Abstract
Background
Although many studies have identified patient characteristics or chronic diseases associated with medication adherence, the clinical utility of such predictors has rarely been assessed. We attempted to develop clinical prediction rules for adherence with antihypertensive medications in two health care delivery systems.
Methods and Results
Retrospective cohort studies of hypertension registries in an inner-city health care delivery system (N = 17176) and a health maintenance organization (N = 94297) in Denver, Colorado. Adherence was defined by acquisition of 80% or more of antihypertensive medications.
A multivariable model in the inner-city system found that adherent patients (36.3% of the total) were more likely than non-adherent patients to be older, white, married, and acculturated in US society, to have diabetes or cerebrovascular disease, not to abuse alcohol or controlled substances, and to be prescribed less than three antihypertensive medications. Although statistically significant, all multivariate odds ratios were 1.7 or less, and the model did not accurately discriminate adherent from non-adherent patients (C-statistic = 0.606). In the health maintenance organization, where 72.1% of patients were adherent, significant but weak associations existed between adherence and older age, white race, the lack of alcohol abuse, and fewer antihypertensive medications. The multivariate model again failed to accurately discriminate adherent from non-adherent individuals (C-statistic = 0.576).
Conclusions
Although certain socio-demographic characteristics or clinical diagnoses are statistically associated with adherence to refills of antihypertensive medications, a combination of these characteristics is not sufficiently accurate to allow clinicians to predict whether their patients will be adherent with treatment.
Keywords: drugs, hypertension, prevention
Although adherence with medications for hypertension and other chronic health conditions is essential to achieving treatment goals, only 50% of individuals take their long-term medications as prescribed.1 Several recent studies have found statistically significant associations between adherence and a wide array of socio-demographic characteristics such as age, gender, race/ethnicity, socioeconomic status, or insurance payer and clinical conditions such as chronic medical and psychiatric problems.2-9 These associations were typically modest in magnitude, and the findings were often inconsistent between studies. Although multivariable prediction rules that classify patients as adherent or non-adherent on the basis of these characteristics could allow clinicians or care managers in integrated health care delivery systems to focus adherence interventions on individuals who might benefit the most,10 such prediction rules have not been developed or confirmed.
To determine if a combination of socio-demographic and clinical characteristics could accurately classify individuals with respect to adherence, we developed a clinical prediction rule for adherence in refilling antihypertensive medications in the comprehensive hypertension registry of a large, inner-city, “safety net” delivery system. To assess the generalizability of our findings from this analysis, we then repeated our analysis in a hypertension registry of patients from a large managed care organization in the same geographic area.
Methods
Derivation of Prediction Rule
We developed our prediction rule using data from the hypertension registry of Denver Health (DH), a nationally recognized delivery system in inner-city Denver, Colorado, which provided health care to more than 140,000 persons in Denver County in 2007. 11-12 A clinical information system integrates information from all DH community health centers, the hospital, emergency services, pharmacies and the clinical laboratory. Since DH patients without third-party health insurance make minimal or no co-payments for medications from DH pharmacies, DH pharmacy records provide an essentially complete record of medications for those individuals.
Participants for this study were drawn from a registry of patients with hypertension who received care at Denver Health between January 1, 2000, and December 31, 2006. This cohort included all DH patients with one or more ICD9-CM code for hypertension (401, 401.0, 401.1, 401.9, 405, 405.0, 405.01, 405.09, 405.1, 405.11, 405.19, 405.9, 405.91, 405.99, 642.0x, 642.1x, 642.2x, 642.3x, 642.4x, 642.5x, 642.6x, 642.7x, 642.9x) on any outpatient or inpatient claim. We previously found that a single ICD-9 CM code for hypertension had a sensitivity of 88% and a specificity of 78% for hypertension as defined by comprehensive medical record review while alternative definitions that required multiple visits had higher specificity but lower sensitivity.13 We chose the more sensitive definition to maximize inclusion of individuals with hypertension when constructing the registry, recognizing that some of those included would not in fact have the disease. Clinical blood pressure measurements were unavailable for most individuals.
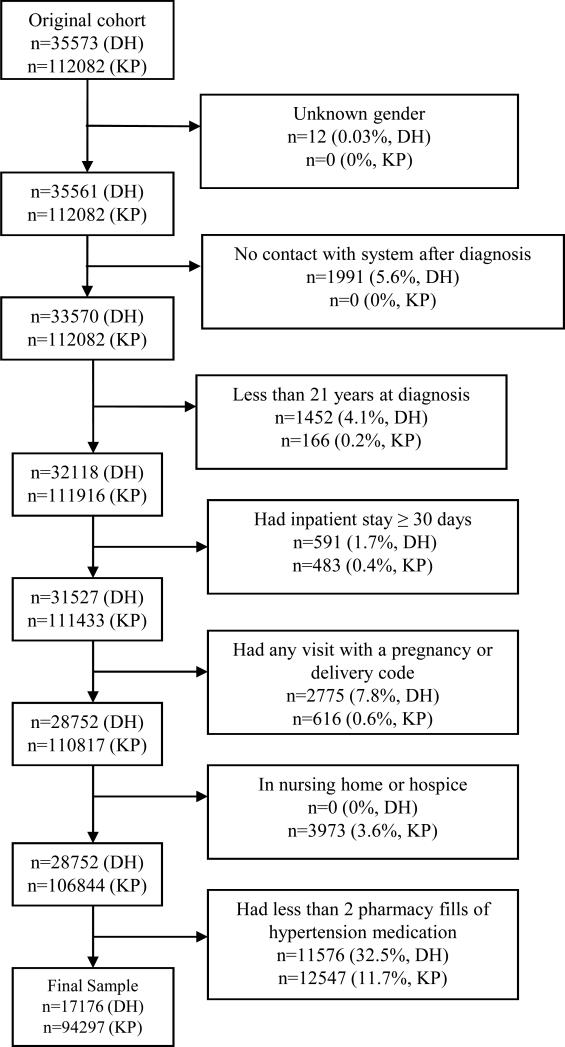
We excluded individuals with missing gender; no contact with the DH system after the date of hypertension diagnosis; age less than 21 years; one or more hospital stays of ≥ 30 days (for whom adherence estimates may have been inaccurate); ICD-9-CM codes associated with pregnancy or delivery at any point after the first encounter for hypertension; nursing home residents; and individuals receiving hospice care. Finally, we excluded patients obtaining 0 or 1 fill of any antihypertensive medication, because their refill adherence could not be calculated. In our prior work, requiring two or more fills along with an ICD-9- CM diagnosis had a specificity of 90.5% for a true diagnosis of hypertension. 13 Figure 1 depicts the number of individuals excluded at each step.
Figure 1.
Inclusion criteria for adherence study, Denver Health (DH) and Kaiser Permanente of Colorado (KP)
Our primary outcome measure was adherence in obtaining medication refills from the DH pharmacy. Although refill adherence is only moderately correlated with adherence in consuming medications,14 refill adherence is an important behavior in its own right, and correlates with other adherence behaviors and with patient outcomes at DH and elsewhere.15, 16 We calculated refill adherence for each antihypertensive drug as the total days’ supply dispensed, divided by the number of days between the first and last fills for that drug.15,17 The proportion of adherence could exceed 100% if patients obtained (and potentially consumed) more medication than prescribed. We calculated patient-level adherence with antihypertensive drugs as the time-weighted average of adherence for each antihypertensive drug.
We limited our selection of possible predictors of adherence to socio-demographic variables and clinical conditions that would be widely available in the electronic medical records of integrated health care delivery systems. For all 35,573 patients in the hypertension registry, we obtained information from registration files (all available socio-demographic variables), claims (site, date, payer, and diagnostic codes), laboratory, and pharmacy (medication name and dosage, dates filled, and days’ supply obtained). For race and ethnicity, 4.4% of subjects had missing data. Because primary language or country of birth were missing in some cases (15.2% and 18.6%, respectively), we defined individuals as having low acculturation in the US if their primary language was not English, or if language was missing and their country of birth was outside the US. Otherwise, acculturation was defined as high; 6.2% of values for this composite variable were missing. In general, we identified clinical conditions that might be associated with adherence, cardiovascular risk, or adverse outcomes of hypertension based on one or more inpatient or outpatient ICD-9 CM codes. A clinical diagnosis of chronic kidney disease within the first 180 days following hypertension diagnosis was based on laboratory findings of two or more estimated GFR values of less than 60 ml/min/1.73m2, calculated from serum creatinine levels more than 90 days apart using the abbreviated MDRD equation,18 or two or more urine albumin to creatinine ratios >0.02 at least 90 days apart. A diagnosis of dyslipidemia was based on the presence of a total fasting cholesterol > 240 mg/dL or a low-density lipoprotein level > 160 mg/dL. Diagnoses of alcohol abuse or substance abuse were based either on ICD-9-CM codes or on a laboratory finding such as an elevated blood alcohol level or an abnormal urine toxicology screen. We previously found that the sensitivity and specificity of these criteria was generally high.13 We counted the overall number of comorbid diagnoses using the Quan index.19
Confirmation of Prediction Rule
To confirm findings from DH, we repeated our analysis using data from the hypertension registry of Kaiser Permanente Colorado (KP).20 This registry included patients who received care over the same time period (January 1, 2000, to December 31, 2006). Hypertensive patients were identified by the presence of 2 or more ICD-9 CM codes, dispensing of one or more fills of an anti-hypertensive medication, and BP measurements. We applied the same exclusion criteria as for the DH cohort; the number of KP patients excluded at each step is shown in Figure 1. Whenever possible, we defined variables identically in both registries. Information on marital status and acculturation was not included in the KP registry. The presence of dyslipidemia, chronic kidney disease, alcohol abuse and substance abuse were identified from ICD9-CM codes alone in the KP registry. Overall 3 of the 4 common sociodemographic variables and 10 of 13 clinical conditions were defined identically in both data sets. Previous research in KP has shown that approximately 95% of patients obtain their medications within the system,21 and has supported the validity of the refill adherence measure.22
Statistical Analysis
We dichotomized refill adherence at a cutoff of 80%.16,22 A secondary analysis using an adherence cutoff of 70% within the DH registry demonstrated similar results, and is not reported here. We conducted bivariate analyses to identify associations between all candidate predictors and refill adherence, using t-tests or Wilcoxon rank-sum tests for continuous variables, and chi-square or Fisher exact tests for dichotomous or categorical predictors. Because the association between age and adherence was linear across age categories, we dichotomized age (≤ 55 years vs. >55 years) for the multivariate analysis. Additional analyses in which the effect of age was assessed in 5-year increments did not differ from the main analyses, and are not reported here.
Using logistic regression, we then developed a multivariable prediction model for high refill adherence. All variables with a p-value < 0.25 from bivariate analysis were entered into the model.23 We assessed the ability of the multivariate model to discriminate between adherent and non-adherent individuals using the c-statistic, which expresses the probability that the model will correctly classify the adherence of pairs of subjects randomly selected from the sample, one of whom is non-adherent and the other of whom is adherent. C-statistics of 0.70-0.75 are generally viewed as providing good discrimination.24 To evaluate the calibration of the models, we calculated the Hosmer-Lemeshow statistic to compare predicted with actual risk of adherence across risk deciles.25 We used the final predictive model at DH to develop a summary score for each subject, multiplying the regression coefficient for each significant predictor by 10 and summing the points for the number of predictors each patient had for high adherence.26 Higher scores represented a higher likelihood of being adherent to anti-hypertensive medications. We then calculated the sensitivity and specificity of the rule at several cutpoints, defining sensitivity as the proportion of adherent patients with scores at or above the cutpoint, and specificity as the proportion of nonadherent patients with scores below the cutpoint.
To determine if our overall conclusions applied to individuals with uncontrolled blood pressure, for whom assessment of adherence would be most clinically beneficial, we repeated our analysis for KP patients who had persistently elevated blood pressure, defined as two initial blood pressure readings and two final blood pressure readings, separated by more than 180 days, of greater than 140/90 mm Hg (or greater than 130/80 mm Hg for individuals with diabetes or chronic renal disease).
This study was approved by the Colorado Multiple Institutional Review Board and the institutional review board of Kaiser Permanente Colorado. All analyses were conducted with SAS Version 9.1 (SAS Institute, Inc., Cary, NC). The authors had full access to the data and take responsibility for its integrity. All authors have read and agreed to the manuscript as written.
Results
The characteristics of the 17176 Denver Health patients included in the final sample are shown in Table 1. Most of these individuals were Hispanic/Latino (45.0%) or African-American (26.7%), and 22.2% had low acculturation. Psychiatric diagnoses, alcohol abuse, and substance abuse were common. Median medication adherence was 70.6% (interquartile range 48.2% to 87.0%). Only 36.3% of these individuals obtained more than 80% of their antihypertensive medications over a median of 2.1 years. In bivariate analyses, individuals who were older, white, or more highly acculturated were more likely to be adherent. Individuals with clinical diagnoses of cerebrovascular disease, dyslipidemia, or peripheral artery disease were more likely to be adherent, as were those without alcohol or substance abuse (data not shown).
Table 1.
Characteristics of patients in hypertension registries of Denver Health and Kaiser Permanente Colorado
| Variable | Denver Health (N = 17176) N (%) | Kaiser Permanente (N = 94297) N (%) | p value |
|---|---|---|---|
| Socio-demographics | |||
| Mean (sd) / Median Age in years | 54.4 (12.1) / 53.9 | 64.9 (13.8) / 65 | <.001 |
| Male gender | 7749 (45.1%) | 43680 (46.3%) | 0.004 |
| Race | <.001 | ||
| African American | 4589 (26.7%) | 2114 (2.2%) | |
| Hispanic/Latino | 7725 (45.0%) | 3116 (3.3%) | |
| Other | 378 (2.2%) | 1618 (1.7%) | |
| White | 4103 (23.9%) | 28267 (30.0%) | |
| Missing | 381 (2.2%) | 59182 (62.8%) | |
| Marital Status* | |||
| Divorced | 2318 (13.5%) | --- | |
| Married | 5458 (31.8%) | ||
| Single | 6873 (40.0%) | ||
| Widowed | 1391 (8.1%) | ||
| Unknown | 1136 (6.6%) | ||
| Country of birth* | |||
| United States | 11381 (66.3%) | --- | |
| Mexico | 3188 (18.6%) | ||
| Other | 1020 (5.9%) | ||
| Missing | 1587 (9.2%) | ||
| Language preference* | |||
| English | 11066 (64.4%) | --- | |
| Spanish | 3187 (18.6%) | ||
| Other | 309 (1.8%) | ||
| Missing | 2614 (15.2%) | ||
| Acculturation† | |||
| High | 13360 (77.8%) | --- | |
| Low | 3816 (22.2%) | ||
| Primary Payer‡ | <.001 | ||
| Colorado Indigent Care Program | 8515 (49.6%) | N/A | |
| Medicaid | 2234 (13.0%) | 377 (0.4%) | |
| Medicare | 4733 (27.6%) | 39488 (41.9%) | |
| Other | 76 (0.4%) | 351 (0.4%) | |
| Commercial | 992 (5.8%) | 50737 (53.8%) | |
| Self-Pay | 603 (3.5%) | 2992 (3.2%) | |
| Missing | 23 (0.1%) | 352 (0.4%) | |
| Baseline coexistent medical conditions | |||
| Diabetes | 5704 (33.2%) | 22616 (24.0%) | <.001 |
| Coronary Artery Disease | 1632 (9.5%) | 13169 (14.0%) | <.001 |
| Congestive Heart Failure | 74 (0.4%) | 982 (1.0%) | <.001 |
| Chronic Kidney Disease§ | 876 (5.1%) | 2008 (2.1%) | <.001 |
| Cerebrovascular Disease | 685 (4.0%) | 4513 (4.8%) | <.001 |
| Dyslipidemia§ | 2514 (14.6%) | 27004 (28.6%) | <.001 |
| Peripheral Artery Disease | 1014 (5.9%) | 3925 (4.2%) | <.001 |
| Baseline mental health conditions | |||
| Depression | 2993 (17.4%) | 12373 (13.1%) | <.001 |
| Bipolar Disorder | 571 (3.3%) | 929 (1.0%) | <.001 |
| Psychotic Disorder | 427 (2.5%) | 1312 (1.4%) | <.001 |
| Baseline alcohol/substance abuse | |||
| Alcohol Abuse∥ | 1953 (11.4%) | 2172 (2.3%) | <.001 |
| Substance Abuse∥ | 615 (3.6%) | 353 (0.4%) | <.001 |
| Total number of Quan Comorbidities 19 | <.001 | ||
| 0 | 3940 (22.9%) | 19857 (21.1%) | |
| 1 | 4797 (27.9%) | 27711 (29.4%) | |
| 2 | 3574 (20.8%) | 19415 (20.6%) | |
| 3 | 2138 (12.5%) | 11330 (12.0%) | |
| 4 | 1238 (7.2%) | 6447 (6.8%) | |
| 5 or more | 1489 (8.7%) | 9537 (10.1%) | |
| Number of Hypertension Medications in Pharmacy data | <.001 | ||
| 1 | 5251 (30.6%) | 33276 (35.3%) | |
| 2 | 4926 (28.7%) | 28307 (30.0%) | |
| 3 or more | 6999 (40.8%) | 32714 (34.7%) |
Variable not present in both registries
High acculturation defined as have English as a primary language, or (if language information missing) being born outside the United States
No patients at Kaiser Permanente were eligible for the Colorado Indigent Care Program; statistical comparison includes other payer categories only.
Variable definition based on laboratory values in Denver Health registry, and on ICD-9 CM codes only in Kaiser Permanente registry
Variable definition based on combination of laboratory values and ICD9-CM codes in Denver Health registry, and on ICD-9 CM codes only in Kaiser Permanente registry
Table 2 shows the results of two logistic regression models for the DH cohort. The first model contains socio-demographic variables that were statistically significant at a bivariate p-value of <0.25. While age, race, marital status, and acculturation were statistically significant predictors of adherence, the c-statistic for the multivariable model was 0.580. Addition of individually significant clinical conditions improved the c-statistic to 0.606. The Hosmer-Lemeshow χ2 statistic to assess the calibration of this model was 14.7 (8 df, p = 0.07). Adding the total number of diagnoses, measured by the Quan index, produced a model with a c-statistic of 0.601 (data not shown). When adherence was treated as a continuous dependent variable rather than using a cutoff of 80%, a multivariable linear regression model including only socio-demographic variables had a R2 of 0.038. Even with the addition of statistically significant clinical conditions, the final model explained only about 5% of the variance in adherence (R2 = 0.053).
Table 2.
Logistic regression models for associations with ≥ 80% adherence to refills, Denver Health and Kaiser Permanente Colorado
| Model Description* | Denver Health OR (95% CI) | Kaiser Permanente OR (95% CI) |
|---|---|---|
| Demographics Only | ||
| Age >55 | 1.38 (1.29-1.48) | 1.57 (1.52-1.63) |
| White Race | 1.65 (1.53-1.78) | 1.17 (1.13-1.21) |
| Married or Widowed | 1.13 (1.06-1.21) | -- |
| High Acculturation | 1.30 (1.19-1.41) | -- |
| Colorado Indigent Care Program Insurance | 1.02 (0.96-1.09) | N/A |
| Kaiser Commercial Insurance | -- | 1.02 (0.98-1.05) |
| Male Gender | -- | --- |
| C statistic for overall model | 0.580 | 0.555 |
| Demographics, Individual Comorbidities, and Number of Antihypertensive Medications Prescribed | ||
| Age >55 | 1.39 (1.30-1.49) | 1.58 (1.52-1.64) |
| White Race | 1.64 (1.52-1.78) | 1.17 (1.14-1.21) |
| Married or Widowed | 1.10 (1.03-1.18) | -- |
| High Acculturation | 1.36 (1.25-1.48) | -- |
| Colorado Indigent Care Program Insurance | 1.01 (0.95-1.08) | N/A |
| Kaiser Commercial Insurance | -- | 0.97(0.94-1.01) |
| Male Gender | -- | --- |
| Diabetes | 1.10 (1.02-1.17) | 0.97 (0.94-1.01) |
| Coronary Artery Disease | 0.98 (0.88-1.11) | 0.93 (0.88-0.97) |
| Congestive Heart Failure | -- | --- |
| Chronic Kidney Disease | 1.06 (0.92-1.23) | 0.76 (0.69-0.84) |
| Cerebrovascular Disease | 1.33 (1.14-1.56) | 0.98 (0.91-1.05) |
| Dyslipidemia | 1.05 (0.96-1.15) | 1.24 (1.19-1.28) |
| Peripheral Artery Disease | 1.04 (0.90-1.20) | --- |
| Depression | 1.03 (0.95-1.12) | 0.89 (0.85-0.93) |
| Bipolar Disorder | -- | 0.94 (0.82-1.09) |
| Psychotic Disorder | -- | 1.31 (1.15-1.49) |
| Substance Abuse | 0.79 (0.65-0.95) | 0.86 (0.69-1.08) |
| Alcohol Abuse | 0.77 (0.69-0.86) | 0.73 (0.67-0.80) |
| <3 Hypertension Meds | 1.56 (1.46-1.67) | 1.22 (1.19-1.26) |
| C - statistic for overall model | 0.606 | 0.575 |
| Hosmer-Lemeshow χ2 statistic (p-value) | 14.7 (8 df, p = 0.07). | 25.2 (8 df, p = 0.001). |
Models for Denver Health and Kaiser Permanente differ due to variables not contained in hypertension registry or not statistically significant at p< 0.25 in bivariate analysis
The lack of ability to predict adherence is further shown in Table 3, in which a simple score was calculated to identify high adherence based on the parameter estimates from the logistic regression model. No cutoff score distinguished high from low adherence with accuracy. For example, a cutoff value of 9 points or higher had a sensitivity of 57% and a specificity of 57% for identifying adherent individuals.
Table 3.
Sensitivity and Specificity of Weighted Scoring System for Predictors of ≥ 80% Refill Adherence in Denver Health
| Number of Points* (Range is 0-24) | Patients n (%) | ≥ 80% Adherent | Sensitivity | Specificity | c-statistic |
|---|---|---|---|---|---|
| Less than 6 | 684 (4.0%) | 22.1% | --- | --- | --- |
| ≥ 6 |
16492 (96.0%) |
36.9% |
0.98 |
0.05 |
0.512 |
| Less than 9 | 3534 (20.6%) | 24.2% | --- | --- | --- |
| ≥ 9 |
13642 (79.4%) |
39.4% |
0.86 |
0.24 |
0.554 |
| Less than 12 | 6086 (35.4%) | 27.6% | --- | --- | --- |
| ≥ 12 |
11090 (64.6%) |
41.1% |
0.73 |
0.40 |
0.567 |
| Less than 15 | 12984 (75.6%) | 32.3% | --- | --- | --- |
| ≥ 15 |
4192 (24.4%) |
48.6% |
0.33 |
0.80 |
0.565 |
| Less than 18 | 16115 (93.8%) | 35.0% | --- | --- | --- |
| ≥ 18 | 1061 (6.2%) | 55.7% | 0.09 | 0.96 | 0.526 |
To calculate a point score for each variable in the final predictive model at DH from Table 2, its regression coefficient was multiplied by 10. The point score for each patient was then calculated by summing the points for the number of predictors each patient had for high adherence. Points = (3 if age>55) + (5 if white race) + (1 if married/widowed) + (3 if acculturation is high) + (1 if diabetic) + (3 if have cerebrovascular disease) - (2 if have substance abuse) - (2 if have alcohol abuse) + (4 if number of antihypertensive medications <3) + 4.
The characteristics of the 94,297 eligible patients from the KP hypertension registry are compared to the DH cohort in Table 1. These groups showed substantial differences in socio-demographic characteristics, and lesser differences in clinical diagnoses. The median medication adherence in KP patients was 92.6% (interquartile range 77.5% to 99.7%). Of these individuals, 72.1% obtained more than 80% of their antihypertensive medications over a median of 3.2 years. In bivariate analyses, older age, white race, the presence of coronary artery disease, dyslipidemia, or a psychotic disorder and the absence of chronic kidney disease, depression, bipolar disorder, substance abuse, or alcohol abuse were associated with higher adherence (data not shown).
Multivariable logistic regression models to identify independent predictors of high adherence in the Kaiser Permanente registry are shown in Table 2. While age, race and gender remained statistically significant predictors of adherence, the c-statistic for the multivariate model was 0.555. Addition of significant clinical conditions improved the c-statistic to 0.575. The Hosmer-Lemeshow χ2 statistic for this model was 25.2 (8 df, p = 0.001). Adding the total number of diagnoses using the Quan index produced a model with a c-statistic of 0.568 (data not shown). When adherence was treated as a continuous dependent variable, a multivariable linear regression model including only socio-demographic variables had a R2 of 0.013. Even with statistically significant comorbid conditions, the model explained less than 2% of the variance in adherence (R2 = 0.015). An analysis limited to the 2010 individuals in KP with persistently elevated blood pressure showed similar findings. In a model including both socio-demographic variables and clinical conditions, only age> 55 years (OR = 1.79, 95% CI 1.45 to 2.23) and dyslipidemia (OR = 1.30, 95% CI 1.05 to 1.60) were associated with high adherence. The c-statistic for the model was 0.585.
Discussion
We found that individuals in both a large “safety-net” delivery system and a health maintenance organization who were younger, white, did not have a diagnosis of alcohol abuse, and received fewer antihypertensive drugs were more likely to be adherent with refills for antihypertensive medications than those without these characteristics. No single socio-demographic or clinical variable discriminated accurately between individuals with higher and lower refill adherence in either system, however. The c-indices for the best multivariable predictive models in both settings were 0.60 or less, suggesting that these models were unable to accurately discriminate adherent from non-adherent patients.
The predictors of high adherence in both models—white race, younger age, and the absence of alcohol abuse—were consistent with the findings of some recent research.2-6 On the basis of such associations, prior studies have often concluded that efforts to improve adherence should focus on specific sub-populations at risk of low adherence.4-6,9 Our findings suggest that such a conclusion is fundamentally mistaken, since it is based on a failure to recognize that risk factors identified at the population level may not translate into clinically useful predictors for individuals.27,28 The clinical utility of any predictor is dependent not only on its statistical significance, but also on the strength of the association. Odds ratios of 16 or greater may be necessary for an individual risk factor to discriminate between those who achieve the outcome of interest and those who do not, since only above that level are the sensitivity and specificity of the risk factor sufficiently high to substantially change the probability of the outcome.27,28 Odds ratios of this magnitude are uncommon in clinical research, and none of the odds ratios in our study or in most prior clinical or behavioral adherence research approach this threshold.
These findings have important implications. First, clinicians cannot accurately predict adherence based on a patient's appearance, background, or “problem list” of physical or mental health conditions. Prior reviews have made this assertion, although it has not previously been supported by information from quantitative clinical prediction rules.1,29-31 In particular, the mistaken assumption that adherence can be predicted on the basis of race or ethnicity may in fact worsen health care disparities if it leads clinicians to withhold effective treatment from minority patients. Such a concern is plausible, since studies have shown that many clinicians hold implicit attitudes that racial minorities or substance abusers are less likely to adhere to treatment.32-35
A striking finding of our study was the difference in refill adherence between individuals with hypertension in DH and KP. Not surprisingly, the two populations differed in many ways. While we assessed some of these differences (Table 1), other potentially important predictors of adherence, such as health beliefs, economic security, social support for adherence and the severity of coexisting clinical conditions, could not be measured from available data. Differences in adherence could also have been due to features of the two delivery systems, although both DH and KP are nationally recognized for the quality of care they provide.12,36 Nevertheless, unmeasured characteristics such as language concordance between patients and providers, access to clinic appointments or pharmacy services, and the presence or effectiveness of chronic disease management programs may in part explain these differences, although prior studies have generally found modest associations between these characteristics and adherence.3 The causes of differences in refill adherence between delivery systems constitute an important avenue for further research.
Strengths and Limitations
Our study has several strengths. Both studies took place in large delivery systems, using comprehensive, multi-year hypertension registries. Both systems provided low copayments as an incentive to obtain medications, improving the ascertainment of refills within the system. The characteristics associated with adherence were similar in the two systems and consistent with prior research, suggesting that our findings may be generalizable to other settings.
Several limitations must also be recognized. Neither delivery system may be representative of other practice settings. Because we used administrative data, we may have misclassified some individuals with respect to race, ethnicity or the presence of clinical conditions. In a prior DH study, we found that ICD9-CM based criteria compared favorably to medical record review for the comorbid conditions assessed in this study, however.13 We used pharmacy refill information to assess adherence, rather than measures of medication consumption. Thus, some individuals may have obtained medication that they did not take, while patients at DH with commercial insurance, Medicare or Medicaid may have obtained medications from other pharmacies. The use of pharmacy refill information also prevented us from identifying individuals whose level of adherence was so low that they obtained 0 or 1 fill of antihypertensive medication. On the other hand, obtaining medications is an important health behavior in its own right, and research in DH, KP, and elsewhere has shown that individuals who are adherent with refills have lower mortality rates, fewer hospitalizations, and are more likely to achieve condition-specific clinical outcomes.15,16,22
While we applied uniform exclusion criteria to both hypertension registries, the criteria for entry into these registries differed, as did the definitions of some variables. Further, missing data (such as race/ethnicity data at Kaiser Permanente) makes the predictive models less directly comparable, while additional characteristics, such as income and education, might improve prediction if available. Finally, we did not include treatment response as measured by blood pressure as part of our predictive model, although clinicians in practice often use blood pressure to identify potential adherence problems.1 This decision reflected our intent to develop a prediction rule using only the socio-demographic and diagnostic information that is commonly available in large delivery systems and insurance plans.
Our findings suggest that individuals with low adherence cannot be accurately identified using information that is routinely collected by health care delivery systems. The only clinical useful approach to assessing adherence is to assess it directly, through discussion with patients or through sources such as pharmacy records.29,37 Instead of using socio-demographic characteristics and concurrent medical conditions to predict the likelihood of adherence with antihypertensive medications, clinicians and population-based disease management programs should assess and reinforce adherence in all their patients.29
What is Known
Prior studies that have attempted to identify sociodemographic and clinical predictors of adherence with medications have produced conflicting findings with relatively weak associations.
The ability of a combination of these characteristics to predict adherence has not been assessed.
What this Article Adds
In both a delivery system for disadvantaged patients and a health maintenance organization, older age, white race, lack of alcohol abuse and use of fewer antihypertensive drugs were statistically significant predictors of higher adherence with antihypertensive drugs, although these associations were weak in magnitude.
Multivariable models based on these predictors were not able to accurately discriminate adherent from non-adherent individuals
These findings suggest that clinicians should not rely on sociodemographic or clinical characteristics of their patients in attempting to predict who will be adherent with their antihypertensive medications.
Acknowledgements
We thank Moises E. Maravi, M.Sc, of the Denver Public Health Department for his excellent programming in developing the hypertension registry.
Source of Funding
Funding was provided by the National Heart, Lung, and Blood Institute (NHLBI) grants 1U01 HL079208 and 1U01 HL079160. This study was also supported, in part, by the Cardiovascular Research Network, sponsored by NHLBI grant 1U19 HL91179-01.
Footnotes
Disclosures
The authors report no financial disclosures.
References
- 1.Haynes RB, McDonald HP, Garg AX. Helping patients follow prescribed treatment: clinical applications. JAMA. 2002;288:2880–2883. doi: 10.1001/jama.288.22.2880. [DOI] [PubMed] [Google Scholar]
- 2.Hyre AD, Krousel-Wood MA, Muntner P, Kawasaki L, DeSalvo KB. Prevalence and predictors of poor antihypertensive medication adherence in an urban health care setting. J Clin Hypertens. 2007;9:179–186. doi: 10.1111/j.1524-6175.2007.06372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mann DM, Allegrante JP, Natarajan S, Halm EA, Charlson M. Predictors of adherence to statins for primary prevention. Cardiovasc Drugs Ther. 2007;21:311–316. doi: 10.1007/s10557-007-6040-4. [DOI] [PubMed] [Google Scholar]
- 4.Bagchi AD, Esposito D, Kim M, Verdier J, Bencio D. Utilization of, and adherence to, drug therapy among Medicaid beneficiaries with congestive heart failure. Clin Ther. 2007;29:1771–1783. doi: 10.1016/j.clinthera.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 5.Hicks PL, Mulvey KP, Chander G, Fleishman JA, Josephs JS, Korthuis PT, Hellinger J, Gaist P, Gebo KA. The impact of illicit drug use and substance abuse treatment on adherence to HAART. AIDS Care. 2007;19:1134–1140. doi: 10.1080/09540120701351888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sajatovic M, Valenstein M, Blow F, Ganoczy D, Ignacio R. Treatment adherence with lithium and anticonvulsant medications among patients with bipolar disorder. Psychiatr Serv. 2007;58:855–863. doi: 10.1176/ps.2007.58.6.855. [DOI] [PubMed] [Google Scholar]
- 7.Akincigil A, Bowblis JR, Levin C, Jan S, Patel M, Crystal S. Long-term adherence to evidence based secondary prevention therapies after acute myocardial infarction. J Gen Intern Med. 2008;23:115–121. doi: 10.1007/s11606-007-0351-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams LK, Joseph CL, Peterson EL, Wells K, Wang M, Chowdhry VK, Walsh M, Campbell J, Rand CS, Apter AJ, Lanfear DE, Tunceli K, Pladevall M. Patients with asthma who do not fill their inhaled corticosteroids: a study of primary nonadherence. J Allergy Clin Immunol. 2007;120:1153–1159. doi: 10.1016/j.jaci.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 9.Jackevicius CA, Li P, Tu JV. Prevalence, predictors and outcomes of primary nonadherence after acute myocardial infarction. Circulation. 2008;117:1028–1036. doi: 10.1161/CIRCULATIONAHA.107.706820. [DOI] [PubMed] [Google Scholar]
- 10.Laupacis A, Sekar N, Stiell IG. Clinical prediction rules: a review and suggested modifications of methodological standards. JAMA. 1997;277:488–494. [PubMed] [Google Scholar]
- 11.Gabow P, Eisert S, Wright R. Denver Health: a model for the integration of a public hospital and community health centers. Ann Intern Med. 2003;138:143–149. doi: 10.7326/0003-4819-138-2-200301210-00016. [DOI] [PubMed] [Google Scholar]
- 12.Nuzum R, McCarthy D, Gauthier A, Beck C. Denver Health: a high-performance public health care system. The Commonwealth Fund. 2007 July; [Google Scholar]
- 13.Hanratty R, Estacio RO, Dickinson LM, Chandramouli V, Steiner JF, Havranek EP. Testing electronic algorithms to create disease registries in a safety net system. J Health Care Poor Underserved. 2008;19:452–465. doi: 10.1353/hpu.0.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choo PW, Rand CS, Inui TS, Lee MT, Cain E, Cordeiro-Breault M, Canning C, Platt R. Validation of patient reports, automated pharmacy records, and pill counts with electronic monitoring of adherence to antihypertensive therapy. Med Care. 1999;37:846–857. doi: 10.1097/00005650-199909000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Steiner JF, Prochazka AV. The assessment of refill compliance using pharmacy records: methods, validity, and applications. J Clin Epidemiol. 1997;50:105–116. doi: 10.1016/s0895-4356(96)00268-5. [DOI] [PubMed] [Google Scholar]
- 16.Batal HA, Krantz MJ, Dale RA, Mehler PA, Steiner JF. Impact of prescription size on statin adherence and cholesterol levels. BMC Health Services Research. 2007;7:175. doi: 10.1186/1472-6963-7-175. doi:10.1186/1472-6963-7-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hess LM, Raebel MA, Conner DA, Malone DC. Measurement of adherence in pharmacy administrative databases: a proposal for standard definitions and preferred measures. Ann Pharmacother. 2006;40:1280–1288. doi: 10.1345/aph.1H018. [DOI] [PubMed] [Google Scholar]
- 18.National Kidney Foundation K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl 1):S1–266. [PubMed] [Google Scholar]
- 19.Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi J, Saunders LD, Beck CA, Feasby TE, Ghali WA. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 20.Shetterly SM, Tavel H, Ho PM, Crounse L, Maddox TM, Havranek EP, Magid DJ. Assessing potential inclusion criteria for a hypertension disease registry using administrative clinical databases. Circulation. 2008;117:e464. [Abstract] [Google Scholar]
- 21.Selby JV, Smith DH, Johnson ES, Raebel MA, Friedman GD, McFarland BH. Kaiser Permanente Medical Care Program. In: Strom BL, editor. Pharmacoepidemiology. 4th ed. John Wiley & Sons; New York, NY: 2005. pp. 241–259. [Google Scholar]
- 22.Ho PM, Rumsfeld JS, Masoudi FA, McClure DL, Plomondon ME, Steiner JF, Magid DJ. Effect of medication nonadherence on hospitalization and mortality among patients with diabetes mellitus. Arch Intern Med. 2006;166:1836–1841. doi: 10.1001/archinte.166.17.1836. [DOI] [PubMed] [Google Scholar]
- 23.Hosmer DW, Lemeshow S. Applied Logistic Regression. John Wiley & Sons; New York, NY: 1989. p. 86. [Google Scholar]
- 24.Lemeshow S, Le Gall J. Modeling the severity of illness of ICU patients: a systems update. JAMA. 1994;272:1049–1055. [PubMed] [Google Scholar]
- 25.Cook NR. Use and misuse of the receiver operating characteristic curve in risk prediction. Circulation. 2007;115:92–935. doi: 10.1161/CIRCULATIONAHA.106.672402. [DOI] [PubMed] [Google Scholar]
- 26.Moons KGM, Harrell FE, Steyerberg EW. Should scoring rules be based on odds ratios or regression coefficients? J Clin Epidemiol. 2002;55:1054–1055. doi: 10.1016/s0895-4356(02)00453-5. [DOI] [PubMed] [Google Scholar]
- 27.Pepe MS, Janes H, Longton G, Leisenring W, Newcomb P. Limitations of the odds ratio in gauging the performance of a diagnostic, prognostic, or screening marker. Am J Epidemiol. 2004;159:882–890. doi: 10.1093/aje/kwh101. [DOI] [PubMed] [Google Scholar]
- 28.Ware JH. The limitations of risk factors as prognostic tools. New Engl J Med. 2006;355:2615–2617. doi: 10.1056/NEJMp068249. [DOI] [PubMed] [Google Scholar]
- 29.Stephenson BJ, Rowe BH, Haynes RB, Macharia WM, Leon G. Is this patient taking the treatment as prescribed? JAMA. 1993;269:2779–2781. [PubMed] [Google Scholar]
- 30.Vik SA, Maxwell CJ, Hogan DB. Measurement, correlates, and health outcomes of medication adherence among seniors. Ann Pharmacother. 2004;38:303–312. doi: 10.1345/aph.1D252. [DOI] [PubMed] [Google Scholar]
- 31.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 32.Bogart LM, Catz SL, Kelly JA, Benotsch EG. Factors influencing physicians’ judgments of adherence and treatment decisions for patients with HIV disease. Med Decis Making. 2001;21:28–36. doi: 10.1177/0272989X0102100104. [DOI] [PubMed] [Google Scholar]
- 33.Sabin JA, Rivara FP, Greenwald AG. Physician implicit attitudes and stereotypes about race and quality of medical care. Med Care. 2008;46:678–685. doi: 10.1097/MLR.0b013e3181653d58. [DOI] [PubMed] [Google Scholar]
- 34.van Ryn M, Burke J. The effect of patient race and socio-economic status on physicians’perceptions of patients. Soc Sci Med. 2000;50:813–828. doi: 10.1016/s0277-9536(99)00338-x. [DOI] [PubMed] [Google Scholar]
- 35.Lutfey KE, Ketcham JD. Patient and provider assessments of adherence and the source of disparities: evidence from diabetes care. Health Serv Research. 2005;40:1803–1817. doi: 10.1111/j.1475-6773.2005.00433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berwick DM, Nolan TW, Whittington J. The triple aim: care, health and cost. Health Aff. 2008;23:759–769. doi: 10.1377/hlthaff.27.3.759. [DOI] [PubMed] [Google Scholar]
- 37.Steiner JF, Earnest MA. The language of medication-taking. Ann Intern Med. 2000;132:926–930. doi: 10.7326/0003-4819-132-11-200006060-00026. [DOI] [PubMed] [Google Scholar]