Abstract
Light sensitive rhabdoms in the octopus retina increase in cross-sectional area in the dark and shrink in the light. Growth in the dark is due to the formation of microvilli in an avillar region of the photoreceptor cell membrane and lengthening of rhabdomere microvilli already present. Diminution in the light is the result of the disassembly and shortening of the same microvilli. Each microvillus contains an actin filament core that must be assembled or disassembled in the dark or light, respectively. To understand the regulation of the construction and breakdown of rhabdomere microvilli in the light and dark, we used centrifugation to separate the rhabdom membranes followed by Western blotting and Rho pull-down assays to investigate the role of Rho GTPases in this process. Western blotting showed a difference in the distribution of Rho in rhabdom membrane and supernatant fractions. In the light, Rho was mostly present in the supernatant but in the dark it was found in the fraction enriched with rhabdom membranes. Complementing these results, pull-down assays showed that Rho is activated in the dark but in the light, Rho is mostly inactive. We believe that in the dark, activated Rho binds to the rhabdom membrane and initiates signaling pathways, leading to growth of rhabdomere microvilli. In the light, Rho is present in the soluble fraction, is inactivated, and is likely bound to a Rho GDI. Receptors involved in the activation of Rho in the dark are undetermined and may involve rhodopsin or another membrane protein.
Keywords: cytoskeleton, rhabdoms, Rho pull-down assay
The cytoskeleton, consisting of microtubules, microfilaments, and intermediate filaments, is a three-dimensional infrastructure within cells responsible for a myriad of functions, including cell movement, cytokinesis, and organization of the cytoplasm. At any particular point in the cell cycle, the cytoskeleton has a unique architecture, which can undergo rapid reorganization in response to environmental or internal signals. Microtubules and microfilaments, composed of tubulin and actin subunits, respectively, generally reorganize by the addition or subtraction of their respective protein subunits, leading to lengthening or shortening of the tubules or filaments and consequent changes in cell shape or movement (Maekawa et al. 1999). Intricate signaling cascades activated in response to environmental or internal cues trigger these changes in cytoskeletal organization and are regulated by the Ras superfamily of small GTPases, including the Rho family GTPases (Ridley 2001, Raftopoulou and Hall 2004).
The Rho family GTPases, which currently includes 22 members such as the well known Rho, Rac, and Cdc42, shuttle between an active GTP-bound state and an inactive GDP-bound state (Takai et al. 1995, 2001, Hall 1998, 2005, Ridley 2001, 2006, Wheeler and Ridley 2004). In the activated state, Rho GTPases interact with specific downstream kinases that affect the state of actin polymerization. Rho and Rac indirectly affect actin polymerization by targeting ROCK (Rho-associated serine-threonine protein kinases) or Pak 1 (p21-activated kinase) that in turn activate LIM kinase 1 or 2 (LIM motif containing kinase). LIM kinases phosphorylate and inactivate actin binding/filament severing proteins, such as cofilin, which leads to an increase in actin polymerization (Naruyima et al. 1997, Arber et al. 1998, Maekawa et al. 1999, Sumi et al. 1999, Ohashi et al. 2000, Ridley 2006).
Photoreceptors of vertebrate and invertebrate retinas contain cytoskeletons that reorganize in the light and dark and may be regulated by the Rho family of GTPases (Miller et al. 2005). This reorganization is necessary to achieve maximum absorption of light by the photoreceptors by either increasing the membrane area containing the photopigment rhodopsin or a mechanical re-orientation of the photoreceptors so the light sensitive area sits more advantageously in light path, such as occurs in teleosts (Ali 1975). In either case, the cytoskeleton is involved in membrane growth or re-orienting the cell but the mechanisms leading to these changes are not well understood in any photoreceptor.
The octopus retina can serve as a model system to dissect these mechanisms since the photoreceptors of octopus species are large and only two cell types are present in the retina, photoreceptors and supportive cells, facilitating microscopic and biochemical studies. In Octopus bimaculoides (Pickford and McConnaughey, 1949), distinct changes in photoreceptor shape occur in the light and dark and may be directly attributable to changes in the organization of the cytoskeleton. Torres et al. (1997) compared the relative cross-sectional area of light- and dark-adapted rhabdoms, the light sensitive region of the cell, and outer segment core cytoplasm and found that the rhabdoms of light-adapted photoreceptors are reduced in cross-sectional area when compared to those maintained in the dark. Electron microscopy showed that the rhabdomeric microvilli in light-adapted rhabdoms partially disappeared, leaving behind an avillar membrane connecting the microvilli on opposite sides of the rhabdoms. In the dark, this avillar membrane was replaced with additional microvilli making the rhabdoms larger.
The biochemical mechanisms leading to increased microvillar formation in the dark and diminution in the light are not well understood. Miller et al. (2005) studied the presence of Rho GTPases in octopus retinas. Immunoblot analyses of whole retinal extracts confirmed the presence of Rho in light- and dark-adapted retinas and confocal microscopy localized Rho in the rhabdoms and showed its co-localization with actin.
The presence of Rho in the rhabdom suggests that it is involved in a signaling transduction pathway leading to rhabdom changes in the light and dark. If Rho were activated in the dark, it could indirectly inactivate the filament severing protein cofilin leading to rhabdom growth. To test this hypothesis we have performed detailed immunoblot analyses on isolated rhabdom compartments and Rho-GTP pull-down assays on light- and dark-adapted octopus retina tissue. Our work will lead to a better understanding of mechanisms leading to retinal changes in the dark and light in octopus species which affect their ability to absorb light. Furthermore, understanding cytoskeletal dynamics are important for all species and may lead to understanding specific types of retinal degeneration in humans attributed to cytoskeletal protein mutations such as Usher syndrome type 1B (Weil et al. 1995).
MATERIALS AND METHODS
Eye structure
Eye tissue was prepared for microscopic observations according to previously published methods and immunostained with antibodies to rhodopsin (Robles et al. 1995). Micrographs were made using an Olympus Vanox or BX461 fluorescence microscope.
Rho immunoblots
Adult specimens of Octopus bimaculoides were dark- or light-adapted for 2–3 hours. Afterwards they were anesthetized on ice, whole eye cups removed, and the sclera and lenses discarded. To isolate the rhabdom compartment from the remaining retinal tissue, we used previously described centrifugation procedures (Robles et al. 1984). These centrifugation experiments were repeated five times using a total of 20 octopuses per lighting condition. Results were consistent throughout each experiment.
Equal amounts of total protein supernatant from the first centrifugation, crude membrane pellet, and final pellet were diluted 1:1 with Laemmli reducing buffer (Bio-Rad Laboratories, Inc., Hercules, California), boiled for 5 minutes, and electrophoresed on 12% sodium dodecylsulfate polyacrylamide gels (SDS-PAGE) at 100V for 2–3 hours (Laemmli 1970). The proteins were blotted onto polyvinyl-difluoride (PVDF) or nitrocellulose membranes and incubated overnight in 2.5% blocking solution (non-fat dry milk and gelatin in phosphate buffer saline [137 mM NaCl, 2.7 mM KCl, 4.3 mM Na2HPO4, 1.4 mM KH2PO4 0.1% Tween-20] Bio-Rad). The membranes were incubated overnight with polyclonal rabbit anti-Rho (-A-B-C) (1:1000 and 1:500, Upstate Cell Signaling Solutions) either overnight at 4 °C or for one hour at room temperature, followed by incubation with AP-GAR secondary antibodies (1:3000, Bio-Rad) for two hours at room temperature. All antibodies were diluted in PBST-BSA (Bio-Rad). An Opti-4CN Substrate kit (Bio-Rad) was used for colorimetric band detection, and the Bio-Rad VersaDoc™ 3000 imaging system was used for molecular weight analyses. Quantitative analysis of Rho concentrations in dark- and light-adapted octopus retinal fractions was obtained via densitometric analysis using the Quantity One 1–D Analysis software on the VersaDoc™.
Rho activation
At the end of light- or dark-adaptation, the eye cups from one octopus in each lighting condition were dissected to use as controls. The remaining octopuses were moved to the opposite lighting condition and sacrificed at 5, 15, 30, 45, and 60 minute time points. The preparation of retinal lysates from the controls and the time points was carried out using a dry ice-ethanol bath to snap freeze the tissue. Whole eye cups were removed and placed into a Petri dish in the dry ice-ethanol bath. Retinal tissue was liberated from the eye cups and added to 0.5 mL of cell lysis buffer (50 mM Tris-HCL pH 7.4; 1% NP-40; 0.25% sodium deoxycholate; 150 mM NaCl; 1 mM EDTA; 1 mM PMSF; 1 µg/mL each Aproptinin, Leupeptin, Pepstatin; 1 mM Na3VO4; 1 mM NaF) and homogenized. Samples were clarified using centrifugation for 5 minutes, 4 °C at 8,000 rpm and Rho activation assayed using the Rho Activation Biochem Kit (Cytoskeleton, Inc.). Supernatants were collected and pellets were discarded. Positive and negative controls were processed by adding a 1:10 volume of loading buffer. A nonhydrolyzable form of GTP (GTPγS) was added to the mixture in a 1:100 volume to a final concentration of 200 µM. This positive control sample was incubated for 15 minutes at 30 °C. The reaction was stopped by transferring the tube to 4 °C and adding a 1:10 volume of stop buffer. The same processing was carried out for the negative control, substituting GDP for GTPγS.
The supernatants and controls were added to 60 µl aliquots of GST tagged Rhotekin-RBD beads. Each reaction tube was incubated for one hour at 4 °C with gentle agitation. Next, the supernatant was removed and the pellet was rinsed with 500 µL 1X lysis buffer. The beads were pelleted at 5,000 g for 3 minutes at 4 °C. The supernatant was removed again and the pellet washed in 500 µL 1X wash buffer. Again, the mixture was centrifuged at 5,000 g for 3 minutes at 4 °C. The pellet was resuspended in a 3:1 ratio with Laemmli reducing buffer, boiled 5 minutes, and subjected to 15% SDS-PAGE at 120V for 2 hours.
The proteins were blotted onto nitrocellulose membranes and incubated for 45 minutes to one hour in Super-Block + 0.05% Tween (Pierce) with gentle agitation. The membranes were incubated for one hour at room temperature or overnight at 4 °C with RhoA monoclonal antibody (1:500, Cytoskeleton) and for one hour at room temperature with HRP-GAM secondary antibody (1:20,000–1:50.000, Pierce ImmunoPure Peroxidase Conjugated GAM IgG(H+L)) at room temperature. All antibodies were diluted in TBS-Tween. The Pierce SuperSignal West Pico Chemiluminescent Substrate Kit was used for chemiluminescent detection. All blots were developed and visualized with the Bio-Rad VersaDoc™ imaging system. Quantitative analysis of the RhoA activation blots was performed using the Quantity One 1–D Analysis software previously described.
RESULTS
Eye structure
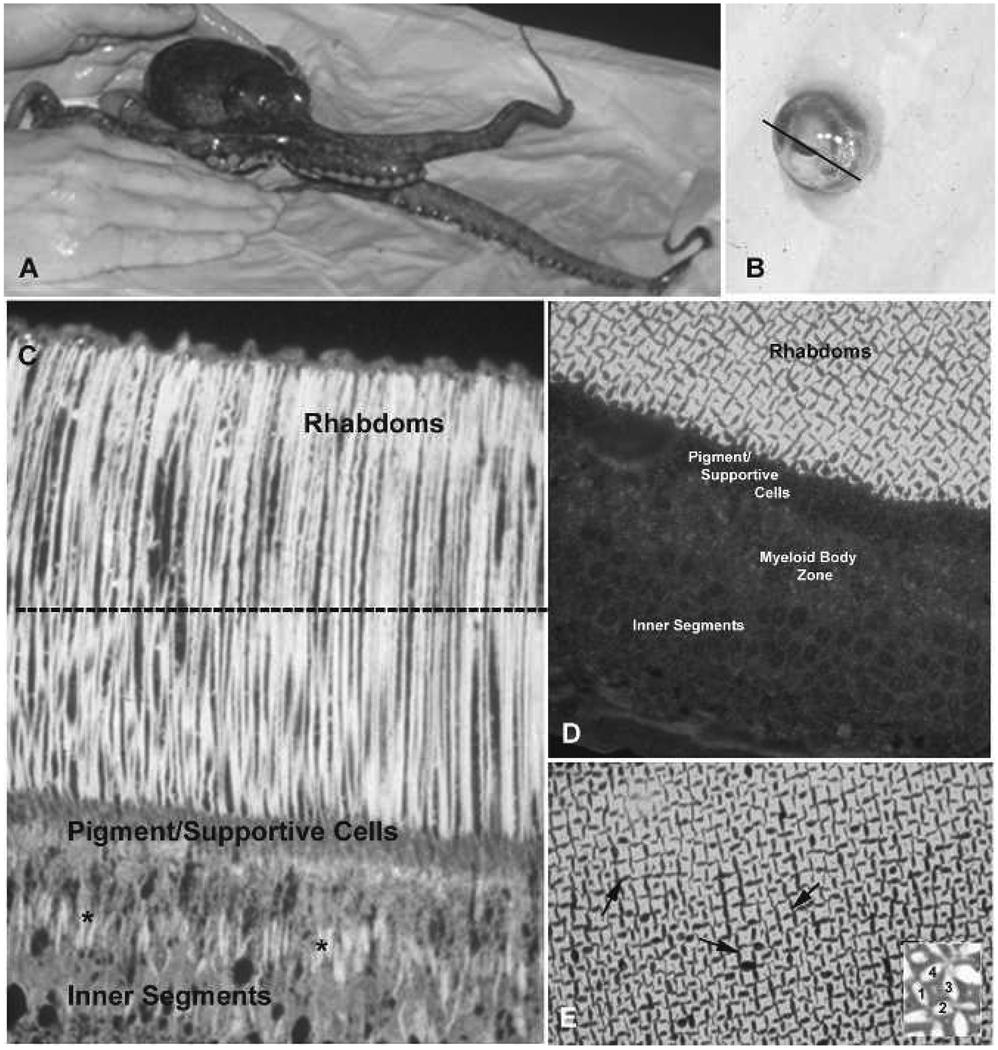
The octopus eye is similar in its external appearance to other camera-like eyes. The slit-shaped iris permits light to pass through the spherical lens and focus on the retina at the back of the eye (Figs. 1A–E). The light sensitive retina of Octopus bimaculoides, as well as those of other octopus species, consists of a layer of photoreceptors and supportive cells (Fig. 1C). Optic nerves exit the photoreceptors at their base, form bundles, and extend to the optic ganglia where they synapse on ganglion cells. The photoreceptors span the entire retina and are compartmentalized into the inner segments, a middle region which passes through the pigment/supportive cell layer, and the rhabdoms. The inner segments contain the biosynthetic machinery of the photoreceptor as well as organelles called myeloid bodies which store a second photopigment retinochrome. The photoreceptors narrow above the myeloid body region and pass between the supportive cells giving rise to the outer segments and rhabdoms. The outer segments consist of a cytoplasmic core and two sets of microvilli on opposite sides of the core which run from the base of the outer segments to their tips. These microvilli are called rhabdomeres, and rhabdomeres from four adjacent cells point toward each other to form the rhabdoms (Figs. 1D–E, see inset on 1E). The membrane of each rhabdomere microvillus contains rhodopsin and signal transduction proteins necessary to process the visual signal after light absorption by rhodopsin as well as a core of actin filaments and accessory proteins (Robles et al. 1995, De Velasco et al. 1999). As mentioned, the rhabdoms increase in size in the dark, by the addition and lengthening of the rhabdomere microvilli, and decrease in the light by the disassembly and shortening of the same microvilli (Torres et al. 1997).
Figure 1.
Retinal structure in Octopus bimaculoides. A, O. bimaculoides. B, Dissected eye from O. bimaculoides. Line through the iris marks the plane of sectioning, after removal of the iris and lens, to obtain retinal image shown in C. C, Longitudinal section through retina showing photoreceptor structure. The dotted line indicates plane of sectioning to obtain tangential to cross-sections through rhabdoms shown in D–E. D, Tangential section through retina to show details of rhabdom structure. E, Arrows highlight individual rhabdoms. In the inset, 1–4 denote the cytoplasm of four individual cells. Each side of the cells contributes one rhabdomere, composed of microvilli, which form the rhabdom.
Rho localization
Rhabdoms are easily separated from the rest of the retina using centrifugation techniques (see Materials and Methods). The rhabdom membranes can be separated from the outer segment cytoplasm using sucrose solutions and higher speed centrifugation. We obtained rhabdom membrane and supernatant (cytoplasm) fractions from light- and dark-adapted retinal homogenates of Octopus bimaculoides and performed immunoblot analyses on the rhabdom membrane enriched and supernatant fractions. We identified Rho GTPase in rhabdom membrane fractions and supernatants of light- and dark-adapted animals.
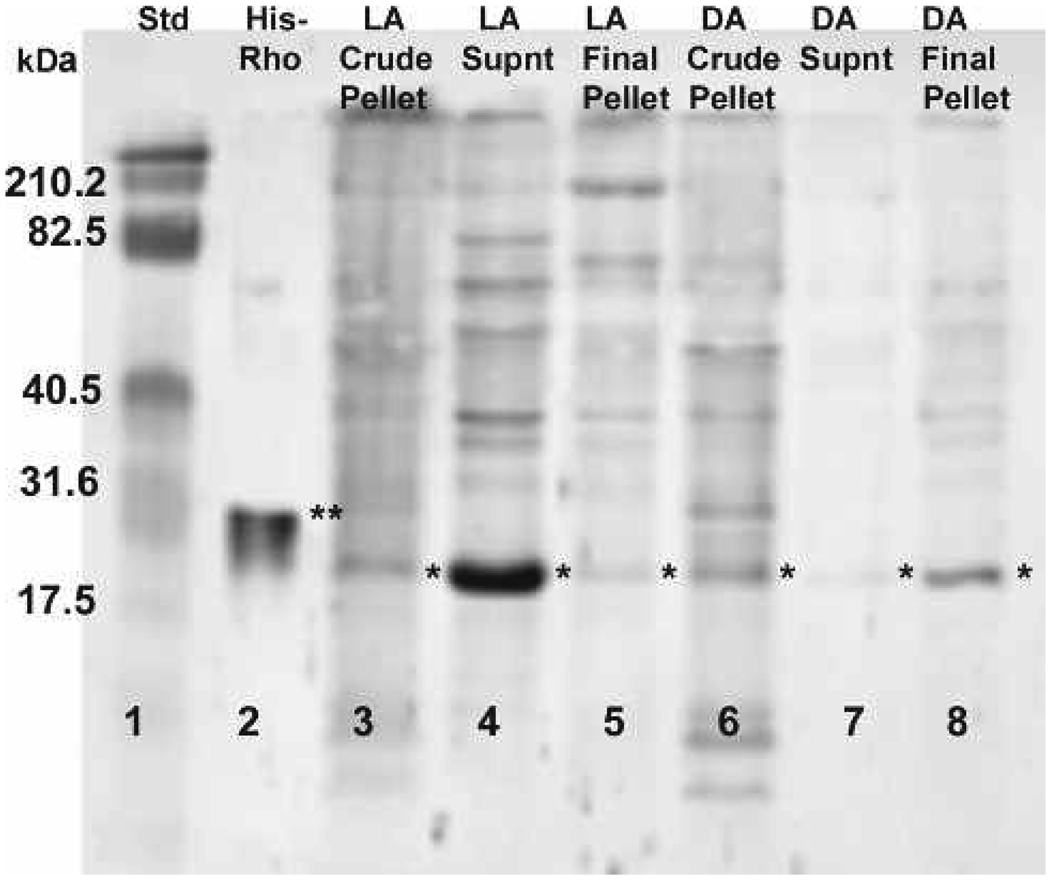
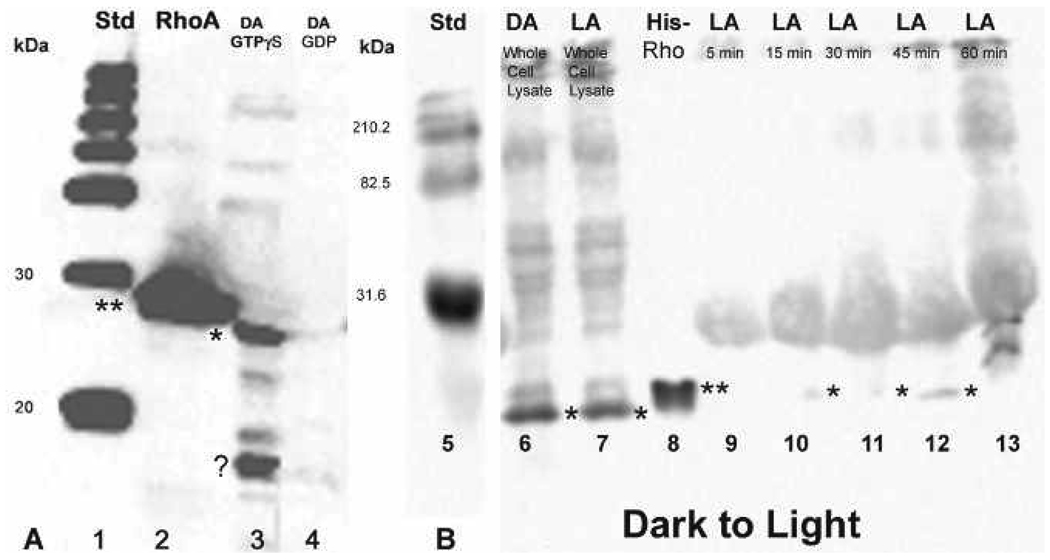
In homogenates obtained from light-adapted animals, faint Rho bands were visible in the crude and final enriched rhabdom membrane pellets, but a strong signal was detected in the supernatant after incubation with polyclonal anti-Rho-A-B-C (Fig. 2, lanes 3–5). In dark-adapted animals, bands are present in the crude and final rhabdom membrane pellet, but only a faint band is visible in the supernatant (Fig. 2, lanes 6–8). Control Rho (Fig. 2, lane 2, double asterisk) is His-tagged (Cytoskeleton, Inc.) and runs at 25 kDa compared to the endogenous Rho in our samples with a molecular weight of approximately 21 kDa. These results were consistent throughout repeated experiments. Background bands are likely due to non-specific binding and insufficient blocking of membrane before overnight incubation with anti-Rho to intensify the bands.
Figure 2.
Western blot localizing Rho in purified light- and dark-adapted rhabdom membrane and supernatant fractions. Rho (⋆) is present in dark-adapted (DA) rhabdom membrane fractions (lanes 6 and 8) but not in the supernatant (lane 7). In the light-adapted (LA) supernatant fraction (lane 4), Rho is present while there is little or no detection of Rho in the LA rhabdom membrane fractions (lanes 3 and 5). The double asterisk denotes the His-tagged RhoA control protein (lane 2). The molecular weight sizes correspond to the fragments of the Kaleidoscope pre-stained standard (lane 1).
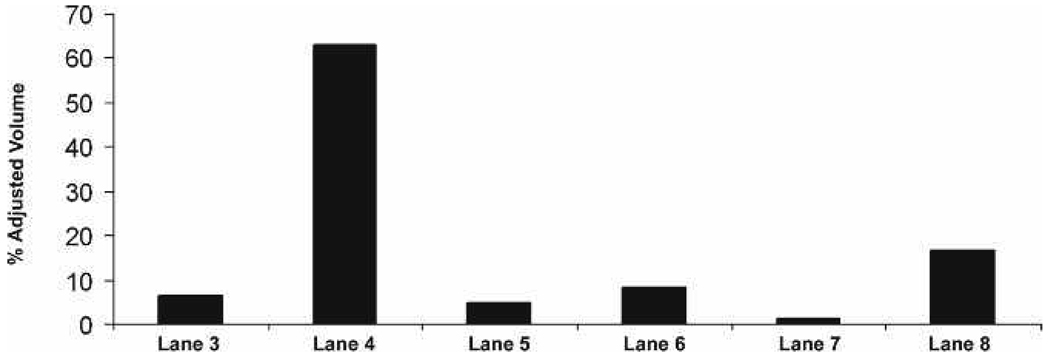
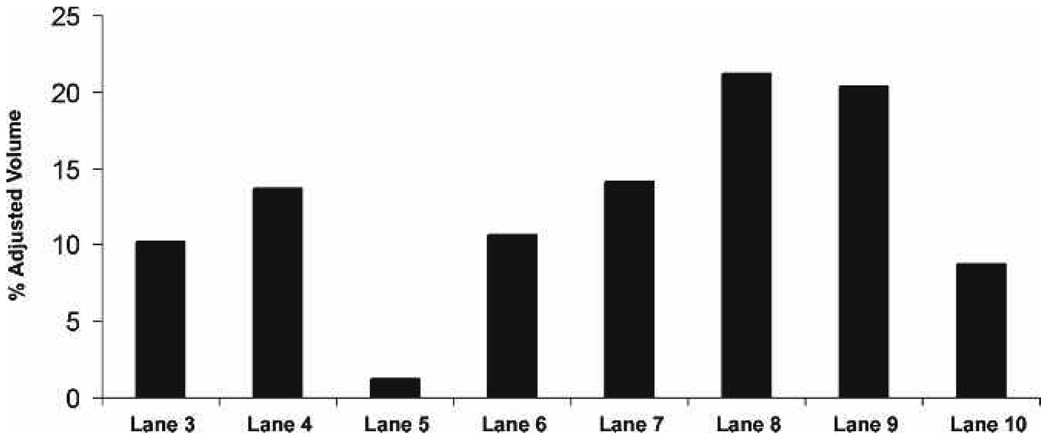
Quantitative analysis of Rho concentrations (adjusted percent volumes) on the blots reveal that Rho is approx. 10-fold more abundant in the light-adapted supernatant fractions (Fig. 3, lane 4) than in rhabdom membrane fractions (Fig. 3, lanes 3, 5) of light-adapted retinas. Rho appears most abundant in the rhabdom membrane fractions (Fig. 3, lanes 6, 8) than in the supernatant fractions (Fig. 3, lane 7) of dark-adapted retinas.
Figure 3.
Quantitative analysis of membrane and supernatant fractions verifies the presence of Rho in the LA supernatant and DA membrane fraction. Lanes correspond to lanes in Fig. 2.
Rho activation
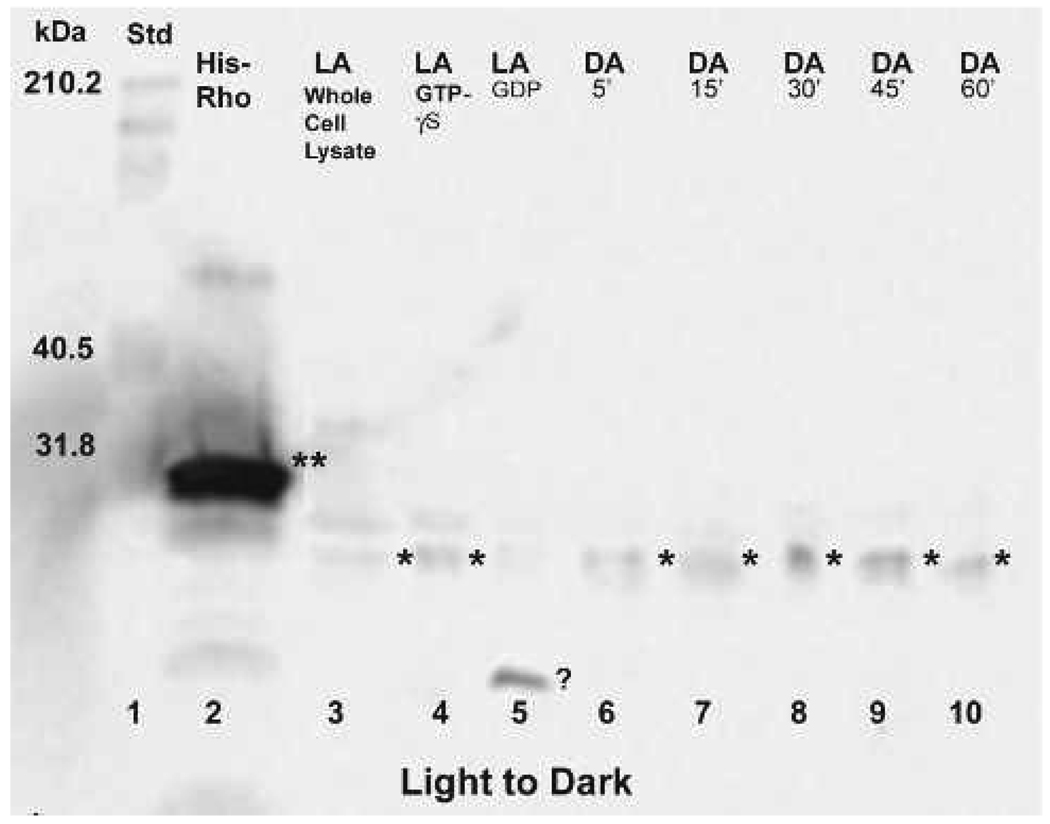
Rho pull-down assays were performed (Cytoskeleton, Inc.) to determine if Rho was activated in the light, dark, or in both lighting conditions and when the activation reaches its peak. After light- or dark-adaptation, octopuses were moved to the opposing lighting condition and sacrificed at 5, 15, 30, 45, and 60-minute time points. Pull-down assays performed at each time point confirmed the presence or absence of endogenous Rho-GTP with a molecular weight of approximately 20–21 kDa in dark to light or light to dark animals (Fig. 4, Fig 6A–B). In addition to the His-tagged RhoA protein control (Cytoskeleton, Inc.) having a molecular weight of approx. 25–27 kDa, positive and negative controls included GTPγS and GDP loaded samples, respectively. Light- and dark-adapted GTPγS samples revealed the presence of activated RhoA while GDP-loaded samples showed little or no activated RhoA (Fig. 4, lanes 4, 5; Fig. 6A, lanes 3, 4). We also included whole cell lysates from light-adapted or dark-adapted animals to compare the activated RhoA signal with that of the total native RhoA (active and inactive) signal (Fig. 4, lane 3; Fig. 6, lanes 6, 7).
Figure 4.
Rho pull-down assay showing Rho activation in light-adapted retinas that were moved to the dark and sampled at 5, 15, 30, 45, and 60-minute time points after the shift. Activated Rho, migrating between 20–22 kDa (⋆) is visible at all time points, most notably and strongly at 30 and 45 minutes. For comparison, Rho can be seen in the whole cell lysate (lane 3). There is little or no activation in the light-adapted GDP control (lane 5), but there is a strong band below 20 kDa (question mark) whose identity has not been determined. His-tagged RhoA control protein (⋆⋆) migrating between 25–27 kDa is in lane 2.
Figure 6.
A, Western blot showing controls for the Rho pull-down assay for octopuses dark-adapted and then moved to the light (lanes 1–4). There is no activation in the dark-adapted GDP control (lane 4), but there is a strong band in the GTPγS control (lane 3, ⋆⋆). The strong band migrating below 20 kDa, is also present (⋆). Lane 2 contains the His-tagged RhoA control protein and standards are in lane 1. B, Western blot after Rho pull-down assay showing the detection of weak, residual Rho activation in dark-adapted retinas moved to the light and sampled at 5, 15, 30, 45, and 60 minutes after the shift. The detection of activated Rho (⋆) is visible at 15, 30, and 45 minutes after the shift to the light (lanes 10–12). Rho in whole cell lysates (activated and inactivated) is shown in lanes 6 and 7 and standards in lanes 1 and 5.
Light to dark
In animals that were light-adapted and then moved to the dark, Western blot analysis of pull-down products with anti-RhoA confirmed the presence of Rho-GTP at the 5, 15, 30, 45, and 60-minute time points, with peak activation at 30 and 45 minutes (Fig. 4, lanes 6–10). Rho is also present in the whole cell lysates and GTPγS control as expected (Fig. 4, lanes 3, 4). The GDP control (Fig. 4, lane 5) was negative except for a band at a very low molecular weight, possibly resulting from proteolysis of which the identity has not yet been determined.
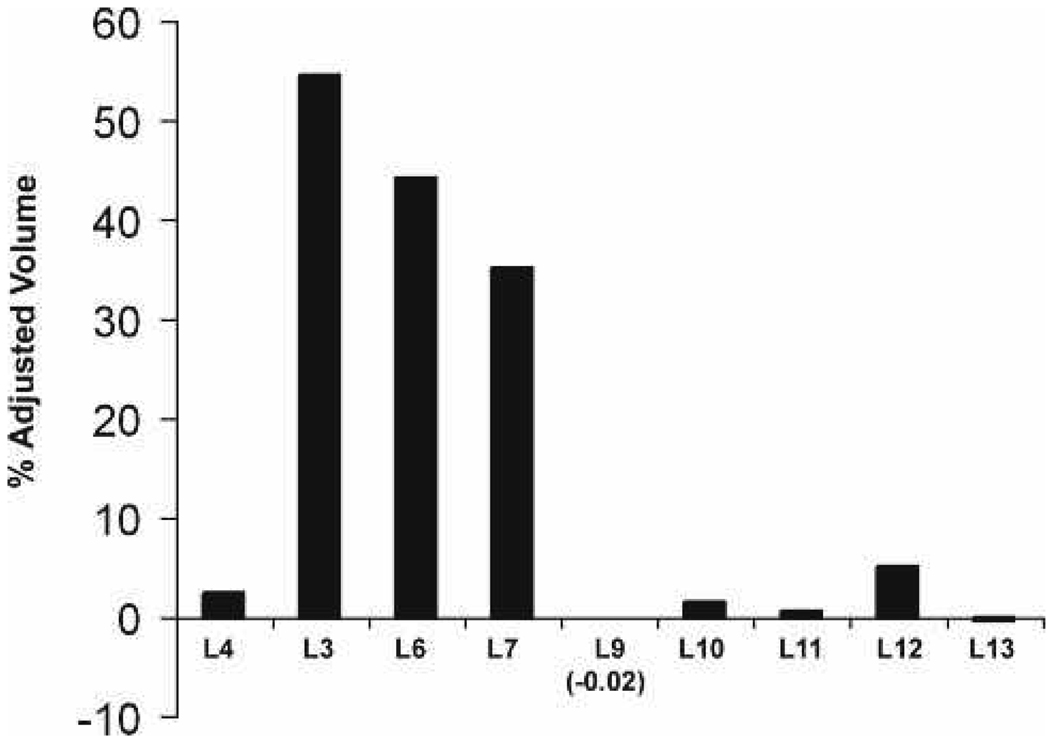
Quantitative analysis of the light to dark blot revealed that the increase in activated RhoA is approx. 2-fold greater at 30 and 45 minutes after light-adapted retinas are moved to the dark (Fig. 5, lanes 6–10).
Figure 5.
Quantitative analysis of the blot shown in Fig. 4 verifies the strong presence of activated Rho with the highest signal at 30 and 45 minutes.
Dark to light
In animals that were dark-adapted and then moved to the light, Rho-GTP was detected faintly at 15 and 30 minutes but was more prominent after 45 minutes in the light (Fig. 6, lanes 9–13). Quantitative analysis of the dark to light blot revealed that RhoA is activated at much lower levels after 15, 30, and 45 minutes of light exposure (Fig. 7, lanes 9–13) when compared to retinas that were light-adapted and moved to the dark (Fig. 4, Fig 5).
Figure 7.
Quantitative analysis of blot in B verifies the weak, residual Rho in dark-adapted retinas moved to the light.
The increased background and non-specific binding seen (Fig. 4) are attributed to overnight incubation with primary antibody at 4 °C to increase the Rho signal on the blots. Also, there is an increase in the number of bands above the targeted Rho protein band in Fig. 6B: the globular bands above 24–25 kDa are because of dimerization of the Rho protein with the Rhotekin beads used in the pull-down assay.
DISCUSSION
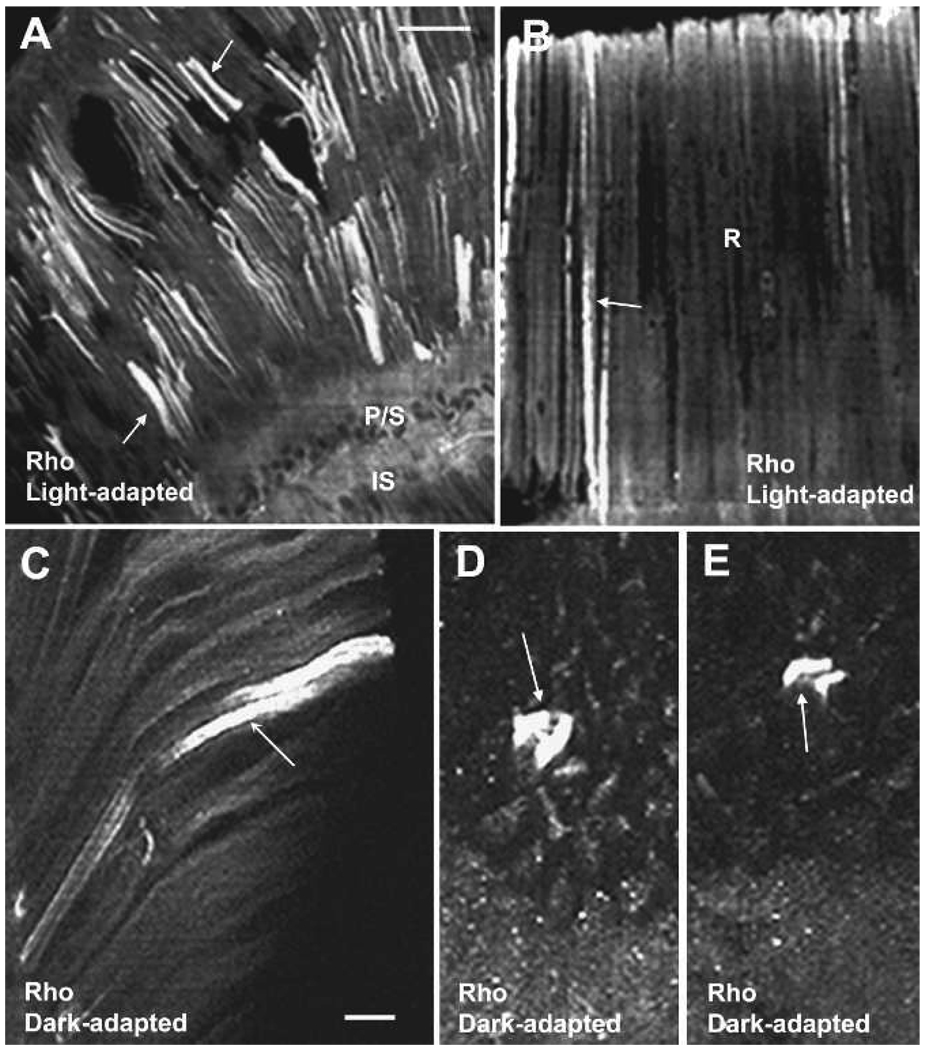
We previously reported the immunocytochemical localization of Rho in the rhabdom compartment of photoreceptors from Octopus bimaculoides (Miller et al. 2005). Rho, as well as actin, were present along the length of the rhabdomere and suggested that the Rho GTPases were candidates for the regulation of rhabdomere growth and diminution in the dark and light, respectively. Rho is present in the rhabdoms of light- and dark-adapted O. bimaculoides (Fig. 8 A–E). Using biochemical methods to localize Rho in the membrane fraction and pull-down assays to demonstrate Rho activation after light-dark-adaptation, the results reported here expand our earlier studies showing the presence of Rho in specific regions of the rhabdom and its activation in the dark or light.
Figure 8.
Rho localization of the retina of Octopus bimaculoides. A–C, Rho is present in the rhabdoms (arrows) of light- and dark-adapted photoreceptors. D–E, Cross-section through rhabdoms showing that Rho robustly labels some rhabdoms and not others. Scale bar = 100 µm A–B, 25 µm C–E. Reprinted with permission from Miller et al. (2005) Visual Neuroscience 22: 295–304.
The centrifugation techniques we used separate the rhabdom membranes from other retinal components and then further divide the compartment into membrane and soluble fractions (Robles et al. 1984). In tissue obtained from light-adapted animals, Western blotting showed that Rho was enriched in the supernatant and very little was found associated with the membranes. In the dark, Rho was mostly associated with the membrane fraction and little was found in the soluble fraction (Fig. 2–Fig 3).
Pull-down assays are used to confirm the presence or absence of a specific protein and are similar to an affinity-binding column. Specifically, Rho pull-down assays are designed to show the presence or absence of GTP-bound Rho in the tissue sample. Our assays on retinal homogenates from light- and dark-adapted Octopus bimaculoides showed that RhoA is mostly activated in membranes obtained from animals in the dark, with peak activation at 30–45 minutes, while RhoA activation was reduced in samples obtained from animals in the light.
Rhabdom cross-sectional areas increase in size in the dark and diminish in the light (Torres et al. 1997). This increase can be explained by either the addition of new microvilli or increase in size of microvilli already present. Addition or growth require membrane assembly and assembly of the actin core contained within each microvillus. The question is what factors initiate rhabdom growth. Our results are consistent with our hypothesis that in the light, RhoA is sequestered by a GDI (Guanine Nucleotide Dissociation Inhibitor) present in the photoreceptor cytoplasm (supernatant fraction), preventing Rho from associating with the rhabdom membranes.
We postulate that in the light, Rho is sequestered in the cytoplasm bound to a GDI of which three are known in mammals (Fukumoto et al. 1990, Olofsson 1999, Dovas and Couchman 2005). We further postulate that rhodopsin inactivation in the dark leads to translocation of the GDI-Rho complex to the rhabdom membrane and release of Rho (Bokoch et al. 1994, Boukharov and Cohen 1998, Michaelson et al. 2001). GDIs form complexes with the Rac/Rho proteins, which are translocated when cells are activated (Seabra 1998, Kaibuchi et al. 1999). Moreover, GDIs are able to block GDP dissociation, inhibit protein phosphorylation, and increase the solubility of the Rac/Rho protein into the cytosol (Bokoch et al. 1994). Since we observed that Rho was mostly associated with the membrane fraction in the dark and that it is activated, we believe that Rho could then initiate a signaling pathway controlling acting filament assembly. Whether or not the signal to initiate the Rho signaling pathway comes from rhodopsin remains undetermined. More work is needed to better understand Rho signaling pathways governing cytoskeletal changes in the retina of Octopus bimaculoides and other species.
ACKNOWLEDGMENTS
The authors thank students in the NIH MBRS RISE program at California State University Dominguez Hills for inspiration and assistance with this work. Supported by NIH NIGMS/MBRS GM08156 and GM062252.
Footnotes
From the symposium “Molluscan models: Advancing our understanding of the eye” presented at the World Congress of Malacology, held from 15 to 20 July 2007 in Antwerp, Belgium. Co-sponsored by the National Science Foundation and the American Malacological Society.
LITERATURE CITED
- Ali MA. Retinomotor responses. In: Ali MA, editor. Vision in Fishes. New York: Plenum Press; 1975. pp. 313–355. [Google Scholar]
- Arber S, Barbayannis FA, Hanser H, Schneider C, Stanyon C, Bernard O, Caroni P. Regulation of actin dynamics through phosphorylation of cofilin by LIM-kinase. Nature. 1998;393:805–809. doi: 10.1038/31729. [DOI] [PubMed] [Google Scholar]
- Bokoch GM, Bohl BP, Chuang TH. Guanine nucleotide exchange regulates membrane translocation of Rac/Rho GTP-binding proteins. Journal of Biological Chemistry. 1994;269:31674–31679. [PubMed] [Google Scholar]
- Boukharov AA, Cohen CM. Guanine nucleotide-dependent translocation of RhoA from cytosol to high affinity membrane binding sites in human erythrocytes. Biochemistry Journal. 1998;330:1391–1398. doi: 10.1042/bj3301391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Velasco B, Martinez JM, Ochoa GH, Miller AM, Clark YM, Matsumoto B, Robles LJ. Identification and immunolocalization of actin cytoskeletal components in light- and dark-adapted octopus retinas. Experimental Eye Research. 1999;68:725–737. doi: 10.1006/exer.1999.0654. [DOI] [PubMed] [Google Scholar]
- Dovas A, Couchman JR. RhoGDI: Multiple functions in the regulation of Rho family GTPase activities. Biochemical Journal. 2005;390:1–9. doi: 10.1042/BJ20050104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto Y, Kaibuchi K, Hori Y, Fujioka H, Araki S, Ueda T, Kikuchi A, Takai Y. Molecular cloning and characterization of a novel type of regulator protein (GDI) for the rho proteins, ras p21-like small GTP-binding proteins. Oncogene. 1990;5:1321–1328. [PubMed] [Google Scholar]
- Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–513. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- Hall A. Rho GTPases and the control of cell behaviour. Biochemical Society Transactions. 2005;33:891–895. doi: 10.1042/BST20050891. [DOI] [PubMed] [Google Scholar]
- Hart MJ, Shinjo K, Hall A, Evans T, Cerione RA. Identification of the human platelet GTPase activating protein for the CDC42Hs protein. Journal of Biological Chemistry. 1991;266:20840–20848. [PubMed] [Google Scholar]
- Kaibuchi K, Kuroda S, Amano M. Regulation of the cytoskeleton and cell adhesion by the Rho family GTPases in mammalian cells. Annual Review of Biochemistry. 1999;68:459–486. doi: 10.1146/annurev.biochem.68.1.459. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;277:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maekawa M, Toshimasa I, Boku S, Watanabe N, Fujita A, Iwamatsu A, Obinata T, Ohashi K, Mizuno K, Narumiya S. Signaling from Rho to the actin cytoskeleton through protein kinases ROCK and LIM-kinase. Science. 1999;285:895–898. doi: 10.1126/science.285.5429.895. [DOI] [PubMed] [Google Scholar]
- Michaelson D, Silletti J, Murphy G, D’Eustachio P, Rush M, Philips MR. Differential localization of rho GTPases in live cells: Regulation by hypervariable regions and rhoGDI binding. Journal of Cellular Biology. 2001;152:111–126. doi: 10.1083/jcb.152.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AM, Ramirez T, Zuniga F, Ochoa G, Gray S, Kelly S, Matsumoto B, Robles L. Rho GTPases regulate rhabdom morphology in octopus photoreceptors. Visual Neuroscience. 2005;22:295–304. doi: 10.1017/S0952523805223052. [DOI] [PubMed] [Google Scholar]
- Narumiya S, Ishizaki I, Watanabe N. Rho effectors and reorganization of actin cytoskeleton. Federation of European Biochemical Societies. 1997;410:68–72. doi: 10.1016/s0014-5793(97)00317-7. [DOI] [PubMed] [Google Scholar]
- Ohashi K, Hosoya T, Takahashi K, Hing H, Mizuno K. A Drosophila homolog of LIM-kinase phosphorylates cofilin and induces actin cytoskeleton reorganization. Biochemical and Biophysical Research Communication. 2000;276:1178–1185. doi: 10.1006/bbrc.2000.3599. [DOI] [PubMed] [Google Scholar]
- Olofsson B. Rho guanine dissociation inhibitors: Pivotal molecules in cellular signaling. Cell Signaling. 1999;11:545–554. doi: 10.1016/s0898-6568(98)00063-1. [DOI] [PubMed] [Google Scholar]
- Raftopoulou M, Hall A. Cell migration: Rho GTPases lead the way. Developmental Biology. 2004;265:23–32. doi: 10.1016/j.ydbio.2003.06.003. [DOI] [PubMed] [Google Scholar]
- Ridley AJ. Rho GTPases and cell migration. Journal of Cell Science. 2001;114:2713–2720. doi: 10.1242/jcs.114.15.2713. [DOI] [PubMed] [Google Scholar]
- Ridley AJ. Rho GTPases and actin dynamics in membrane protrusions and vesicle trafficking. Trends in Cell Biology. 2006;16:533–529. doi: 10.1016/j.tcb.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Robles LJ, Cabebe CS, Aguilo JA, Anyakora PA, Bok D. Autoradiographic and biochemical analysis of photoreceptor membrane renewal in octopus retina. Journal of Neurocytology. 1984;13:145–164. doi: 10.1007/BF01148323. [DOI] [PubMed] [Google Scholar]
- Robles LJ, Camacho JL, Torres SC, Flores A, Fariss RN, Matsumoto B. Retinoid cycling proteins redistribute in light-/dark-adapted octopus retinas. Journal of Comparative Neurology. 1995;358:605–614. doi: 10.1002/cne.903580412. [DOI] [PubMed] [Google Scholar]
- Seabra MC. Membrane association and targeting of prenylated Ras-like GTPases. Cellular Signaling. 1997;10:167–172. doi: 10.1016/s0898-6568(97)00120-4. [DOI] [PubMed] [Google Scholar]
- Sumi TK, Matsumoto K, Takai Y, Namkamura T. Cofilin phosphorylation and actin cytoskeleton dynamics regulated by Rho- and Cdc42-activated LIM-kinase 2. Journal of Cell Biology. 1999;147:1519–1532. doi: 10.1083/jcb.147.7.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai Y, Sasaki T, Tanaka K, Nakanishi H. Rho as a regulator of the cytoskeleton. Trends in Biochemical Sciences. 1995;20:227–231. doi: 10.1016/s0968-0004(00)89022-2. [DOI] [PubMed] [Google Scholar]
- Takai Y, Takura S, Takashi M. Small GTP-binding proteins. Physiological Reviews. 2001;81:153–208. doi: 10.1152/physrev.2001.81.1.153. [DOI] [PubMed] [Google Scholar]
- Torres SC, Camacho JL, Matsumoto B, Kuramoto RT, Robles LJ. Light-/dark-induced changes in rhabdom structure in the retina of Octopus bimaculoides. Cell and Tissue Research. 1997;290:167–174. doi: 10.1007/s004410050918. [DOI] [PubMed] [Google Scholar]
- Weil D, Blanchard S, Kaplan J, Guilford P, Gibson F, Walsh J, Mburu P, Varela A, Levilliers J, Weston MD, Kelley PM, Kimberling WJ, Wagenaar M, Levi-Acobas F, Larget-Piet D, Munnich A, Steel KP, Brown SDM, Petit C. Defective myosin VIIA gene responsible for Usher syndrome type 1B. Nature. 1995;374:60–61. doi: 10.1038/374060a0. [DOI] [PubMed] [Google Scholar]
- Wheeler AP, Ridley AJ. Why three Rho proteins? RhoA, RhoB, RhoC and cell motility. Experimental Cell Research. 2004;301:43–49. doi: 10.1016/j.yexcr.2004.08.012. [DOI] [PubMed] [Google Scholar]