide spread in biological research, nanoparticles should either fluoresce or change their optical properties. Nanoparticles usually form the core of nano-biomaterial. It can be used as a convenient surface for molecular assembly, and may be composed of inorganic or polymeric materials. It can also be in the form of nano-vesicle surrounded by a membrane or a layer. The core itself might have several layers and be multifunctional. For example, combining magnetic and luminescent layers one can both detect and manipulate the particles. The size and size distribution are becoming extremely critical when quantum-sized effects are used to control material properties, or if penetration through a pore structure of a cellular membrane is required. In addition, nanoparticle systems have multifaceted advantages in drug delivery. They may be used to 1) provide targeted cellular or tissue delivery of drugs; 2) improve oral bioavailability [6, 7]; 3) sustain drug or gene effect in target tissue [8]; 4) carry various functional groups on the surface of nanoparticle [9]; 5) solubilize drugs for intravascular delivery; 6) improve the stability of therapeutic agents (e.g., against pH and enzymatic degradation); and 7) control drug release rate in target tissue for required duration of treatment for optimal therapeutic efficacy [10, 11].
Physicochemical and molecular complexity of drugs and in vivo inaccessibility of most drug targets presents the most challenging– to deliver specific drugs to their site of action at therapeutically relevant levels. Drug targeting has evolved as the most desirable but elusive goal in drug delivery science. At present, about 10% of drugs under investigation have bioavailability problems due to poor solubility. It is estimated that about 40% of newly developed drugs will be poorly soluble in the future [12]. Poor drug solubility makes it very difficult to perform high-throughput screening of compounds for potential drug effects. Therefore, there is an urgent need for intelligent drug formulations to achieve sufficient bioavailability. Many different approaches have been developed to overcome the solubility problem of poorly soluble drugs including solubilisation, inclusion compounds, and complexation. An alternative to these methods developed was drug nanoparticle formulation. The basic advantage of nanonization is increases in surface area and concentration gradient of these poorly soluble compounds followed by an increased dissolution rate of the compounds according to the Noyes-Whitney equation [13]. In addition, the saturation solubility is also increased after nanonization. All these may benefit oral bioavailability of poorly soluble drugs by enhancing drug transport through a gut wall into the systemic circulation. Various approaches for nanonization of pharmaceutical particles and how nanoparticle formulation enhances oral bioavailability of poorly soluble drugs by using in vitro and in vivo models are summarized as follows.
NANOPARTICLE PROCESSES
Development of processing techniques for consistent and economical production of solid nanoparticles, in either suspension or powder forms, represents a significant challenge because of the physical limitation for sub-micron sizing, physicochemical stability, purity, and concerns about the large-scale cGMP-compliant manufacturing of such products. Pharmaceutical manufacturing nanoparticles can be achieved through a variety of methods: some have been around for many years; others are more recent. Each method can result in materials with different properties depending on the route chosen to produce them:
Wet Chemical Processes
Most of the solution-based nanoparticles use surfactant or polymer as protective agents for defined control of the particle size and size distribution. Solutions of different ions are usually mixed in well-defined quantities and under controlled conditions of heating, temperature, and pressure to promote the formation of insoluble compounds, which precipitate out of solution. These precipitates are then collected through filtering and/or spray drying to produce a dry and fine powder. The advantages of the wet chemical processes are that a variety of compounds can be fabricated, including inorganic and organic compounds, and some metals, in fairly inexpensive equipment and significant quantities. Another important advantage of these processes is the ability to control particle size and to produce highly mono-disperse materials. However, there are limitations to these processes: bound water molecules can be problematic, and the yields can be quite low. New processes are being developed to solve these problems.
Media Milling
This patent-protected technology (also named NanoCrystals) was filed by Liversider et al. in 1992 [34], and owned by NanoSystems and Elan Nanosystems, respectively. In this method the nanoparticles are produced using high-shear media mills or pearl mills. The high energy and shear forces generated as a result of the impaction of the milling media with the drug provide the energy input to break the microparticle drug into nanoparticles. The milling medium is composed of glass, zirconium oxide, or highly cross-linked polystyrene resin. They are considered as non-toxic. The batch time required to obtain dispersions with unimodal distribution and mean diameters< 200 nm is 30–60 min. Depending on the hardness of the drug material and the required fineness of the particle, the milling times range from hours to days, and the size range is below 400 nm. The media milling process can process micronized and non-micronized drug materials with little batch-to-batch variation. The major concern, however, is the generation of residues of milling media, which may be introduced in the final product as a result of erosion. This problem can be partially overcome by using cross-linked polystyrene resin-based milling medium.
High Pressure Homogenization
This technology was initially developed by Müller and Böhm using high-pressure homogenizers (also called Disso Cubes) [33]. There are at least five companies owning the similar technology: Drug Delivery Services, SkyePharma, APV Deutschland GmbH, Avestin, and Stansted. This procedure starts with dispersing drug powder in a surfactant solution by a high speed stirrer, and followed by milling drug size to microparticle range. The microparticle suspension passes under high pressure (usually ranging from 100 bar to a maximum of 2000 bar) a small homogenization gap of a diameter of 25 μm, which leads to a high streaming velocity. In the homogenization gap, the dynamic pressure of the fluid increases with the simultaneous decrease in static pressure below the boiling point of water at room temperature. In consequence, water starts boiling at room temperature, leading to the formation of gas bubbles, which implode when the suspension leaves the gap (called cavitation) and normal air pressure is reached again. The cavitation forces are sufficiently high to disintegrate the microparticles to drug nanoparticles. Additional disintegration effects are the high shear forces in the gap and particle collision. To improve the efficiency of nanonization, the addition of viscosity enhancers is advantageous because that could result in increase in the powder density within the homogenization gap. Due to collision effects it is efficient to homogenize a highly-concentrated suspension, e.g., 10% of solid content. Suspensions from 1% up to 15% solid content can normally be processed.
Gas-Phase Synthesis
These include flame pyrolysis, laser ablation, and high temperature evaporation techniques. Flame pyrolysis has been used for many years in the fabrication of simple materials such as carbon black and fumed silica, and is being used in manufacturing many more compounds. Laser ablation is capable of making almost any nanomaterial since it utilizes a mix of physical erosion and evaporation. However, the production rates are extremely slow and suited only for research purposes. High temperature evaporation has been used successfully to make a wide range of materials. The heat source is very clean and controllable, which means that even highly refractory materials can be processed. However, this also means that the technique is unsuitable for processing organic materials.
The production of fullerences and carbon nanotubes [3, 15] is a specific subset of gas-phase synthesis techniques. Many variations have been explored and patented since they were discovered. All the techniques essentially involve the controlled growth of a nanotube on a catalyst particle through the cracking of carbon-rich gases such as methane.
It is possible to make low purity nanotubes using electric discharge technique, but this would result in wide variations in materials within a batch. Most techniques are focused on the production of either single- or multi-walled nanotubes to increase the purity and yields. There are currently no large-scale production facilities in operation, but a number of companies in the USA, Japan, and Europe are planning to install significant production capacity [4].
Form-in-Place Processes
These include lithography, vacuum deposition processes such as physical vapor deposition, chemical vapor deposition, and spray coatings. Synthesis of carbon nanotubes by chemical vapor deposition over patterned catalyst arrays leads to nanotubes grown from specific sites on surfaces. The growth directions of the nanotubes can be controlled by van der Waals self-assembly forces and applied electric fields. Controlled synthesis of nanotubes opens up exciting opportunities for coupling single-walled carbon nanotubes with peptide nucleic acid [3] enzymes and proteins [16]. These processes are more geared to the production of nanostructured layers and coatings, and can be used to fabricate nanoparticles by scraping the deposits from the collector. However, they tend to be quite inefficient and are generally not used for the production of dry powders although some laboratories are exploiting these processes.
However, there are problems with the above-mentioned nanoparticle preparations such as agglomeration of the powders, broad particle size distributions, and contamination from the process equipment itself. Special concern is placed on the degree of particle agglomeration and its control. It is desirable that nanoparticles are stable as aqueous dispersions without having the need to make a lyophilized or spray-dried product. Lyophilization (also called hydrosols) can make products stable longer. Insufficient stability of suspensions can lead to crystal growth and/or particle aggregation. To avoid the growth of the nanoparticle, an appropriate surfactant such as Tween 80 and Phospholipon (0.6%), is needed to stop particle agglomeration. Utilizing electrostatic and steric stabilizers and coating the nanoparticles can prevent them from agglomeration and ensure pharmaceutical stability [35]. Renaud et al. [14] recently reported an important advance in controlling and monitoring the growth of nanometer-scale particles. They demonstrated that a grazing incidence small-angle x-ray scattering (GISAXS) experiment can be configured to reveal details of nanoparticle growth in situ and in real time. Such real-time monitoring of a nanoparticle ensemble during growth has not been realized before.
PHYSIOLOGICAL BASIS OF PARTICLE TRANSLOCATION FROM INTESTINAL TRACT INTO SYSTEMIC CIRCULATION
Intestinal Physiology
The intestinal tract is a complex organ with both barrier and exchange functions. From the stomach, only small molecules, especially those compounds soluble in acidic conduction of stomach (pH= 2), can diffuse through the gastric epithelium. The intestinal epithelium is in close contact with ingested materials so that a mixture of disaccharides, peptides, fatty acids, monoglycerides, and drugs in small intestine can be further transformed and taken in the villi. Villi are covered with micro-villi, resulting in multiple increases of total absorption area in small intestine to 200 m2. In intestinal tract, the ingested materials are stressed from gastric acidic condition to intestinal basic (pH= 6–8, depending on the anatomic sections of intestine) condition. The shift in pH markedly changes the solubility and the ionic state of the drugs.
Translocation from Intestinal Tract into Systemic Circulation
Digested nutrients and dissolved drugs translocate from the lumen of the intestinal tract via aggregations of intestinal lymphatic tissue (also called Peyer’s patches), containing M-cells (specialized phagocytic enterocytes). Particle and nutrient uptake happens not only via the M-cells in the Peyer’s patches and the isolated follicles of the gut-associated lymphoid tissue, but also via the normal intestinal enterocytes [19, 20]. Uptake of inert particles has been shown to occur transcellularly through normal enterocytes and Peyer’s patches via M-cells, and across para-cellular pathways [21]. Initially it was assumed that the Peyer’s patches did not discriminate clearly in the type and size of the absorbed particles. Later it was recognized that modified characteristics, such as particle size [22] the surface charge of particles [23], attachment of ligands [24, 25] or coating with surfactants [26], offers possibilities of site-specific targeting to different regions of the gastrointestinal tract, including the Peyer’s patches [27].
The kinetics of particle translocation in the intestine depends on diffusion and accessibility through mucus, initial contact with enterocyte or M-cell, cellular trafficking, and post-translocation events. Charged particles, such as carboxylated polystyrene nanoparticles [23] or those composed of positively charged polymers exhibit poor oral bioavailability through electrostatic repulsion and mucus entrapment. Szentkuti [28] determined the rate of particle diffusion across the mucus layer to the enterocyte surface with respect to both size and surface charge of the particles, and found that cationic nanometer-sized latex particles became entrapped in the negatively charged mucus, whereas repulsive carboxylated fluorescent latex nanoparticles were able to diffuse across this layer. Transit through the intestinal tract is a relatively fast process. The smaller the particle diameter the faster they could permeate the mucus to reach the colonic enterocytes: 14 nm diameter permeated within 2 min, 415 nm particles took 30 min, while 1000-nm particles were unable to translocate this barrier. Within, the time of the experiment (30 min) none of the particles was endocytosed by the enterocytes despite the fact that the latex nanoparticles preferentially bound the cell surface more strongly than the mucus. After a longer time window (oral gavage for several days) a sparse accumulation of charged particulates in the lamina propria (connective tissue under the epithelia) was found compared to uncharged latex nanoparticles in the same size range [23].
Particles, once in the sub-mucosal tissue, are able to enter both lymphatic and capillaries. Particles entering the lymphatic are probably important in the induction of secretory immune responses while those which enter the capillaries become systemic and can reach different organs. In one study [29], the body distribution after translocation of polystyrene particles was examined in some detail. Polystyrene spheres (ranging from 50 nm to 3 micron) were fed by gavage to female Sprague Dawley rats daily for 10 days at a dose of 1.25 mg/kg. As much as 34 % and 26% of the 50 and 100 nm particles were absorbed respectively. Those larger than 300 nm were absent from blood.
IN VITRO AND IN VIVO EXPERIMENTAL EVIDENCES
Recent reports have demonstrated that nanonization of poorly soluble drugs can improve cellular uptake, biodistribution, and oral bioavailability of the drugs [36–38]. We also conducted both in vitro and in vivo experiments to test the hypothesis that nanonization of poorly soluble drugs improves the drugs’ oral bioavailability. Two investigational drugs were tested: a thiadiazole derivative (301029) that inhibits bovine viral diarrhea virus [6] and carbendazim [7]. The mean particle size of the two drugs was reduced by 25-folds from the initial size of 7 μm to 280 nm by pearl milling.
In vitro Experiment
The human colon adenoma derived cell line Caco-2 was used as a more relevant in vitro model for investigation of intestinal absorption. The Caco-2 cells show high degree of enterocytic differentiation and spontaneous dome formation [30]. Drug permeability across Caco-2 monolayer has been widely accepted as in vitro tools for prediction of human intestinal drug absorption [31, 32]. The cells were grown in Dulbecco’s Modified Eagle’s Medium (DMEM) and maintained in flasks at 37°C and 5% CO2 until cell confluence was reached. Appropriate amounts of cells were then transferred onto clear Transwell inserts at a density of 106 cells/cm2. Cells were maintained in a 5% CO2 incubator at 37°C for 7–10 days until a monolayer was formed on the Transwell membrane. With the inserts suspended in the wells of 12-well plates, microparticle and nanoparticle 301029 at the same concentration (100 μM) were separately added to apical side of the Caco-2 monolayer, and the medium samples were collected from basolateral side at different intervals for quantitative analysis by liquid chomatography/mass spectrometry (LC/MS) of the drug permeated across the Caco-2 monolayer. Transwells without cells seeded were run concurrently as a control to measure maximal permeation rate of test drugs. The apparent permeability coefficient (Papp) of the test drugs was expressed by cm/sec.
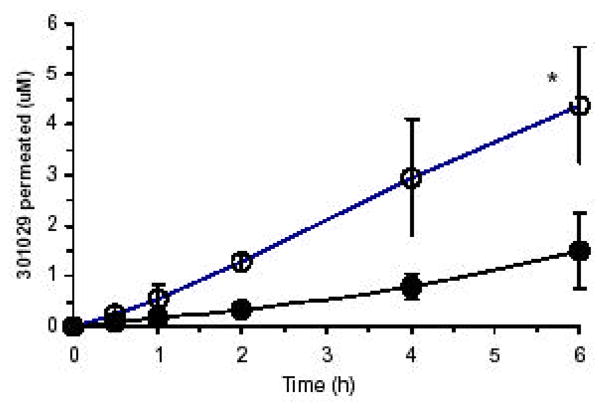
The permeation profile of 301029 across Caco-2 monolayer is shown in (Fig. 1). Cell free control experiments conducted in the same Transwell plate showed that the drug was freely permeable through the microporous membrane and supporting matrix. Therefore, any delay in permeability rate of 301029 was provided by Caco-2 monolayer only. Under well-controlled conditions, we found the Papp (cm/s) for nanoparticle 301029 was 2.94 ×10−6, and microparticle 301029 8.08 ×10−7. The permeability and permeated amounts of 301029 in nanoparticle were about four times higher than those in microparticle, indicating that nanonization increases absorption rate and amount of the drug. The relatively low permeability of microparticle 301029 may be due to its poor solubility and slow dissolution rate. Indeed, we observed undissolved 301029 particles in the micrometric formulation on the surface of the Caco-2 monolayer under a microscope (x100 magnification). Whereas, the 301029 particles after nanonization were hardly seen on the surface of the Caco-2 monolayer.
Fig. 1.
In vivo Experiments
SCID mice and Sprague-Dawley rats were used to evaluate size-dependent pharmacokinetic profiles of the two drugs, 301029 and carbendazim. The drugs were diluted with water until the appropriate concentrations were achieved before oral administration. The fasted mice and rats were orally administered with 500 mg/kg of 301029 in microparticles and nanoparticles, respectively. Animals were also given intravenous 301029 in order to determine the absolute bioavailability. In a separate study the fasted rats were orally given carbendazim at 1000 mg/kg of microparticle formulation, or 516 mg/kg of nanoparticle formulation. The animals were then euthanized by CO2 asphyxiation at various intervals for blood sampling after oral administration. Blood samples were prepared for quantitative analysis of drug concentrations by LC/MS.
Drug serum concentration-time curves (Fig. 2, 3) after oral administration were analyzed with the WinNonlin PK software version 3.2 (Pharsight Co., Mountain View, CA) using a non-compartmental model for pharmacokinetic analysis. Calculated parameters included: Tmax, time to maximum serum concentration; Cmax, maximum serum concentration; AUC, area under the serum concentration-time curve after each single dose. The absolute bioavailability of 301029 was calculated as (AUC oral/AUCi.v.) × 100, while the relative bioavailability was calculated as [AUC(nanoparticle)/AUC(microparticle)] ×[Dose (microparticle)/Dose (nanoparticle)]× 100.
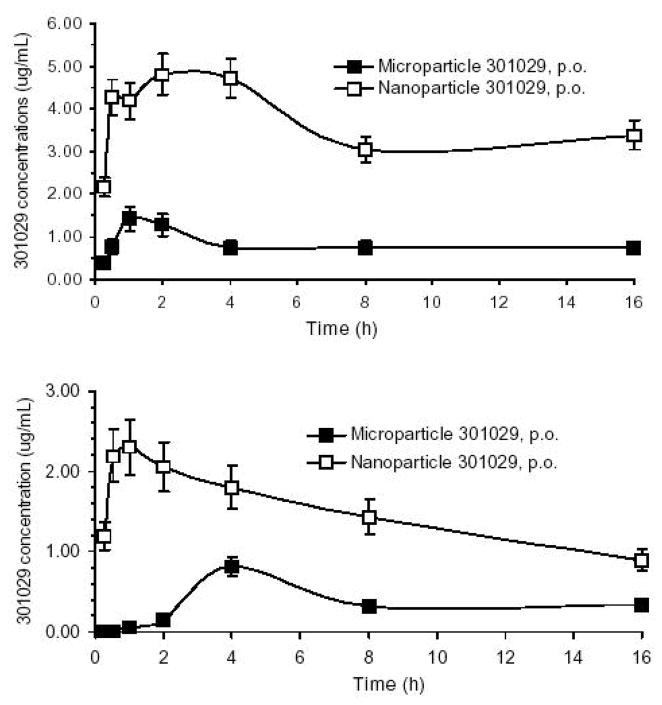
Fig. 2.
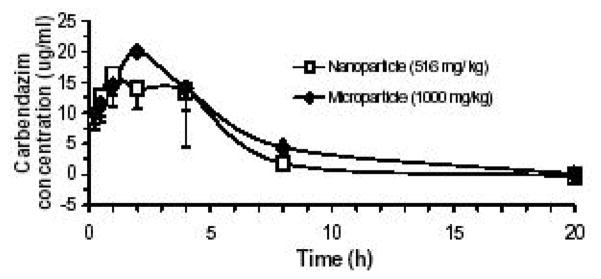
Fig. 3.
There were marked differences in the pharmacokinetic profile between the microparticle 301029 and nanoparticle 301029. In the mouse experiment (Fig. 2 upper), the average maximal serum levels of 301029 (Cmax) reached 4.8 μg/mL and 1.4 μg/mL, respectively, for nanoparticle and microparticle 301029 after single oral administration with Tmax being 1–2 h. In addition, the AUC0→16h was 58.4 and 13 μg/mL·h, respectively, for the nanoparticle and microparticle 301029 in the mice, which resulted in 449% of the relative oral bioavailability of nanoparticle 301029 versus the microparticle.
In the rat experiment (Fig. 2, lower), the mean Cmax reached 2.3 and 0.8 μg/mL, respectively, for nanoparticle and microparticle 301029 after single oral administration. The Tmax for nanoparticle was 1 h, and for microparticle 4 h. The fast absorption of 301029 after nanonization was likely due to increases in dissolution rate and saturation solubility of the drug, as well as an increased adhesiveness of nanoparticles to intestinal mucosa. Nanoparticle 301029 was well absorbed with an AUC0→8h 14.2 μg/mL·h, which is about four times more than that of micrometric size of 301029 (AUC0→8h 3.4 μg/mL·h). The large AUC value resulted in 99% of absolute oral bioavailability for nanoparticle 301029, and only 23% for microparticle 301029.
Taking carbendazim as the second example to demonstrate advantages of nanonization (Fig. 3): the micrometric carbendazim reached maximum blood concentration in rats around 2 h after oral administration. Whereas, the nanoparticle formulation seems to have absorption rate faster than the micrometric carbendazim because the calculated Tmax for nanoparticle carbendazim is about 1.5 h. The relative oral bioavailability of nanoparticle carbendazim was 166% on the basis of AUC0→20h of the micrometric one.
As demonstrated above, nanoparticle formulation provides a plausible pharmaceutical basis for enhancing oral bioavailability and therapeutic efficacy of the drugs that are poorly soluble, and cannot be injected as a drug solution. Beneficial effects of drug nanonization on bioavailability are primarily based on the fundamentals that nanonization increases surface area of poorly soluble drugs. Consequently, one can predict the following: 1. An increase in adhesion surface area between nanoparticles and the mucin layer coating the intestinal epithelium of villi facilitates the nanonized drug to traverse the mucin layer and the epithelial cells with the result of an increase in oral absorption of the nanonized drug. 2. Compared to large particles, nanoparticles in general possess a stronger curvature of the surface, which produces more dissolution pressure with a corresponding increase in saturation solubility [33]. The increased saturation solubility, in turn, favors an increase in concentration gradient between intestinal epithelial cells and the mesenteric circulation beneath. 3. An increased dissolution rate of the drug, which overcomes this rate-limiting step in the drug absorption process. In addition, the diffusion distance on the surface of drug nanoparticles is decreased, causing an increased concentration gradient [12]. An increase in surface area and concentration gradient leads to a more pronounced increase in dissolution rate compared to the micronized product. Saturation solubility and dissolution rate are important parameters affecting the bioavailability of orally administered drugs. Drug nanonization can reduce erratic drug absorption so the adhesion process of drug nanoparticles to mucosal surface can be improved. It has been reported that smaller particles of drugs are taken up more easily by macrophages, and obtain a higher deposition rate, and hence a better therapeutic index [11].
Because of the “nano” in nanoparticles, size is a primary focus of the analysis, but other properties, such as crystallization, morphology, structure, and surface chemistry are equally important since performance is dependent upon these properties as well. Drug carriers and disease conditions may also affect pharmacokinetic profiling and efficacy of the same drugs. The extraordinarily large surface area on the nanoparticle presents diverse opportunities to place functional groups on the surface. Particles can be created to expand or contract with changes in pH, or interact with antibodies, or second drugs used for combination therapy, in special ways to provide an increased dissolution velocity. Based on these favorable characteristics, nanonization has the potential to overcome absorption limitations of poorly soluble drugs. The present data taken from the above-mentioned experiments demonstrated the beneficial effects of drug nanonization by showing a consistent increase of the nanonized drugs in permeability across the in vitro Caco-2 model and in in vivo oral absorption.
References
- 1.Seeman NC. Nature. 2003;421:427–431. doi: 10.1038/nature01406. [DOI] [PubMed] [Google Scholar]
- 2.Liao S, Seeman NC. Science. 2004;306:2072–2074. doi: 10.1126/science.1104299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams KA, Veenhuizen PTM, de la Torret BG, Eritja R, Dekker C. Nature. 2002;420:761. doi: 10.1038/420761a. [DOI] [PubMed] [Google Scholar]
- 4.Jia L. Current NanoScience. 2005;1 doi: 10.2174/157341305774642939. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pitkethly MJ. Nanotoday. 2004;1:20–29. [Google Scholar]
- 6.Jia L, Wong H, Cerna C, Weitman SD. Pharm Res. 2002;19:1090–1095. doi: 10.1023/a:1019829622088. [DOI] [PubMed] [Google Scholar]
- 7.Jia L, Wong H, Garza M, Weitman SD. J Pharm Sci. 2003;92:161–172. doi: 10.1002/jps.10272. [DOI] [PubMed] [Google Scholar]
- 8.Prabha S, Labhasetwar V. Pharmaceutics. 2004;1:211–219. doi: 10.1021/mp049970+. [DOI] [PubMed] [Google Scholar]
- 9.Weber C, Coester C, Kreuter J, Langer K. Int J Pharm. 2000;194:91–102. doi: 10.1016/s0378-5173(99)00370-1. [DOI] [PubMed] [Google Scholar]
- 10.Lode J, Fichtner I, Kreuter J, Berndt A, Diederichs JE, Reszka R. Pharm Res. 2001;18:1613–1619. doi: 10.1023/a:1013094801351. [DOI] [PubMed] [Google Scholar]
- 11.Lamprecht A, Schafer U, Leh CM. Pharm Res. 2001;18:788–793. doi: 10.1023/a:1011032328064. [DOI] [PubMed] [Google Scholar]
- 12.Radtek M. New Drugs. 2001;3:62–68. [Google Scholar]
- 13.Noyes A, Whitney W. J Am Chem Soc. 1897;19:930–934. [Google Scholar]
- 14.Renaud G, Lazzari R, Revenant C, Barbier A, Noblet M, Ulrich O, Leroy F, Jupille J, Borensztein Y, Henry CR, Deville J, Scheurer F, Mane-Mane J, Fruchart O. Science. 2003;300:1416–1419. doi: 10.1126/science.1082146. [DOI] [PubMed] [Google Scholar]
- 15.Dai H. Acc Chem Res. 2002;35:1035–1044. doi: 10.1021/ar0101640. [DOI] [PubMed] [Google Scholar]
- 16.Wong SS, Joselevich E, Woolley AT, Cheung CL, Lieber CM. Nature. 1998;394:52–55. doi: 10.1038/27873. [DOI] [PubMed] [Google Scholar]
- 17.Sinani VA, Koktysh DS, Yun BG, Matts RL, Pappas TC, Motamedi M, Thomas SN, Kotov NA. Nano Lett. 2003;3:1177–1182. [Google Scholar]
- 18.Zhang Y, Kohler N, Zhang M. Biomaterials. 2002;23:1553–1561. doi: 10.1016/s0142-9612(01)00267-8. [DOI] [PubMed] [Google Scholar]
- 19.Florence AT, Hussain N. Adv Drug Deliv Rev. 2001;50:S69–89. doi: 10.1016/s0169-409x(01)00184-3. [DOI] [PubMed] [Google Scholar]
- 20.Hussain N, Jaitley V, Florence AT. Adv Drug Deliv Rev. 2001;50:107–142. doi: 10.1016/s0169-409x(01)00152-1. [DOI] [PubMed] [Google Scholar]
- 21.Aprahamain M, Michel C, Humbert W, Devissaguet JP, Damge C. Biol Cell. 1987;61:69–76. doi: 10.1111/j.1768-322x.1987.tb00571.x. [DOI] [PubMed] [Google Scholar]
- 22.Hillyer JF, Albrecht RM. J Pharm Sci. 2001;90:1927–1936. doi: 10.1002/jps.1143. [DOI] [PubMed] [Google Scholar]
- 23.Jani P, Halbert GW, Langridge J, Florence AT. J Pharm Pharmcol. 1989;41:809–812. doi: 10.1111/j.2042-7158.1989.tb06377.x. [DOI] [PubMed] [Google Scholar]
- 24.Hussain N, Florence AT. Pharm Res. 1998;15:153–156. doi: 10.1023/a:1011981610840. [DOI] [PubMed] [Google Scholar]
- 25.Hussain N, Jani PU, Florence AT. Pharm Res. 1997;14:613–618. doi: 10.1023/a:1012153011884. [DOI] [PubMed] [Google Scholar]
- 26.Hillery AM, Jani PU, Florence AT. J Drug Target. 1994;2:151–156. doi: 10.3109/10611869409015904. [DOI] [PubMed] [Google Scholar]
- 27.Woodley JF. J Drug Target. 2000;7:325–333. doi: 10.3109/10611869909085515. [DOI] [PubMed] [Google Scholar]
- 28.Szentkuti L. J Control Release. 1997;46:233–242. [Google Scholar]
- 29.Jani P, Halbert GW, Langridge J, Florence AT. J Pharm Pharmacol. 1990;42:821–826. doi: 10.1111/j.2042-7158.1990.tb07033.x. [DOI] [PubMed] [Google Scholar]
- 30.Audus KL, Bartel RL, Hidalgo IJ, Borchardt RT. Pharm Res. 1990;7:435–451. doi: 10.1023/a:1015800312910. [DOI] [PubMed] [Google Scholar]
- 31.Moutardier V, Tosini F, Vlieghe P, Cara L, Delpero JR, Clerc T. Int J Pharm. 2003;260:23–38. doi: 10.1016/s0378-5173(03)00231-x. [DOI] [PubMed] [Google Scholar]
- 32.Bailey CA, Bryla P, Malick AW. Adv Drug Delivery Rev. 1996;22:85–103. [Google Scholar]
- 33.Müller RH, Böhm BHL. In: Emulsions and Nanosuspensions for the Formulation of Poorly Soluble Drugs. Müller RH, Benita S, Böhm BHL, editors. Medpharm Scientific Publishers; Stuttgart: 1998. pp. 149–174. [Google Scholar]
- 34.Liversidge GG, Cundy KC, Bishop JF, Czekai DA. 145,684 US Patent . 1992;5
- 35.Rabinow BE. Nature Rev Drug Discovery. 2004;3:785–796. doi: 10.1038/nrd1494. [DOI] [PubMed] [Google Scholar]
- 36.Yi Y, Kim JH, Kang HW, Oh HS, Kim SW, Seo MH. Pharm Res. 2005;22:200–208. doi: 10.1007/s11095-004-1187-1. [DOI] [PubMed] [Google Scholar]
- 37.Hu L, Tang X, Cui F. J Pharm Pharmacol. 2004;56:1527–1535. doi: 10.1211/0022357044959. [DOI] [PubMed] [Google Scholar]
- 38.Dai J, Nagai T, Wang X, Zhang T, Meng M, Zhang Q. Int J Pharm. 2004;280:229–240. doi: 10.1016/j.ijpharm.2004.05.006. [DOI] [PubMed] [Google Scholar]